Synopsis
For people with diabetes, hypoglycemia remains the limiting factor in achieving glycemic control. This article reviews recent advances in how the brain senses and responds to hypoglycemia. Novel mechanisms by which people with insulin treated diabetes develop hypoglycemia unawareness and impaired counterregulatory responses are outlined. Prevention strategies for reducing the incidence of hypoglycemia are discussed
Keywords: hypoglycemia, unawareness, glucose, diabetes, counterregulation, brain, hypothalamus, hypoglycemia-associated autonomic failure (HAAF)
Hypoglycemia - the clinical problem
Poorly-controlled diabetes is associated with vascular complications including renal failure, peripheral vascular disease, neuropathy, blindness, amputations, coronary artery disease and stroke. Multiple large clinical trials have shown the benefits of intensifying glycemic control in preventing or delaying microvascular complications. These trials, however, consistently report significantly higher rates of hypoglycemia in patients that intensify their glycemic control1-5. Thus, hypoglycemia becomes the limiting factor for blood glucose management in patients with diabetes and precludes the attainment of microvascular benefits associated with tight glycemic control.
Incidence of Hypoglycemia
Compared to earlier self-reported and glucometer-based studies, continuous glucose monitor based studies more accurately assess the true incidence of hypoglycemia. In reasonably well-controlled patients with Type 1 diabetes (HbA1c 7.6%), biochemical hypoglycemia (<60mg/dl) averaged a disconcertingly high 2.1 times per 24 hours6. Of note, even in the group of people, who reported intact hypoglycemia awareness, biochemically confirmed hypoglycemia failed to elicit symptoms 62% of the time6. Thus, symptomatic hypoglycemia underestimates the true incidence of hypoglycemia and hypoglycemia awareness is not an “all or none” phenomena.
As discussed below, episodes of moderate hypoglycemia are not without clinical consequences. Recurrent episodes of moderate hypoglycemia can lead to decreased sympathoadrenal responses and decreased awareness of hypoglycemia, collectively termed hypoglycemia associated autonomic failure (HAAF)7, which leads to an increased risk of more frequent and more severe episodes of hypoglycemia.
Severe Hypoglycemia
Severe hypoglycemia is defined clinically as occurring when the patient requires assistance from another individual to correct hypoglycemia. For insulin treated diabetic patients, severe hypoglycemia has a high prevalence (46 and 25%) and high incidence (3.2 and 0.7 episodes per person-year) for people with Type 1 and Type 2 diabetes, respectively8. Severe hypoglycemia is associated with excess morbidity and mortality7. It can alter brain structure9 and cause brain damage10,11_ENREF_9, cognitive dysfunction12,13 and even sudden death14. It is estimated that between 6 and 10% of patients with Type 1 diabetes die from hypoglycemia15-17_ENREF_13. The mechanisms by which low glucose levels lead to sudden death has not been entirely worked out, but appears to be related to intensive sympathetic activation leading to fatal cardiac arrhythmias18,19.
The Counterregulatory Response to Hypoglycemia
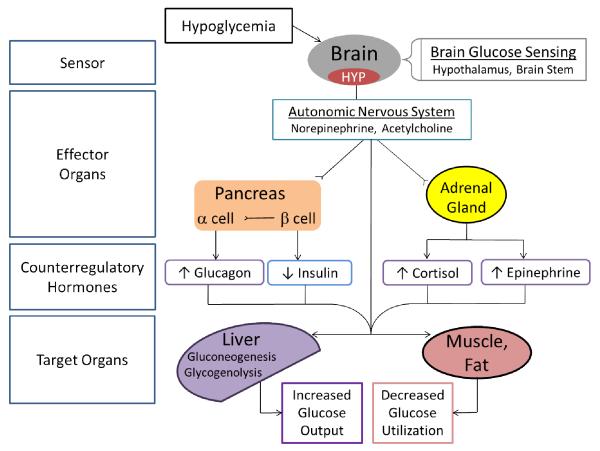
Since the brain is continuously dependent on peripheral glucose for metabolism, robust counterregulatory mechanisms exist to rapidly increase blood glucose levels in order to protect the body from the pathological consequences of hypoglycemia. In the setting of absolute or relative hyperinsulinemia, the counterregulatory response (CRR) is normally initiated when glucose levels fall below 80 mg/dl. The CRR to hypoglycemia normally includes suppression of endogenous insulin secretion and increase the secretion of glucagon, catecholamines (epinephrine, norepinephrine), cortisol, and growth hormone, which together act to increase plasma glucose levels by stimulating hepatic glucose production and limiting glucose utilization in peripheral tissues (Figure 1).
Figure 1. The counterregulatory response to hypoglycemia.
Hypoglycemia is first sensed in various brain regions including the hypothalamus and brain stem. Low glucose in these brain regions stimulates the autonomic nervous system to release norepinephrine and acetylcholine at postganglionic nerve terminals and induce symptoms of hypoglycemia (hypoglycemia awareness). A principal counterregulatory response is the secretion of glucagon which may be stimulated by various mechanisms including independent α-cell glucose sensing, autonomic innervation, epinephrine stimulation, and a reduction of intra-islet insulin secretion. Via autonomic stimulation, epinephrine is released from the adrenal medulla. Not shown is the hypothalamic-pituitary-adrenal axis by which the release of ACTH from the pituitary stimulates cortisol release from the adrenal cortex. The cumulative effect of the sympathetic nervous system and counterregulatory hormones at the level of the liver is to increase hepatic gluconeogeneis and glycogenolysis while the effect at muscle and adipose tissue is to decrease peripheral glucose utilization.
Glucagon Response to Hypoglycemia
Normally, as blood glucose levels fall, increased glucagon secretion from the pancreatic alpha cells along with decreased insulin secretion are the primary counterregulatory mechanisms by which hepatic glucose production is increased. Insulin-deficient diabetes results in an acquired defect of the glucagon response20. Several mechanisms have been proposed to explain this phenomenon, including defective alpha cell glucose sensing, absent decrements in insulin secretion (intra-islet crosstalk), and reduced autonomic stimulation (recently reviewed21 22). Elevated intra-islet somatostatin within the diabetic pancreas may also play a role in limiting the glucagon response to hypoglycemia because pharmacological antagonism of the somatostatin receptor can restore the glucagon response to hypoglycemia in diabetic rats23.
Sympathetic and Adrenal Medullary Counterregulatory Response
In response to hypoglycemia, patients with Type 1 diabetes and advanced Type 2 diabetes are not able to suppress circulating (exogenous) insulin levels or increase glucagon secretion20. Thus, patients with diabetes rely extensively on the sympathoadrenal system as their primary counterregulatory defense against hypoglycemia19. Adrenergic activation leads to the release of norepinephrine at nerve terminals located throughout the periphery. Adrenergic stimulation of the adrenal glands stimulates epinephrine release. Activation of the adrenergic system combats falling glucose levels by increasing glucose production, reducing peripheral glucose utilization, and eliciting symptoms of hypoglycemia (figure 1).
Hypoglycemia Awareness
Exigent hypoglycemia is usually accompanied by characteristic signs and symptoms, which prompt the patient to take corrective action. Neurogenic symptoms occur as a result of activation of the autonomic nervous system by hypoglycemia, and lead to perception of hypoglycemia (hypoglycemia awareness) by the patient. These symptoms include sweating, hunger, tingling, tremors, palpitations, nervousness and anxiety. Interestingly, as characterized in adrenalectomized patients, these neurogenic symptoms of hypoglycemia are chiefly the result of sympathetic neural system activation, rather than epinephrine or norepinephrine release from the adrenal gland24. Thus the unawareness of hypoglycemic symptoms in patients with diabetes is inexorably linked to exiguous sympathetic neuronal activation. On the other hand, neuroglycopenic symptoms result from brain’s deprivation of glucose, and may be exhibited as warmth, weakness, altered mental status, drowsiness, seizures, or even coma or death25. Autonomic symptoms of hypoglycemia tend to occur at higher thresholds (58 mg/dL) than neuroglycopenic symptoms, which tend to occur at 51 mg/dL26. In the setting of progressive hypoglycemia, awareness of autonomic symptoms generally occurs prior to the development of profound neuroglycopenic symptoms, allowing the patient a window of opportunity to initiate corrective action to ameliorate hypoglycemia before global cognitive impairments limit behavioral responses and hypoglycemia becomes life-threatening.
Anatomy of Brain Glucose sensing
Although all brain cells utilize glucose, only a few specialized neurons in the brain truly sense and respond to reduced glucose supply. Rodent studies indicate that glucose is sensed in brain regions known to be important in metabolism and energy homeostasis, particularly in the hypothalamus, where glucose sensing neurons are located27,28. Most studies to date indicate that it is the hypothalamus that principally initiates (or at least coordinates) the CRR to stimulate hormone secretion in the pituitary gland, pancreas, and adrenal glands resulting in a coordinated response. Clinical studies confirm the importance of the hypothalamus as a critical glucose sensing area because blood flow to the hypothalamus increases significantly during hypoglycemia, even before counterregulatory hormones rise29. Within the hypothalamus, key regions that respond to changes in circulating glucose levels are the ventromedial hypothalamus (VMH) which contains the ventromedial nucleus (VMN) and the arcuate nucleus (ARC)30. Studies show that glucose infusion into the VMH in the setting of peripheral hypoglycemia blunts the epinephrine response to hypoglycemia31,32. These studies indicate that decreases in glucose are detected by the VMH and are required to fully activate the sympathoadrenal response to hypoglycemia (Figure 2).
Figure 2. Afferent glucose sensing pathways, neural integration, and efferent autonomic pathways that mediate the counterregulatory response to hypoglycemia.
During hypoglycemia, the initiation and coordinatation of the counterregulatory response is mediated by a network of glucose sensing neurons in the hypothalamus, including the ventromedial nucleus (VMN), arcuate nucleus (ARC) and lateral hypothalamus (LH). Glucose sensing also occurs in the nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus (DVN) in the hindbrain. The NTS also receives afferent information from peripheral glucose sensors, including the portal vein. Hypothalamic and hindbrain neural networks project to the paraventricular nucleus of the hypothalamus (PVN) in order to elicit autonomic and neuroendocrine counterregulatory responses. Although not directly glucose sensing, parvicellular neurons in the PVN initiate sympathetic autonomic responses via pre-ganglionic spinal efferents. Parasympathetic (vagal) innervation is relayed from the PVN to the dorsal vagal nucleus (DVN) which then relays to peripheral organs. In addition, the hypothalamic-pituitary-adrenal axis is also initiated from a distinct set of medial parvicellar neurons within the PVN that secretes corticotropin releasing hormone (CRH). CRH acts on the anterior pituitary (Pit) to stimulate the secretion of adrenocorticotropic hormone (ACTH) which circulates to the adrenal cortex to increase cortisol secretion. Interneurons involved in the neural glucose sensing and autonomic response network shown in black. Afferent glucose sensing pathways are shown in green. Efferent autonomic responses to hypoglycemia shown in brown. Post-ganglionic vagal parasympathetic efferent pathway shown in red. Hypothalamic-Pituitary-Adrenal axis shown in blue.
The VMH contains glucose excitatory (GE, increase neuronal activity in response to glucose) and glucose inhibited (GI, decrease neuronal activity in response to glucose) neurons33. Similarities and differences between GI and GE neurons with regard to glucose metabolic and electrophysiological properties have been reviewed34. It is thought, that in response to hypoglycemia, concerted activation of GI neurons and suppression of GE neurons, as an initiating part of a neural network, result in a coordinated efferent process that activate the sympathoadrenal response (Figure 3).
Figure 3. Glucose sensing neurons of the ventromedial hypothalamus (VMH).
A) VMH glucose excited (GE) neurons are activated in response to increasing glucose. In a setting of low glucose, decreased glucose entry into the GE neuron through glucose transporters (GLUT) leads to decreased phosphorylation by glucokinase (GK) leading to an increase in the AMP/ATP ratio, thus increasing the activity of AMPK and stimulating KATP channel activation. Activation of KATP channels leads to decreased membrane depolarization and decreased action potential frequency and neurotransmitter release, in particular GABA, thus leading to activation of the hypoglycemic counterregulatory response (CRR). B) VMH glucose inhibited (GI) neurons are activated in response to decreasing glucose. Decreased glucose entry into neurons leads to an increase in the AMP/ATP ratio, activation of AMPK which activates formation of nitric oxide (NO) which can act as a neurotransmitter. Increased AMP/ATP also inhibits a chloride channel, thought to be the cystic fibrosis transmembrane conductance regulator, (CFTR), leading to membrane depolarization, increased action potential frequency, and neurotransmitter release, including glutamate, which leads to activation of the counterregulatory response. GABA and glutamate can potentially be secreted from both GE and GI neurons upon their activation but a decrease in GABA levels is required for full activation of the counterregulatory response.
Other glucose sensing neurons have been discovered in regions outside the hypothalamus. Within the brainstem, the nucleus tractus solitarius (NTS), the area postrema (AP), the dorsal motor nucleus of the vagus (DVN), and the subfornical region have been shown to contain glucose sensing neurons35-37. Other peripheral glucose sensing neurons (ie, within the portal/mesenteric vein, gut, and carotid bodies) ascend via afferent neurons to the brainstem (NTS) which projects anteriorly to the paraventricular nucleus (PVN) of the hypothalamus30 (Figure 2). _ENREF_35
Activation of the PVN appears to be critically important for activating several aspects of the stress counterregulatory responses including, 1) CRH release which acts on the anterior pituitary to release ACTH, 2) innervation of the dorsal motor nucleus of the vagus (DVN) to regulate the vagal parasympathetic efferent neurons, and 3) perhaps most importantly, direct activation of sympathetic nerves in the spinal cord (Figure 2). Failure to activate PVN neurons is a common finding in animal models that exhibit impaired counterregulatory responses to hypoglycemia38,39_ENREF_35.
Cellular Biology of Brain Glucose sensing
The brain contains different isoforms of glucose transporters responsible for neuronal and astrocyte glucose uptake. The ubiquitous GLUT1 and the neural specific GLUT3 are the primary isoforms present in the brain that are responsible for glucose transport across the blood brain barrier and into the brain. Based on discrete regional expression, the potential contribution of other glucose transporters has been investigated. A role for GLUT2 in mediating CNS glucose sensing has been proposed40. Also, a role for insulin-sensitive GLUT4 in mediating brain glucose sensing and the counterregulatory response to hypoglycemia was evident in recent reports from our lab showing that in response to hypoglycemia, brain GLUT4 knockout mice have 1) impaired epinephrine and glucagon responses, 2) impaired neuronal activation within the hypothalamus, and 3) impaired glucose sensing in GI neurons41. Further research is needed to understand how differential regional expression of the various glucose transporters regulate the CRR to hypoglycemia.
Once glucose enters the cells, it is phosphorylated by glucokinase, an enzyme which has been shown to play an important role in mediating hypothalamic glucose sensing42,43. Metabolism of glucose increases the intracellular ATP to AMP ratio, which, in turn, regulates the activity of a key metabolic sensor, AMP-activated protein kinase (AMPK). During acute hypoglycemia, increased AMPK activity is hypothesized to lead to chloride ion (cystic fibrosis transmembrane conductance regulator, CFTR) channel closure, neuronal depolarization, and neurotransmitter release in GI neurons44,45 (Figure 3). In GE neurons, downstream of glucose metabolism, however, it is the activation of K-ATP channels that increases the sympathoadrenal response to hypoglycemia33,34,46. Thus pharmacologic or genetic disruption of the metabolic41,42,44 or electrophysiological33,34,45 properties in these critical VMH glucose sensing neurons can lead to impaired hypoglycemic counterregulation.
Hormonal and neuro-hormonal regulation of CNS glucose sensing
The intrinsic glucose sensing/metabolism properties of glucose sensing neurons in the VMH can be acutely modified by the action of circulating hormones. Both leptin and insulin cross the blood-brain barrier and have been shown to acutely activate K-ATP channels in glucose sensing hypothalamic neurons47. In the setting of hypoglycemia, insulin has been shown to act in the brain to augment the counterregulatory response48. Furthermore, neuronal insulin receptor knockout (NIRKO) mice have impaired hypoglycemic counterregulation, characterized by a blunted epinephrine and norepinephrine response39. Consistent with insulin exerting its effect primarily in the hypothalamus, NIRKO mice have impaired glucose sensing in VMH GI neurons and impaired activation of PVN neurons in response to hypoglycemia39. Together, these studies demonstrate that central insulin signaling plays an important role in regulating the normal counterregulatory response to hypoglycemia. Other, slower-acting hormones, including nuclear receptor transcription activators such as cortisol (discussed below), may also exert more long-term effects in regulating hypothalamic glucose sensing.
As blood glucose level fall, glucose levels within the brain also decrease. A fall in glucose levels within the VMH is associated with increased norepinephrine release in the VMH and the initiation of the counterregulatory response to hypoglycemia49,50. Adrenergic receptors within the brain, particularly the VMH, are important mediators that trigger the sympathoadrenal response to hypoglycemia51. It is likely that increased or decreased catecholaminergic neurotransmitter output from other glucose sensing neurons (perhaps portal-mesenteric glucose-sensitive afferents52) are integrated in the VMH to either amplify or suppress, respectively, the sympathoadrenal response to hypoglycemia.
Defective Counterregulation and Hypoglycemia Unawareness in People with Diabetes and Recurrent Hypoglycemia
As noted above, in people with Type 1 and advanced type 2 diabetes, impaired hypoglycemic counterregulation is noted by a failure to both suppress circulating insulin and increase glucagon secretion which _ENREF_45 leads to an increased reliance on the sympathoadrenal response as the primary counterregulatory responses53,54_ENREF_46. Recurrent hypoglycemia further impairs the sympathoadrenal response as part of a viscous cycle, making diabetic patients vulnerable to more severe episodes of hypoglycemia7,20. In addition, recurrent hypoglycemia induces impairments in brain glucose sensing on both a cellular basis in glucose sensing VMH neurons55 as well as on a whole body basis, noted clinically by both impaired sympathoadrenal responsiveness and hypoglycemia unawareness (Figure 4). Hypoglycemia unawareness increases the incidence of severe hypoglycemia 6-fold for Type 1 diabetic patients56 and 17-fold for Type 2 diabetic patients57. Although approximately 20% of Type 1 diabetic patients report hypoglycemia unawareness58, this value is most certainly an underestimation, as even diabetic patients who have intact hypoglycemia awareness are often unaware of biochemically confirmed hypoglycemia 6.
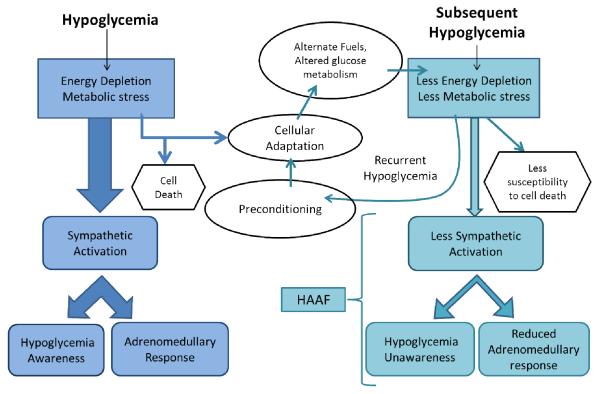
Figure 4. Preconditioning through recurrent hypoglycemia leads to cellular adaptation and HAAF.
Hypoglycemia is a state of energy depletion that leads to metabolic stress. Sympathetic activation leads to symptoms of hypoglycemia awareness and the adrenomedullary response. The normal response to hypoglycemia is a cellular adaptation, assuming the energy depletion and metabolic stress was not enough to induce cell death. The mechanism by which cellular adaptation occurs is unclear but may include the use of alternate fuels (such as lactate) and/or an enhanced glucose transport/phosphorylation/metabolism. During a subsequent hypoglycemic episode, the adapted cell experiences less marked energy depletion and less metabolic stress thus making the cells less susceptible to death. Less intracellular energy depletion leads to impaired sympathetic activation resulting in hypoglycemia unawareness and a reduced adrenomedullary response to subsequent hypoglycemia, collectively known as hypoglycemia associated autonomic failure (HAAF). Preconditioning through recurrent hypoglycemia, paradoxically, acts to render an individual more prone to, but less vulnerable to, an episode of severe hypoglycemia.
The mechanisms by which recurrent hypoglycemia leads to altered CNS glucose sensing, impaired sympathoadrenal activation, and hypoglycemia unawareness remain an active area of investigation59. Several potential mediators being investigated include 1) actions of hormones released during hypoglycemia (cortisol, epinephrine, opioids), 2) cell autonomous changes in substrate metabolism, and 3) altered neuronal circuitry/neurotransmitter release. These mechanisms are not mutually exclusive. Better understanding of these mechanisms will aid in developing therapeutic strategies to prevent hypoglycemia unawareness in insulin treated patients with diabetes.
Peripheral Mediators of HAAF and Hypoglycemia Unawareness
Cortisol
The hypothalamic-pituitary-adrenal axis involves a family of neuropeptides that regulate glucocorticoid (cortisol) secretion during stress. Corticotropin releasing hormone agonists have been shown to impair the counterregulatory response to subsequent hypoglycemia suggesting a possible mechanistic role in mediating HAAF60. The hypoglycemia associated rise in systemic corticosteroids has been proposed to feedback to the hypothalamus and thereby potentially contribute to HAAF61-63. It remains controversial as to whether the endogenous hypercortisolemia associated with the counterregulatory response is of sufficient magnitude to blunt the CRR to hypoglycemia64,65.
Catecholamines
It has been well established that antecedent hypoglycemia induces a blunted epinephrine response to a subsequent episode of hypoglycemia. A study by Ramanathan et al.66 showed that intravenous infusion of adrenergic blockers (phentolamine and propranolol) on day 1 of hypoglycemia prevented the induction of counterregulatory failure in the subsequent response on day 2 of hypoglycemia. This study implicates that HAAF is induced by antecedent sympathoadrenal responses to hypoglycemia, and possibly the antecedent sympathoadrenal response to exercise as discussed below. Extending these findings to their potential pharmacologic and therapeutic implications, an apparent dichotomy emerges whereby blocking the action of catecholamines (presumably within the CNS) may protect against subsequent hypoglycemic bouts by limiting the development of HAAF; but unfortunately, blocking the action of catecholamines in the periphery would tend to increase the severity of acute hypoglycemia. Perhaps future pharmacological treatment of recurrent severe hypoglycemia will involve development of selective adrenergic receptor modulators that favorably alter CNS mediated counterregulatory responses without adversely altering the beneficial peripheral effects of the sympathoadrenal response.
Opioids
Opioid signaling in the CNS has also been implicated in the development of impaired sympathoadrenal responses in people with Type 1 diabetes. Naloxone, an opioid receptor blocker, augmented the sympathoadrenal response to hypoglycemia67 and when infused during antecedent hypoglycemia, it prevented the development of the hypoglycemia-associated reduced epinephrine response68. Mechanistically, naloxone may preserve the counterregulatory response to hypoglycemia by reprogramming metabolic genes to use alternate fuels instead of glucose69. Together, these pre-clinical and clinical studies implicate that the rise in endogenous opioids that occurs during hypoglycemia have a pathological role in mediating the adrenomedullary defect associated with HAAF and a potential therapeutic role for opioid receptor blockade to protect against HAAF.
Exercise
The inability to decrease circulating insulin during exercise puts people with Type 1 diabetes at increased risk for hypoglycemia during or after exercise. Furthermore, antecedent exercise can decrease sympathoadrenal responses to subsequent hypoglycemia70. It is interesting that despite this reduced epinephrine responses to hypoglycemia following exercise, symptoms of hypoglycemia are not reduced, suggesting that there can be a distinction between hypoglycemia awareness and the counterregulatory adrenal response. Additionally, during stress, including hypoglycemia and exercise, the opioid β-endorphin is released to activate the sympathoadrenal response. In a recent study, healthy volunteers who exercised and had elevated endorphin levels had impaired epinephrine and norepinephrine responses during hypoglycemia the following day71. Thus, the impaired adrenomedullary response to hypoglycemia induced by antecedent hypoglycemia or exercise may be mediated by endogenous opioid action within the CNS. Thus, from a therapeutic perspective, blocking the actions of endogenous opioids may protect against exercise-induced autonomic failure.
Sleep
Nocturnal hypoglycemia is very common in patients with Type 1 Diabetes. It has a prevalence rate of up to 68%72 and has an incidence of once every third night73. While sleeping, subjects with Type 1 diabetes have a significantly reduced epinephrine response to hypoglycemia74 and reduced awakening from sleep during hypoglycemia, compared to non-diabetics75. Thus the state of sleep induces a transient, additional, HAAF-like syndrome, characterized by hypoglycemia unawareness and an impaired adrenomedullary response to hypoglycemia.
If a diabetic patient is unable to wake up and take corrective actions, nocturnal hypoglycemia can be potentially life-threatening. Tattersall and Gill have reported the unexplained overnight deaths of otherwise healthy young people with Type 1 diabetes76, often referred to as the ‘Dead in Bed syndrome’. Recently, the overnight death of a young man with Type 1 diabetes confirmed with continuous glucose monitoring that the dead in bed syndrome is associated with severe hypoglycemia14,76_ENREF_71.
Alternative Substrate Metabolism
Glycogen Supercompensation
It has been hypothesized that increased glycogen stores in astrocytes might contribute to hypoglycemia unawareness and impaired sympathoadrenal responses by supplementing glucosyl units for CNS metabolism during periods of systemic hypoglycemia. Studies in rats and humans have shown that brain glycogen content is increased following one or more episodes of hypoglycemia77,78 or 2-deoxyglucose induced neuroglycopenia79. Currently, much controversy exists in this field since subsequent studies have shown that glycogen content in the rat brain is not elevated after acute or recurrent hypoglycemia80 and that lower, not higher, glycogen levels exist in diabetic patients81. Hopefully improved techniques in measuring brain glycogen turnover in vivo both during and after hypoglycemia will resolve these apparent discrepant results. The more important question to be addressed is whether changes to brain glycogen levels (induced via physiological or pharmacological means) will offer people who suffer from recurrent hypoglycemia a beneficial therapeutic advantage in preserving both sympathoadrenal responses and hypoglycemia awareness.
Enhanced Glucose Metabolism
Alterations in glucose transport or glucose metabolism as a result of repeated exposure to hypoglycemia have been postulated to be a potential mediator of HAAF. Increased glucose transport to sustain metabolic demands during hypoglycemia is supported by studies in rats that show increased expression of glucose transporters in the brain after acute hypoglycemia82 and after recurrent hypoglycemia83. The next important metabolic regulator in glucose sensing neurons is the enzyme glucokinase the expression level of which is also upregulated in the setting of recurrent hypoglycemia42. Consistent with an upregulation of glucose transport or glucokinase activity, recurrent hypoglycemia has been shown to increase hypothalamic glucose phosphorylation84. Therefore, repeated exposure to hypoglycemia may upregulate the capacity for glucose metabolism (including increased glucose transporters, glucokinase activity, and glucose phosphorylation) during subsequent hypoglycemia. This process simultaneously limits neuroglycopenia and induces hypoglycemia unawareness at the cellular level in glucose sensing neurons, ultimately resulting in a diminished sympathoadrenal response.
While supported in some42,83,84 but not all85 rodent models, altered glucose transport/metabolism as a cause for hypoglycemia unawareness is less well substantiated in humans. During hypoglycemia, Type 1 diabetic patients had similar glucose metabolism in the brain as compared to healthy controls86. Patients with hypoglycemia unawareness seem to have normal global brain glucose metabolism; although several studies have identified specific brain regions that exhibit decreased glucose uptake, including the subthalamic brain region involving the hypothalamus87, the prefrontal cortex88, the amygdala and orbitforntal cortex89. Iatrogenic hypoglycemia that induces HAAF also did not increase global brain glucose transport in healthy patients90, but was associated with significantly greater synaptic activity in the dorsal midline thalamus91. Further clinical studies utilizing enhanced PET and MRI technologies to examine regional brain glucose uptake/metabolism in patients with Type 1 diabetes with and without HAAF will help define the brain regions pathologically linked to this clinical syndrome.
Alternative Fuel Hypothesis
In the setting of reduced glucose supply from the periphery, the brain may be able to decrease its reliance on circulating glucose and maintain its metabolic processes by increasing uptake of alternate carbon fuels, such as ketones or lactate92. Lactate from astrocytes can be taken up into neurons via monocarboxylate transporters (MCT) and is hypothesized to support oxidative phosphorylation during times of glucose deficit85,92,93. During a hypoglycemic clamp, Type 1 diabetic patients had twofold higher brain lactate concentrations than control subjects indicating increased brain uptake of lactate94. If, in response to recurrent hypoglycemia, the brain has adapted in such a way as to meet its metabolic demands by utilizing relatively more lactate rather than circulating glucose, then the neurons’ sufficed metabolic demands likely blunt its ability to sense and respond to subsequent hypoglycemia, resulting in impaired sympathetic activation and hypoglycemia unawareness (Figure 4).
Altered neuronal communication
Gamma-Aminobutyric Acid (GABA) is a potent inhibitory neurotransmitter. Hypothalamic GABA levels normally decrease during hypoglycemia95, thereby relinquishing a tonic inhibitory effect on VMH neurons and allowing the generation of the counterregulatory response. Both diabetic rats and recurrently hypoglycemia rats have higher basal levels of VMH GABA that fail to decrease normally during subsequent hypoglycemia, which correlates with the reduced glucagon and epinephrine responses96 97. These results indicate that altered GABA tone may be an important common mediator in the development of HAAF, especially in diabetic patients, and drugs that selectively target GABA secretion or receptor binding may improve sympathoadrenal responses to hypoglycemia.
Adaptations to Recurrent Hypoglycemia - adaptive or maladaptive?
Repetitive hypoglycemia induces a state of hypoglycemic tolerance, in which lower and lower blood glucose levels are needed to elicit symptomatic and counterregulatory responses. From a teleological perspective, brain adaptations that occur in response to repetitive hypoglycemia endeavor to maintain neuronal/cognitive function via sufficing CNS metabolic needs in the setting of another episode of hypoglycemia. Unfortunately, these adaptations ultimately reduce neuronal efferent signals, thereby limiting sympathoadrenal responses and induce a state of hypoglycemia unawareness7. Metabolic adaptions in patients with hypoglycemia unawareness allow cognitive function at dangerously low blood sugar levels, but do so at the perilous risk of a precipitous neuroglycopenic coma. By reducing awareness and counterregulation to subsequent hypoglycemia, HAAF jeopardizes patient safety and should therefore be considered a maladaptive response7,98. However, hypoglycemic tolerance induced by recurrent hypoglycemia may induce some unexpected beneficial adaptations. Our laboratory has shown that adaptations associated with recurrent antecedent hypoglycemia protect the brain against severe hypoglycemia-induced brain damage and cognitive decline11. Thus, similar to the phenomena of pre-conditioning, recurrent bouts of moderate hypoglycemia might, paradoxically, render an individual more prone to, but less vulnerable to, an episode of severe hypoglycemia. If a neuroprotective effect of hypoglycemic pre-conditioning were to be extrapolated to the clinical setting, it may explain the seemingly incongruous clinical findings that intensively treated patients, who experience recurrent hypoglycemia may be paradoxically protected from severe hypoglycemia-induced brain damage and cognitive dysfunction16. Of course recurrent hypoglycemia should not be advocated clinically, but defining the mechanisms of how recurrent hypoglycemia leads to beneficial adaptations could lead to the development of pharmacologic agents that will help protect against the morbidity and mortality associated with severe hypoglycemia11,99.
Prevention of Hypoglycemia, Defective Counterregulation, and Hypoglycemia Unawareness
Identification of patients with risk factors for severe hypoglycemia is a critical step in the prevention of hypoglycemia. Type 1 diabetic patients with hypoglycemia unawareness and impaired counterregulation are more likely to be older, have diabetes of longer duration, and have lower HbA1c100,101. Gender may also contribute to risk for hypoglycemia. The blunted counterregulatory response to hypoglycemia in women102, likely mediated by estrogen103, uniquely increases the risk for hypoglycemia in women104.
Strategies for the prevention of hypoglycemia includes frequent self-monitoring of blood glucose levels and patient education regarding insulin analogs, dose adjustments for anticipated exercise and carbohydrate consumption, and the use of technology for insulin administration and blood glucose monitoring (ie. insulin pumps and continuous glucose sensors).
Avoidance of Hypoglycemia
Recurrent antecedent hypoglycemia induces impaired sympathoadrenal responses and hypoglycemia unawareness. Fortunately, avoidance of hypoglycemia can completely restore hypoglycemia awareness, and partially restore the adrenomedullary response to hypoglycemia105-107. Studies showed an improved awareness after 3 days, and normalized hypoglycemia awareness with improved evidence of counterregulation in as little as 3 weeks of hypoglycemia avoidance. Thus, the adaptations that occur in response to recurrent hypoglycemia are reversible. As hypoglycemia begets hypoglycemia, so does hypoglycemia avoidance avoid hypoglycemia.
Patient Education
There are two types of patient education strategies that aim to decrease the incidence of hypoglycemia. Psychological-based instructional programs aim to improve the patients’ accuracy in detecting hypoglycemia108,109. Other educational programs advocate hypoglycemia avoidance strategies, dietary education, and flexible insulin dosing strategies to account for varied diet and activity levels110,111_ENREF_100. Efficacy of these educational programs is noteworthy. In the setting of intensified blood glucose control (reducing HgbA1C from 8.1 to 7.3%), when rates of hypoglycemia would be expected to increase, educational programs markedly reduced the incidence of severe hypoglycemia from 0.37 to 0.14 events per patient per year111. Thus, patient education appears to break the bond that hitherto invariably linked intensive glycemic control to increased incidence of hypoglycemia.
Choice of insulin analog therapy
Multiple clinical trials demonstrate that the newer insulin analogues reduce the incidence of hypoglycemia. With a shorter duration of action as compared to regular insulin, rapid acting insulin analogs decrease the incidence of post-prandial hypoglycemia112,113. By not rising in the middle of the night, the flatter pharmacokinetics of long-acting insulin glargine help to decrease incidence of nocturnal hypoglycemia as compared with NPH insulin114. The newer ultra-long acting basal insulin degludec, appears to be particularly effective in lowering the risk of nocturnal hypoglycemia115.
Choice of Insulin delivery - use of continuous subcutaneous insulin infusion (CSII) pumps
If a patient’s hemoglobin A1C<8.5% is not obtainable via multiple doses of injectable insulin, a clinical decision to initiate a trial of a continuous subcutaneous insulin infusion pump is reasonable. A review analysis of 26 observational studies noted that the majority of CSII studies demonstrated significantly decreased rates of severe hypoglycemia116. A recent prospective study specifically recruiting patients with hypoglycemia unawareness showed that transitioning patients from multiple daily injections to CSII halved the hypoglycemic events rate and, remarkably, virtually eliminated the rate of severe hypoglycemia, from 1.25 to 0.05 events per year117. Thus, the use of CSII may reduce the amount of human error that occurs with multiple daily insulin injections and thereby reduce episodes of hypoglycemia.
Self-Monitoring of blood glucose - Glucose Continuous Glucose Monitors
A meta-analysis of nineteen trials indicate that continuous glucose monitors (CGM) improve glycemic control in adults with diabetes, but, in spite of hypoglycemia alarms, the effect on reducing incidence of hypoglycemia is marginal118. A more positive interpretation would be that the use of CGM improves HbA1C without increasing the incidence of hypoglycemia119, results very much unlike the DCCT3. Therefore, CGM technology may help dissociate intensive glycemic control from increased hypoglycemia.
Establishing closed-loop communication between CGM and insulin pumps may offer new technology-based opportunities to decrease the burden of hypoglycemia. For example, programing of insulin pumps to automatically suspend basal insulin infusion for two hours after a low blood sugar detected by CGM significantly decreases the duration of hypoglycemia120. In addition to suspending insulin delivery, perhaps technology in the near future will allow dual chamber pumps to automatically infuse glucagon in response to a low or falling blood glucose in order to limit the incidence, duration, or severity of hypoglycemia121.
Whole Pancreas and Islet cell transplantation
Continuous glucose monitoring systems have confirmed that transplant recipients either have significant decreased (in insulin requiring subjects) or completely eradicated (in insulin independent subjects) the amount of time spent in the hypoglycemic range (<60 mg/dL)122. The mechanisms for this striking reduction in the incidence of hypoglycemia include, 1) the elimination (or marked reduction) in exogenous insulin administration reduces the risk of iatrogenic hypoglycemia, 2) the provision of a regulatory decrement in endogenous insulin secretion, 3) the partial restoration of the glucagon counterregulatory response, and 4) the recovery of sympathoadrenal response and hypoglycemia awareness due to the avoidance of iatrogenic hypoglycemia123,124. For patients with intractable, recurrent, severe hypoglycemia, whole pancreas or islet cell transplantation should be considered as a viable therapeutic option.
Relaxed A1C goals
Although the ADA and AACE differ with regards to HbA1c goals (<7.0 or <6.5, respectively) both societies acknowledge that these goals should only be attempted if they can be achieved safely “without significant/substantial hypoglycemia”. Given that significant/substantial, temporarily disabling, severe hypoglycemia occurs so frequently in people with both Type 1 and Type 2 diabetes8, it could be argued that these idyllic HbA1c goals are not appropriate for a relatively large percentage of people with diabetes. To decrease the incidence of hypoglycemia, both societies advocate less-stringent A1C goals (such as 8%) as being appropriate for patients with a history of severe hypoglycemia. Also, for young children who are often unable to recognize the symptoms of hypoglycemia, relaxed glycemic control guidelines are recommended125. Additionally, relaxed HbA1C goals may be appropriate for patients in whom one severe hypoglycemic reaction might be particularly catastrophic (ie. frail elderly people with diabetes, patients with extensive comorbid conditions or advanced macrovascular complications). Finally, less-strict A1C goals may be appropriate for individuals in whom the benefits of intensive glycemic control may not be realized (ie. patients with advanced microvascular complications or limited life expectancy). Therefore, ideal HbA1c target goals should not be generalized for all individuals with diabetes; but rather, healthcare practitioners should individualize realistic HbA1c goals on a case by case basis in order to minimize the potential morbidity and mortality of hypoglycemia.
Summary
Until a cure for diabetes is found, hypoglycemia will continue to be a major barrier for the achievement of long-term glucose control and will cause recurrent morbidity in individuals with diabetes. Numerous sedulous research studies have begun to uncover the mechanisms by which the CNS responds and adapts to hypoglycemia. Understanding these mechanisms will undoubtedly lead to better management and therapies that reduce the risk for hypoglycemia, while still allowing patients to achieve the benefits associated with tight glycemic control. Given this pervasive barrier of hypoglycemia for the treatment of diabetes, physicians should discuss hypoglycemia prevention strategies with their patients, so that they can have a better chance of achieving their glucose controls goals while avoiding the morbidity and mortality associated with hypoglycemia.
KEY POINTS.
Hypoglycemia continues to be a major barrier for the achievement of long-term glucose control and will cause recurrent morbidity in individuals with diabetes.
Numerous sedulous research studies have begun to uncover the mechanisms by which the CNS responds and adapts to hypoglycemia.
Understanding these mechanisms will undoubtedly lead to better management and therapies that reduce the risk for hypoglycemia, while still allowing patients to achieve the benefits associated with tight glycemic control.
Given this pervasive barrier of hypoglycemia for the treatment of diabetes, physicians should discuss hypoglycemia prevention strategies with their patients, so that they can have a better chance of achieving their glucose controls goals while avoiding the morbidity and mortality associated with hypoglycemia.
Table 1. Clinical Indications for less strict HbA1c Goals (ie, goal HbA1c in 7-8% range).
| Advanced Age |
| Pediatric Patients |
| Hypoglycemia Unawareness |
| Frequent Severe Hypoglycemia |
| Life Expectancy <5 years |
| Advanced Macrovascular Complications |
| Renal Failure |
| Extensive Comorbidities |
Table 2. Recommended Review of Hypoglycemia at Clinic Visits.
| Monitoring | Recent history of symptomatic or severe hypoglycemia Recent history of hypoglycemia unawareness Recommend frequent blood glucose measurements Monitor carbohydrate intake and insulin dosage, special events/considerations Review data: assess for patterns of hypoglycemia (time of day, association with types of meals/activities/exercise, weekdays versus weekends, post-menstrual) Recommend checking blood sugar before meals, at bedtime, and before driving Consider referral for diabetes education If nocturnal hypoglycemia is suspected, wake patient at 3am for a few nights to check blood glucose Consideration of a continuous glucose monitoring system with hypoglycemia alarms |
| Meals | Adjust insulin-to-carbohydrate ratio to avoid post-meal hypoglycemia Proper assessment of portion size and carbohydrate content Ideal insulin-to-carbohydrate ratio should reach target blood sugar three to four hours after meals Pre-meal glycemia may influence the timing delay between pre-meal insulin dosage and initiating of meal If pre-meal glucose is below target, reduce pre-meal insulin dose appropriately Alcohol has glucose-lowering effects and can mask symptoms of hypoglycemia; consider reducing basal insulin doses when consuming alcohol |
| Insulin | Insulin Basal long-acting + rapid-acting pre-meal insulin combinations less likely to cause hypoglycemia than intermediate acting + regular insulin preparations Consider less aggressive correction insulin doses. Use the ‘1,800 rule‘ rather than the ‘1,500 rule’ (i.e. estimated mg/dl drop in glycemia per unit of insulin=1,800/total daily dose) Avoid repetitive, or stacking of, correction doses |
| Exercise | Consider type of exercise (timing, duration and intensity) Check blood sugars before and during prolonged exercise; snack if necessary Consider reducing basal insulin dosage prior to anticipated period of prolonged exercise Make adjustments for increased insulin sensitivity for 24 hours after exercise |
| Treatment | Readily available emergency supplies including sugar tablets, candy, sugar-paste in tube Prescription glucagon kits (non-expired) readily available People who have regular contact with patient (family members, colleagues, teachers, etc.) need to know signs of hypoglycemia and how to treat it Notification of emergency medical services (i.e. 911) |
| Prevention | Discussion between patient and physician regarding a period of less intensive glycemic management goals (i.e. relaxed/higher glycated hemoglobin goal, higher blood glucose targets pre-/post-meals, and when calculating correction factor, etc.) Review comorbidities that cause hypoglycemia including malabsorption (celiac or pancreatic insufficiency), renal failure, liver disease, adrenal insufficiency,hypothyroidism Scrupulous avoidance of hypoglycemia to restore hypoglycemia awareness Medical identification bracelet or necklace indicating that patient has diabetes and takes insulin |
Table 3. Investigational and Advanced and therapies for hypoglycemia prevention.
| Interventions | Rational | Reference |
|---|---|---|
Bedtime Snacks:
|
Prevention of early nocturnal hypoglycemia |
126,127 |
Insulin Pump & Continuous glucose monitors
|
Prevention of iatrogenic insulin induced hypoglycemia |
121,128 |
| Alert Dogs | Dogs trained to recognize hypoglycemia and alert owners |
129 |
| Islet cell or whole pancreas transplantation | Restoration of the counterregulatory response |
123,124 |
Medications:
|
Increase hypoglycemic counterregulatory response and patient perception of hypoglycemia |
126,130,131 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. New England Journal of Medicine. 2009;360:129–U162. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 2.Terry T, Raravikar K, Chokrungvaranon N, et al. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012;14:79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of lon-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329:977–996. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Turner RC, Holman RR, Stratton IM, et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 5.The ADVANCE collaborative group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2008:358. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Kubiak T, Hermanns N, Schreckling HJ, et al. Assessment of hypoglycaemia awareness using continuous glucose monitoring. Diabetic Medicine. 2004;21:487–490. doi: 10.1111/j.1464-5491.2004.1136.x. [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. AmJPhysiol EndocrinolMetab. 2001;281:E1115–E1121. doi: 10.1152/ajpendo.2001.281.6.E1115. [DOI] [PubMed] [Google Scholar]
- 8.Heller SR, Choudhary P, Davies C, et al. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 9.Perantie DC, Wu J, Koller JM, et al. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 10.Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55:1280–1286. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- 11.Puente EC, Silverstein J, Bree AJ, et al. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes. 2010;59:1055–1062. doi: 10.2337/db09-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northam EA, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asvold BO, Sand T, Hestad K, et al. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care. 2010;33:1945–1947. doi: 10.2337/dc10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. EndocrPract. 2010;16:244–248. doi: 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 15.Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes - Results from the Yorkshire Register of Diabetes in Children and Young Adults. Diabetes Care. 2008;31:922–926. doi: 10.2337/dc07-2029. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. NEnglJMed. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skrivarhaug T, Bangstad HJ, Stene LC, et al. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298–305. doi: 10.1007/s00125-005-0082-6. [DOI] [PubMed] [Google Scholar]
- 18.Reno CM, Daphna-Iken D, Fisher SJ. Adrenergic blockade prevents life threatening cardiac arrhythmias and sudden death due to severe hypoglycemia. Diabetes. 2012;61:A46. [Google Scholar]
- 19.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and Cardiovascular Risks. Diabetes Care. 2011;34:S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 21.Cryer PE. Minireview: Glucagon in the Pathogenesis of Hypoglycemia and Hyperglycemia in Diabetes. Endocrinology. 2012;153:1039–1048. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taborsky GJ, Mundinger TO. Minireview: The Role of the Autonomic Nervous System in Mediating the Glucagon Response to Hypoglycemia. Endocrinology. 2012;153:1055–1062. doi: 10.1210/en.2011-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue JTY, Burdett E, Coy DH, et al. Somatostatin Receptor Type 2 Antagonism Improves Glucagon and Corticosterone Counterregulatory Responses to Hypoglycemia in Streptozotocin-Induced Diabetic Rats. Diabetes. 2012;61:197–207. doi: 10.2337/db11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeRosa MA, Cryer PE. Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. American Journal of Physiology-Endocrinology and Metabolism. 2004;287:E32–E41. doi: 10.1152/ajpendo.00539.2003. [DOI] [PubMed] [Google Scholar]
- 25.Zammitt NN, Streftaris G, Gibson GJ, et al. Modeling the consistency of hypoglycemic symptoms: high variability in diabetes. Diabetes Technol Ther. 2011;13:571–578. doi: 10.1089/dia.2010.0207. [DOI] [PubMed] [Google Scholar]
- 26.Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of Glycemic Thresholds for Counterregulatory Hormone-Secretion, Symptoms, and Cerebral-Dysfunction. American Journal of Physiology. 1991;260:E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 27.Borg MA, Borg WP, Tamborlane WV, et al. Chronic hypoglycemia and diabetes impair counterregulation induced by localized 2-deoxy-glucose perfusion of the ventromedial hypothalamus in rats. Diabetes. 1999;48:584–587. doi: 10.2337/diabetes.48.3.584. [DOI] [PubMed] [Google Scholar]
- 28.Tong QC, Ye CP, McCrimmon RJ, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page KA, Arora J, Qiu M, et al. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58:448–452. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts AG, Donovan CM. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Frontiers in Neuroendocrinology. 2010;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borg MA, Sherwin RS, Borg WP, et al. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. Journal of Clinical Investigation. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2005;289:R977–R981. doi: 10.1152/ajpregu.00403.2005. [DOI] [PubMed] [Google Scholar]
- 33.Kang L, Routh VH, Kuzhikandathil EV, et al. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- 34.Routh VH. Glucose Sensing Neurons in the Ventromedial Hypothalamus. Sensors. 2010;10:9002–9025. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros N, Dai L, Ferguson AV. Glucose-responsive neurons in the subfornical organ of the rat--a novel site for direct CNS monitoring of circulating glucose. Neuroscience. 2012;201:157–165. doi: 10.1016/j.neuroscience.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Dallaporta M, Himmi T, Perrin J, et al. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport. 1999;10:2657–2660. doi: 10.1097/00001756-199908200-00040. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro RE, Miselis RR. The Central Neural Connections of the Area Postrema of the Rat. Journal of Comparative Neurology. 1985;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 38.Paranjape SA, Briski KP. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience. 2005;130:957–970. doi: 10.1016/j.neuroscience.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Diggs-Andrews KA, Zhang X, Song Z, et al. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes. 2010;59:2271–2280. doi: 10.2337/db10-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marty N, Dallaporta M, Foretz M, et al. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocytedependent glucose sensors. Journal of Clinical Investigation. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puente E, Daphna-Iken D, Bree AJ, et al. Impaired counterregulatory response to hypoglycemia and impaired glucose tolerance in brain glucose transporter 4 (GLUT4) knockout mice. Diabetes (Abstract) 2009:A13. [Google Scholar]
- 42.Dunn-Meynell AA, Routh VH, Kang L, et al. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 43.Levin BE, Becker TC, Eiki J, et al. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2008;57:1371–1379. doi: 10.2337/db07-1755. [DOI] [PubMed] [Google Scholar]
- 44.McCrimmon RJ, Shaw M, Fan XN, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 45.Fioramonti X, Marsollier N, Song ZT, et al. Ventromedial Hypothalamic Nitric Oxide Production Is Necessary for Hypoglycemia Detection and Counterregulation. Diabetes. 2010;59:519–528. doi: 10.2337/db09-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCrimmon RJ, Evans ML, Fan XN, et al. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes. 2005;54:3169–3174. doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- 47.Spanswick D, Smith MA, Mirshamsi S, et al. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nature Neuroscience. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 48.Davis SN, Colburn C, Dobbins R, et al. Evidence That the Brain of the Conscious Dog Is Insulin-Sensitive. Journal of Clinical Investigation. 1995;95:593–602. doi: 10.1172/JCI117703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beverly JL, De Vries MG, Bouman SD, et al. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;280:R563–R569. doi: 10.1152/ajpregu.2001.280.2.R563. [DOI] [PubMed] [Google Scholar]
- 50.Barnes MB, Lawson MA, Beverly JL. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2011;301:R1815–R1820. doi: 10.1152/ajpregu.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szepietowska B, Zhu WL, Chan OW, et al. Modulation of beta-Adrenergic Receptors in the Ventromedial Hypothalamus Influences Counterregulatory Responses to Hypoglycemia. Diabetes. 2011;60:3154–3158. doi: 10.2337/db11-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saberi M, Bohland M, Donovan CM. The locus for Hypoglycemic rate of fall in glycemia - The role of portal-superior mesentieric vein glucose sensing. Diabetes. 2008;57:1380–1386. doi: 10.2337/db07-1528. [DOI] [PubMed] [Google Scholar]
- 53.Cryer PE. Elimination of Hypoglycemia From the Lives of People Affected by Diabetes. Diabetes. 2011;60:24–27. doi: 10.2337/db10-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cryer PE. Hypoglycaemia: The limiting factor in the glycaemic management of Type I and Type II Diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 55.Song ZT, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2006;291:R1283–R1287. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- 56.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25:501–504. doi: 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 57.Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87:64–68. doi: 10.1016/j.diabres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabetic Medicine. 2008;25:501–504. doi: 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 59.McCrimmon RJ, Sherwin RS. Hypoglycemia in Type 1 Diabetes. Diabetes. 2010;59:2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCrimmon RJ, Song ZT, Cheng HY, et al. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. Journal of Clinical Investigation. 2006;116:1723–1730. doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. American Journal of Physiology-Endocrinology and Metabolism. 2002;282:E770–E777. doi: 10.1152/ajpendo.00447.2001. [DOI] [PubMed] [Google Scholar]
- 62.Davis SN, Shavers C, Davis B, et al. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. Journal of Clinical Investigation. 1997;100:429–438. doi: 10.1172/JCI119550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis SN, Shavers C, Costa F, et al. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. Journal of Clinical Investigation. 1996;98:680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raju B, McGregor VP, Cryer PE. Cortisol elevations comparable to those that occur during hypoglycemia do not cause hypoglycemia-associated autonomic failure. Diabetes. 2003;52:2083–2089. doi: 10.2337/diabetes.52.8.2083. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg PA, Weiss R, McCrimmon RJ, et al. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes. 2006;55:1121–1126. doi: 10.2337/diabetes.55.04.06.db05-1169. [DOI] [PubMed] [Google Scholar]
- 66.Ramanathan R, Cryer PE. Adrenergic Mediation of Hypoglycemia-Associated Autonomic Failure. Diabetes. 2011;60:602–606. doi: 10.2337/db10-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caprio S, Gerety G, Tamborlane WV, et al. Opiate Blockade Enhances Hypoglycemic Counterregulation in Normal and Insulin-Dependent Diabetic Subjects. American Journal of Physiology. 1991;260:E852–E858. doi: 10.1152/ajpendo.1991.260.6.E852. [DOI] [PubMed] [Google Scholar]
- 68.Vele S, Milman S, Shamoon H, et al. Opioid Receptor Blockade Improves Hypoglycemia-Associated Autonomic Failure in Type 1 Diabetes Mellitus. Journal of Clinical Endocrinology & Metabolism. 2011;96:3424–3431. doi: 10.1210/jc.2011-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poplawski MM, Mastaitis JW, Mobbs CV. Naloxone, but Not Valsartan, Preserves Responses to Hypoglycemia After Antecedent Hypoglycemia Role of Metabolic Reprogramming in Counterregulatory Failure. Diabetes. 2011;60:39–46. doi: 10.2337/db10-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galassetti P, Mann S, Tate D, et al. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab. 2001;280:E908–917. doi: 10.1152/ajpendo.2001.280.6.E908. [DOI] [PubMed] [Google Scholar]
- 71.Milman S, Leu J, Shamoon H, et al. Magnitude of Exercise-Induced beta-Endorphin Response Is Associated with Subsequent Development of Altered Hypoglycemia Counterregulation. Journal of Clinical Endocrinology & Metabolism. 2012;97:623–631. doi: 10.1210/jc.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmet A, Dagenais S, Barrowman NJ, et al. Prevalence of Nocturnal Hypoglycemia in Pediatric Type 1 Diabetes: A Pilot Study Using Continuous Glucose Monitoring. Journal of Pediatrics. 2011;159:297–U385. doi: 10.1016/j.jpeds.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 73.Wiltshire EJ, Newton K, McTavish L. Unrecognised hypoglycaemia in children and adolescents with type 1 diabetes using the continuous glucose monitoring system: Prevalence and contributors. Journal of Paediatrics and Child Health. 2006;42:758–763. doi: 10.1111/j.1440-1754.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 74.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. New England Journal of Medicine. 1998;338:1657–1662. doi: 10.1056/NEJM199806043382303. [DOI] [PubMed] [Google Scholar]
- 75.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes - Reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195–1203. doi: 10.2337/diabetes.52.5.1195. [DOI] [PubMed] [Google Scholar]
- 76.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. DiabetMed. 1991;8:49–58. doi: 10.1111/j.1464-5491.1991.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 77.Oz G, Kumar A, Rao JP, et al. Human Brain Glycogen Metabolism During and After Hypoglycemia. Diabetes. 2009;58:1978–1985. doi: 10.2337/db09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canada SE, Weaver SA, Sharpe SN, et al. Brain glycogen supercompensation in the mouse after recovery from insulin-induced hypoglycemia. J Neurosci Res. 2011;89:585–591. doi: 10.1002/jnr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alquier T, Kawashima J, Tsuji Y, et al. Role of hypothalamic adenosine 5′-monophosphate activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology. 2007;148:1367–1375. doi: 10.1210/en.2006-1039. [DOI] [PubMed] [Google Scholar]
- 80.Herzog RI, Chan O, Yu S, et al. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oz G, Tesfaye N, Kumar A, et al. Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab. 2012;32:256–263. doi: 10.1038/jcbfm.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mastaitis JW, Wurmbach E, Cheng H, et al. Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes. 2005;54:952–958. doi: 10.2337/diabetes.54.4.952. [DOI] [PubMed] [Google Scholar]
- 83.Koranyi L, Bourey RE, James D, et al. Glucose transporter gene expression in rat brain: Pretranslational changes associated with chronic insulin-induced hypoglycemia, fasting, and diabetes. Molecular and Cellular Neuroscience. 1991;2:244–252. doi: 10.1016/1044-7431(91)90051-o. [DOI] [PubMed] [Google Scholar]
- 84.Osundiji MA, Hurst P, Moore SP, et al. Recurrent hypoglycemia increases hypothalamic glucose phosphorylation activity in rats. Metabolism-Clinical and Experimental. 2011;60:550–556. doi: 10.1016/j.metabol.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang LH, Herzog RI, Mason GF, et al. Recurrent Antecedent Hypoglycemia Alters Neuronal Oxidative Metabolism In Vivo. Diabetes. 2009;58:1266–1274. doi: 10.2337/db08-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van de Ven KC, van der Graaf M, Tack CJ, et al. Steady-State Brain Glucose Concentrations During Hypoglycemia in Healthy Humans and Patients With Type 1 Diabetes Mellitus. Diabetes. 2012 doi: 10.2337/db11-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cranston I, Reed LJ, Marsden PK, et al. Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes. 2001;50:2329–2336. doi: 10.2337/diabetes.50.10.2329. [DOI] [PubMed] [Google Scholar]
- 88.Bingham EM, Dunn JT, Smith D, et al. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [C-11]-3-O-methyl-D-glucose PET study. Diabetologia. 2005;48:2080–2089. doi: 10.1007/s00125-005-1900-6. [DOI] [PubMed] [Google Scholar]
- 89.Dunn JT, Cranston I, Marsden PK, et al. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic Hypoglycemia in type 1 diabetes - A new player in Hypoglycemia unawareness? Diabetes. 2007;56:2766–2773. doi: 10.2337/db07-0666. [DOI] [PubMed] [Google Scholar]
- 90.Segel SA, Fanelli CG, Dence CS, et al. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50:1911–1917. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]
- 91.Arbelaez AM, Powers WJ, Videen TO, et al. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition - A mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–475. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herzog RI, Jiang L, Mason G, et al. Increased Brain Lactate Utilization Following Exposure to Recurrent Hypoglycemia. Diabetes. 2009;58:A14–A14. [Google Scholar]
- 93.Mason GF, Petersen KF, Lebon V, et al. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55:929–934. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feyter HD, Shulman G, Rothman D, et al. Increased brain uptake of lactate in type I diabetic patients with hypoglycemia unawareness. Diabetes. 2012;61:A33. [Google Scholar]
- 95.Oz G, Moheet A, Emir U, et al. Hypothalamic GABA drops in response to acute hypoglycemia in healthy humans. Diabetes. 2012;61:A32. [Google Scholar]
- 96.Chan O, Paranjape S, Czyzyk D, et al. Increased GABAergic Output in the Ventromedial Hypothalamus Contributes to Impaired Hypoglycemic Counterregulation in Diabetic Rats. Diabetes. 2011;60:1582–1589. doi: 10.2337/db10-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan O, Cheng HY, Herzog R, et al. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory reponses after antecedent Hypoglycemia. Diabetes. 2008;57:1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boyle PJ, Nagy RJ, O’Connor AM, et al. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reno CM, Tanoli T, Puente EC, et al. Deaths due to severe hypoglycemia are exacerbated by diabetes and ameliorated by hypoglycemic preconditioning. Diabetes. 2011;60:A81. [Google Scholar]
- 100.Smith CB, Choudhary P, Pernet A, et al. Hypoglycemia Unawareness Is Associated With Reduced Adherence to Therapeutic Decisions in Patients With Type 1 Diabetes Evidence from a clinical audit. Diabetes Care. 2009;32:1196–1198. doi: 10.2337/dc08-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135–141. doi: 10.2337/diacare.20.2.135. [DOI] [PubMed] [Google Scholar]
- 102.Davis SN, Shavers C, Costa F. Gender-related differences in counterregulatory responses to antecedent hypoglycemia in normal humans. J Clin Endocrinol Metab. 2000;85:2148–2157. doi: 10.1210/jcem.85.6.6641. [DOI] [PubMed] [Google Scholar]
- 103.Sandoval DA, Ertl AC, Richardson MA, et al. Estrogen blunts neuroendocrine and metabolic responses to hypoglycemia. Diabetes. 2003;52:1749–1755. doi: 10.2337/diabetes.52.7.1749. [DOI] [PubMed] [Google Scholar]
- 104.McGill JB, Vlajnic A, Knutsen PG, et al. Effect of gender on outcomes in patients with T2DM treated with insulin glargine vs comparators. Diabetes. 2011;60:A602. [Google Scholar]
- 105.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 106.Cranston I, Lomas J, Maran A, et al. Restoration of Hypoglycemia Awareness in Patients with Long-Duration Insulin-Dependent Diabetes. Lancet. 1994;344:283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 107.Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous Prevention of Hypoglycemia Normalizes the Glycemic Thresholds and Magnitude of Most of Neuroendocrine Responses to, Symptoms of, and Cognitive Function during Hypoglycemia in Intensively Treated Patients with Short-Term Iddm. Diabetes. 1993;42:1683–1689. doi: 10.2337/diab.42.11.1683. [DOI] [PubMed] [Google Scholar]
- 108.Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood glucose awareness training (BGAT-2) - Long-term benefits. Diabetes Care. 2001;24:637–642. doi: 10.2337/diacare.24.4.637. [DOI] [PubMed] [Google Scholar]
- 109.Cox DJ, Kovatchev B, Koev D, et al. Hypoglycemia anticipation, awareness and treatment training (HAATT) reduces occurrence of severe hypoglycemia among adults with type 1 diabetes mellitus. International Journal of Behavioral Medicine. 2004;11:212–218. doi: 10.1207/s15327558ijbm1104_4. [DOI] [PubMed] [Google Scholar]
- 110.Hopkins D, Lawrence I, Mansell P, et al. Improved Biomedical and Psychological Outcomes 1 Year After Structured Education in Flexible Insulin Therapy for People With Type 1 Diabetes The U.K. DAFNE experience. Diabetes Care. 2012;35:1638–1642. doi: 10.2337/dc11-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samann A, Muhlhauser I, Bender R, et al. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia. 2005;48:1965–1970. doi: 10.1007/s00125-005-1905-1. [DOI] [PubMed] [Google Scholar]
- 112.Heller SR, Colagiuri S, Vaaler S, et al. Hypoglycaemia with insulin aspart: a double-blind, randomised, crossover trial in subjects with Type 1 diabetes. Diabetic Medicine. 2004;21:769–775. doi: 10.1111/j.1464-5491.2004.01244.x. [DOI] [PubMed] [Google Scholar]
- 113.Brunelle RL, Llewelyn J, Anderson JH, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726–1731. doi: 10.2337/diacare.21.10.1726. [DOI] [PubMed] [Google Scholar]
- 114.Home PD, Fritsche A, Schinzel S, et al. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obesity & Metabolism. 2010;12:772–779. doi: 10.1111/j.1463-1326.2010.01232.x. [DOI] [PubMed] [Google Scholar]
- 115.Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 116.Cummins E, Royle P, Snaith A, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technology Assessment. 2010;14:1. doi: 10.3310/hta14110. [DOI] [PubMed] [Google Scholar]
- 117.Gimenez M, Lara M, Conget I. Sustained Efficacy of Continuous Subcutaneous Insulin Infusion in Type 1 Diabetes Subjects with Recurrent Non-Severe and Severe Hypoglycemia and Hypoglycemia Unawareness: A Pilot Study. Diabetes Technology & Therapeutics. 2010;12:517–521. doi: 10.1089/dia.2010.0028. [DOI] [PubMed] [Google Scholar]
- 118.Gandhi GY, Kovalaske M, Kudva Y, et al. Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized trials. J Diabetes Sci Technol. 2011;5:952–965. doi: 10.1177/193229681100500419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Beck RW, Hirsch IB, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garg S, Brazg RL, Bailey TS, et al. Reduction in Duration of Hypoglycemia by Automatic Suspension of Insulin Delivery: The In-Clinic ASPIRE Study. Diabetes Technology & Therapeutics. 2012;14:205–209. doi: 10.1089/dia.2011.0292. [DOI] [PubMed] [Google Scholar]
- 121.El-Khatib FH, Russell SJ, Nathan DM, et al. A Bihormonal Closed-Loop Artificial Pancreas for Type 1 Diabetes. Science Translational Medicine. 2010:2. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paty BW, Senior PA, Lakey JRT, et al. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technology & Therapeutics. 2006;8:165–173. doi: 10.1089/dia.2006.8.165. [DOI] [PubMed] [Google Scholar]
- 123.Leitao CB, Tharavanij T, Cure P, et al. Restoration of Hypoglycemia Awareness After Islet Transplantation. Diabetes Care. 2008;31:2113–2115. doi: 10.2337/dc08-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rickels MR. Recovery of Endocrine Function After Islet and Pancreas Transplantation. Curr Diab Rep. 2012 doi: 10.1007/s11892-012-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 126.Raju B, Arbelaez AM, Breckenridge SM, et al. Nocturnal Hypoglycemia in type 1 diabetes: An assessment of preventive bedtime treatments. Journal of Clinical Endocrinology & Metabolism. 2006;91:2087–2092. doi: 10.1210/jc.2005-2798. [DOI] [PubMed] [Google Scholar]
- 127.Axelsen M, Wesslau C, Lonnroth P, et al. Bedtime uncooked cornstarch supplement prevents nocturnal hypoglycaemia in intensively treated type 1 diabetes subjects. Journal of Internal Medicine. 1999;245:229–236. doi: 10.1046/j.1365-2796.1999.00432.x. [DOI] [PubMed] [Google Scholar]
- 128.Weinzimer SA. Closed-loop artificial pancreas: current studies and promise for the future. Current Opinion in Endocrinology Diabetes and Obesity. 2012;19:88–92. doi: 10.1097/MED.0b013e3283514e6b. [DOI] [PubMed] [Google Scholar]
- 129.Hardin D, Hillman D, Cattet J. Hypoglycemia alert dogs-Innovative assistance for people with type I diabetes. Diabetes. 2012;61:A99. [Google Scholar]
- 130.Watson JM, Jenkins EJE, Hamilton P, et al. Influence of caffeine on the frequency and perception at hypoglycemia in free-living patients with type 1 diabetes. Diabetes Care. 2000;23:455–459. doi: 10.2337/diacare.23.4.455. [DOI] [PubMed] [Google Scholar]
- 131.de Galan BE, Tack CJ, Lenders JW, et al. Effect of 2 weeks of theophylline on glucose counterregulation in patients with type 1 diabetes and unawareness of hypoglycemia. Clinical Pharmacology & Therapeutics. 2003;74:77–84. doi: 10.1016/S0009-9236(03)00093-6. [DOI] [PubMed] [Google Scholar]