Abstract
The dynamics of most healthy physiological processes are complex, in that they are comprised of fluctuations with information-rich structure correlated over multiple temporospatial scales. Lipsitz and Goldberger (1992) first proposed that the aging process may be characterized by a progressive loss of physiologic complexity. We contend that this loss of complexity results in functional decline of the organism by diminishing the range of available, adaptive responses to the innumerable stressors of everyday life. From this relationship, it follows that rehabilitative interventions may be optimized by targeting the complex dynamics of human physiology, and by quantifying their effects using tools derived from complex systems theory. Here, we first discuss several caveats that one must consider when examining the functional and rehabilitative implications of physiologic complexity. We then review available evidence regarding the relationship between physiologic complexity and system functionality, as well as the potential for interventions to restore the complex dynamics that characterize healthy physiological function.
Keywords: Complexity, Postural Control, Cardiovascular, Multiscale Entropy, Nonlinear
1. INTRODUCTION
A hallmark of healthy physiologic function is the capacity to detect, respond and adapt to the innumerable perturbations and stressors of daily life. This capacity is achieved via complex interactions between multiple control systems, feedback loops, and regulatory processes that operate over multiple scales of time and space (Lipsitz and Goldberger, 1992), and interact with one another in nonlinear fashion (Goldberger et al., 2002a). As a result of this rich organization, the seemingly irregular dynamics of most physiological outputs are “complex;” i.e., they contain “meaningful structural richness (Grassberger, 1991)” marked by a degree of non-random fluctuations over multiple temporospatial scales (Costa et al., 2002; Goldberger et al., 2002a). In recent years, the study of physiologic complexity, using the theory and quantitative tools derived from complex systems biology, has shown great promise for improving our understanding of aging, monitoring senescence, and evaluating novel interventions that treat age-related disease and promote healthy aging.
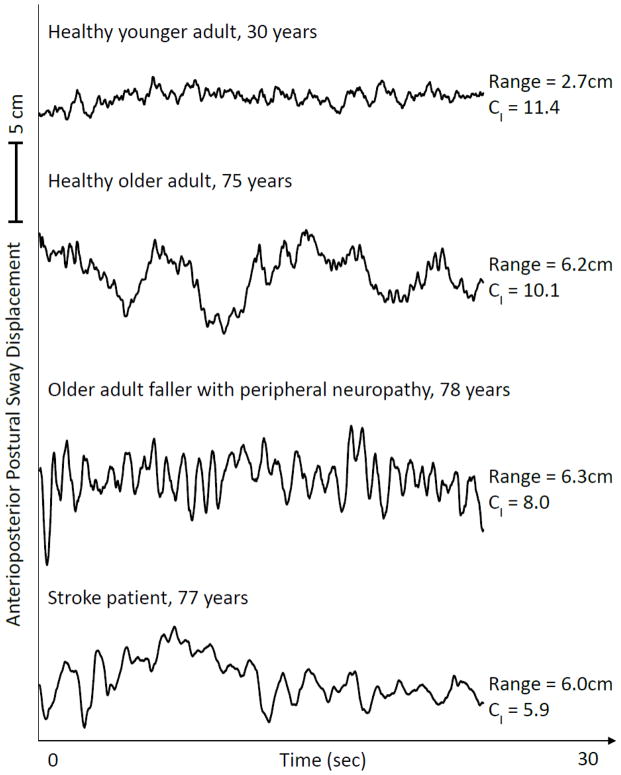
The conventional view of aging is that it is a linear process of physical and cognitive decline that occurs over time as one progresses from adulthood into senescence. Lipsitz and Goldberger (1992) first proposed that the aging process can be defined by a progressive loss of complexity within the dynamics of physiologic outputs. Although important exceptions have been reported and are described elsewhere (Duarte and Sternad, 2008; Hartman et al., 1994; Vaillancourt and Newell, 2002; Vaillancourt and Newell, 2003), numerous studies have since demonstrated that biological aging and numerous age-related diseases and syndromes are characterized by a loss of physiologic complexity in the dynamics of the cardiovascular (Beckers et al., 2006; Costa et al., 2008; Iyengar et al., 1996; Kaplan et al., 1991; Pikkujamsa et al., 1999), respiratory (Peng et al., 2002), central nervous (Yang et al., 2012) and motor control (Costa et al., 2007; Manor et al., 2010; Thurner et al., 2002) systems, among others. Importantly, this loss of information content is often independent of age- and/or disease-related changes in signal variability (Manor et al., 2010). Figure 1, as an example, illustrates the dynamics of anterioposterior (i.e., fore-aft) postural sway recorded as four individuals stood with their eyes open on a stationary force platform. Compared to the healthy young adult, the fluctuations in postural sway were less complex in each older adult and in particular those suffering from peripheral or central nervous system impairment.
Figure 1. Representative anterioposterior postural sway time-series during standing with eyes open.
Age- and disease-related changes to the neuromuscular system are often associated with unique alterations to the dynamics of postural sway. Presented here are unfiltered postural sway (i.e., center-of-pressure) dynamics of four individuals differing in age and/or disease status. Multiscale entropy analysis (of high-pass filtered data) was used to derive the complexity index (CI), for which higher values reflect a greater degree of irregularity across multiple scales of time (i.e., greater complexity). The CI was highest in the healthy young adult and lowest in the older adult with chronic brain damage due to a history of a hemispheric middle cerebral artery infarction (i.e., stroke). It is also of note that CI was independent of the traditional measure of maximum sway range.
An age-related loss of physiologic complexity is believed to stem from gradual deterioration of underlying structural components of physiological systems, as well as alterations within the nonlinear coupling between these systems (Lipsitz, 2002; Lipsitz, 2004). We therefore contend that 1) relatively low physiologic complexity in the dynamics of a system under basal conditions (i.e., resting or free-running) underlies the diminished capacity of that system to respond and adapt to stressors, and 2) preventative and/or rehabilitative interventions may be optimized by targeting the physiologic complexity that often characterizes healthy system function.
In this paper we aim to provide empirical evidence regarding the relationship between measured physiologic complexity and system functionality, as specifically defined by the capacity to adapt to physiologic stresses or perturbations. We then examine the potential for and functional implications of interventions designed to restore the loss of physiologic complexity with advancing age. First, however, we discuss several important caveats regarding measurement and task constraints that one must consider when interpreting this research.
2. PHYSIOLOGIC COMPLEXITY: MEASUREMENT ISSUES AND TASK CONSTRAINTS
When examining the relationship between the complexity of a system’s dynamics and the functionality of that system, one must consider 1) the metric(s) used to quantify complexity, 2) the sampling frequency and window of observation, 3) the impact of task constraints, and 4) the type of stimulus, stressor or perturbation being examined.
First, there are numerous metrics available that each quantify different aspects of the complex, nonlinear properties of physiologic time-series, including entropy (Pincus, 1991) and multiscale entropy analyses (MSE) (Costa et al., 2002), detrended fluctuation analysis (Peng et al., 1995), fractal dimension (Higuchi, 1988) and recurrence plot analysis (Webber, Jr. and Zbilut, 1994), among others. It is of note that traditional entropy-based metrics estimate the degree of regularity or orderliness of a time-series on a single scale of time. As discussed in depth by Costa et al (2002), these metrics do not capture the structural characteristics of a signal over multiple scales of time, and thus, may fail to characterize physiologic complexity. To overcome this issue, new metrics have been developed such as MSE, which utilizes a technique called “coarse-graining” to enable estimation of a signal’s regularity over multiple time scales (Figure 2). Still, Goldberger et al (2002b) has argued that no single statistical measure can fully capture the complexity of a physiological system. Insensitivity of a particular metric to the effects of group, experimental condition or intervention does not necessarily imply that other metrics will also lack meaningful relationships to the functionality or rehabilitative potential of the system in question.
Figure 2. Schematic illustration of the coarse-graining procedure utilized in multiscale entropy analysis (Adapted from Costa et al., 2002).
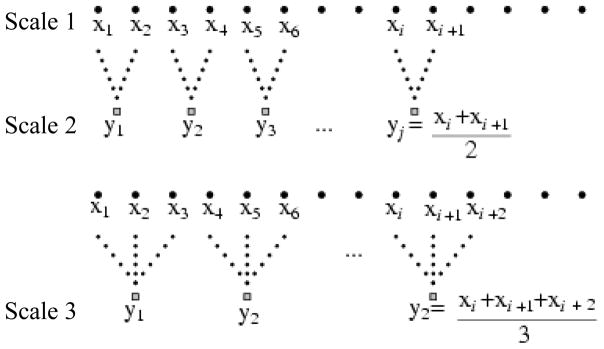
Consecutive time-series are constructed from the original time-series (scale 1) by averaging successively increasing number of data points in non-overlapping windows. Here, coarse-grained time-series capturing time scale two and three are shown. Entropy of each coarse-grained series is then calculated to estimate the degree of irregularity over multiple scales of time. See Figure 3 for an example of multiscale entropy analysis of physiological data.
Second, estimation of a signal’s complexity is dependent upon both the sampling frequency and window of observation. The contribution of high-frequency fluctuations may be omitted if the sampling frequency is not sufficiently high, whereas the contribution of low-frequency fluctuations may be overlooked if the measurement window is not sufficiently long. An example of the latter can be seen in the regulation of heart rate. Endogenous circadian rhythms influence heart rate on time scales of approximately 24 hours. When measured over days or weeks, these low-frequency fluctuations contribute to the physiologic complexity of heart rate dynamics (Hu et al., 2008). On the other hand, if heart rate is observed over an observation window of several hours, circadian influences will cause a “drift” or “nonstationarity” in heart rate; i.e., the statistical distribution of the signal will change over time. Such nonstationarities—whether stemming from important physiological processes, measurement error or noise—significantly affect complexity metrics and should be detrended (Peng et al., 2009). In addition to clouding inter-study comparisons, therefore, these issues must be considered when drawing conclusions regarding the functional implications of complexity as estimated from finite physiological time-series.
Third, the constraints within which a system operates may influence the functional implications of physiologic complexity. For example, in several studies examining the dynamics of force output of the finger (Sosnoff and Newell, 2008; Vaillancourt and Newell, 2002; Vaillancourt and Newell, 2003), subjects were asked to match either constant or time-varying target forces with their index finger by pressing on a load cell, and were provided with real-time continuous visual feedback from a computer screen. Younger adults performed each task with less error than older adults. Yet, compared to older adults, the force dynamics produced by younger adults were also more complex in the constant force condition, and less complex in the time-varying force condition, as quantified by approximate entropy analysis. In the constant force condition, this relatively high complexity likely stemmed from the numerous regulatory processes interacting over multiple time scales to effectively dampen output fluctuations about the fixed target value. At the same time, this high-functioning control system may have also enabled younger adults to more reliably produce oscillatory forces during the time-varying force condition, resulting in force dynamics with lower complexity. Thus, it is important to consider whether a system is operating under resting or free-running conditions, or responding to a given stimulus.
Fourth, the innumerable stressors and perturbations that a system may experience can be either acute or chronic in nature, and may also change system constraints. Quantification of the physiological response to an acute stressor may be relatively straight-forward; for instance, measuring the magnitude of blood pressure drop during the head-up tilt test or the increase in heart rate during an exercise test. Quantifying the response to a chronic perturbation, on the other hand, may be clouded by time-dependent physiological adaptation to that stimulus. When standing, for example, closing one’s eyes creates a continuous visual perturbation that results initially in increased size and speed of postural sway. However, this increase tends to diminish over time as standing posture is sustained, a phenomenon attributed to dynamic “re-weighting” of available sensory feedback (Hafstrom et al., 2002). It is also important to remember that in some cases, introducing a stimulus may alter the task constraints within which the system must operate. In the aforementioned studies on force production, for example, (Sosnoff and Newell, 2008; Vaillancourt and Newell, 2002; Vaillancourt and Newell, 2003), a stimulus was introduced by adding acute changes to target force output values. And, as previously discussed, the functional implications of complexity may be different between these two conditions.
3. RELATIONSHIP BETWEEN SYSTEM COMPLEXITY AND FUNCTIONALITY
Much of the available evidence regarding the link between the complexity of a system’s output over time and its capacity to adapt to stressors stem from indirect, cross-sectional studies relating physiologic complexity to conditions, diseases, or outcomes commonly associated with impaired adaptive capacity. Only a handful of studies offer direct, intra-subject comparisons of physiologic complexity and the capacity to adapt to an experimentally-induced perturbation. Although examples from other physiological systems exist, here we examine available evidence from the cardiovascular, central nervous and motor control systems.
3.1. Cardiovascular System
Heart rate is influenced by numerous interacting factors operating across a variety of time scales, including the parasympathetic and sympathetic branches of the autonomic nervous system, hormonal and temperature variation, bouts of physical activity, digestion and circadian rhythms. As such, healthy variations in heart rate are exceedingly complex (Kaplan et al., 1991). Biological aging from adulthood into senescence alters this multi-scale organization, resulting in a loss of complexity in cardiovascular signals (Beckers et al., 2006; Iyengar et al., 1996; Lipsitz, 1995; Pikkujamsa et al., 1999).
A large and growing body of literature has linked the degree of physiologic complexity contained within heart rate time-series to cardiac disease and mortality in older adults. Specifically, the age-related loss of complexity in heart rate variability is accelerated in those with heart murmur (Gomez-Garcia et al., 2011), atrial fibrillation (Costa et al., 2002; Vikman et al., 1999), and heart failure (Angelini et al., 2007; Costa et al., 2002; Ho et al., 1997; Ho et al., 2011; Norris et al., 2008). Relatively low heart rate complexity is also predictive of suffering post-surgical complications (Makikallio et al., 1997; Makikallio et al., 1999). In a recent study, Ho et al (2011) analyzed the variability and complexity of 24 hour heart rate time-series in 40 older adults suffering from heart failure. Whereas traditional, linear metrics of heart rate variability did not predict survival, those patients with greater heart rate complexity, as estimated by MSE analysis, were more likely to survive the follow-up period.
3.2. Central Nervous System
The healthy human brain comprises remarkable complexity in both its structural architecture and functional communication networks (Bullmore and Sporns, 2009). As measured by electroencephalography, magnetoencephalography (MEG) and most recently blood-oxygen level dependent (BOLD) MRI, the degree of long-range correlation and multiscale organization in brain activity appears to decrease with advancing age (Raja Beharelle et al., 2012; Sun et al., 2012; Takahashi et al., 2009; Yang et al., 2012). The relationship between functional complexity and mental disorders, however, has been debated and is seemingly dependent upon the type of disorder in question, the metric utilized to estimate complexity and the potential interactive influences of “normal” biological aging (for a recent review, see Takahashi, 2012).
Several recent papers indicate that the degree of complexity contained within the temporal fluctuations of brain activity may relate to cognitive function. Raja Beharelle and colleagues (2012) completed MSE analysis of MEG signals recorded as traumatic brain injury patients and healthy controls completed a visual feature-matching task. As compared to controls, the patient group exhibited lower complexity within multiple brain regions. Lower signal complexity was in turn correlated with greater trial-to-trial variability in cognitive task performance. Yang et al (2012) studied healthy younger and older adults and examined the link between MSE-derived complexity of spontaneous, resting-state BOLD activity and performance in a battery of cognitive tests in. An age-related loss of complexity was present in the temporal fluctuations of BOLD signals from multiple brain regions. Within older adults, the degree of complexity (and not traditional measures of variability) estimated from numerous brain regions within the default mode network was positively correlated with numerous cognitive functions, including attention, orientation, memory and verbal fluency.
3.3. Motor Control System
3.3.1. Postural Control
The postural control system is comprised of somatosensory, visual and vestibular sensory feedback networks, numerous brain regions and the musculo-skeletal system (Horak and MacPherson JM, 1996; Maki and McIlroy, 1996). This system regulates the body’s postural sway with respect to its base of support, thereby enabling both upright stance and the capacity to adapt to stressors in unpredictably changing environments. Postural sway is most commonly assessed by recording center-of-pressure fluctuations as an individual stands on a force plate (Winter et al., 1990). Similar to other physiological signals, postural sway dynamics during quiet, upright standing are complex; i.e., they contain a degree of correlated fluctuations over multiple time scales (Lipsitz, 2004; Riley and Clark, 2003; Sabatini, 2000).
Although the longitudinal effects of aging are unclear (Duarte and Sternad, 2008; Seigle et al., 2009; Thurner et al., 2002), cross-sectional studies have indicated that several age-related conditions linked to poor balance are also marked by reduced postural sway complexity. Costa et al (2007) demonstrated that older adults with a history of falling had diminished postural sway complexity (as quantified by MSE) when standing quietly with eyes-open as compared to older adult non-fallers and healthy younger adults. Using the same complexity metric in a large sample of older adults, lower postural sway complexity has also been independently associated with frailty, a complex syndrome characterized by weakness, unintentional weight loss, slow gait, exhaustion and low daily activity (Kang et al., 2009).
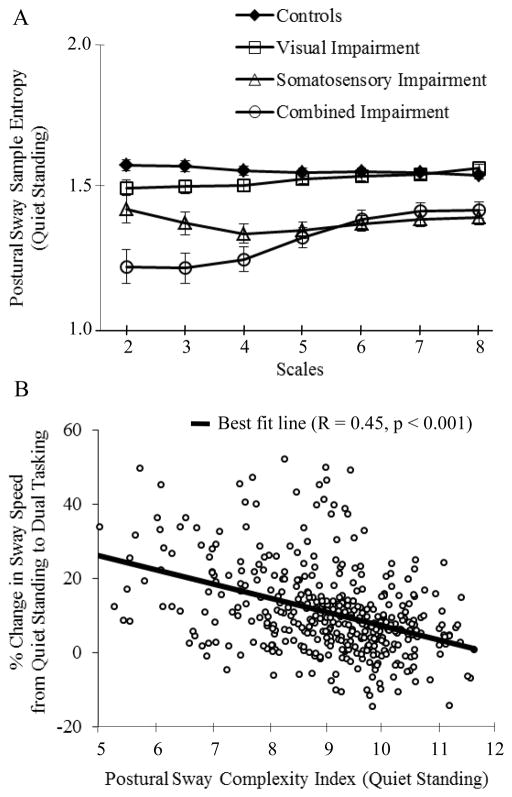
Recently, we studied the direct link between postural sway complexity and the adaptive capacity of the postural control system in older adults (Manor et al., 2010). Specifically, we examined the effects of visual and lower-extremity somatosensory impairment on postural sway complexity during quiet standing, and its relationship to postural adaptation to a serial-subtraction cognitive dual task. Older adults participating in the MOBILIZE Boston study (Leveille et al., 2008) were classified into four mutually-exclusive groups: normal controls, visual impairment only (< 20/40 vision), somatosensory impairment only (inability to perceive the 5.07 monofilament on the plantar hallux), or combined impairments. A complexity index was quantified using MSE analysis. The traditional metrics of postural sway speed and magnitude, which did not correlate with complexity, were also computed. During quiet standing, the complexity index was highest in controls and successively lower in the visual, somatosensory, and combined impairment groups (Figure 3A). The stress of performing serial subtractions while standing (i.e., dual tasking) resulted in decreased complexity, but increased speed and magnitude of postural sway. Importantly, with all participants combined, lower complexity during quiet standing correlated with greater absolute and percent increases in postural sway speed from quiet standing to dual tasking conditions (Figure 3B). Together, these observations indicate that chronic sensory impairments contribute to decreased complexity in standing balance dynamics, which in turn is associated with reduced adaptive capacity of the postural control system during dual task conditions. Relatively low baseline complexity, therefore, may indicate control systems that are more vulnerable to dysfunction during cognitive and other stressors.
Figure 3. Analysis of postural sway complexity and its relationship to the adaptive capacity of the postural control system.
(Adapted from Manor et al., 2010). Multiscale entropy was computed from postural sway (i.e., center-of-pressure) dynamics by plotting the sample entropy of anterioposterior postural sway displacement as a function of time scale (A). It is apparent that chronic sensory impairments alter the regularity of postural sway over numerous unique time scales. Across all subjects, those with lower complexity index values, as calculated by the area beneath the MSE curve, were less able to adapt their postural sway to a cognitive dual task (B).
3.3.2 Locomotor system
The capacity to maintain balance when walking through unpredictably changing environments is critical to independent living. Locomotor system output, which is most commonly quantified by recording the temporal and/or spatial variations in kinematic variables from one stride to the next, is complex (Hausdorff et al., 1995; Jordan et al., 2006). To the authors’ knowledge, no study to date has investigated the direct relationship between the complexity of gait dynamics and an individual’s capacity to overcome physical (i.e., a slip, trip or push) or cognitive stressors. However, both healthy aging and disease have been associated with diminished complexity within the kinematic properties of locomotion. For instance, the fractal-like, multi-scale variations in stride duration as one walks over ground is lower in healthy older adults as compared to their younger counterparts (Hausdorff et al., 1997b; Scafetta et al., 2009). In patients with Parkinson’s disease, this fractal-scaling is further diminished, such that stride-to-stride fluctuations closely resemble uncorrelated white noise (Herman et al., 2005). Finally, in older adults with “higher-level gait disorders,” diminished fractal scaling of gait dynamics is significantly lower in those with a history of falling (Hausdorff et al., 1997a).
3.3.3 Manual Force Production
Multiple studies have examined the dynamics of force output of the fingers during force-matching tasks (Sosnoff and Newell, 2008; Vaillancourt and Newell, 2002; Vaillancourt and Newell, 2003). Vaillencourt and Newell (2003) asked older adults to match both constant and time-varying target forces using visual feedback from a computer screen. In the constant force condition, the physiologic complexity of force output was greatest in young adults, lower in old adults (aged 60–69 years), and lowest in old-old adults (aged 75–90 years). In the time-varying force condition, the results were opposite; i.e., complexity was lowest in the young group and highest in the old-old group. These results suggest that the complexity of a system may be dependent upon the specific task and/or environmental constraints in question. (Vaillancourt and Newell, 2002). They also suggest that the degree of complexity associated with baseline dynamics (i.e., the constant force condition) may predict the capacity of the system to adapt to a more difficult condition (i.e., the time-varying force condition).
3.4 Implications and future directions
Mounting evidence from the physiologic systems described above suggests a link between the degree of complexity contained within resting-state or “free-running” system dynamics and the functionality of that system, as defined by the capacity to respond and/or adapt to stressors. We therefore contend that a loss of information content in the dynamics of system output underlies the diminution of adaptive capacity that often accompanies the aging process and is characteristic of frailty. As such, complexity-based metrics appear to hold strong clinical potential and may compliment traditional biomarkers of health and disease. Future research is warranted to strengthen our understanding of the mechanisms through which complexity changes over time, as well as the sensitivity and specificity with which various complexity metrics predict the resilience of an organism.
4. PHYSIOLOGIC COMPLEXITY: IMPLICATIONS FOR REHABILITATION
The growing appreciation of the link between a biological system’s complexity and its functionality has exciting implications for the prevention and rehabilitation of functional loss associated with age and disease. First, metrics that quantify characteristics of physiologic complexity may serve as sensitive markers of an intervention’s effectiveness. Second, new interventions may be derived from the theoretical underpinnings of complexity theory that aim to improve outcomes via restoration of healthy system dynamics. As previously detailed by Lipsitz (2004), interventions with the greatest potential to restore healthy dynamics in biological systems may include those that 1) have effects on multiple systems (i.e., single interventions with multi-system effects such as mind-body exercise, medications, etc.), 2) identify and treat numerous risk factors that contribute to a particular disease or disability (i.e., multifaceted interventions), and 3) include noise-based devices that augment the information readily available to the system of interest.
To date, several studies have reported the effects of various interventions on dynamic complexity, primarily with respect to the cardiovascular and postural control systems.
4.1. Cardiovascular System
Both exercise and pharmacological interventions appear to influence the degree of complexity contained within the heart rate dynamics of healthy older adults and/or patients suffering from cardiovascular disease. Resistance training is one example (Heffernan et al., 2007; Millar et al., 2012). Most recently, Millar et al (2012) published a randomized, controlled trial examining the effects of a two-month progressive isometric hand-grip training program on blood pressure and several markers of heart rate complexity in older adults with treated hypertension. Compared to controls, the exercise group demonstrated decreased systolic and mean blood pressure following the intervention. Whereas no changes were observed in traditional spectral and time-domain measures of heart rate variability, the HR signals of participants of the exercise program showed an increase in sample entropy (i.e., decreased predictability) and decrease in the fractal distance score (i.e., the HR signal moved closer toward fractal-like, 1/f noise)—two important, nonlinear markers of complexity.
Resistance training, and detraining, also appears to alter cardiovascular dynamics in healthy young adults (Heffernan et al., 2007). Sample entropy, Lempel-Ziz entropy, and traditional heart rate variability measures were calculated from heart rate time-series acquired during supine rest with paced-breathing (12 breaths/min) before, immediately after, and four weeks following a six week progressive full-body resistance training program in fourteen young men (mean age = 25 years). Despite no change in any spectral heart rate variability parameter, training was associated with a significant increase in each entropy measure. Moreover, after only four weeks of detraining, these variables returned to their pre-training levels.
Although results are mixed, aerobic exercise may also beneficially affect cardiovascular dynamics (Kanaley et al., 2009; Tulppo et al., 2001). Tulppo et al (2001) reported that two months of moderate to high-intensity aerobic training increased the fractal-like correlations contained within heart rate dynamics of previously sedentary older adults. On the other hand, however, a four month moderate-intensity aerobic training program did not affect the sample entropy of heart rate dynamics in obese adults (aged 40–60 years) with or without type 2 diabetes mellitus (Kanaley et al., 2009). Thus, while promising, much more work is warranted to determine the exercise mode, intensity and amount needed to optimize cardiovascular dynamics, keeping in mind that effects may be specific to both the population studied and the metric(s) used to estimate system complexity. Furthermore, direct correlations between exercise-related changes in system dynamics and clinical outcomes will be critical to furthering our understanding of the functional implications of system dynamics in aging and disease.
A small but growing body of research also suggests that certain medications may affect cardiovascular dynamics. Single doses of the beta-blocking drug propranolol (Castiglioni et al., 2011; Lepoluoto et al., 2005), as well as the β2-adrenoceptor agonist terbutaline (Jartti et al., 1998), induce acute increases in the fractal dimension of heart rate dynamics in healthy young adults. Recently, Ho et al (2011) examined the effects of beta-blocker therapy on heart rate dynamics in older adults suffering from heart failure. Conventional measures of heart rate variability were not affected by beta-blocker therapy (either carvedilol or metoprolol). Compared to controls, however, subjects receiving the therapy exhibited increased multiscale entropy of heart rate dynamics and moreover, were more likely to survive the follow-up period.
4.2. Postural Control System
Several interventions aimed at improving postural control appear to induce beneficial changes in the complexity of postural sway. First, Collins, Priplata and others have reported that in healthy older adults (Collins et al., 2003; Priplata et al., 2002) and in patients with type 2 diabetes mellitus or stroke (Priplata et al., 2006), the application of stochastic, sub-sensory vibrations to the foot soles decreased the magnitude of postural sway and altered several nonlinear characteristics derived from stabilogram diffusion analysis. Costa et al (2007) extended this work by demonstrating that this mechanical vibration also increased the MSE-derived complexity of postural sway in healthy older adults. Although the exact mechanism is unclear, the authors theorized that the addition of sub-sensory mechanical noise may have increased the complexity of postural control via “stochastic resonance,” a phenomenon in which non-zero levels of random noise lower the response threshold of receptors and thus, increase input to the control system.
Tai Chi training may also increase the complex dynamics of postural sway. As a multi-component therapeutic intervention stemming from the Chinese martial and healing arts, Tai Chi combines physical movement, breathing techniques and cognitive imagery (Wayne and Kaptchuk, 2008a). It is widely purported to improve clinical measures of balance in older adults both with and without movement disorders (Wayne and Kaptchuk, 2008b). In order to determine if complexity-based metrics inform Tai Chi’s impact on the postural control system, we recently examined the effects of a 12-week, group-based Tai Chi training program, as compared to an education-based control intervention, on standing postural sway and physical function in frail older adults residing in supportive housing facilities. Although no changes were observed in the traditional metrics of postural sway speed or magnitude, the Tai Chi group increased postural sway complexity when standing with eyes open and eyes closed as compared to controls. Intriguingly, these training-related increases in complexity correlated closely with functional improvements in both gait speed and mobility (Lough et al., 2012).
4.3 Implications and Future Directions
Limited yet intriguing evidence suggests that physiologic complexity is a modifiable property of system dynamics. Yet, many important questions remain unanswered. For example, it is unclear whether multifaceted interventions have greater impact upon complexity as compared to single-component interventions. Although multifaceted interventions may be intuitively superior, complex systems theory suggests that modifying only a single component of a system may also lead to holistic effects on system behavior. Furthermore, as both random noise (e.g., stochastic resonance) and goal-directed therapies (e.g., medications, Tai Chi) have resulted in increased physiologic complexity, future research should strive to uncover the mechanisms through which different forms of perturbations and/or stressors are integrated over multiple temporospatial scales and reflected in altered system behavior.
5. Conclusions
The degree of physiologic complexity associated with a system’s resting dynamics may be fundamentally related to the adaptive capacity of that system. Numerous studies have linked the age-related loss of complexity to diseases, conditions and syndromes that are often associated with diminished adaptive capacity, and mounting evidence also points to positive, intra-subject relationships between physiologic complexity and the capacity of a system to adapt to experimentally-imposed stressors. While encouraging, future studies are needed to further our understanding of the functional implications of physiologic complexity. When designing these studies, considerable care should be taken regarding experimental design and the quantification of physiologic complexity.
The theoretical underpinnings of the complexity theory of aging suggest that interventions designed to restore healthy system dynamics may optimize functional improvements in older adults. Available evidence from exercise, pharmacological and noise-based interventions suggests that physiologic complexity is modifiable and may compliment traditional, linear-based approaches in the prediction of adverse outcomes or assessment of intervention effectiveness. This promising yet relatively young body of research should therefore serve as grounds for future research to examine the rehabilitative implications of physiologic complexity across the lifespan.
Highlights.
Physiologic signals contain a degree of information-rich structure, or complexity
Aging and disease are often linked to reduced physiologic complexity
Diminished physiologic complexity may underlie loss of system functionality
Interventions designed to restore complexity may optimize outcomes in older adults
Acknowledgments
This work was conducted with the support of a KL2 Medical Research Investigator Training (MeRIT) award (1KL2RR025757-04) from Harvard Catalyst | The Harvard Clinical and Translational Science Center (UL 1RR025757), and a grant from the National Institutes of Health (R37-AG025037), Bethesda, MD. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Abbreviations
- MSE
multi-scale entropy
- MEG
magnetoencephalography
- BOLD
blood-oxygen level dependent
- MRI
magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelini L, Maestri R, Marinazzo D, Nitti L, Pellicoro M, Pinna GD, Stramaglia S, Tupputi SA. Multiscale analysis of short term heart beat interval, arterial blood pressure, and instantaneous lung volume time series. Artif Intell Med. 2007;41:237–250. doi: 10.1016/j.artmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Beckers F, Verheyden B, Aubert AE. Aging and nonlinear heart rate control in a healthy population. Am J Physiol Heart Circ Physiol. 2006;290:H2560–H2570. doi: 10.1152/ajpheart.00903.2005. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Castiglioni P, Parati G, Di RM, Carabalona R, Cividjian A, Quintin L. Scale exponents of blood pressure and heart rate during autonomic blockade as assessed by detrended fluctuation analysis. J Physiol. 2011;589:355–369. doi: 10.1113/jphysiol.2010.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Priplata AA, Gravelle DC, Niemi J, Harry J, Lipsitz LA. Noise-enhanced human sensorimotor function. IEEE Eng Med Biol Mag. 2003;22:76–83. doi: 10.1109/memb.2003.1195700. [DOI] [PubMed] [Google Scholar]
- Costa M, Ghiran I, Peng CK, Nicholson-Weller A, Goldberger AL. Complex dynamics of human red blood cell flickering: alterations with in vivo aging. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:020901. doi: 10.1103/PhysRevE.78.020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa M, Priplata AA, Lipsitz LA, Wu Z, Huang NE, Goldberger AL, Peng CK. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M, Sternad D. Complexity of human postural control in young and older adults during prolonged standing. Exp Brain Res. 2008;191:265–276. doi: 10.1007/s00221-008-1521-7. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002a;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002b;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Garcia JA, Martinez-Vargas JD, Castellanos-Dominguez G. Complexity-based analysis for the detection of heart murmurs. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2728–2731. doi: 10.1109/IEMBS.2011.6090748. [DOI] [PubMed] [Google Scholar]
- Grassberger P. Information Dynamics. Plenum; New York: 1991. [Google Scholar]
- Hafstrom A, Fransson PA, Karlberg M, Ledin T, Magnusson M. Visual influence on postural control, with and without visual motion feedback. Acta Otolaryngol. 2002;122:392–397. doi: 10.1080/00016480260000076. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997a;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol. 1997b;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J Appl Physiol. 1995;78:349–358. doi: 10.1152/jappl.1995.78.1.349. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am J Physiol Heart Circ Physiol. 2007;293:H3180–H3186. doi: 10.1152/ajpheart.00648.2007. [DOI] [PubMed] [Google Scholar]
- Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Approach to an irregular time series on the basis of the fractal theory. Physical D. 1988;31:277–283. [Google Scholar]
- Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- Ho YL, Lin C, Lin YH, Lo MT. The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One. 2011;6:e18699. doi: 10.1371/journal.pone.0018699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom A, Fransson PA, Karlberg M, Ledin T, Magnusson M. Visual influence on postural control, with and without visual motion feedback. Acta Otolaryngol. 2002;122:392–397. doi: 10.1080/00016480260000076. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997a;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol. 1997b;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J Appl Physiol. 1995;78:349–358. doi: 10.1152/jappl.1995.78.1.349. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am J Physiol Heart Circ Physiol. 2007;293:H3180–H3186. doi: 10.1152/ajpheart.00648.2007. [DOI] [PubMed] [Google Scholar]
- Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Approach to an irregular time series on the basis of the fractal theory. Physical D. 1988;31:277–283. [Google Scholar]
- Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- Ho YL, Lin C, Lin YH, Lo MT. The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One. 2011;6:e18699. doi: 10.1371/journal.pone.0018699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, MacPherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepard JT, editors. Handbook of Physiology. Oxford University Press; New York: 1996. pp. 255–92. [Google Scholar]
- Hu K, Scheer FA, Buijs RM, Shea SA. The endogenous circadian pacemaker imparts a scale-invariant pattern of heart rate fluctuations across time scales spanning minutes to 24 hours. J Biol Rhythms. 2008;23:265–273. doi: 10.1177/0748730408316166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–R1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- Jartti TT, Kuusela TA, Kaila TJ, Tahvanainen KU, Valimaki IA. The dose-response effects of terbutaline on the variability, approximate entropy and fractal dimension of heart rate and blood pressure. Br J Clin Pharmacol. 1998;45:277–285. doi: 10.1046/j.1365-2125.1998.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Long range correlations in the stride interval of running. Gait Posture. 2006;24:120–125. doi: 10.1016/j.gaitpost.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Goulopoulou S, Franklin RM, Baynard T, Holmstrup ME, Carhart R, Jr, Weinstock RS, Fernhall B. Plasticity of heart rate signalling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obes (Lond) 2009;33:1198–1206. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Costa MD, Priplata AA, Starobinets OV, Goldberger AL, Peng CK, Kiely DK, Cupples LA, Lipsitz LA. Frailty and the Degradation of Complex Balance Dynamics During a Dual-Task Protocol. J Gerontol A Biol Sci Med Sci. 2009;64:1304–1311. doi: 10.1093/gerona/glp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DT, Furman MI, Pincus SM, Ryan SM, Lipsitz LA, Goldberger AL. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepoluoto A, Nino J, Tahvanainen K, Ylitalo R, Kuusela T, Kahonen M, Kaila T. Propranolol increases the complexity of heart rate fluctuations--a mode of antiarrhythmic action? Int J Clin Pharmacol Ther. 2005;43:101–108. doi: 10.5414/cpp43101. [DOI] [PubMed] [Google Scholar]
- Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, Kang HG, Samelson EJ, Gagnon M, Freeman M, Lipsitz LA. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz LA. Age-related changes in the “complexity” of cardiovascular dynamics: A potential marker of vulnerability to disease. Chaos. 1995;5:102–109. doi: 10.1063/1.166091. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ. 2004;2004:e16. doi: 10.1126/sageke.2004.16.pe16. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. [PubMed] [Google Scholar]
- Lough M, Manor B, Gagnon M, Iloputaife I, Wayne P, Lipsitz L. Tai Chi training increases the complexity of standing postural control in frail older adults. Journal of the American Geriatrics Society. 2012;60:S102–S103. [Google Scholar]
- Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12:635–658. [PubMed] [Google Scholar]
- Makikallio TH, Koistinen J, Jordaens L, Tulppo MP, Wood N, Golosarsky B, Peng CK, Goldberger AL, Huikuri HV. Heart rate dynamics before spontaneous onset of ventricular fibrillation in patients with healed myocardial infarcts. Am J Cardiol. 1999;83:880–884. doi: 10.1016/s0002-9149(98)01068-6. [DOI] [PubMed] [Google Scholar]
- Makikallio TH, Seppanen T, Airaksinen KE, Koistinen J, Tulppo MP, Peng CK, Goldberger AL, Huikuri HV. Dynamic analysis of heart rate may predict subsequent ventricular tachycardia after myocardial infarction. Am J Cardiol. 1997;80:779–783. doi: 10.1016/s0002-9149(97)00516-x. [DOI] [PubMed] [Google Scholar]
- Manor B, Costa MD, Hu K, Newton E, Starobinets O, Kang HG, Peng CK, Novak V, Lipsitz LA. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol. 2010;109:1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar PJ, Levy AS, McGowan CL, McCartney N, Macdonald MJ. Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- Norris PR, Stein PK, Morris JA., Jr Reduced heart rate multiscale entropy predicts death in critical illness: a study of physiologic complexity in 285 trauma patients. J Crit Care. 2008;23:399–405. doi: 10.1016/j.jcrc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Peng CK, Costa M, Goldberger AL. Adaptive data analysis of complex fluctuations in physiologic time series. Advances in Adaptive Data Analysis. 2009;1:61–70. doi: 10.1142/S1793536909000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus JE, Liu Y, Lee C, Hausdorff JM, Stanley HE, Goldberger AL, Lipsitz LA. Quantifying fractal dynamics of human respiration: age and gender effects. Ann Biomed Eng. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- Pikkujamsa SM, Makikallio TH, Sourander LB, Raiha IJ, Puukka P, Skytta J, Peng CK, Goldberger AL, Huikuri HV. Cardiac interbeat interval dynamics from childhood to senescence : comparison of conventional and new measures based on fractals and chaos theory. Circulation. 1999;100:393–399. doi: 10.1161/01.cir.100.4.393. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priplata A, Niemi J, Salen M, Harry J, Lipsitz LA, Collins JJ. Noise-enhanced human balance control. Phys Rev Lett. 2002;89:238101. doi: 10.1103/PhysRevLett.89.238101. [DOI] [PubMed] [Google Scholar]
- Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- Raja Beharelle A, Kovacevic N, McIntosh AR, Levine B. Brain signal variability relates to stability of behavior after recovery from diffuse brain injury. Neuroimage. 2012;60:1528–1537. doi: 10.1016/j.neuroimage.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MA, Clark S. Recurrence analysis of human postural sway during the sensory organization test. Neurosci Lett. 2003;342:45–48. doi: 10.1016/s0304-3940(03)00229-5. [DOI] [PubMed] [Google Scholar]
- Sabatini AM. Analysis of postural sway using entropy measures of signal complexity. Med Biol Eng Comput. 2000;38:617–624. doi: 10.1007/BF02344866. [DOI] [PubMed] [Google Scholar]
- Scafetta N, Marchi D, West BJ. Understanding the complexity of human gait dynamics. Chaos. 2009;19:026108. doi: 10.1063/1.3143035. [DOI] [PubMed] [Google Scholar]
- Seigle B, Ramdani S, Bernard PL. Dynamical structure of center of pressure fluctuations in elderly people. Gait Posture. 2009;30:223–226. doi: 10.1016/j.gaitpost.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Age-related loss of adaptability to fast time scales in motor variability. J Gerontol B Psychol Sci Soc Sci. 2008;63:344–352. doi: 10.1093/geronb/63.6.p344. [DOI] [PubMed] [Google Scholar]
- Sun J, Tong S, Yang GY. Reorganization of Brain Networks in Aging and Age-related Diseases. Aging Dis. 2012;3:181–193. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.05.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Murata T, Mizuno T, Kikuchi M, Mizukami K, Kosaka H, Takahashi K, Wada Y. Age-related variation in EEG complexity to photic stimulation: a multiscale entropy analysis. Clin Neurophysiol. 2009;120:476–483. doi: 10.1016/j.clinph.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner S, Mittermaier C, Ehrenberger K. Change of complexity patterns in human posture during aging. Audiol Neurootol. 2002;7:240–248. doi: 10.1159/000063740. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Hughson RL, Makikallio TH, Airaksinen KE, Seppanen T, Huikuri HV. Effects of exercise and passive head-up tilt on fractal and complexity properties of heart rate dynamics. Am J Physiol Heart Circ Physiol. 2001;280:H1081–H1087. doi: 10.1152/ajpheart.2001.280.3.H1081. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23:1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol. 2003;94:903–912. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
- Vikman S, Makikallio TH, Yli-Mayry S, Pikkujamsa S, Koivisto AM, Reinikainen P, Airaksinen KE, Huikuri HV. Altered complexity and correlation properties of R-R interval dynamics before the spontaneous onset of paroxysmal atrial fibrillation. Circulation. 1999;100:2079–2084. doi: 10.1161/01.cir.100.20.2079. [DOI] [PubMed] [Google Scholar]
- Wayne PM, Kaptchuk TJ. Challenges inherent to t’ai chi research: part I--t’ai chi as a complex multicomponent intervention. J Altern Complement Med. 2008a;14:95–102. doi: 10.1089/acm.2007.7170a. [DOI] [PubMed] [Google Scholar]
- Wayne PM, Kaptchuk TJ. Challenges inherent to t’ai chi research: part II-defining the intervention and optimal study design. J Altern Complement Med. 2008b;14:191–197. doi: 10.1089/acm.2007.7170b. [DOI] [PubMed] [Google Scholar]
- Webber CL, Jr, Zbilut JP. Dynamical assessment of physiological systems and states using recurrence plot strategies. J Appl Physiol. 1994;76:965–973. doi: 10.1152/jappl.1994.76.2.965. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS. Assessment of balance control in humans. Med Prog Technol. 1990;16:31–51. [PubMed] [Google Scholar]
- Yang AC, Huang CC, Yeh HL, Liu ME, Hong CJ, Tu PC, Chen JF, Huang NE, Peng CK, Lin CP, Tsai SJ. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.05.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]