Abstract
Introduction
In September 2007, Northwestern University’s Feinberg School of Medicine received a $21.1 million dollar, five-year grant from the National Institutes of Health (NIH) to fund the Oncofertility Consortium (OFC). Over the course of the grant, those engaged with the psychological, legal, social, and ethical issues arising from oncofertility provided recommendations to the OFC. The inclusion of serious, real-time consideration of ethical issues as a funded focus of the grant, and the work senior scholars in law, bioethics and economics was a key part of the process of research. Now that this grant has ended, this commentary points to some of the issues that came forward during the five years of this project. Because of the emerging status of oncofertility, these issues are ones that need continued discussion and clarification, prompting our call for an oversight mechanism to provide guidance for how this technology should proceed.
Methods
An initial draft of this commentary arose from notes taken during a small colloquium held to discuss the oversight of oncofertility following the conclusion of the grant. This colloquium occurred in the fall of 2011. Using these notes as a starting point, the draft was then sent to other researchers who had been involved with the OFC in considering the psychological, legal, social, and ethical issues related to fertility preservation for cancer patients during the course of the grant. Finally, this commentary was further framed by the authors’ review of existing published and grey literature regarding issues concerning fertility preservation for cancer patients.
Results
We provide several points to consider and then offer two suggestions for an oversight mechanism for research as it continues.
Conclusions and Implications for persons who survivor cancer
The circumstances in which fertility preservation should be discussed and the patients for whom fertility preservation would be most suitable are important guideline issues for people who survive cancer and for their treatment team. Oversight of the field of oncofertility would strengthen the rights of cancer patients and help protect them from abuses as well as alert health care professionals to their correlative duties to these vulnerable patients and families.
Keywords: oncofertility, fertility preservation, guidelines, regulations
Introduction
In September 2007, Northwestern University’s Feinberg School of Medicine received a $21.1 million dollar, five-year grant from the National Institutes of Health (NIH) to fund the Oncofertility Consortium (OFC). In addition to researchers at Northwestern, the OFC originally comprised researchers from the University of California-San Diego, the University of Pennsylvania, the University of Missouri-Columbia, and the Oregon Health and Sciences University. The purpose of the grant and the OFC was to use basic science/bench research to improve understanding of ovarian function with an eye toward fertility preservation for cancer patients. More specifically, researchers wanted a deeper understanding of how ovaries produced eggs, how to preserve ovarian tissue, and how to preserve eggs at various stages of development. This essential work is tied to preserving the fertility of those diagnosed with cancer who are in their reproductive years - or who have not yet entered puberty - should their potential fertility be compromised or destroyed as a result of their cancer treatment. Since men have had the option of sperm cryopreservation for decades, of particular interest for the OFC was preserving the reproductive potential of women and girls. “We’re trying to create a total shift in how we interact with female cancer patients to anticipate their lives as survivors and their ability to bear children,” reproductive scientist Teresa Woodruff said at the time the grant was awarded [1]. Headed by Dr. Woodruff, the interdisciplinary OFC proposed to examine not just the scientific quandaries of how to preserve the fertility of those diagnosed with cancer, but also some of the psychological, legal, social, and ethical issues of doing so. A separate portion of the grant was directed to support the research of senior scholars in bioethics, law, economics and communication, and post-doctoral research was independently funded to support their scholarly inquiries.
Over the course of the five years of the grant, those engaged with the psychological, legal, social, and ethical issues arising from oncofertility provided ongoing, real time recommendations to the OFC and saw their questions, critiques and suggestions incorporated into the project itself. Now that this grant has ended, this commentary points to some of these issues.
Because of the emerging status of oncofertility, these issues need continued discussion and clarification, prompting our call for an oversight mechanism to provide guidance for how this technology should proceed. We note that this commentary’s scope, however, is limited to fertility preservation options and related services for cancer patients, per the original NIH grant. It is beyond the scope to address issues deeper and more challenging such as the disposition of the collected tissue, gametes, and embryos or the ethics of these issues. We recognize the broader implications of these concerns and have explicitly bracketed these issues in this commentary. After outlining the scope, applicability, and methods of this statement, we provide several points to consider and then offer two suggestions for what an oversight mechanism could look like and how it could be formed.
Scope, Applicability, and Methods
A draft of this commentary arose from the notes of the discussion during a small colloquium held in the fall of 2011. This was the last of three national meetings which brought together leading experts in bioethics, law, economic, religion, and policy to consider both immediate and long term implications of the research. Of those attending the final meeting, one person was the principal investigator for the ethics section and part of the OFC since its beginning, one was on the original ethics advisory board, two were paid post-doctoral researchers on the grant for two years, and one was new to the field of oncofertility, although not to the issue of reproductive ethics. During the colloquium, we used the “Points to Consider” of the Recombinant DNA Advisory Committee as a model for the discussion. [3] Everyone at the colloquium agreed that some sort of oversight mechanism would be beneficial to the research and application of oncofertility. Then, the draft was sent to other researchers who had been involved with the OFC in considering the psychological, legal, social, and ethical issues of fertility preservation for cancer patients during the course of the grant. Finally, the authors reviewed existing published literature from their respective fields regarding issues concerning fertility preservation for cancer patients to establish these points for oversight consideration.
Points to Consider for Oncofertility (Table 1)
Table 1.
Points to Consider for Oncofertility Oversight
| An oncofertility body should… | |
|---|---|
| Privacy and confidentiality | …consider measures to protect the privacy and confidentiality of cancer patients undergoing fertility preservation and their families. |
| Consent/assent | …ensure that appropriate measures are being taken to obtain consent or assent of patients, especially minors. |
| Safety and efficacy | …consider how to address the experimental status of fertility preservation technologies with unknown efficacy, low viability, and safety risks. |
| Creation of a registry | …maintain a centralized registry of those who preserve their tissue, gametes, or embryos either before or as part of their therapy |
| Conflict of interest | …be aware of possible conflicts of interest between the needs of patients and the financial and other incentives of the researchers and/or clinicians. |
| Age restrictions | …consider minimum and maximum ages at which patients may be allowed to preserve their fertility. |
| Future use of stored tissue, gametes, and embryos | …address limiting the number of children who may be conceived using frozen tissue, gametes, or embryos |
| Emerging areas | …provide thoughtful recommendations and oversight regarding the points outlined above and others that arise in the coming years. |
Privacy and Confidentiality
Matters of privacy and confidentiality are inherent in reproductive activities because of their highly personal nature. An oncofertility oversight body should carefully consider measures to protect the privacy and confidentiality of cancer patients undergoing fertility preservation, as well as those of their families and, possibly, of the progeny who will be generated from stored materials.
Consent/Assent
An oncofertility oversight body should ensure that appropriate measures are being taken to obtain consent or assent of patients, in particular those patients who are minors and who are a particularly vulnerable population [4–6]. In addition, an oncofertility body should consider the psychologically refractory state many patients or family members have after learning they have or their child has cancer. As such, informed consent meeting traditional Belmont criteria may not be psychologically possible during this emotionally charged time [7–9]. Moreover, since many of the options presently available are considered experimental and not therapeutic, oversight guidelines should include safeguards against the conflation of research and therapy, and guidelines for obtaining informed consent when there is no decisive evidence to establish the likely outcomes of a particular method of fertility preservation, or the impact of a fertility- preservation-based delay on cancer treatment [10–14]. Consent may be further hampered by the fact that it is not possible to give accurate estimates on the probability of infertility resulting from cancer treatment for individual patients [11]. That is, the success rates for even established technologies are not based on treatments in patients who were infertile due to cancer or its treatments.
Factors for consideration in the consent include: the financial costs for patients and/or their families; the need for long-term medical and psychological follow-up as part of the survivorship process; and acknowledgment of the inability to provide survival estimates to those using fertility preservation and the possibility the patient will not survive long enough to use stored tissues, gametes, and/or embryos. In addition, an oncofertility oversight body should consider whether such disclosures are sufficient, and to anticipate scenarios arising when a potential conflict of interest is so significant that the clinician or researcher must not simply just disclose that conflict, but have limitations to their involvement.
Experimental Status - Safety and Efficacy
Though ovarian transposition, radical trachelectomy (removal of the cervix), and sperm and embryo freezing are considered established technologies, other forms of fertility preservation - particularly those available for pre-pubertal boys and girls, or those available for women without an acceptable reproductive partner or for whom ovarian transposition/radical trachelectomy are not viable options - are presently considered experimental [12,15].i Because of the largely liquid composition of the egg, human ova remain difficult to thaw while retaining viability [16]. Experimental options include cryopreservation of testicular tissue in boys or ovarian tissue in girls or women, with the hope that one day such tissues might yield viable gametes and could be used to create a child through assisted reproductive technologies (ART) [12,17]. An oncofertility body should consider how to address the experimental status of these fertility preservation technologies, with their currently unknown efficacy, low viability, and safety risks, including risks that may occur years later, to survivors or their offspring. In addition, an oncofertility body should consider how to address those technologies that are considered viable clinically, such as embryo freezing, but that have not been studied for safety in women with cancer [11].
Conflict of Interest
As in other areas of science and medicine, attention should be paid to all possible conflicts of interest between the needs of patients and the personal or institutional financial and other incentives of the researchers and/or clinicians. Funding sources for researchers and clinicians, per NIH conflict of interest regulations, ought to be fully disclosed to those seeking to use fertility preservation options.
Age Restrictions
By necessity, certain aspects of medical care should be left to clinical assessment. However, considerations for oversight are minimum and maximum ages at which patients may be allowed to preserve their fertility. Maximum age restrictions are not new; five years ago, the OFC issued a consensus statement setting an age maximum for ovarian tissue cryopreservation [21]. Because age adversely affects not just potential offspring but also the health of the pregnant woman, oversight should be considered in this aspect of oncofertility, with the understanding that social and medical understandings of maximum age may change and need reconsideration. Of additional consideration is a minimum age for fertility preservation, again with the understanding that social and medical understandings of a minimum age may change and need reconsideration.
Future Use of Stored Tissue, Gametes, and Embryos
An individual female or male may store more tissue, eggs, or embryos than they choose to use. An oversight oncofertility body could address limiting the number of children who may be conceived using frozen tissue, gametes, or embryos. Such a limit could be modeled after the policy in the United Kingdom, where the gametes of sperm and egg donors are allowed to produce a maximum of ten children per donor [22]. Additionally, guidelines should be established about the unused materials, should the woman or man die prior to using them without leaving a legal indication of how they may be used [23]. Guidelines should further consider whether stored tissues, gametes, and embryos be labeled as coming from a person who has had cancer, in the event that the person who stored them does not want or need them all of and wishes to donate them to science. This then could give rise to the question of whether she or he may be allowed to donate these materials to another person for reproductive intent without such disclosure. Further, considering that fertility preservation may be attempted for a very young child, guidelines should potentially include whether there is a limit to the number of years which frozen embryos, gametes, and tissues must be stored by a facility [24].
Creation of a Registry
Of additional consideration for an oncofertility body is the maintenance of a centralized registry of those who preserve their tissue, gametes, or embryos either before or as part of their cancer therapy (or therapies for other non-cancer conditions which may result in fertility loss), as well as a registry of their stored materials. Such a registry would be beneficial for several reasons. First, because those with possibly compromised health conditions will access fertility preservation, and since, at present, the safety of fertility preservation for women with cancer is unknown, monitoring will potentially provide relevant information. For example, the short- and long-term implications of fertility preservation could be monitored on a macro scale through a registry so that any adverse outcomes may be recognized, attributed, taken into account, and, to whatever degree possible, corrected, in individual patients. Second, if tissue, gametes, and embryos were on a registry as separate entities, each entity could be tracked to monitor health and viability, again, potentially allowing for intervention on an individual patient level (be they the donor or the progeny from the stored tissue, gametes, or embryos) if a problem is seen on the macro level. Further, such a registry could provide evidence-based guidance for physicians to offer counsel and recommendations, based on prior cases and outcomes. A handful of studies have begun tracking this information, and in addition, such a registry could be joined with, for example, the existing Pregnancy and Cancer Registry at Cooper University Hospital [18–20].
Two Options for an Oversight Mechanism
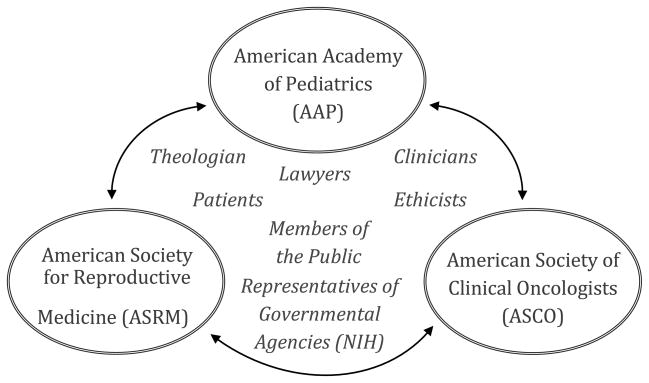
We recommend the creation of a mechanism dedicated to continue discussions, such as those outlined above, regarding oncofertility and to provide additional oversight beyond what currently exists. While other entities have called for greater regulation of the research and clinical practices surrounding all fertility services, such a regulatory frame does not currently exist in the United States. One idea for what we here refer to as an “oncofertility body” would be a regulatory structure parallel to the United Kingdom’s Human Fertilization and Embryology Authority, which oversees all fertility services in the UK. A different model would constitute a working group from representatives of the American Society of Clinical Oncologists (ASCO), the American Society for Reproductive Medicine (ASRM), and the American Academy of Pediatrics (AAP), all of which currently have individual practice guidelines regarding oncofertility. An oncofertility body formed using this latter mechanism could also include patients, members of the public, ethicists, lawyers, theologians, clinicians, and representatives of governmental agencies such as the NIH (see Figure 1).
Figure 1.
Oversight Mechanism
Creation of an Oversight Mechanism
Given that we see it as more likely that an oversight mechanism would take the latter form of the two we have suggested, such an oncofertility body would need the buy-in from the AAP, ASCO, and ASRM, as well as a governmental agency such as the NIH. This might be similar in intent and purpose the existing Recombinant DNA Advisory Committee (The RAC). An oncofertility body would also need the approval of cancer patient support and activism groups, such as the I’m Too Young for This! Foundation. To create such a body, the AAP, ASCO, and ASRM could each designate a person (staff or board member) to be their member representative. Collectively, these bodies could then put a call out to the NIH and perhaps three affected patient population groups, such as the I’m Too Young for This! Foundation, to solicit representatives from these groups. A call could as well go out to legal, psychological, social, ethical, and theological scholars who have worked in the area of fertility preservation among cancer patients. Representatives from each of these areas, as well as from the public and from clinicians, could form the oncofertility body. Such a body could be supported by, in terms of providing the help of a staff member and hosting the meetings, the AAP, ASRM, and ASCO. For example, possibly a part of a staff person’s time at one of these organizations could be dedicated to supporting the oncofertility body, with the other two organizations then alternatively hosting the once a year meetings.
Conclusion
Though clinical practice guidelines have recently been issued from ASCO, AAP, and ASRM, these bodies focused more on the circumstances in which fertility preservation should be discussed, and the patients for whom fertility preservation would be most suitable [12,15,25]. Discussion of such information - how it is discussed and with whom it should be discussed - is an important component of the future of fertility preservation, and much work has been done to improve these discussions to develop best-practice guidelines for clinicians [26,27].
This statement, however, calls not just for guidelines for the clinical practice of oncofertility, but oversight of the scope of this emerging technology because of its profound implications. We recognize that many long to a have a child and form a family, and that for some cancer interrupts this path. We encourage all options - including adoption, fostering children, and other family-by-choice options such as avuncular relationships - to be part of the scope of family formation. However, we also recognize that the restoration of the bodily capacity to have children biologically after cancer therapy has negatively affected it, is one of the important goals of oncofertility, and that the subject is deserving of attention from our larger society just as other devastating side effects of cancer and its treatments have become more recognized. With the conclusion of this grant, we call for the creation of a permanent oversight mechanism to thoughtfully and earnestly consider how to guide oncofertility to allow this emerging technology to be carefully considered as it develops.
Acknowledgments
Research for this paper was supported by Award Number RL1HD058296 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. The authors also want to thank Linda Emanuel, Farr Curlin, Paul Lauritzen, and Karrie Snyder for their comments, as well as the editorial assistance of Kelly Werner, Maria Battaglia, and Ivana Sehovic.
Footnotes
As of October 22, 2012, the American Society for Reproductive Medicine (ASRM) no longer considers egg freezing experimental.
Contributor Information
Sarah B. Rodriguez, Email: srodriguez@northwestern.edu, Research Assistant Professor, Medical Humanities + Bioethics Program, Northwestern University, Feinberg School of Medicine, 312-503-2887
Lisa Campo-Engelstein, Email: campoel@mail.amc.edu, Assistant Professor, Alden March Bioethics Institute & Department of OBGYN, Albany Medical College, 47 New Scotland Avenue, MC 153, Albany, New York 12208, P: 518.262.0239, F: 518.262.6856
Marla L. Clayman, Email: m-clayman@northwestern.edu, Assistant Professor, Division of General Internal Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, 251 East Huron Street, Galter Suite 3-150, Chicago, IL 60611, (312) 926-6895.
Caprice Knapp, Email: caprice1@ufl.edu, Associate Professor, Department of Health Outcomes and Policy, College of Medicine, University of Florida, 1329 SW 16th Street, Room 5130, Gainesville, Florida 32610, (352) 265-2517, (352) 265-7299 (Fax).
Gwendolyn Quinn, Email: gwendolyn.quinn@moffitt.org, Moffitt Cancer Center, tel: 813.745.1359|fax: 813.449.8019
Laurie Zoloth, Email: lzoloth@northwestern.edu, McCormick Professor 2009, Professor of Medical Humanities and Bioethics, Professor of Religious Studies, Director, The Brady Program in Ethics and Public Life, Crowe Hall 4140, Weinberg College of Arts and Sciences, Feinberg College of Medicine, Northwestern University, Evanston, Illinois, 847-644 8807 cell, 847-491-2615 office
Linda Emanuel, Email: l-emanuel@northwestern.edu, Director of the Buehler Center on Aging, 750 North Lake Shore Drive Suite 601, Chicago, IL 60611, 312-503-3087, 312-503-5868 (fax).
References
- 1.Paul M. Feinberg awarded grant for research in cancer patients’ fertility. Northwestern University; 2007. [Accessed 15 Oct 2011]. http://www.northwestern.edu/newscenter/stories/2007/09/oncofertility.html. [Google Scholar]
- 2.Cahn NR. Test tube families: Why the fertility market needs legal regulation. New York: New York University Press; 2009. [Google Scholar]
- 3.Regulatory issues: the revised “points to consider” document. Hum Gene Ther. 1990;1:93–103. doi: 10.1089/hum.1990.1.1-93. [DOI] [PubMed] [Google Scholar]
- 4.Patrizio P, Caplan A. Ethical issues surrounding fertility preservation in cancer patients. Clin Obstet Gynecol. 2010;53:717–26. doi: 10.1097/GRF.0b013e3181f96a70. [DOI] [PubMed] [Google Scholar]
- 5.Nisker J, Baylis F, McLeod C. Choice in fertility preservation in girls and adolescent women with cancer. Cancer. 2006;107:1686–9. doi: 10.1002/cncr.22106. [DOI] [PubMed] [Google Scholar]
- 6.Quinn G, Stearsman D, Campo-Engelstein L, Murphy D. Preserving the right to future children. Am J Bioeth. 2012 doi: 10.1080/15265161.2012.673688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardino SL, Emanuel LL. Choosing life when facing death: understanding fertility preservation decision-making for cancer patients. In: Woodruff TK, Zoloth L, Campo-Engelstein L, Rodriguez S, editors. Oncofertility: ethical, legal, social, and medical perspectives. New York: Springer; 2010. pp. 447–58. [Google Scholar]
- 8.Jeruss JS. Discussing fertility preservation with breast cancer patients. In: Woodruff TK, Zoloth L, Campo-Engelstein L, Rodriguez S, editors. Oncofertility: ethical, legal, social, and medical perspectives. New York: Springer; 2010. pp. 461–6. [Google Scholar]
- 9.Gavin KM, Clayman ML. Whose future is it? Ethical family decision making about Daughters’ treatment in the oncofertility context. In: Woodruff TK, Zoloth L, Campo- Engelstein L, Rodriguez S, editors. Oncofertility: ethical, legal, social, and medical perspectives. New York: Springer; 2010. pp. 429–45. [Google Scholar]
- 10.Cohn F. Oncofertility and Informed Consent. In: Woodruff TK, Zoloth L, Campo- Engelstein L, Rodriguez S, editors. Oncofertility: ethical, legal, social, and medical perspectives. New York: Springer; 2010. pp. 249–58. [PubMed] [Google Scholar]
- 11.Jungheim ES, Carson KR, Brown D. Counseling and consenting women with cancer on their oncofertility options: a clinical perspective. In: Woodruff TK, Zoloth L, Campo- Engelstein L, Rodriguez S, editors. Oncofertility: ethical, legal, social, and medical perspectives. New York: Springer; 2010. pp. 403–12. [Google Scholar]
- 12.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 13.Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 14.Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2001;97:1260–8. doi: 10.1016/j.fertnstert.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–28. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Because of changes in freezing technique, some practitioners no longer consider it as experimental Leslie M. Melting Opposition to Frozen Eggs. Science. 2007;316:388–9. doi: 10.1126/science.316.5823.388.
- 17.ACOG Committee Opinion. Ovarian tissue and oocyte cryopreservation. Obstet Gynecol. 2008;111:1255–6. doi: 10.1097/AOG.0b013e31817578d1. [DOI] [PubMed] [Google Scholar]
- 18.Reproductive Health Research Studies. UC San Diego: Moore Cancer Center; [Accessed 22 May 2012]. http://cancer.ucsd.edu/coping/fertility/Pages/studies.aspx. [Google Scholar]
- 19.FIRST Registry. [Accessed 22 May 2012];The Oncofertility Consortium at Northwestern University. http://oncofertility.northwestern.edu/first-registry.
- 20.For pregnant women who find out they have cancer. Cooper University Hospital; [Accessed 1 Mar 2012]. www.cancerandpregnancy.com. [Google Scholar]
- 21.Backhus LE, Kondapalli LA, Chang RJ, Coutifaris C, Kazer R, Woodruff TK. Oncofertility consortium consensus statement: guidelines for ovarian tissue cryopreservation. In: Woodruff TK, Snyder KA, editors. Oncofertility. New York: Springer; 2007. pp. 235–9. [DOI] [PubMed] [Google Scholar]
- 22.Report of the Committee of Inquiry into Human Fertilisation and Embyology. London: 1984. [Accessed 21 Nov 2011]. http://www.hfea.gov.uk/docs/Warnock_Report_of_the_Committee_of_Inquiry_into_Human_Fertilisation_and_Embryology_1984.pdf. [Google Scholar]
- 23.Dolin G, Roberts DE, Rodriguez LM, Woodruff TK. Medical hope, legal pitfalls: potential legal issues in the emerging field of oncofertility. Santa Clara Law Rev. 2009;49:673–716. doi: 10.1007/978-1-4419-6518-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Report of the Committee of Inquiry into Human Fertilisation and Embyology [22], for example, recommends the limit for frozen embryos be a decade - potentially too short a time for people who are quite young when they freeze tissue, gametes, or embryos.
- 25.Fallat ME, Hutter J American Academy of Pediatrics Committee on Bioethics, Section on Hematology/Oncology, Section on Surgery. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121:e1461–9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 26.Snyder KA, Pearse W. Discussing fertility preservation options with patients with cancer. JAMA. 2011;306:202–3. doi: 10.1001/jama.2011.973. [DOI] [PubMed] [Google Scholar]
- 27.Quinn GP, Vadaparampil ST, Lee J, Jacobsen PB, Bepler G, Lancaster J, Keefe DL, Albrecht TL. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 28.ASRM Lifts Experimental Label from Technique. American Society for Reproductive Medicine; 2012. [Accessed 29 October 2012]. Fertility Experts Issue New Report on Egg Freezing. http://www.asrm.org/news/article.aspx?id=10324&terms=(+%40Publish_To+Both+Sites+or+%40Publish_To+ASRM+Only+)+and+Mature+Oocyte+Cyryopreservation. [Google Scholar]