Abstract
Purpose
Cefazolin/tobramycin, Cefuroxime/gentamicin, and moxifloxacin were compared using bacterial keratitis isolates to determine whether empiric therapy constituted optimal anti-bacterial treatment.
Methods
Based on percent incidence of corneal infection, 27 Staphylococcus aureus, 16 Pseudomonas aeruginosa, 10 Serratia marcescens, 4 Moraxella lacunata, 3 Haemophilus influenzae, 9 coagulase-negative Staphylococci, 7 Streptococcus viridans, 6 Streptococcus pneumoniae, 7 assorted Gram-positive isolates, and 11 assorted Gram-negative isolates were tested for MICs to cefazolin, tobramycin, cefuroxime, gentamicin, and moxifloxacin using E-tests to determine susceptibility and potency.
Results
The in vitro coverage (susceptible to at least one antibiotic) of cefuroxime/gentamicin (97%) was statistically equal to cefazolin/tobramycin (93%) and moxifloxacin (92%) (p=0.29). Double coverage (susceptible to both antibiotics) was equivalent (p=0.77) for cefuroxime/ gentamicin (42%) and cefazolin/tobramycin (40%). The susceptibilities of individual coverage were moxifloxacin (92%), gentamicin (89%), tobramycin (74%), cefazolin (58%), and cefuroxime (52%). Methicillin-resistant Staphylococcus aureus was best covered by gentamicin 100% (9 of 9). Tobramycin was more potent (p=0.00001) than gentamicin for Pseudomonas aeruginosa, while cefazolin was more potent (p=0.0004) than cefuroxime for Staphylococcus aureus.
Conclusions
Although there appears to be no in vitro empiric coverage advantage between cefazolin/tobramycin, cefuroxime/gentamicin, and moxifloxacin monotherapy, potency differences may occur, and optimal treatment can best be determined with laboratory studies.
Keywords: keratitis, antibiotics, empiric topical therapy
Introduction
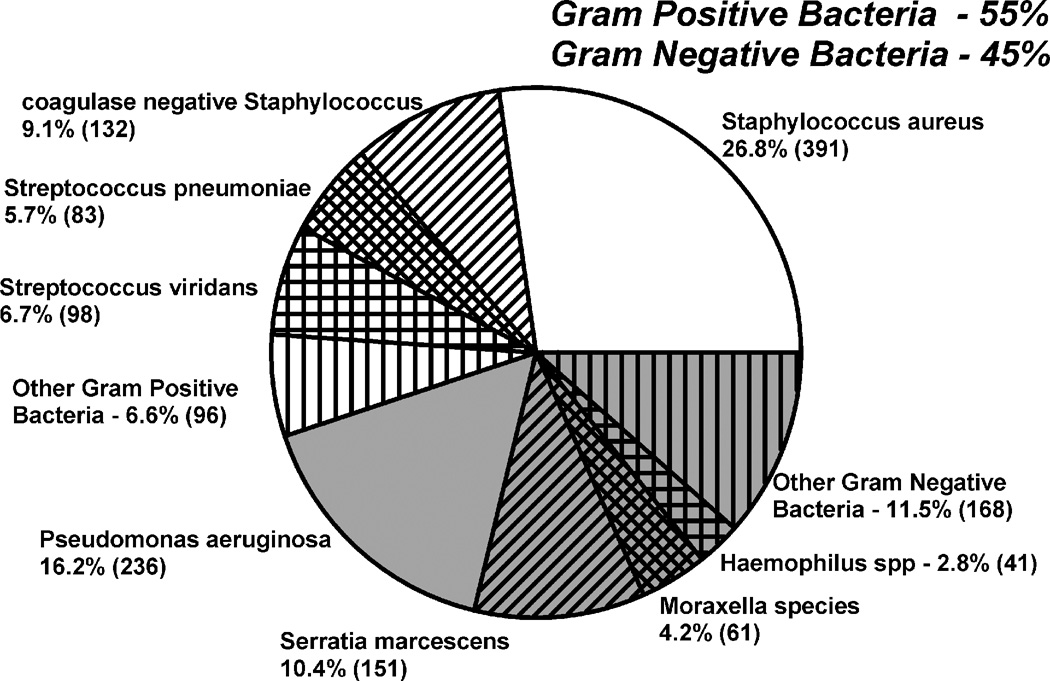
Bacterial keratitis is generally treated empirically without or with the subsequent knowledge of laboratory identification and antibiotic susceptibility studies. Figure 1 provides a distribution of Gram-positive and Gram-negative bacteria that were isolated from the corneas of patients with bacterial keratitis at The Charles T. Campbell Ophthalmic Microbiology Laboratory, University of Pittsburgh Medical Center, Pittsburgh, PA. This distribution of bacterial pathogens supports the use of “broad-spectrum” anti-bacterial drugs to empirically treat suspected bacterial keratitis in a timely manner. Antibiotics for the coverage of Gram-positive and Gram-negative negative are formulated at high concentration to provide broad-spectrum therapy. In the United Kingdom (Dua H, Bacterial Infections, EVER 2010, October 6, Supplement pg 21), cefuroxime, a second-generation cephalosporin and gentamicin, an aminoglycoside, is the favored combination for empiric therapy, while cefazolin, a first-generation cephalosporin, along with tobramycin, another aminoglycoside are commonly used in the United States.1 The first-generation cephalosporins are considered to be more efficacious than the 2nd generation cephalosporins for Gram-positive bacteria2, and tobramycin was an improvement over gentamicin for the coverage of Pseudomonas aeruginosa,3 a Gram-negative bacteria. Neither of these combinations is commercially packaged for keratitis care.
Figure 1.
The distribution of 1457 bacterial keratitis isolates from 1993 to 2010.
A commercially formulated “broad-spectrum” antibiotic to act as monotherapy for empiric bacterial keratitis treatment appears to be advantageous for wide-availability and simplified use. Monotherapy with a fluoroquinolone has been practiced for empiric therapy since the inception of the second-generation and third-generation fluoroquinolones (ciprofloxacin1, ofloxacin4, levofloxacin5) and the fourth-generation fluoroquinolones (moxifloxacin6, gatifloxacin7). Another fourth-generation fluoroquinolone, besifloxacin (Besivance, Bausch and Lomb, Rochester, NY), also has potential for topical keratitis treatment. The fourth-generation fluoroquinolones have been reported to be more potent and less likely to promote resistance than the second-generation fluoroquinolones.8
We hypothesized that the cefazolin/tobramycin combination would provide broader empiric bacterial keratitis coverage than the cefuroxime/gentamicin combination and monotherapy with a fourth-generation fluoroquinolone. The hypothesis was tested by determining the in vitro susceptibilities of the regimens against a panel of bacterial keratitis pathogens that were chosen on the basis of incidence and chronologic order to represent the correct incidence of infection. We determined whether empiric anti-infective coverage was optimal with potency comparisons within the cephalosporin and aminoglycoside groups.
Methods
The incidence of bacterial keratitis was determined from 1993 to 2010 at The Charles T. Campbell Ophthalmic Microbiology Laboratory at The University of Pittsburgh Medical Center, Pittsburgh, PA (Figure 1). Based on percent incidence of infection, 27 Staphylococcus aureus, 16 Pseudomonas aeruginosa, 10 Serratia marcescens, 4 Moraxella lacunata, 3 Haemophilus influenzae, 9 coagulase-negative Staphylococci, 7 Streptococcus viridans, 6 Streptococcus pneumoniae, 7 assorted Gram-positive isolates, and 11 assorted Gram-negative isolates were tested for MICs using E-tests (AB BioDisk, Dalvagen, Sweden) with NCCLS guidance.8–10 The anti-infectives tested were cefazolin, cefuroxime, tobramycin, gentamicin, and moxifloxacin. The 100 isolates were consecutively retrieved, without patient identification markers, in reverse chronologic order from 3/4/2011. All isolates were collected from the corneas of patients with the clinical presentation of bacterial keratitis. These isolates are part of the clinical bank collection used to validate new antibiotics and diagnostic testing, and monitoring of antibiotic resistance that is necessary for clinical laboratory certification.
Previous 2000 National Committee for Clinical Laboratory Standards (now CSLI, Clinical and Laboratory Standards Institute, Wayne, PA) was used to interpret susceptibility using guidelines based on serum concentrations9. There are no standards for interpreting topical ocular treatment, but the serum standards can be used if it is assumed that the antibiotic concentrations in the ocular tissue are equal or greater than the antibiotic concentrations that can be attained in the serum.8–10 This assumption is based on the high concentration of topical antibiotic that cross the corneal epithelium to reach higher concentration in the ocular tissue than would by systemic therapy. These elevated concentrations are in excess of the required MICs to denote susceptibility in the blood serum. The newer 2009 CLSI standards have changed to accommodate the refinement of serum level susceptibility, but these do not reflect any change for topical therapy and have no bearing on the ophthalmic interpretation.11 Experience (The first author has 35 years in susceptibility testing as an ophthalmic microbiologist) and the lack of supportive data for intermediate susceptibility has dictated that intermediate susceptibility has no meaning for ocular isolates, and high antibiotic concentrations in the ocular tissue should allow for intermediate values to be interpreted as susceptible. It is important to interpret susceptibility with the same standards between time periods (2000 versus 2009) for non-bias comparisons.
The in vitro coverage (susceptible to at least one antibiotic) and (susceptible to both antibiotics) were determined for the cefuroxime/gentamicin and cefazolin/tobramycin combinations, and these were further compared with moxifloxacin monotherapy. The susceptibilities of individual coverage for moxifloxacin, gentamicin, tobramycin, cefazolin, and cefuroxime were also determined.
In addition, the MICs were used to compare the potencies within the aminoglycoside and cephalosporin drug classes. The anti-infective in a drug class with the lower MICs is deemed the more potent anti-infective. Potency can not be compared between classes (i.e. tobramycin versus cefazolin) of anti-infectives using MIC data.12
Statistical Analysis
All in vitro susceptibilities for the combinations and individual anti-infectives were tested for differences using the Chi-Square analysis (MiniTab™, State College, PA). The Mann- Whitney test (MiniTab™, State College, PA) for non-parametric comparisons was used to determine any MIC differences for gentamicin and tobramycin against Gram-negative bacteria, and cefazolin and cefuroxime against Gram-positive bacteria. A p-value of 0.05 or less was used to define a significant difference.
Results
The bacterial corneal isolates were retrieved during the following periods: Staphylococcus aureus (11/28/2009 to 2/4/2011), Pseudomonas aeruginosa (10/18/2009 to 11/19/2010), Serratia marcescens (9/24/2006 to 12/28/2010), Moraxella lacunata (11/12/2010 to 2/21/2011), Haemophilus influenzae (7/27/2010 to 11/12/2010), coagulase-negative Staphylococci (11/19/2008 to 2/18/2011), Streptococcus viridans (9/28/2009 to 3/4/2011), Streptococcus pneumoniae (6/12/2009 to 12/29/2010), assorted Gram-positive isolates (4/4/2005 to 10/28/2010), and assorted Gram-negative isolates (1/21/2009 to 11/9/2010).
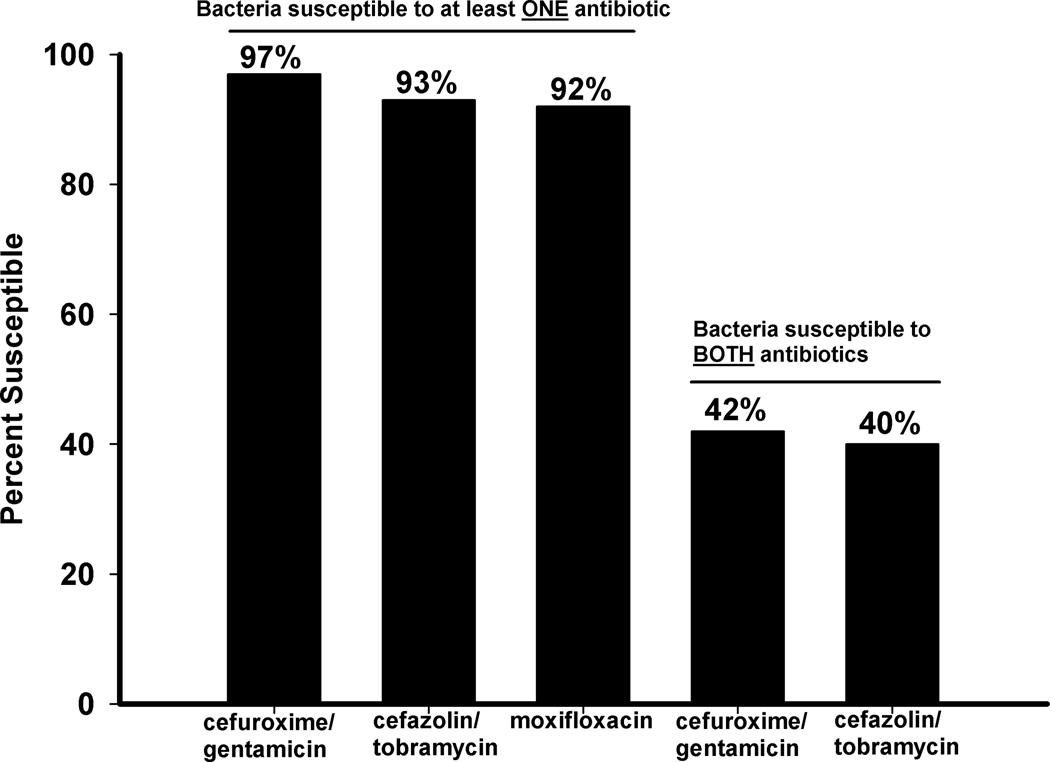
Figure 2 depicts the percent of bacterial keratitis isolates covered by at least one antibiotic. Cefuroxime/gentamicin (97%, 97 of 100) was statistically equal to cefazolin/tobramycin (93%, 93 of 100) and moxifloxacin (92%, 92 of 100) (p=0.29, Chi-square). Cefuroxime/gentamicin did not cover one isolate each of Haemophilus influenzae, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. Cefazolin/tobramycin did not cover two isolates of Staphylococcus aureus, and one isolate each of Haemophilus influenzae, Stenotrophomonas maltophilia, Serratia marcescens, Streptococcus viridans, and Chryseobacterium indologenes. Moxifloxacin did not cover three isolates of coagulase-negative Staphylococcus, and one isolate each of Staphylococcus aureus, Haemophilus influenzae, Streptococcus viridans, Morganella morganii, and Enterococcus species.
Figure 2.
The percent susceptibility of 100 bacterial isolates from keratitis to at least one antibiotic or two antibiotics.
Figure 2 depicts the percent of bacterial keratitis isolates covered by both antibiotics for each regimen. Double coverage was equivalent (p=0.77, Chi-square) for cefuroxime/ gentamicin (42%, 42 of 100) and cefazolin/tobramycin (40%, 40 of 100).
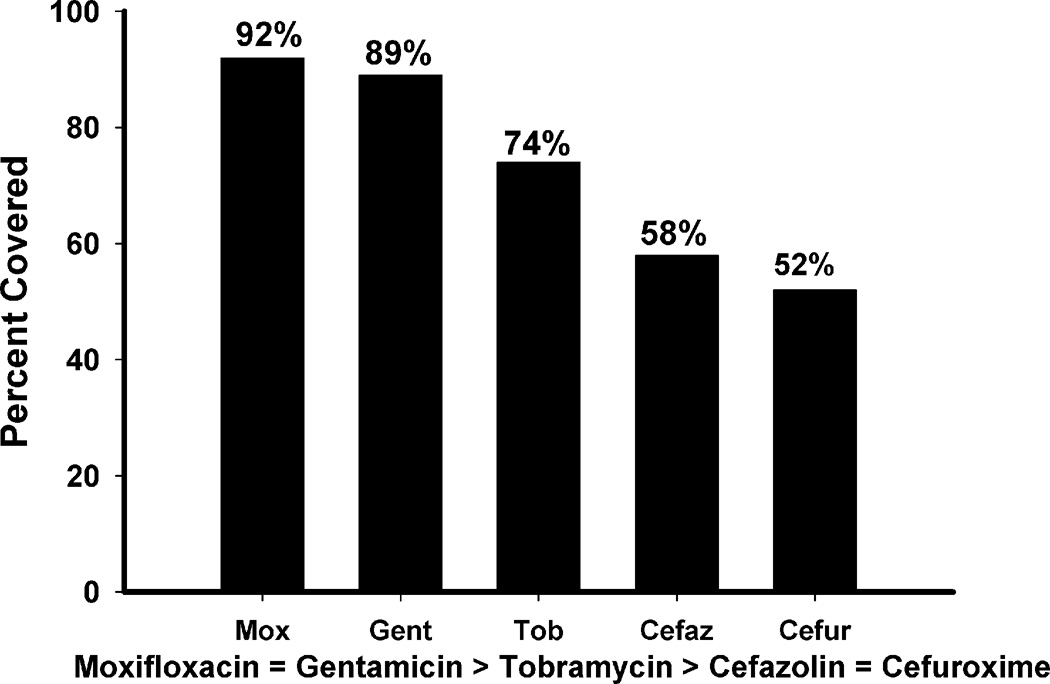
Figure 3 depicts the individual coverage for all five anti-infectives. Individual coverage determined that moxifloxacin (92%) was equal to gentamicin (89%) (p=0.47, Chi-square), while both covered more than tobramycin (74%) (p=0.001, Chi-square). Tobramycin (74%) covered more (p=0.035, Chi-square) than cefazolin (58%), and cefuroxime (52%) which were statistically equal (p=0.494, Chi-square).
Figure 3.
Individual susceptibility of 100 bacterial isolates from keratitis to moxifloxacin, gentamicin, tobramycin, cefazolin, and cefuroxime.
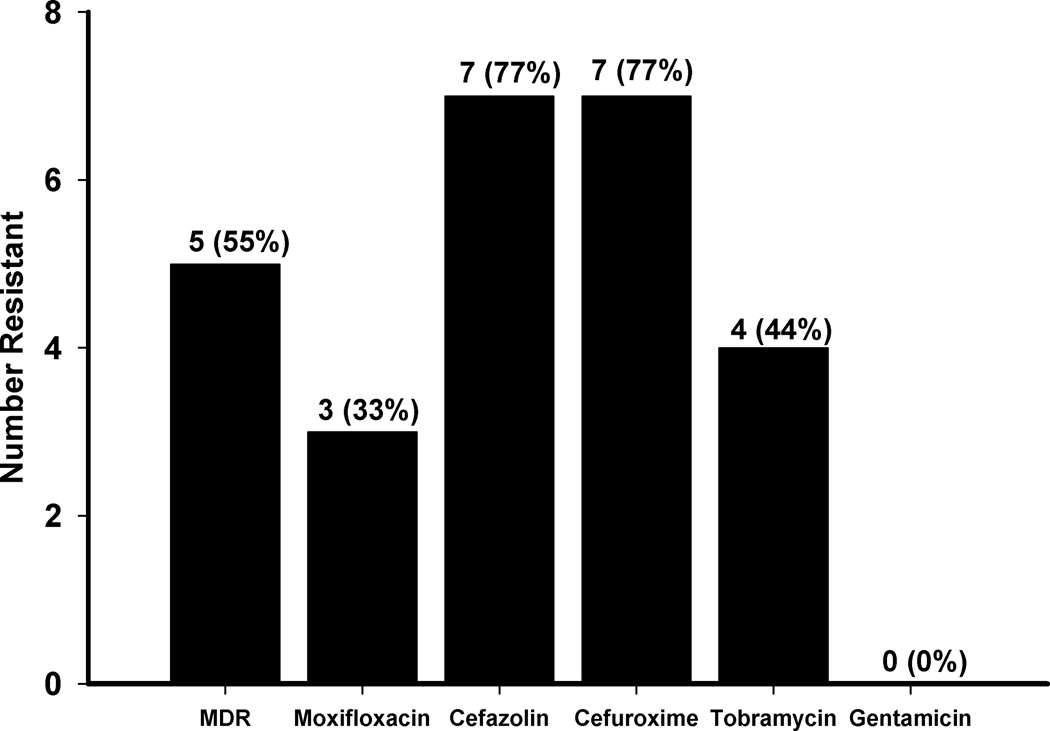
Figure 4 depicts the distribution of methicillin-resistant Staphylococcus aureus (MRSA) antibiotic susceptibility among nine isolates. All nine MRSA isolates were susceptible to gentamicin whereas only five were susceptible to tobramycin. Only two MRSA isolates were susceptible to both cefazolin and cefuroxime. Five MRSA isolates were susceptible to moxifloxacin.
Figure 4.
Distribution of nine methicillin resistant Staphylococcus aureus (MRSA) isolates for resistance to moxifloxacin, gentamicin, tobramycin, cefazolin, and cefuroxime. The nine isolates represent 33% of 27 Staphylococcus aureus isolates. MDR is the abbreviation for multiple drug resistant.
Potency analysis for Gram-negative bacteria determined that Pseudomonas aeruginosa MICs (n=16) for tobramycin (median = 1.0 µg/ml) were significantly lower (more potent) than gentamicin (median = 3.0 µg/ml) (p=0.00001, Mann-Whitney). The Serratia marcescens MICs (n=10) for gentamicin (median = 1.75 µg/ml) were significantly lower (more potent) than tobramycin (median = 3.0 µg/ml) (p=0.0065, Mann-Whitney). For other Gram-negative bacteria (n=11), the MICs were equivalent (equally potent) to gentamicin (median = 1.5 µg/ml) and tobramycin (median = 1.5 µg/ml) (p=0.79, Mann-Whitney).
Potency analysis for Gram-positive bacteria determined that Staphylococcus aureus MICs (n=27) for cefazolin (median = 1.0 µg/ml) were significantly lower (more potent) than cefuroxime (median = 2.0 µg/ml) (p=0.0004, Mann-Whitney). The Streptococcus pneumoniae MICs (n=6) for cefuroxime (median = 0.0195 µg/ml) were significantly lower (more potent) than cefazolin (median = 0.094 µg/ml) (p=0.005, Mann-Whitney). The MICs for coagulase-negative Staphylococcus (n=9) to cefazolin (median = 0.75 µg/ml) and cefuroxime (median =1.5 µg/ml) were equivalent (equally potent) (p=0.21, Mann-Whitney). The MICs for Streptococcus viridans (n=7) to cefazolin (median = 0.38 µg/ml) and cefuroxime (median =0.19 µg/ml) were equivalent (equally potent) (p=0.12, Mann-Whitney). The MICs for other Gram-positive bacteria (n=7) to cefazolin (median = 0.8 µg/ml) and cefuroxime (median =1.5 µg/ml) were equivalent (equally potent) (p=0.52, Mann-Whitney).
Discussion
The empiric treatment of bacterial keratitis is an important issue especially with the lack of laboratory support for confirming adequate coverage. In general, small corneal defects with infiltrates less than 2 mm precipitated by contact lens use may not be candidates for laboratory studies since most are either sterile or respond nicely to empiric therapy. Smaller defects can become larger defects, and if these are not adequately covered by empiric therapy, these defects could result in corneal ulceration and extended therapy
The strength of our study is that the corneal isolates were selected consecutively on the basis of a weighted incidence of infection and not on a bias of select antibiotic susceptibility. The ideal study would be a controlled clinical trial, but no trial could compare multiple antibacterial regimens on single patients. It is highly unlikely that a clinical trial for comparing bacterial keratitis treatment regimens would be funded through governmental sources or by industry. A reasonable position by government is that industry should provide evidence of the efficacy of antibacterial drugs, and a pharmaceutical company would prefer a guaranteed favorable demonstration of their drug product.
In vitro studies have been used successfully to guide in vivo therapy. Although we do not have clinical data for the in vivo efficacy of the regimens used to treat the bacterial infections in the present study, we assume that all of the patients resolved their bacterial keratitis with empiric and subsequent directed therapy. As an in vitro study, other factors such as drug penetration, dosing schedule, innate immunity, virulence factors, adjunctive measures, and patient compliance, were not accounted in the critical management of acute bacterial keratitis.
In the present in vitro study, we rejected the hypothesis that empiric therapy of bacterial keratitis would be best covered with cefazolin and tobramycin rather than cefuroxime and gentamicin. In addition, neither combination provided wider coverage than moxifloxacin monotherapy. It appeared that topical empiric therapy with either combination or moxifloxacin monotherapy was equivalent. The next question would be whether empiric therapy is optimal therapy based on laboratory testing.
Potency studies indicate that the empiric combination of cefazolin and tobramycin would be best for covering Staphylococcus aureus and Pseudomonas aeruginosa keratitis. Gentamicin may provide an advantage over tobramycin for treating Serratia marcescens and cefuroxime may provide an advantage over cefazolin for treating Streptococcus pneumoniae. As always, changes in therapy after empiric treatment should be considered based on the ophthalmologist’s clinical impression and experience.
We did not include vancomycin and ciprofloxacin as candidates for empiric therapy. Vancomycin is an excellent Gram-positive antibiotic and is the agent of choice in the treatment of MRSA. Vancomycin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus have not been yet a problem in the treatment of ocular infections. Some reserve vancomycin for select ocular infections to avoid possible acquired resistance. Vancomycin should be considered as empiric therapy in bacterial keratitis cases where MRSA is a possibility or highly suspected (i.e. patient exposure, health-care worker).
Ciprofloxacin is a second-generation fluoroquinolone that could be used for the treatment of Pseudomonas aeruginosa keratitis when topical therapy using tobramycin or moxifloxacin are not providing satisfactory results. Ciprofloxacin has been reported to be the most potent fluoroquinolone for covering Pseudomonas aeruginosa, however it is less potent and therefore more at risk for selection of resistance with Staphylococcus aureus.8,10
Although vancomycin is the first-line antibiotic to treat MRSA keratitis, gentamicin appeared to be an alternative therapy in the present study. Although gentamicin tested to provide 100% coverage for MRSA keratitis infection, we highly advocate that laboratory susceptibility testing support this coverage for each keratitis case. It must also be noted that our present in vitro study does not support the use of topical cefazolin for the treatment of MRSA, but prior data from our laboratory13, reports by others14–16, and success in our animal model17 indicate coverage is possible. Cefazolin can bind to the penicillin binding 2 prime protein (PBP2a) which is coded by the mecA gene and thereby prevents cell wall formation.
In the present study, moxifloxacin demonstrated to be an adequate choice for empiric therapy, but laboratory confirmation should support treatment because of reports of fluoroquinolone resistance among Pseudomonas aeruginosa and Staphylococcus aureus isolates.18–22 All fluoroquinolones are not the same, and one should not assume that there is equal coverage with the second- and fourth-generation fluoroquinolones. Bacterial resistance is more likely to occur with the second-generation fluoroquinolones because a single mutation (DNA gyrase or topoisomerase IV) is sufficient to confer resistance, whereas two mutations (DNA gyrase and topoisomerase IV ) are necessary to confer resistance to the fourth generation fluoroquinolone anti-infectives such as moxifloxacin.18 It can be reasoned that increased resistance to the fluoroquinolones can be compounded: with widespread surgical prophylaxis; improper dosing with a concentration-dependent anti-infective; mixing the usage of second and fourth-generation fluoroquinolones to create step mutations; and chronic dosing.
In summary, cefazolin/tobramycin, cefuroxime/gentamicin, and moxifloxacin monotherapy, appeared to be equivalent for the empiric topical treatment of bacterial keratitis. Although in general broad-spectrum, empiric therapy may not represent optimal management and laboratory studies should guide best possible therapy.
Acknowledgements
We are grateful to the Pennsylvania Lions Club, The Charles T. Campbell Foundation, and a core grant for Vision Research NIH EY008098 for continued financial support. RMQS has received a career development award from Research to Prevent Blindness (AI085570).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no “Conflict of Interests” to disclose for the completion of this study as determined by the University of Pittsburgh, Pittsburgh, PA.
References
- 1.Hyndiuk RA, Eiferman RA, Caldwell DR, et al. Comparison of ciprofloxacin ophthalmic solution 0.3% to fortified tobramycin-cefazolin in treating bacterial corneal ulcers. Ciprofloxacin bacterial keratitis study group. Ophthalmology. 1996;103:1854–1862. doi: 10.1016/s0161-6420(96)30416-8. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien TP. Foster C Stephen, azar Dimitri T, Dohlman Claes H., editors. Bacterial Keratitis. Chapter 13.1. “The Cornea – Smolin and Thoft’s” Scientific Foundations & Clinical Practice 4th edition. :261–262. [Google Scholar]
- 3.O’Brien TP. Foster C Stephen, Azar Dimitri T, Dohlman Claes H., editors. Bacterial Keratitis. Chapter 13.1. “The Cornea – Smolin and Thoft’s” Scientific Foundations & Clinical Practice 4th edition. :263–264. [Google Scholar]
- 4.Khokhar S, Sindhu N, Mirdha BR. Comparison of topical 0.3% ofloxacin to fortified tobramycin-cefazolin in the therapy of bacterial keratitis. Infection. 2000;28:149–152. doi: 10.1007/s150100050068. [DOI] [PubMed] [Google Scholar]
- 5.Kasetsuwan N, Tanthuvanit P, Reinprayoon U. The efficacy and safety of 0.5% levofloxacin versus fortified cefazolin and amikacin ophthalmic solution for the treatment of suspected and culture-proven cases of infectious bacterial keratitis: A comparative study. Asian Biomedicine. 2011;5:77–83. [Google Scholar]
- 6.Constantinou M, Daniell M, Snibson GR, et al. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114:1622–1629. doi: 10.1016/j.ophtha.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Parmar P, Salman A, Kalavathy CM, et al. Comparison of topical gatifloxacin 0.3% and ciprofloxacin 0.3% for the treatment of bacterial keratitis. Am J Ophthalmol. 2006;141:282–286. doi: 10.1016/j.ajo.2005.08.081. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski RP, Dhaliwal DK, Karenchak LM, et al. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol. 2003;136:500–505. doi: 10.1016/s0002-9394(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards: Methods for Dilution Antimicrobials Susceptibility Tests for Bacteria That Grow Aerobically, ed. 4. Approved standard. No.2. vol.20. Villanova, Pennsylvania: National Committee for Clinical Laboratory Standards; 2000. document M7-A5. [Google Scholar]
- 10.Mather R, Karenchak LM, Romanowski EG, Kowalski RP. 4th generation fluoroquinolones: New weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002;133(4):463–466. doi: 10.1016/s0002-9394(02)01334-x. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards: Methods for Dilution Antimicrobials Susceptibility Tests for Bacteria That Grow Aerobically, ed. 8. Approved standard. No.2. vol.29. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2009. document M07-A8. [Google Scholar]
- 12.Kowalski RP, Yates KA, Romanowski EG, et al. An Ophthalmologist's Guide to Understanding Antibiotic Susceptibility and Minimum Inhibitory Concentration (MIC) Data. Ophthalmology. 2005;112:1987–1991. doi: 10.1016/j.ophtha.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski RP, Karenchak LM, Romanowski EG. Infectious Disease: Changing Antibiotic Susceptibility. Ophthalmol Clin N Amer. 2003;16:1–9. doi: 10.1016/s0896-1549(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 14.Chambers HF, Sachdeva M. Binding of b-Lactam Antibiotics to Penicillin-binding Proteins in Methicillin-resistant Staphylococcus aureus. J Inf Dis. 1990;161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 15.Hikida M, Mori M, Yoshida M, Yokota T. Possible usefulness of cephem antibiotics with anti-staphylococcal activity for preventing the spread of methicillin-resistant Staphylococcus aureus. Jpn J Antibiot. 1993;46(9):836–839. [PubMed] [Google Scholar]
- 16.Fallon MT, Shafer W, Jacob E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J Surg Res. 1999;86(1):97–102. doi: 10.1006/jsre.1999.5686. [DOI] [PubMed] [Google Scholar]
- 17.Romanowski EG, Mah FS, Yates KA, et al. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with Zymar (gatifloxacin 0.3%) in a NZW rabbit model. Am J Ophthalmol. 2005;139:867–877. doi: 10.1016/j.ajo.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Hooper DC. Fluoroquinolone resistance among gram-positive cocci. Lancet Inf Dis. 2002;2:530–538. doi: 10.1016/s1473-3099(02)00369-9. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106:1313–1318. [PubMed] [Google Scholar]
- 20.Kowalski RP, Pandya AN, Karenchak LM, et al. An in vitro resistance study of levofloxacin, ciprofloxacin and ofloxacin using keratitis isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Ophthalmology. 2001;108:1826–1829. doi: 10.1016/s0161-6420(01)00724-2. [DOI] [PubMed] [Google Scholar]
- 21.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106:1319–1323. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhry NA, Flynn HW, Murray TG, et al. Emerging ciprofloxacin-resistant Pseudomonas aeruginosa. Am J Ophthalmol. 1999;128:509–510. doi: 10.1016/s0002-9394(99)00196-8. [DOI] [PubMed] [Google Scholar]