Abstract
Background & Aims
The type III interferons (IFN-λs: interleukin [IL]-28a, IL-28b, and IL-29) have important roles in hepatitis C virus (HCV) infection, but little is understood about what cells produce these cytokines or how production is activated. We investigated whether human immune cells recognize HCV-infected cells and respond by producing IFN-λ.
Methods
We cultured healthy human peripheral blood mononuclear cells (PBMCs) with different populations of immune cells and JFH-1 HCV-infected Huh7.5 (HCVcc/Huh7.5) cells.
Results
Human PBMCs recognized HCVcc/Huh7.5 cells responded by producing IFN-α, IFN-γ, and IFN-λ. A rare subset of myeloid dendritic cells (mDCs), which are BDCA3+, (also called mDC2 cells), were the major source of IL-28 and IL-29 production in response to HCVcc/Huh7.5 cells. Plasmacytoid DCs (pDCs) produced IFN-α, whereas natural killer and natural killer T cells were the main source of IFN-γ production in co-culture experiments. Of the endosomal toll-like receptors (TLRs)3, 7, 8, and 9, only TLR3 or double-stranded HCV RNA induced production of IL-28 and IL-29 by mDC2s; endosomal maturation was required. Production of IFN-α and IFN-λ were linked—IFN-λ increased production of IFN-α by pDCs and IFN-α significantly increased production of IFN-λ.
Conclusions
mDC2s are a major source of IFN-λs production by PBMCs in response to HCVcc/Huh7.5 cells. mDC2s are activated through the TLR3 pathway, indicating that human DCs can efficiently initiate and immune response against HCV infection. IFN-λ therefore has an important role in HCV infection.
Keywords: IL28B SNP; NK cells, NKT cells; viral immune regulation
Introduction
Hepatitis C virus (HCV) infection affects 120 million people worldwide, typically resulting in chronic infection. Early and high induction of interferon-stimulated genes (ISGs) in HCV infection is a marker of decreased response to therapy,1 however, the coordinated role of interferons (IFNs) during HCV infection and the signaling pathways leading to their production are only partially understood. The IFNs are divided into type I (IFN-α and β), II (IFN-γ) and III subtypes (IFN-λs: IL-28a, IL-28b and IL-29) and have immunomodulatory and antiviral activities through their respective receptors that induce IFN-stimulated genes (ISGs).2 All IFNs contribute to the coordination of antiviral immunity in the control of HCV. IFN-α has anti-HCV activity and is widely used to treat chronic HCV infection.3 IFN-γ is produced by NK cells or HCV-specific T cells and orchestrates anti-HCV immune responses.4 IFN-λs may play a unique role in clearing HCV infection because several single nucleotide polymorphisms (SNPs) near the IL-28b region predict the outcome of natural HCV infection or IFN-α therapy.5-8 Despite using different receptors, IFN-λ shares a common downstream JAK-STAT pathway with type I IFNs and induces very similar anti-viral immune responses.2 IFN-λ is currently in clinical trials for treatment of HCV infection.9
Although recent reports suggest that human hepatocytes produce type I and III IFNs in response to HCV infection,10, 11 it is also well recognized that HCV disrupts IFN synthesis though NS3-4 mediated cleavage of two crucial adaptor proteins, mitochondrial antiviral-signaling protein (MAVS) and TIR-domain-containing adapter-inducing interferon-β (TRIF), in infected hepatocytes.12 Thus, it is crucial for host immune cells to recognize ongoing HCV infection and initiate an immune response. Human dendritic cells (DCs) bridge the innate and adaptive immune systems and play an irreplaceable role in immune defense against viral infections. According to their distinct phenotype and functional characteristics, human peripheral DCs can be divided into three subsets: mDC1 (myeloid CD1c+ DC), mDC2 (myeloid CD141+ DC or myeloid BDCA3+ DC) and pDC (plasmacytoid DC).13 The mDC1 (conventional DC) represents the largest mDC population in the blood which produce inflammatory cytokines and chemokines.14 The mDC2, a minor subset, is the human homologue of the mouse CD8+ DC subset15, 16 that produces IL-12 and cross–presents antigen for CD8 class 1 restricted CTL responses under TLR3 ligation.17 Plasmacytoid DCs (pDCs), natural type 1 interferon producing cells, produce high levels of IFN-α and have a central role in anti-viral immune responses.18
Since HCV cannot effectively infect immune cells,19 it is speculated that immune cells must recognize HCV-virions and/or HCV-infected cells. Previous studies found that HCVcc (cell culture derived HCV particles) cannot or only weakly induce mDC or pDC activation.20, 21 A recent report showed that pDCs can be triggered to produce type I IFNs by Huh-7.5 cells containing replicating HCV RNA.22 We hypothesized that human immune cells can recognize HCV-infected hepatoma cells and produce IFNs or inflammatory cytokines in response. We found that all three types of IFNs were produced in co-cultures of human PBMCs and HCV-infected hepatoma cells without significant inflammatory cytokine induction. We determined that induction of the different IFNs by HCV-infected hepatoma cells was cell-specific and involved different pattern recognition receptors (PRRs). We identified mDC2s as the main producer of IFN-λs and confirmed IFN-α production by pDCs and IFN-γ production by NK/NKT cells. These findings broaden our view of anti-HCV immunity and reveal unique roles for different human dendritic cells in initiating immune responses in HCV-infection.
Materials and methods
Cells, replicons, and viruses
JFH-1 genomic RNA was derived from pUC-vJFH-1 plasmid using in vitro transcription. JFH-1 virions were produced by JFH-1-RNA transfected Huh7.5 cells. Detailed information and protocols are described in Supplementary Materials and Methods.
Preparation of human PBMCs, DC subsets and co-culture experiments
Human PBMCs were isolated from peripheral blood from normal human volunteers and chronic HCV-infected patients after informed consent was obtained according to procedures approved by the Committee for Protection of Human Subjects in Research at the University of Massachusetts Medical School. Detailed protocols for DC isolation and co-culture experiments are described in Supplementary Materials and Methods.
Reagents, Bioplex, ELISA, RNA quantification, Flow cytometry, HCV dsRNA generation, IL28B SNP genotyping and other methods and statistical analysis
Please see Supplementary Materials and Methods.
Results
HCV-infected cells induce Type I, II and III IFNs in human PBMCs
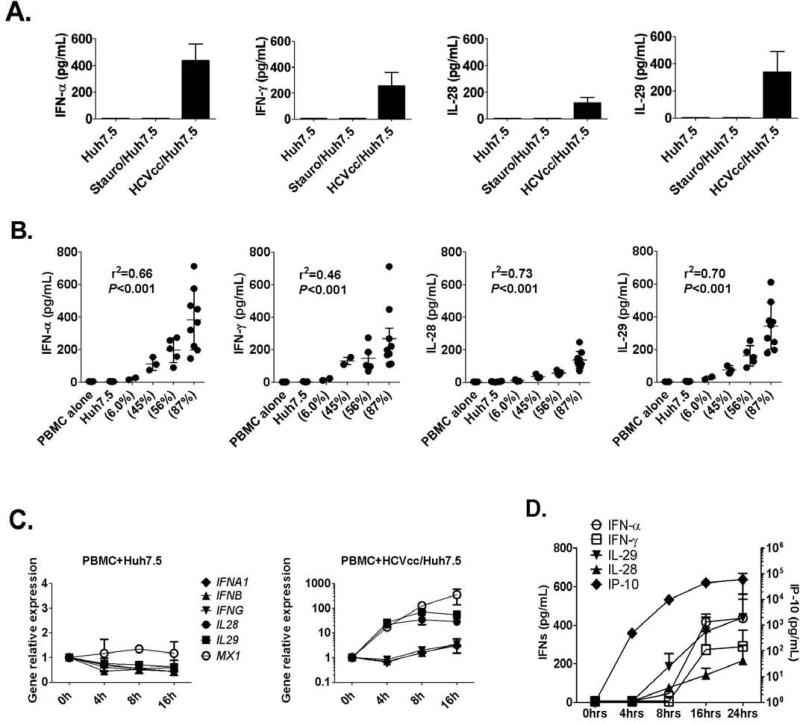
We hypothesized that HCV-infected hepatoma cells represent danger signals and stimulate interferons and inflammatory cytokines in human PBMCs. Using co-cultures between normal human PBMCs and HCVcc/Huh7.5 or control Huh7.5 cells we found that all three types of IFNs (IFN-α, IFN-γ and IFN-λs – IL-28 and IL-29) were produced in the presence of HCVcc/Huh7.5 but not non-infected or apoptotic Huh7.5 cells (Fig. 1A). There was no IFN production by PBMCs, Huh7.5 cells or HCVcc/Huh7.5 cells alone (data not shown). IFN-α, IFN-γ, IL-28 and IL-29 induction was highest in the presence of HCVcc/Huh7.5 cells with the highest percent of HCV infection (87% HCV-infected) and the extent of IFN induction directly correlated with the percent of HCV-infected Huh7.5 cells (Fig. 1B). We found a significant increase in the levels of interferon-inducible gene products, IP-10 (CXCL10) and TRAIL but no induction in inflammatory cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12, RANTES and TNF-α) in co-cultures with HCVcc/Huh7.5 cells (Fig. S1). We also confirmed the induction of CXCL10 and no induction of IL1B, IL6, IL8, IL10 and TNFA at mRNA levels (Fig. S2). Addition of concentrated JFH-1 virions to PBMCs resulted in no IFN-α, -γ or -λ induction (data not shown) suggesting that HCV-infected hepatoma cells and not the isolated virus induced all three types of IFNs without inducing inflammatory cytokines.
Figure 1. All three types of IFNs (IFN-α, IFN-γ and IFN-λs – IL-28 and IL-29) are produced from co-cultures of human PBMCs and HCV-infected cells.
(A) Huh7.5 cells or HCVcc/Huh7.5 cells were co-cultured with PBMCs for 24 hours. IFNs in the supernatants were measured by ELISA (mean±SD; n=6). (B) HCVcc/Huh7.5 cells with different infection percentages (NS3 expression level) were co-cultured with human PBMCs for 24 hours. IFN-levels were measured by ELISA. Mean±SD are shown and every dot represents one data point. (C) Human PBMCs and hepatoma cells were collected before co-culture as 0h control or 4, 8 and 16 hours later. Total RNA was extracted from co-cultured cells and IFNs or MxA (MX1) expression were examined by real-time PCR, GAPDH as internal control (mean±SD; n=3). (D) Huh7.5 cells or HCVcc/Huh7.5 cells were co-cultured with PBMCs. Supernatants were collected 4, 8, 16 and 24 hours later and IFNs or IP-10 levels determined by ELISA (mean±SD; n=3-6).
IL28, IL29 and MX1 gene are rapidly induced from co-cultures between human PBMCs and HCV-infected cells
Time course experiments revealed that IL28, IL29 and ISG (MX1) genes were rapidly induced in co-cultures between human PBMCs and HCVcc/Huh7.5 cells, but not with non-infected Huh7.5 cells (Fig. 1C). None of the type I or II IFNs (including IFNA1, IFNB and IFNG) genes was induced at early time point (4 hours) indicating that the early Type III IFN response against HCV infection was independent of type I/II IFN production. We found increased protein levels of IL-28 and IL-29 at 8 hours after co-culture while IFN-α and IFN-γ levels remained low (Fig. 1D). Furthermore, IP-10, another IFN-inducible gene, was detected at 4 hours, while IFN-α was detectable only at 8 hours after co-culture (Fig. 1D), indicating the existence of IP-10-inducers that were different from pDCs and IFN-α.
Type I, II and III IFNs are produced by different immune cell subsets in response to HCV-infected cells
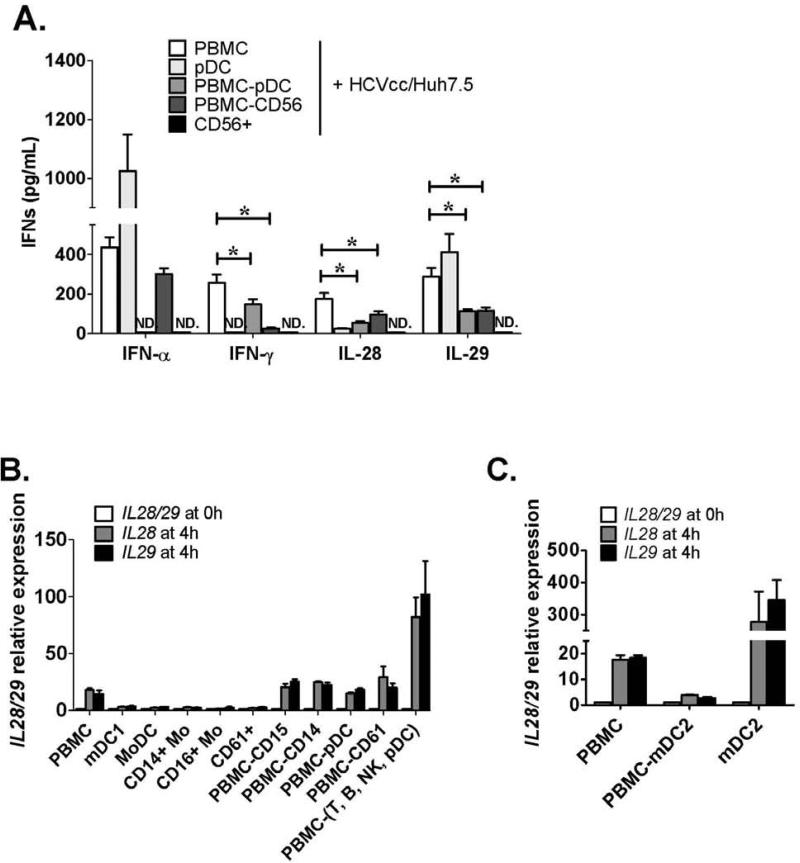
A recent report showed that pDCs produce IFN-α in response to HCV-infected cells.22 To identify the cell types responsible for IFN-γ and IFN-λ production in the PBMC and HCVcc/Huh7.5 co-cultures, we tested isolated pDCs, PBMCs depleted of pDCs, PBMCs depleted of CD56+ cells, and CD56+ cells (NK/NKT cells). We found that isolated pDCs could recognize HCV-infected cells and produce both IFN-α and IL-29, but very little IL-28 and not IFN-γ. IFN-α release was diminished when HCVcc/Huh7.5 cells were co-cultured with PBMCs depleted of pDC (PBMC-pDC) confirming that pDCs were fully responsible for IFN-α production (Fig. 2A). IFN-γ, IL-28 and IL-29 were still produced after pDC depletion, although at lower levels compared with PBMC co-cultures, indicating that IFN-γ, IL-28 and IL-29 production can occur in the absence of pDCs. NK/NKT cells are a major source of IFN-γ production. We found that PBMCs depleted of either NK/NKT cells (PBMC-CD56) or pDCs resulted in a significant decrease in IFN-γ production from co-cultures with HCVcc/Huh7.5 cells. Interestingly, co-culture of purified CD56+ NK/NKT cells and HCVcc/Huh7.5 cells resulted in no IFN-γ production, suggesting that IFN-γ was produced from NK/NKT cells after acquiring help from other accessory cells in the PBMC population.
Figure 2. IFN-α is produced by pDC, IFN-γ is produced by NK/NKT, while IFN-λ is produced by mDC2.
(A) HCVcc/Huh7.5 cells were co-cultured with human PBMCs, purified pDCs, PBMCs depleted of pDCs (PBMC-pDC), PBMCs depleted of NK/NKT (PBMC-CD56) or purified NK/NKT (CD56+) cells. IFNs in the supernatants were measured by ELISA after 24 hours (mean±SD; n=3-6 and (*) indicates p<0.05). (B) HCVcc/Huh7.5 cells were co-cultured with PBMCs, purified CD1c+ myeloid DCs (mDC1), monocyte derived DCs (MoDC), purified monocytes (CD14+ monocytes), CD16+ monocytes, platelet associated cells (CD61+), PBMCs depleted of granulocytes (PBMC-CD15), PBMCs depleted of monocytes (PBMCCD14), PBMCs depleted of pDC (PBMC-pDC), PBMCs depleted of platelet-associated cells (PBMCCD61) or PBMCs depleted of lymphocytes [PBMC-(T,B,NK,pDC)]. Co-cultured cells were preserved before co-culture as 0h control or collected after 4 hours. Total RNA was extracted from the cells and IL28 or IL29 expression examined by real-time PCR (mean±SD; n=2). (C) HCVcc/Huh7.5 cells were co-cultured with PBMCs, PBMCs depleted of mDC2 cells (PBMC-mDC2), or purified mDC2s. IL28 and IL29 expression from co-cultures was examined by real-time PCR (mean±SD; n=2).
mDC2s (BDCA3+ dendritic cells) are the major immune cell subset that initiates a Type III IFN response
Our data suggested a pDC-independent recognition of HCV-infected cells and an early induction of IL-28 and IL-29 in human PBMCs. To identify the immune cell subset responsible for IL-28 and IL-29 production, isolated subsets of mDC1 (CD1c+ dendritic cells), monocyte-derived dendritic cells (MoDC), CD14+ monocytes, CD16+ monocytes and platelet-associated cells (CD61+ cells) were co-cultured respectively with HCVcc/Huh7.5 cells. However, none of these cell populations induced IL28 and IL29 at mRNA levels (Fig. 2B). Depletion of granulocytes (PBMC-CD15), monocytes (PBMC-CD14), pDCs (PBMC-pDC) or contaminated platelets (PBMC-CD61) from human PBMCs by magnetic bead separation revealed that the remaining cells still retained the capacity to induce IL28 and IL29 expression when co-cultured with HCVcc/Huh7.5 cells (Fig. 2B). Furthermore, after depletion of all lymphocytes and pDCs (PBMC-(T,B,NK,pDC) by a cocktail of antibody labeled beads (CD3+, CD19+, CD16/56+ and CD123+), the remaining cells produced high levels of IL28 and IL29, indicating the presence of an immune subset of IFN-λ producing cells.
Recently, human mDC2s (also known as BDCA3+ mDCs) were identified as the counterpart of murine CD8α+ DCs15-17 and one report showed that both murine CD8α+ DCs and human BDCA3+ mDCs produce IFN-λs in response to poly I:C stimulation.23 Thus, we tested whether human mDC2 was responsible for IL28 and IL29 induction by HCV-infected cells. We found robust IL28 and IL29 induction from co-cultures of enriched mDC2 with HCVcc/Huh7.5 cells, while IL28 and IL29 induction was diminished when PBMCs were depleted of mDC2 (Fig. 2C) indicating that mDC2 was the major cell population producing IFN-λs in response to HCV-infected cells.
mDC2s respond to HCV-infected cells or the TLR3-ligand, Poly I:C, and produce high levels of IL-29
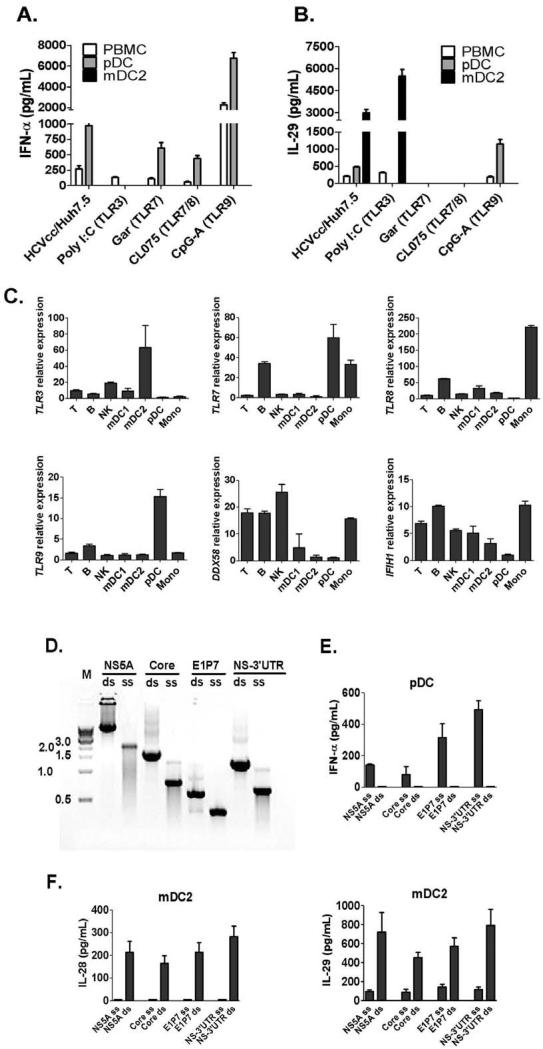
To evaluate the involvement of pattern recognition receptors (PRRs) in IFN-α and IFN-λ induction in pDCs and mDC2s, we tested TLR3, 7, 8 or 9 ligands that mimic viral PAMPs. We did not detect IFN-α release from mDC2s, while pDCs produced IFN-α after co-culture with HCVcc/Huh7.5 cells or stimulation with TLR7, 8 or 9 ligands (Fig. 3A). We found high levels of IL-29 in co-cultures of HCVcc/Huh7.5 cells and mDC2s. In contrast, pDC produced low levels of IL-29 in response to HCVcc/Huh7.5 cells (Fig. 3B). We further determined that mDC2s produced high levels of IL-29 in response to TLR3-ligand (Poly I:C) and not to TLR7-, 8- or 9-ligand stimulation (Fig. 3B). IL-29 production in pDCs required TLR9 (CpG-A) stimulation and TLR3-, 7- or 8-ligands failed to induce IL-29 in isolated pDCs (Fig. 3B). Consistent with these findings, we found that mDC2s had the highest level of TLR3 mRNA expression among PBMCs, while pDCs specifically expressed TLR7 and TLR9 mRNA (Fig. 3C).
Figure 3. mDC2s specifically express TLR3 and produce IFN-λs in response to HCV-dsRNA or poly I:C stimulation.
(A-B) Human PBMC, purified pDCs, or mDC2s were co-cultured with HCVcc/Huh7.5 cells, or stimulated with different TLR ligands: TLR3 - Poly I:C (10μg/mL), TLR7 – Gardiquimod (1μg/mL), TLR7/8 - CL075 (2.5μg/mL) and TLR9 - CpG-A (2μM) for 24 hours. IFNs in the supernatants was measured by ELISA (mean±SD; n=3). (C) mRNA expression of TLRs (TLR3, 7, 8 and 9) and RLRs (DDX58 and IFIH1) was examined by real-time PCR from different immune subsets in PBMCs using GAPDH as internal control. Relative mRNA expressions are shown (mean±SD; n=3). (D) Gel electrophoresis of in vitro synthesized HCV ssRNA and dsRNA derived from the indicated HCV genome region. M: 1 kb DNA ladder (New England Biolabs Inc). (E-F) Isolated pDCs or mDC2s were stimulated by 10 μg/mL HCV-ssRNA or dsRNA mixed with lipofectamine 2000 for 24 hours. IFN levels are measured by ELISA (n=3). IFN-α production in pDCs was shown in (E), while IL-28 and IL-29 production in mDC2s was shown in (F).
Because of their specific expression of either TLR3 or TLR7, we suspected that mDC2s and pDCs responded to ssRNA-ligand or dsRNA-ligand in HCV-infected cells and produced IFN-α and IFN-λ respectively. To test this hypothesis, a series of HCV-ssRNAs and ds-RNAs with different lengths were generated to stimulate human pDCs or mDC2s (Fig. 3D). We found that mDC2 preferentially responded to synthesized HCV-dsRNA rather than ssRNA, produced IL-28 and IL-29 and increased expression of CD80 and CD86 (Fig. 3E) while pDCs preferentially responded to synthesized HCV-ssRNAs with IFN-α production and increased co-stimulatory molecule expression (CD80 and CD86) (Fig. 3F and Fig. S3). We also examined the involvement of PPRs in IFN-λ induction by primary human hepatocytes (PHHs) in response to HCV-infection. We found that TLR3 and TLR7/8 ligands failed to stimulate IFN-λ production in PHHs, while JFH-1 virions or RIG-I ligand induced both IL-28 and IL-29 in PHHs (Fig. S4).
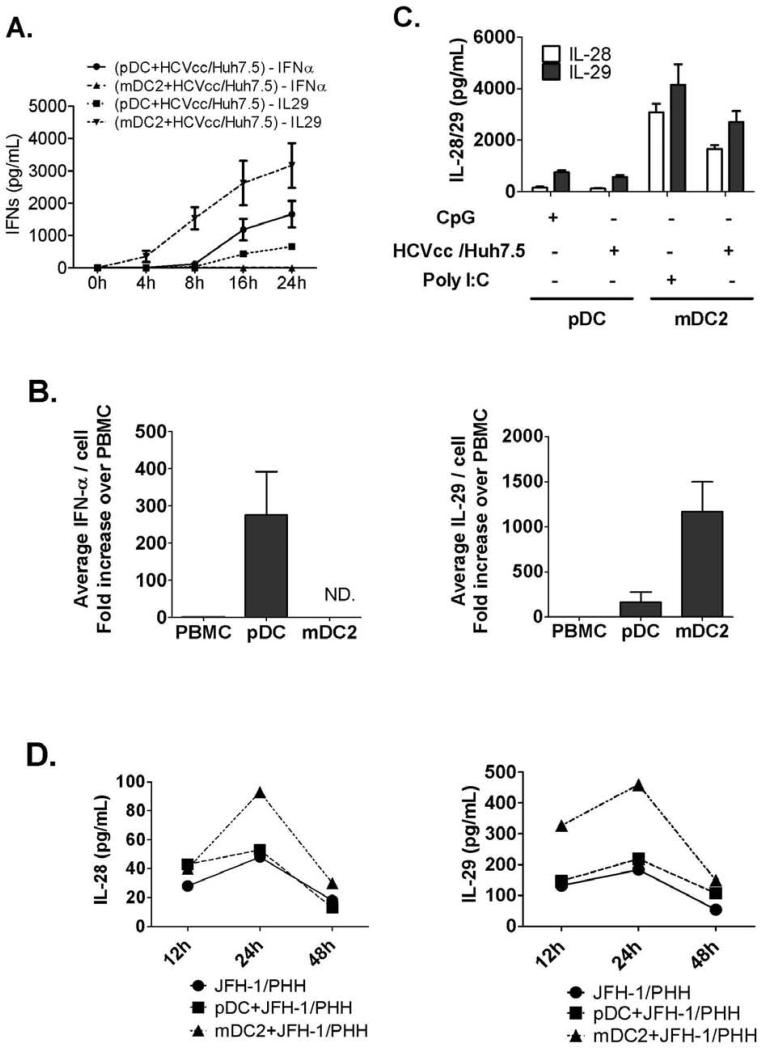
Further analysis of the pDC and mDC2 responses after recognition of HCV-infected cells revealed unique immunological features of mDC2. First, mDC2 showed rapid IL-29 production compared to pDCs, indicating rapid recognition and response in mDC2s after exposure to HCV-infected hepatoma cells (Fig. 4A). Second, we determined that on an average, the same number of mDC2s produced 10 times more IL-29 than pDCs in response to HCVcc/Huh7.5 cells (Fig. 4B). We also confirmed that pDCs produced large amounts of IFN-α (Fig. 4B). Next, we showed for the first time that the IFN-λ response by mDC2s included both IL-28 and IL-29 production in response to HCVcc/Huh7.5 cells or Poly I:C stimulation. In contrast, pDCs produced low levels of IL-29 and very little IL-28 when activated by HCVcc/Huh7.5 cells or CpG-A (Fig. 4C). Finally, we found that mDC2s and not pDCs produced both IL-28 and IL-29 in response to JFH-1-infected PHHs (Fig. 4D). These observations identified mDC2s as the major cellular source of IL-28 and IL-29 production in response to HCV-infected hepatocytes.
Figure 4. Functional dichotomy of pDCs and mDC2s in IFN production.
(A) Purified pDC or mDC2 populations were co-cultured with HCVcc/Huh7.5 cells and culture supernatants were collected at different time points for IFN-α and IL-29 measurement by ELISA (mean±SD; n=2). (B) PBMC-IFN production per cell was artificially set as 1 unit, then average IFN release from pDCs or mDC2s was calculated and compared (mean±SD; n=3). (C) Purified pDCs or mDC2s were co-cultured with HCVcc/Huh7.5 cells, or stimulated with CpG-A or Poly I:C respectively. 24 hours later, IL-28 or IL-29 in culture supernatants was measured by ELISA (mean±SD; n=3). (D) JFH-1 infected PHHs were collected 48h post infection for co-culture with human pDCs or mDC2s. Co-culture supernatants were collected at different timepoints and IL-28 or IL-29 levels were measured by ELISA. Representative data of one experiment was shown.
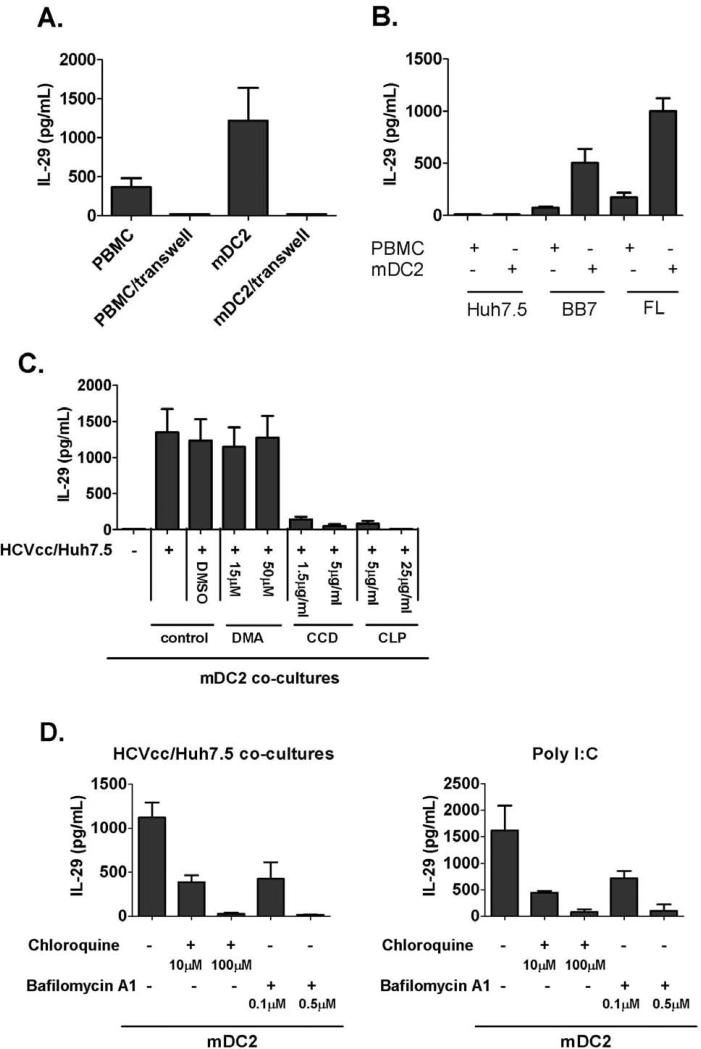
Recognition of HCV-infected cells by mDC2s requires cell-cell contact and involves the endocytosis pathway
The liver tissue environment allows close contact between hepatocytes and immune cells. We found that separation of mDC2s and HCV-infected hepatoma cells with a transwell insert fully prevented IL-29 induction, indicating that cell-cell contact was necessary for mDC2 activation (Fig. 5A). We also found that concentrated HCV virions failed to induce IL-29 production in mDC2 (Fig. S5). To further exclude the possibility that HCV-virions coming from infected cells cause mDC2-activation, HCV-replicon cell lines (BB7 and FL) which do not produce infectious virions were co-cultured in co-cultures with human mDC2. Robust IL-29 induction was present at both mRNA levels and protein levels in mDC2 co-cultures with replicon cells (Fig. 5B and Fig. S6). We next hypothesized that mDC2-IFN-λ induction from HCV-infected cells occurs through an endocytosis-mediated pathway. We determined that inhibitors of endocytosis (CCD and CLP) significantly diminished IL-29 release from mDC2-hepatoma co-cultures, while inhibitors of macropinocytosis (DMA) did not (Fig. 5C). Finally, since TLR3 signaling induced IFN-λ production in mDC2, we tested whether endosome function was required for mDC2 activation. We found that pretreatment of mDC2 with inhibitors of endosome acidification (choloroquine and bafilomycin) abolished the IL-29 production from mDC2 and HCV-infected hepatoma co-cultures (Fig. 5D), indicating the signaling transduction pathway involved endosome acidification. IL-29 production was also abolished in endosomal-inhibitor treated mDC2s stimulated with the TLR3-ligand, poly I:C.
Figure 5. HCV-induced IFN-λ induction requires cell-to-cell contact and endosomal acidification.
(A) HCVcc/Huh7.5 cells were co-cultured with human PBMCs or purified mDC2s in a normal plate or a plate with a transwell insert. IL-29 in the supernatants was measured by ELISA after 24 hours (mean±SD; n=3), (B) Huh7.5 cells and HCV replicon cells – BB7 and FL cells were co-cultured with human PBMCs or purified mDC2s. IL-29 in culture supernatants was measured by ELISA (mean±SD; n=2), (C) HCVcc/Huh7.5 cells were co-cultured with purified mDC2 in the absence or presence of DMA, CCD or CLP. IL-29 production was measured by ELISA (mean±SD; n=2), (D) purified human mDC2s were co-cultured with HCVcc/Huh7.5 cells or stimulated with Poly I:C in the presence of chloroquine or bafilomycin as indicated. IL-29 production was measured by ELISA after 24 hours (mean±SD; n=2).
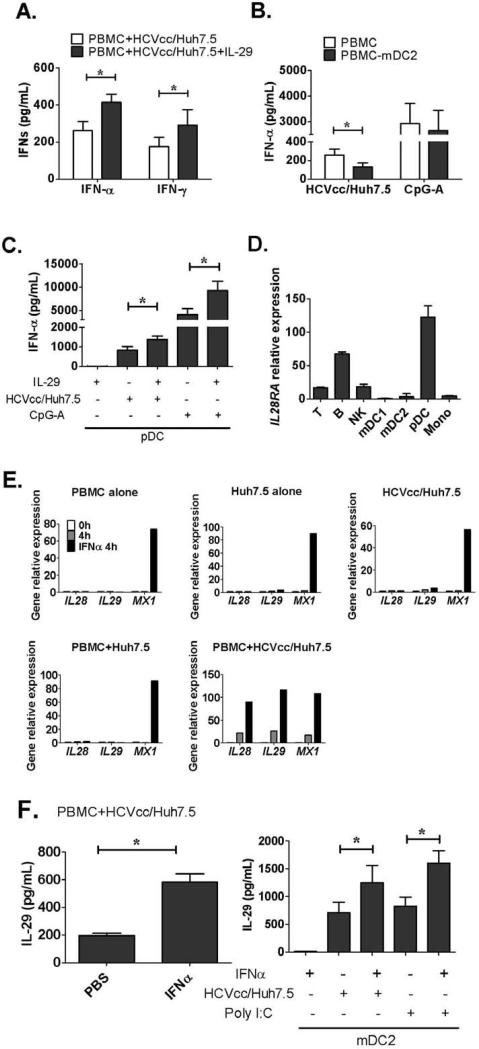
IFN-λ and IFN-α amplify antiviral IFN induction
Recently, SNP studies revealed a close association between the IL28B gene and the outcome of HCV-infected individuals, however, the molecular basis for this finding is unknown.5-8 We hypothesized that type I and type III IFN mutually amplify their antiviral effects during HCV infection. Since mDC2-IFN-λ production preceded pDC-IFN-α production in co-cultures, we first examined the possible effect of IFN-λ on IFN-α production. We found that addition of recombinant IL-29 significantly increased IFN-α and IFN-γ release in PBMC plus HCVcc/Huh7.5 co-cultures (Fig. 6A), while depletion of mDC2 from PBMCs (PBMC-mDC2) significantly reduced IFN-α production (Fig. 6B). In contrast, depletion of mDC2 failed to significantly affect PBMC IFN-α production after CpG-A stimulation suggesting that the presence of mDC2 in HCV infection promotes pDC function (Fig. 6B). Next, we tested whether IFN-λ had a direct effect on pDC-IFN-α production. We found that addition of recombinant IL-29 significantly enhanced pDC IFN-α production in response to HCV-infected hepatoma cells or CpG-A stimulation (Fig. 6C). There was no IFN-α induction by IFN-λ alone (Fig. 6C). Furthermore, we determined that among all cell populations in our study pDCs showed the highest expression of IL28RA which is the specific receptor for IFN-λ (Fig. 6D). We also confirmed that human pDCs expressed both the functional and no-functional isoform of IL28RA mRNA, while hepatoma cells, Huh7 and Huh7.5 cells, mainly expressed the functional isoform (Fig. S7).
Figure 6. Cross-regulations exist between IFNs induction in co-cultures.
(A) HCVcc/Huh7.5 cells were co-cultured with human PBMCs in the absence or presence of recombinant IL-29 (25ng/mL). IFN-α and IFN-γ levels were measured by ELISA (mean±SD; n=3), (B) Human PBMCs or PBMC depleted of mDC2s (PBMC-mDC2) were co-cultured with HCVcc/Huh7.5 cells or stimulated with CpG-A. IFN-α production in the supernatants was measured by ELISA (mean±SD; n=3-4), (C) purified human pDCs were co-cultured with HCVcc/Huh7.5 cells or stimulated with CpG-A in the absence or presence of IL-29. IFN-α production was measured by ELISA (mean±SD; n=3), (D) total RNA was extracted from different immune subsets in human PBMCs. IL28RA expression was examined by real-time PCR and normalized to GAPDH (mean±SD; n=3), (E) Human PBMCs, Huh7.5 cells, HCVcc/Huh7.5 cells, co-culture of PBMCs and Huh7.5 cells or co-culture of PBMCs and HCVcc/Huh7.5 cells were preserved before culture as 0h control or collected after 4 hours culturing in the absence or presence of IFN-α-2a (100U/mL). IL28, IL29 and MX1 induction were measured be real-time PCR, one representative experiment of three is shown. (F) Human PBMCs or purified mDC2s were co-cultured with HCVcc/Huh7.5 cells or stimulated with Poly I:C in the absence or presence of IFN-α-2a. IL-29 production in the supernatants was measured by ELISA (mean±SD; n=3). (*) indicates p<0.05.
Next, to examine the effect of IFN-α on IFN-λ production, we added recombinant IFN-α to human PBMC or to HCV-infected cells and observed no induction of IL28 and IL29 in spite of increases in MX1, an IFN-α-induced gene (Fig. 6E). However, IFN-α treatment in human PBMC co-cultures with HCVcc/Huh7.5 cells resulted in significantly increased IL28 and IL29 induction at the mRNA level (Fig. 6E) and the protein level (Fig. 6F). Finally, to test whether IFN-α could directly affect mDC2 function, we added recombinant IFN-α to human mDC2s and found a significant increase in mDC2-IFN-λ production in co-cultures or after Poly I:C stimulation (Fig. 6F), indicating that IFN-α can augment immune responses against HCV infection through regulating Type III IFNs production by mDC2s. IFN-α alone did not induce IL-28 and IL-29 production in mDC2s (Fig. 6F).
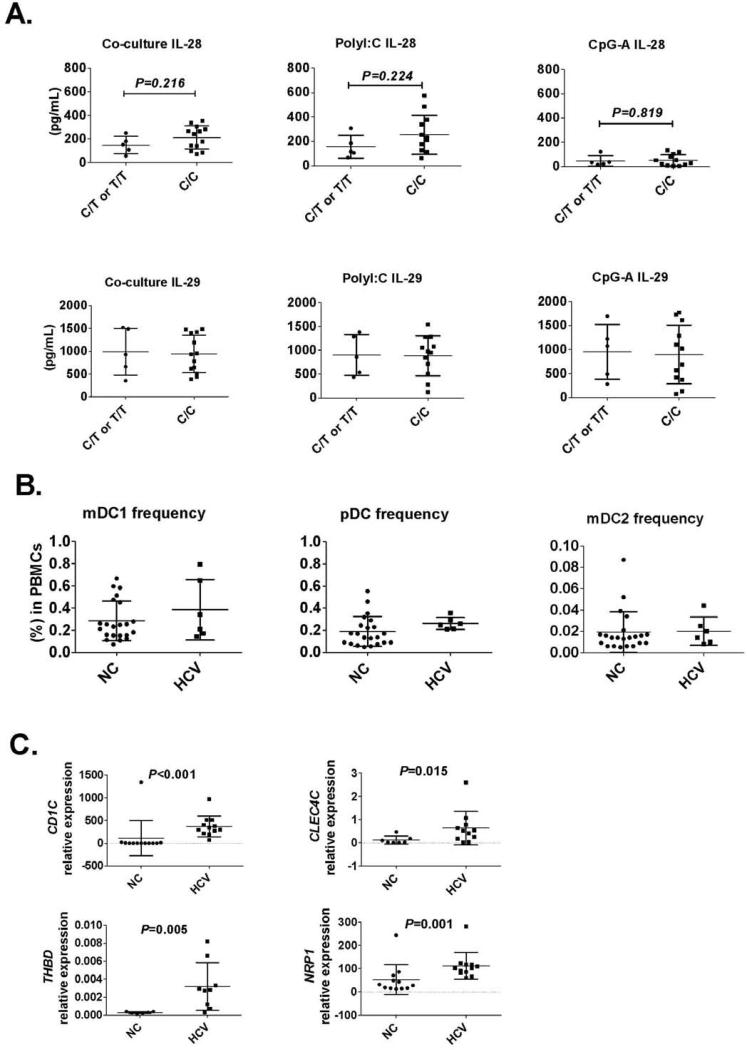
Genotype of rs12979860, chronic hepatitis C and IFN-λ production in PBMCs
Given the importance of IL-28 SNPs in clinical outcomes, we examined the effect of rs12979860 genotype on PBMCs-IFN-λ production in normal donors with “C/C” and “C/T or T/T” genotypes. We found no significant difference in IL-28 and IL-29 production in PBMCs from T/T, C/T or C/C groups in response to JFH-1 infected hepatoma cells, poly I:C or CpG-A stimulation (Fig. 7A). We found comparable frequencies of circulating mDC1, mDC2 and pDC in chronic HCV-infected patients and that their PBMCs showed no defect in IFN-λ induction in response to JFH-1 infected hepatoma cells or poly I:C stimulation (Fig. 7B and Fig. S8).
Figure 7.
Genotype of rs12979860 does not correlate with IL-28 and IL-29 induction in human PBMCs by HCV-infected cells. (A) Human PBMCs were co-cultured with JFH-1 infected Huh7.5 cells or stimulated with PolyI:C (20μg/mL) or CpG-A (2μM) for 24 hours. Co-culture supernatants were collected and IL-28 or IL-29 levels determined by ELISA. Each dot represents one individual in the figures. (B) Circulating mDC1, mDC2 and pDC frequencies from normal control (NC) or naïve HCV patients (HCV) were determined by flow cytometry. (C) BDCA1 (CD1C), BDCA2 (CLEC4C), BDCD3 (THBD) and BDCA4 (NRP1) mRNA expression in human livers were determined by realtime PCR using GAPDH as internal control.
Finally, we found significantly higher expression of DC markers, BDCA1 (CD1C), BDCA2 (CLEC4C), BDCA3 (THBD) and BDCA4 (NRP1), in chronic HCV-infected livers compared to non-infected livers (Fig. 7C), indicating the recruitment of mDCs and pDCs into the liver during HCV infection. We also confirmed that chronic HCV-infected livers had significantly higher ISGs mRNA expression, including ISG15, ISG56, MX1 and CXCL10 compared to controls (Fig. S9).
Discussion
HCV infection stimulates an early IFN response in the liver and paradoxically, high interferon-stimulated gene expression correlates with poor viral clearance.1, 4 However, the exact mechanisms by which immune cells recognize HCV and/or HCV-infected hepatocytes are only partially understood. In this study, using co-cultures of human PBMCs and HCV-infected hepatoma cells, we demonstrated that all three types of IFNs (IFN-α, IFN-γ and IFN-λ) are induced. We identified the mDC2 population as a source of IFN-λ production in HCV infection. We further demonstrated that IFN induction is TLR- and cell-specific. IFN-λ production required TLR3 activation in mDC2s that responded to HCV-dsRNA in contrast to IFN-α production by pDCs that required TLR7 activation by HCV-ssRNA. Our novel data indicated that IFN-λ induction in mDC2s was dependent on direct cell contact with the HCV-infected hepatoma cells and both endocytosis and endosomal acidification were involved. Finally, we showed for the first time that IFN-α and IFN-λ have mutual cross-regulatory effects and amplify IFN production in response to HCV-infected hepatoma cells.
A recent report indicates that pDCs recognize HCV-infected cells through cell-cell contact.22 We show here that mDC2s recognize HCV-infected cells using a cell-cell contact dependent mechanism. We found that only HCV-infected cells and not HCV virions activated mDC2s. In support of this, HCV replicon cells, which do not produce HCV virions, also stimulated mDC2s. Furthermore, we demonstrated that mDC2s capture their ligands from HCV-infected cells through an endocytosis and not macropinocytosis mediated pathway. Interestingly, earlier reports indicated that this phenomenon also occurred during other human viral infections, such as HIV and influenza infection.24, 25
Compared with type I IFNs, the cellular source and induction pattern of IFN-λs, including IL-28a, IL-28b and IL-29, are undefined. Recently, it was reported that human hepatocytes produce IFN-λs in vitro after HCV-infection.10 Here we also found IFN-λ induction by HCV infection in primary human hepatocytes and our novel data show an interesting paradigm of IFN-λ production in human immune cells after recognition of HCV-infected cells where mDC2s produce high levels of IL-28 and IL-29 while pDCs only produce low levels of IL-29. Our findings support the hypothesis that mDC2s are specialized IFN-λ producers inresponse to HCV infection; this is in agreement with a recent study where mouse CD8α+ DCs, the murine homologue of human mDC2, were found to produce IL-28 and IL-29 during poly I:C injection in vivo.23 Although IFN-λs are speculated to be induced through similar signaling pathways as type I IFNs during viral infection,26 we found that IFN-α and IFN-λ had distinct cellular sources in pDCs and mDC2s respectively, indicating that the mechanism of controlling induction of type I IFNs and IFN-λs induction is cell- and stimulation-specific. In other studies, IFN-α induction was limited to a small fraction of pDCs in a similar system22 and at this time we cannot rule out the possibility that within the mDC2 population only a subset of cells produces very large amounts of IL-28 and/or IL-29. Our results suggested that the IFN-production profile is finely tuned during immune response to HCV and depends on differential usage of PRRs or transcriptional factors in different cell types.
The mammalian immune system mainly utilizes two groups of PRRs to sense viral infection, RLRs (RIG-I like receptors) and TLRs (TLR3, 7, 8 or 9).27 RLRs localize in the cytosol and usually detect intracellular viruses. Since HCV does not effectively infect immune cells, it is speculated that immune cells recognize HCV infection through TLR pathways. Indeed, human pDC can recognize HCV through a TLR7-mediated pathway.22 We present novel evidence that human mDC2s recognize HCV infection and that IFN-λ induction is mediated by the dsRNA-sensing, TLR3-mediated pathway. Consistent with earlier reports,28 mDC2s specifically expressed high levels of TLR3 and very low levels of TLR7 and 9 mRNA in our experiments; in addition, only the TLR3 ligand, poly I:C or HCV-dsRNA, and not TLR7-, 8- and 9-ligands or HCV-ssRNA, stimulated mDC2-IFN-λ production. We also found that inhibitors of endosome acidification abolished mDC2-IFN-λ production in co-cultures, further indicating that a TLR and not an RLR pathway was responsible for recognition of HCV by mDC2.
SNP studies revealed that IL-28b (IFN-λ3) is closely associated with the recovery of natural HCV infection or after IFN-α therapy;5-8 however, the molecular basis is unclear. Although we found no defects in IFN-λ production in donors with unfavorable SNP genotype, our results provide other key information for reconsidering the underlying mechanisms as we revealed inter-regulatory relationships between type I IFN and type III IFN. In our study, IFN-λ was released earlier than IFN-α in the PBMC-HCV-infected hepatoma co-cultures and IFN-λ could directly regulate pDC to increase their IFN-α production. In contrast to earlier reports suggesting that minimal expression or a spliced form of IL-28Ra, the specific receptor for IFN-λ, on hematopoietic cells that results in their low responsiveness to IFN-λ,29, 30 we found that pDCs express higher level of IL-28Ra compared with other cell populations in PBMCs, indicating that this responsiveness to IFN-λ is unique and important for pDC. Conversely, we also found that IFN-α could directly affect mDC2 function and significantly increase IFN-λ production. It is tempting to speculate that exogenous IFN-α would increase IFN-λ production by mDC2s during IFN-α therapy and this could provide a potential explanation why SNP types of IL-28b predict the outcome of IFN-α therapy.
The changes of cell number and function of mDC1s (CD1c+ mDCs) and pDCs in acute and chronic HCV infection had been characterized in several studies, however, the study of mDC2 was hindered by its previous unknown function and low frequency.31 Here we found that the frequency and IFN-λ production by circulating mDC2s from chronic HCV-infected patients was comparable to normal controls. However, the expression of mDC2 marker, BDCA3, was significantly increased in chronic HCV-infected livers indicating an enriched mDC2 population in the local liver environment in chronic HCV infection. Importantly, since IFN-λ induction in chronically HCV-infected hepatocytes could be inhibited due to MAVS and TRIF cleavage, we suspect that liver resident mDC2s and pDCs might represent an alternative or necessary resource of IFNs when hepatocyte-derived IFN production is disabled by HCV.
In conclusion, our novel data identifies a unique DC population, mDC2s, as an important source of IFN-λ production in response to HCV-infected hepatoma cells. These observations also indicate that innate immune cells can efficiently initiate immune responses against HCV infection.
Acknowledgements
This work was supported by NIH grant R37AA014372 (GS) and AI069285 (KL). The authors thank Drs Charles M.Rice and Takaji Wakita for kindly providing reagents. PHH cultures and human liver samples were provided by the National Institutes of Health-funded Liver Tissue Procurement and Cell Distribution System (N01-DK-7-0004/HHSN26700700004C) (principal investigator: Stephen Storm, University of Pittsburgh).
Abbreviations used in this paper
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- ISG
interferon-stimulated gene
- MAVS
mitochondrial antiviral-signaling protein
- mDC
myeloid dendritic cell
- NK
natural killer
- NKT
natural killer T
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- pDC
plasmacytoid dendritic cell
- PHH
primary human hepatocytes
- PRR
pattern recognition receptor
- RLR
RIG-I like receptor
- SNP
single nucleotide polymorphism
- TLR
toll-like receptor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have declared that no competing interests exist.
Author Contributions-list: Study concept and design: GS SZ. Acquisition, analysis and interpretation of data: SZ KK GS. Drafting of the manuscript: SZ KK GS. Critical revision of the manuscript for intellectual content: SZ KL GS. Administrative, technical, or material support: KK KL GS. Obtained funding: GS.
References
- 1.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105:7034–9. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60:1284–93. doi: 10.1136/gut.2010.222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang KR, Wu JJ, Huang DB, et al. Biological and clinical basis for molecular studies of interferons. Methods Mol Med. 2005;116:1–23. doi: 10.1385/1-59259-939-7:001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 7.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–4. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir AJ, Shiffman ML, Zaman A, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822–32. doi: 10.1002/hep.23743. [DOI] [PubMed] [Google Scholar]
- 10.Marukian S, Andrus L, Sheahan TP, et al. Hepatitis C virus induces interferon-lambda and interferon-stimulated genes in primary liver cultures. Hepatology. 2011;54:1913–23. doi: 10.1002/hep.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K, Li NL, Wei D, et al. Activation of chemokine and inflammatory cytokine response in HCV-infected hepatocytes depends on TLR3 sensing of HCV dsRNA intermediates. Hepatology. 2011;55:666–75. doi: 10.1002/hep.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–7. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 14.Piccioli D, Tavarini S, Borgogni E, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–9. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 15.Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulin LF, Salio M, Griessinger E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reizis B, Bunin A, Ghosh HS, et al. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marukian S, Jones CT, Andrus L, et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48:1843–50. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–95. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 21.Gondois-Rey F, Dental C, Halfon P, et al. Hepatitis C virus is a weak inducer of interferon alpha in plasmacytoid dendritic cells in comparison with influenza and human herpesvirus type-1. PLoS One. 2009;4:e4319. doi: 10.1371/journal.pone.0004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Asabe S, Wieland S, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431–6. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauterbach H, Bathke B, Gilles S, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–17. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepelley A, Louis S, Sourisseau M, et al. Innate sensing of HIV-infected cells. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui G, Manches O, Angel J, et al. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS One. 2009;4:e7111. doi: 10.1371/journal.pone.0007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iversen MB, Paludan SR. Mechanisms of type III interferon expression. J Interferon Cytokine Res. 2010;30:573–8. doi: 10.1089/jir.2010.0063. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki C, Miyamoto R, Hoshino K, et al. Conservation of a chemokine system, XCR1 and its ligand, XCL1, between human and mice. Biochem Biophys Res Commun. 2010;397:756–61. doi: 10.1016/j.bbrc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Sommereyns C, Paul S, Staeheli P, et al. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witte K, Gruetz G, Volk HD, et al. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–14. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
- 31.Dolganiuc A, Szabo G. Dendritic cells in hepatitis C infection: can they (help) win the battle? J Gastroenterol. 2011;46:432–47. doi: 10.1007/s00535-011-0377-y. [DOI] [PubMed] [Google Scholar]