1. Brown Adipose Tissue and Thermoregulation
In mammals, thermogenesis is necessary to maintain body temperature or generate fevers that fight infection. Thermogenesis also affects energy homeostasis, partially via sympathetic control of brown adipose tissue (BAT) thermogenesis 1. The importance of BAT thermogenesis in human body weight control has been debated 2;3 and only recently exciting advances in the field of BAT development has opened new avenues for pharmacological induction of brown adipocytes from progenitor cells within muscle and white adipose tissue 4. Most importantly, several studies demonstrated substantial amounts of functional BAT in adult humans 5–8, so that BAT thermogenesis has been rediscovered as a potential target to treat obesity.
1.1. Introduction Brown Adipose Tissue
Brown adipose tissue (BAT) is a specialized tissue that is able to generate heat and thus enables homoiotherm mammals to maintain body temperature largely independent of the environmental temperature. BAT generates heat with the BAT-specific expression of uncoupling protein 1 (UCP1), a proton channel of the inner mitochondrial membrane that allows proton influx into the mitochondrial lumen. The mitochondrial respiratory chain maintains a proton gradient across the inner mitochondrial membrane for ATP production, which is uncoupled by UCP1 to release energy as heat instead 9–11. This uncoupling process is highly regulated and depends on sympathetic stimulation of β3-adrenergic receptors (β3-AR), but is also regulated by fatty acids and thyroid hormone upon other stimulants 12–15. Stimulation of β3-ARs increases UCP1 gene expression, UCP1 activity and β-oxidation, the latter is of major importance to fuel the energy demanding thermogenic process. The induction of BAT thermogenesis is also termed adaptive thermogenesis or non-shivering thermogenesis to distinguish this highly regulated process from other non-regulated (e.g. thermic effects of metabolic processes) or mechanical (e.g. shivering) processes that also contribute to heat production.
1.2. Induction of BAT thermogenesis
Exposure to a cold environment robustly stimulates BAT thermogenesis (cold-induced thermogenesis -CIT), and is particularly important for small mammals such as rodents and newborns. This is because small mammals have a larger surface relative to their body volume compared to large mammals and thus loose more heat in a cold environment 16;17. BAT thermogenesis is also required for normal fever responses (fever-inducing thermogenesis) and is mediated by endogenous pyrogenes like prostaglandin E2 18. It is also well known that the ingestion of food per se as well as caloric dense diets increases body temperature as well as energy expenditure (Diet-induced thermogenesis -DIT)19. However, the fact that any meal generally induces energy expenditure and heat production as a mere by product of metabolic processes (non-regulated) has elicit some controversial discussions whether DIT is indeed an adaptive, regulated process or rather an indirect metabolic effect 2;3.
1.3. Controversial views and why we should mind
In the 70th BAT thermogenesis was considered as an important target for anti-obesity drugs and launched intensive studies on DIT in humans. The insensitivity of β3-AR agents in humans, safety issues with drugs that increased BAT thermogenesis (e.g. sibutramine)20 and finally the lack of evidence for substantial amounts of BAT in adult humans led to a cease of research support for DIT related drug targets. Just recently, a series of publications demonstrated functional and inducible amounts of BAT in at least a subpopulation of adult humans 5–8, which revived the discussion of adaptive thermogenesis as a potential obesity drug target. Coupled with new insights into the developmental origin of brown adipocytes, central regulation and metabolic dynamics important to induce BAT thermogenesis dramatically highlighted that we were/are still lacking major knowledge about this peculiar heat generating tissue as well as other potential heat generating mechanisms in rodents and humans. Thus, there is reasonable hope that continued efforts to understand the role of BAT function in energy homeostasis will boost our approaches to find suitable drug targets to support energy homeostasis control in western societies.
2. Central control of BAT thermogenesis
2.1. Neuronal circuits
Much of the recent research has focused on BAT function per se, however, BAT thermogenesis is generally controlled by central mechanisms. Neuroanatomical and pharmacological approaches based on cold and pyrogen induced thermogenesis have identified several CNS sites as key players in the control of BAT thermogenesis 21.
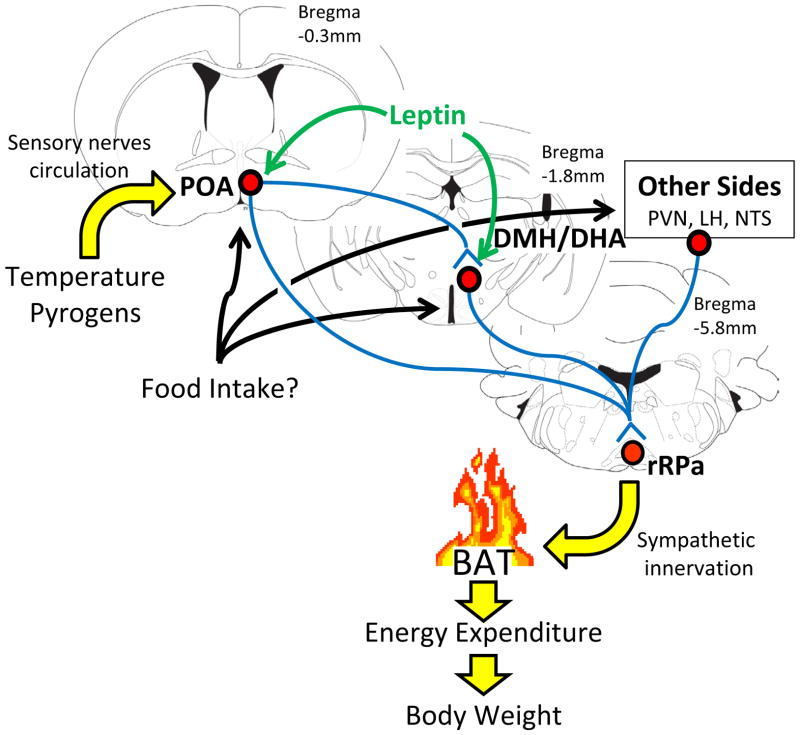
The preoptic area (POA) acts as a temperature sensor and integrates temperature information from the CNS, peripheral and deep-body thermoreceptors 21. POA neurons provide at least inhibitory inputs to the dorsomedial hypothalamus/dorsal hypothalamic area (DMH/DHA)22–26. Neuronal activation of DMH/DHA neurons, likely representing glutamatergic neurons 23, is critical for further stimulation of downstream premotor neurons in the rostral raphe pallidus (rRPa)25;27 to control sympathetic BAT activity (Figure 1). Indeed, typical BAT-inducing stimuli such as cold or pyrogens like lipopolysaccharide (LPS) result in robust stimulation of neuronal activity in rRPa-innervating DMH/DHA neurons 26;28. The rRPa also receives direct inputs from the POA 29 and orexin neurons in the lateral hypothalamus (LHA) 30; and inputs from the nucleus of the solitary tract (NTS) have been hypothesized 31, even though these circuits have been less well studied.
Figure 1.
Central pathways controlling brown fat thermogenesis
Neurons in the preoptic area (POA) receive sensory information about ambient temperature (from skin) or pyrogens (from circulation) that is relayed to neurons in the dorsomedial hypothalamus/dorsal hypothalamic area (DMH/DHA). Neuronal activation of DMH/DHA neurons stimulates sympathetic premotor neurons in the rostral raphe pallidus to control sympathetic inputs to the brown adipose tissue (BAT). It is still unclear if and where feeding related signals could integrate into this central thermoregulatory pathway to promote diet-induced thermogenesis.
The VMH had been historically associated with thermoregulatory control 32–34, but recent work suggested that the anatomically large size of deletions and injections may have confounded the original conclusions and the observed thermogenic effects could have resulted from leakage into the nearby DMH/DHA structure 27;35. Indeed, the DMH/DHA has been well demonstrated to play a crucial role in the regulation of BAT thermogenesis and responds to thermoregulatory drugs more sensitively than the VMH 36. However, the DMH is also known for its exceptional interconnection with virtually all other hypothalamic sites, including the VMH 37, so that it is still possible that VMH neurons regulate thermoregulation indirectly through innervation of the DMH/DHA.
2.2. Sensing processes for BAT thermogenesis: from cold and pyrogens to ingested diets
Thermoregulatory control is initiated via sensory neurons in the skin, abdomen, spinal cord, but also via thermosensing neurons in the CNS, e.g. warm sensing neurons in the POA 38. Fever responses to LPS can be initiated by prostaglandin receptor (PGE2) expressing POA neurons 39, that also regulate BAT thermogenesis via the DMH/DHA and rRPa neurons 26. However, very little is known about the sensory systems that could connect changes in dietary content with thermoregulatory control mechanisms (diet-induced thermogenesis) and it is not entirely clear if cold- and diet-induced thermogenesis are indeed regulated by identical systems.
Temperature sensing mechanisms have been studied in POA neurons, where warm- and cold-sensing neurons can be distinguished due to increased firing rates with hypothalamic warming or cooling, respectively 40–42. There is strong evidence that neuronal cold-sensitivity is due to (GABAergic) synaptic inhibition from warm-sensing neurons 40;43–46. DMH/DHA neurons have characteristics similar to these cold-sensing neurons, because cold exposure increases their activity 25–27;47;48 and they are inhibited by GABAergic inputs at least from the POA 22;25;26;47;49–51. Neuronal coupling with other sensory systems that also influence body temperature control (e.g. neurons regulated by glucose, fasting or reproductive hormones) may further influence neuronal firing and transmitter release within these systems, even though this has not been investigated yet.
2.3. The Melanocortin System, thermoregulation and energy expenditure
The hypothalamic arcuate nucleus senses and reacts to changes in feeding states (e.g. high fat diet or fasting). Particularly the melanocortin system consists of anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic agouti-related-peptide (AgRP) neurons that are differentially regulated in response to feeding states 52. POMC and AgRP neurons both project broadly within the hypothalamus including the POA and DMH 53. Indeed, melanocortin-4-receptors (MC4R) are found in many neurons associated with the regulation of sympathetic BAT inputs 54 and MC4R function in cholinergic intermediolateral nucleus (IML) neurons is sufficient to recover the low energy expenditure of MC4R-deficient mice 55. Intriguingly, MC4R deficient mice are very prone to gain weight, but fail to raise UCP1 in response to a HFD 54;56, indicating that MC4R signaling is an important component to induce diet-induced thermogenesis. Thus, the melanocortin system would be well positioned to serve as a sensory relay between feeding state and thermoregulatory control in diet-induced obesity.
2.4. The Leptin System thermoregulation and energy expenditure
Leptin is an adipocyte derived hormone which is well known to mediate its anorexigenic effects via the melanocortin system. However, targeted deletion of leptin receptors (LepRb) from AgRP and/or POMC neurons did not substantially modulate energy expenditure 57;58. In contrast, the complete lack of leptin signaling results in hypothermia, cold-sensitivity 59–62, BAT atrophy and decreased uncoupling protein (UCP1) expression 63;64, clearly demonstrating an important function of leptin in thermoregulatory control. Leptin mediates food-independent body weight loss 65, that depends on UCP1 expression 66, suggesting that food-independent body weight control by leptin is mediated via BAT thermogenesis. Also fat oxidation – a key thermoregulatory function to fuel mitochondrial respiration – is centrally controlled by leptin via AMP-kinase (AMPK) pathways 67–69. Similarly, in humans leptin prevents decreased energy expenditure commonly associated with dieting 70, even though if this involves central mechanisms or regulation of peripheral tissues (e.g. BAT, muscle) to increase energy expenditure remains unclear.
Leptin receptors are expressed in the POA and DMH/DHA and these LepRb neurons recapitulate known thermoregulatory circuits: POA and DMH/DHA LepRb neurons are associated with sympathetic BAT innervation and both innervate the rRPa. POA LepRb neurons innervate the DMH/DHA and DMH/DHA LepRb neurons are robustly stimulated by cold-exposure 71. This indicates that leptin utilizes identical circuits as cold or pyrogens to regulate thermogenesis. The effect of leptin on energy expenditure and body temperature is most robust in states of low leptin levels (e.g. leptin deficiency and fasting) and it has been argued that leptin has a rather permissive than actively thermogenic effect. However, other studies also confirm in normal fed rodents that leptin effectively increases sympathetic BAT activity, BAT temperature and body core temperature 72–74. Furthermore, high fat diet induced hyperleptinemia is further associated with increased body temperature, indicating that leptin in fact contributes to the induction of diet-induced thermogenesis 72. Thus, the capacity of this thermoregulatory leptin system to regulate energy expenditure and body weight remains to be tested. Also, the cellular mechanisms involved to regulate neuronal activity e.g. in DMH/DHA LepRb neurons by cold-exposure (and supposedly by leptin) are unknown.
Thermoregulatory leptin action can also be mediated by brainstem mechanisms independent of hypothalamic function, as observed in decerebrated rats 75. Interestingly, while 4th ventricle leptin alone only mildly raises body or brown fat temperature, leptin robustly enhanced thermogenic capacities of thyroid releasing hormone (TRH)31;76. This sensitizing effect was dependent on phospholipase C and inositol-3-phosphate calcium release mechanisms and could be attributed to direct leptin effects on NTS neurons 77. These NTS neurons may further stimulate rRPa neurons to control BAT thermogenesis, even though these connections remain to be validated.
2.5. Other central regulators of thermogenesis and energy expenditure
Central thyroid function and AMPK signaling
Thyroid hormone is well known to regulate body temperature and energy expenditure via its function in peripheral organs 14. Thyroxin (T4) is the predominant form of thyroid hormone in the circulation and is converted to the more potent form T3 by deiodinases in local target tissues like BAT and white adipose tissue, liver and muscle. Such deiodinases are also found in the brain indicating that thyroid hormone may also regulate metabolic function via central mechanisms 78. This has been further confirmed by hypothalamic T3 injections, which increases energy expenditure and body temperature via inactivation of AMPK signaling pathways and further activated rRPa neurons 79. Thus, thyroid hormone may also regulate these typical cold- and pyrogene-induced thermoregulatory circuits.
Leptin also deactivates hypothalamic AMPK signaling, which is well known to modulate feeding behavior, in part via regulation of POMC and AgRP expression 67 and it is possible that thermogenic leptin actions are also mediated via hypothalamic AMPK pathways. Also, bone morphogenetic protein 8B (BMP8B) importantly regulates energy expenditure and body temperature via central mechanisms that involve AMPK signaling and ultimately results in neuronal activation of rRPa neurons 80. However, it remains unclear which cellular mechanisms are regulated by hypothalamic AMPK that would explain neuronal excitation in brainstem rRPa neurons.
In peripheral tissues AMPK is known as a master energy sensor and regulator of lipid metabolism. AMPK is upregulated by increased ATP demand (thus high AMP/ATP ratio) and activation of AMPK enables enhanced β-oxidation and FFA transport to fuel mitochondrial energy generation 81. In the brain AMPK is inversely regulated to the periphery, but how this relates to cellular processes within the brain (e.g. changes in lipid metabolism, gene expression, neuronal activity) is not well understood and requires further investigations.
Thermoregulatory neuropeptide Y (NPY) action in the dorsomedial hypothalamus
The DMH harbors a well described population of neurons that expresses NPY, which is greatly enhanced in states of increased energy demand 82;83 or in rodent models of obesity 84–87. Viral knockdown of NPY mRNA selectively in the DMH increased UCP1 expression in BAT as well as white fat, which resulted in increased energy expenditure 88. This effect was mediated via sympathetic innervation of at least the white fat, even though whether NPY neurons feed into the thermoregulatory circuit – POA>DMH/DHA>rRPa – remains to be tested.
3. Peripheral control of BAT thermogenesis
3.1. Cell types responsible for thermogenesis: The origin of brown and beige (brite) adipocytes
There are multiple fat depots all over the body containing different cell types important for energy storage and thermogenesis 89. Excess energy is stored in white adipocytes in one large lipid droplet, whereas brown adipocytes realize the combination of lipid storage in multilocular lipid droplets and energy combustion by abundant mitochondria. Classical brown adipocytes, located for instance in the interscapular region of mice, share a developmental Myf5-positive precursor with muscle cells 90. Critically involved in brown adipocyte commitment and differentiation are nuclear receptors such as peroxisome proliferator activated receptors (PPARs) and their respective transcriptional co-activators such as PGC1α and PRDM16 (for review see 91. Thermogenic stimuli also induce the appearance of UCP1-expressing brown-like fat cells especially in subcutaneous white adipose tissue depots. These beige or brite (made up of brown in white) adipocytes arise from discrete progenitor cells in white adipose tissue 92;93 in a process which is again dependent on the expression of PGC1α and PRDM16 94. The regulation of browning is regulated by a number of signals including central and endocrine signals which are discussed in more detail in 3.2 – 3.7. Notably, recently it was demonstrated that human brown adipose tissue resembled more closely those of murine beige adipocytes than of classical brown adipocytes, at least when comparing gene expression signatures from human brown adipose tissue with fat depots taken from mice 95. However, it should be emphasized that although the development of these UCP1-positive adipocytes is associated with beneficial effects on body weight and metabolic health 94, it is still unclear whether beige adipocytes arising in white adipose tissue of mice are as powerful as their classical brown adipocyte relatives.
3.2. Hormones controlling BAT development and function
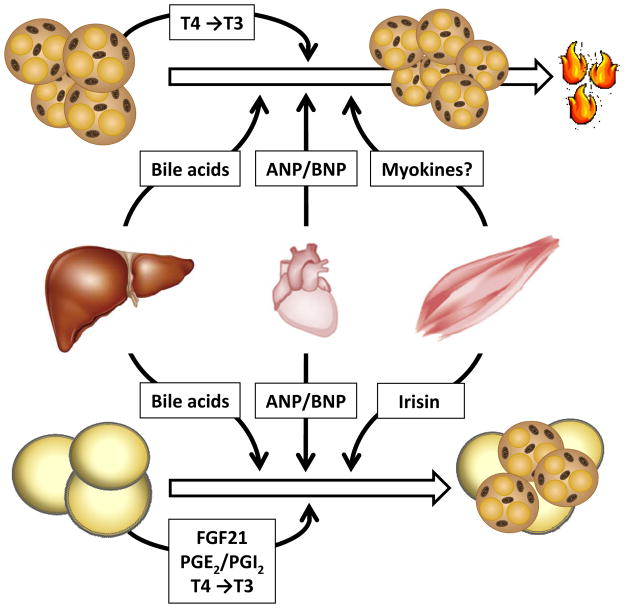
The development of brown as well as beige adipocytes is regulated by diverse factors in an endocrine, paracrine and autocrine fashion (Figure 2). Next to catecholamines, the pleiotropic role of thyroid hormones, sex hormones, bile acids, endocannabinoids and corticosteroids for BAT biology has been summarized in a number of excellent reviews 1;96–98. Recent reports indicate that also prostaglandins, members of the fibroblast growth factor (FGF) family, BMP, cardiac natriuretic peptides and a novel myokine called irisin are important peripheral modulators for BAT development and thermogenesis.
Figure 2.
Peripheral signals controlling brown and brite adipose tissue
Next to activation via the sympathetic nervous system, diverse endocrine signals such as bile acids or natriuretic peptides (ANP and BNP) increase energy expenditure by the induction of thermogenic genes in brown adipose tissue. These hepatocyte- and cardiomyocyte-specific factors also positively influence the development of UCP1-expressing, multilocular so called beige or brite adipocytes, which are characterized by an intermediate phenotype between brown and white adipoyctes. Irisin – a myokine released by exercised muscles – stimulate only specific precursor cells within the white but not the brown adipose tissue to develop a brownish phenotype. Next to endocrine control, specific prostaglandins as well as FGF21 released by white adpocytes initiate the browning in an autocrine and/or paracrine manner. In addition, the deiodinase 2-mediated conversion of thyroxine (T4) into triiodothyronine (T3) further promotes activation and development of brown and brite adipocytes, respectively. Both the activation of BAT but also the occurrence of brite adipose tissue is associated with a beneficial metabolic profile, implying that both processes are important for the maintenance of metabolic health.
3.3. Prostaglandins
The generation of fatty acid derived prostaglandins (PG) is controlled by two cyclooxygenase isoenzymes (COX1 and COX2). The latter is induced by β-adrenergic signaling after long-term cold exposure in white adipose tissue which is associated with an increase in PGE2/PGI2 levels. Interestingly, selective COX2 overexpression in white adipose tissue leads to de novo recruitment of beige adipocytes thereby preventing high fat diet induced obesity by increased energy expenditure 99. Accordingly, cold-induced UCP1 expression is attenuated in white adipose tissue of COX2 deficient mice 100 underlining that the manipulation of this novel signaling pathway might be an alternative strategy to induce slimming by browning 101.
3.4. FGFs
There are a number of FGF family members which have been implicated in the regulation of adipose tissue function. Autocrine and/or paracrine signaling are mediated via the interaction of FGFs with their respective FGF receptors and heparin 102. The prototype of this protein family, FGF1, is increased in obesity and seems to be the main functional FGF protein - at least in human subcutaneous white adipose tissue 103. In line with this observation, as a direct target of the adipose tissue master regulator PPARγ, FGF1 is a critical transducer of environmental signals to maintain white adipose tissue function 104.
Members of the FGF19 subfamily including FGF21 can exert also endocrine functions by activating FGF receptors complexed to klotho proteins 105. In mice, FGF21 expression is induced by either treatment with PPARα agonist and fasting in liver, by treatment with PPARγ agonist and feeding in white adipose tissue or by cold exposure in brown adipocytes 106;107. Since circulating plasma levels of FGF21 corresponds to its hepatic expression, the endocrine function such as the induction of fat oxidation in response to fasting is mediated exclusively by liver-derived FGF21. However, anti-diabetic properties of PPARγ agonist seems to be dependent on FGF21 synthesized in white adipose tissue. Mechanistically, FGF21 prevents PPARγ inactivation by inhibiting its conversion to an inactive, sumoylated form of PPARγ 106. In addition, by enhancing PGC1α protein levels, FGF21 is also critical for the development of beige adipocytes. Consequently, FGF21 deficiency is associated with decreased browning and an impaired adaptation to cold exposure 108. In summary, over the past years FGF21 has evolved as a strong metabolic regulator, positively influencing energy expenditure probably via inducing the development of beige adipocytes. However, to gain a more comprehensive picture of FGF21 biology in humans and to decipher its potential adverse effects on skeleton 109, future work will have to elucidate its suitability to treat metabolic diseases in clinical studies.
3.5. BMPs
BMPs are secreted molecules that are known to induce the differentiation of mesenchymal towards bone-forming cells, the osteoblast 110. Interestingly, BMP7-mediated signaling induces not only the key transcription factor for osteoblast differentiation, RUNX2 111, but also promotes differentiation of brown pre-adipocytes leading to the induction of PRDM16 and PGC1α. Consequently, the lack of BMP7 results in an almost complete absence of UCP1 whereas on the other side BMP7 overexpression increases brown adipose tissue mass and energy expenditure 112. As described above (see chapter 2.5.), another BMP family member, BMP8B, is involved in the regulation of hypothalamic AMPK activity 80. In addition, BMP8B expression is also induced in mature brown adipocytes in response to thermogenic stimuli such as diet and cold exposure, thereby enhancing noradrenaline-mediated signaling via stimulation of P38-MAPK and CREB phosphorylation 80. Consequently, BMP8B-deficient mice display an obesogenic phenotype probably mediated by both central and peripheral actions.
3.6. Cardiac natriuretic peptides
Atrial and brain natriuretic peptides ANP and BNP, respectively, are produced in the heart to maintain the homeostasis of body fluids and blood pressure. It is well established that cold exposure raises blood pressure thereby triggering cardiovascular complications in winter 113. Thus, the release of cardiac hormones to activate thermogenesis for heat production would make physiological sense to prevent cold-induced hypertension. Natriuretic peptides can bind to their respective receptors present on adipocytes to stimulate lipolysis via cGMP-dependent signaling 114, a pathway also known to control brown fat cell differentiation and mitochondrial biogenesis 115. Recently, Bordicchia et al. described that increased levels of both ANP and BNP as a consequence of cold exposure or infusion of recombinant proteins leads to the expression of PGC1α and UCP1 in white and brown adipose tissue 116. This intriguing report clearly demonstrates that cardiac hormones are peripheral regulators for heat production, a process which might - from a evolutionary perspective – be developed to counteract cold-induced harmful effects such as cardiac hypertrophy and hypertension.
3.7. Muscle-derived Irisin
Similarly to the adaptive response of the heart, skeletal muscle should also activate energy expenditure by adipose tissue to circumvent the detrimental effects of shivering thermogenesis. Boström et al. showed that this concept might indeed have evolved since exercised muscle cells increase the expression of FNDC5. After proteolytic cleavage by an unknown shedding enzyme, this membrane protein is released into the circulation as a hormone called irisin, a myokine driving beige adipocyte development within white adipose tissue 117. Despite the attractive hypothesis that beneficial metabolic effects of exercise are in part explained by the browning of white adipose tissue, open questions including the molecular targets of irisin responsible for beige adipocyte differentiation as well as the relevance of irisin for human physiology remain to be solved.
4. BAT mediated regulation of fuel delivery
4.1 BAT as a new player in lipoprotein metabolism
Cold exposure results in the acute break-down of cellular triglyceride stores within brown adipocytes which are used as fuel for heat production 1. To sustain their function, brown adipocytes rely on supply with glucose and fatty acids. In fact, up to 90% of energy for heat production is derived from fatty acids which are the main source for β-oxidation in brown adipocytes 1. Fatty acids are mainly transported as esterified triglycerides by two classes of lipoproteins that are responsible for the transport of energy to the different organs via the circulation: chylomicrons are formed in the intestine to deliver dietary fat in the postprandial phase whereas VLDL are produced in the liver when food supply is low. In the bloodstream, these triglyceride-rich lipoproteins (TRL) are hydrolyzed by lipoprotein lipase (LPL), which results in the release of non-esterified fatty acids and their subsequent internalization by fatty acid transporters into muscle or adipose tissues. LPL activity is controlled by a number of proteins including apolipoproteins and angiopoietin-like proteins (reviewed by 118;119, ensuring that energy is transported into the right tissue dependent on the metabolic conditions. Thus, the regulation of LPL activity fulfills an important gatekeeper function for energy delivery to metabolically active tissues. In addition, LPL is able to facilitate the interaction with lipoprotein receptors to enhance lipoprotein clearance 120. In activated BAT, local LPL activity is enormously induced ensuring fatty acid uptake as a fuel for heat production. Recent work from our group demonstrated that the triglyceride lowering effect of LPL in stimulated BAT is mediated in concert with the fatty acid transporter CD36 121. Interestingly, in addition to NEFAs also whole TRL particles were internalized in a LPL-dependent manner at the vascular endothelium into brown adipocytes. Given that the liver is equipped with a fenestrated endothelium enabling direct contact with plasma lipoproteins, TRL particle uptake into BAT either involves leakage of the endothelial layer or requires transendothelial transport. This study strongly implicates that lipids are – to a large extent – transported by lipoproteins to brown adipocytes. However, the biological importance and the underlying mechanisms of lipoprotein uptake into BAT is still unclear but it seems that these processes are fundamentally different from canonical lipoprotein pathways found in skeletal muscle or white adipose tissue 121–124
4.2 BAT function: Regulated by fatty acids?
Fatty acids not only serve as fuels, they can also act as ligands for transcription factors of the PPAR family 125. For instance, NEFAs released from intracellular triglyceride stores by adipose triglyceride lipase (ATGL) have been shown to promote PPARα function in the heart 126. The molecular program initiated in response to cold in BAT is induced by PPARα, PPARγ, PGC1α as well as the aforementioned PRDM16 127;128. In this light, lipoprotein-mediated delivery of lipids to brown adipocytes could be directly linked to mitochondrial activity and energy expenditure through the PPAR-activating properties of certain triglyceride lipolysis products. Given that 50% of a lipid-rich meal end-up in BAT when mice are exposed to cold 129, it is a conceivable and intriguing concept that diets - especially the composition of ingested fatty acids - could directly influence the thermogenic program of BAT.
5. BAT thermogenesis and Body weight control
5.1. Mouse models
There is no doubt that BAT thermogenesis is the key tissue to maintain homeothermia in small mammals as well as newborns in cold environments. This has been greatly confirmed in mice with genetic deletion of UCP1 (UCP1-KO mice), which are unable to survive acute cold exposure 130. BAT thermogenesis is obviously a very energy demanding process and impacts whole body energy homeostasis as demonstrated by the robust increase in food intake observed during cold exposure, while body weight is maintained. Thus, it was expected that UCP1-KO mice would develop obesity. Surprisingly, UCP1-KO mice were leaner than their wildtype littermates 130, strongly arguing against a role of BAT thermogenesis for body weight control. In contrast, when put on a high fat diet under thermoneutral conditions (eliminating the need for compensatory thermogenic mechanisms that could override the UCP1 deficiency phenotype 131) UCP1-KO mice indeed gain more weight than control littermates 132, even though this was not found by others 130. Furthermore, overexpression of UCP1 in epididymal fat tissue resulted in a marked metabolic improvement beyond the expected heat generating UCP1 function. Indeed, mild UPC1 overexpression that did not cause a change in energy expenditure resulted in a robust leptin and insulin sensitizing effect and involved afferent nerve signals from fat to the brain 133. Thus, while the above described mouse models convincingly connect BAT thermogenesis with body weight loss, there still remains some debate if this can be solely explained by typical heat generating UCP1 mechanisms.
Yet, the main question remains how BAT thermogenesis can be safely modulated without severe side effects (e.g. cardiac dysfunction). This will require a better understanding of central and peripheral regulators of BAT thermogenesis. While the central pathways regulating sympathetic BAT inputs have been well defined, it is not clear if these circuits also control other peripheral tissues (e.g. white fat, liver, skeletal or heart muscle). Similarly, novel hormones like irisin may not be restricted to peripheral actions, but could also engage central circuits to regulate thermogenic function.
5.2. Humans
In order to visualize the metabolism of cancer cells, non-invasive metabolic imaging has been established with radiolabeled substrates such as 18F-desoxyglucose in nuclear imaging modalities, like positron emission tomography (PET). The combination of PET with computed tomography (PET/CT) allows determining the exact localization of glucose uptake. BAT was long thought to be only of relevance in children and small mammals but by using this PET/CT technology to re-evaluate 18F-desoxyglucose uptake patterns, high metabolic activity was found within the fatty tissue of shoulders, neck and thoracic spine especially in underweight patient. Although it was not proven at that time, the authors proposed that these depots could represent activated brown adipose tissue 134. Meanwhile it is accepted that the prevalence of functional BAT ranges from 30–100% depending on the respective cohort analyzed (for review see 135;136) and that BAT activity declines in obese and elderly people 5;135;137;138. However, in comparison to mice, the amount of active BAT in humans is approximately 100 fold lower 122;139, arguing against a significant role of human BAT for energy metabolism. Nevertheless, dedicated studies imply that selective drugs sustaining or increasing BAT activity might have beneficial effects on obesity and metabolic health. For example, based on PET/CT tracer studies, Virtanen et al calculated the weight of supraclavicular BAT depots for one individual with 63 g, which – if fully activated - would be able dissipate an energy equivalent of approximately 4.1 kg during one year138. Characteristic metabolic alterations that commonly accompany obesity and diabetes are low levels of high density lipoproteins (HDL) and increased plasma triglycerides. Notably, LPL and brown fat like gene expression patterns in epicardial adipose tissue correlate positively with HDL cholesterol and negatively with plasma triglyceride levels in dyslipidemic patients 140 suggesting that BAT activation improve plasma lipoprotein profile not only in rodents. This intriguing concept of induced energy expenditure mediated by BAT as a promising strategy to facilitate weight loss received further support by a very elegant study using different radiolabeled tracers 141. The authors showed that BAT activated by acute cold exposure significantly contributes to oxidative metabolism and fuel uptake in humans, emphasizing the potential of BAT as an energy sink to lose weight. In addition, Orava et al showed in cold-exposed healthy individuals a positive association between whole-body energy expenditure and BAT perfusion 142. Interestingly and in contrast to rodents, cold exposure but not sympathomimetic ephedrine activates human BAT 143 accentuating the need to determine molecular targets and pathways in central and peripheral organs which are activated by cold exposure in humans.
6. Summary and outlook
During the last decades obesity research has focused on food intake regulation, while energy expenditure has been mainly measured based on whole body oxygene consumption. With the renaissance of BAT thermogenesis as potential drug target in humans more thought is put into alternative heat producing mechanisms. Also, the interaction of peripheral and central components to regulate thermogenesis require further studies including central control of sympathetic outputs, humoral control of fat browning as well as the importance of BAT activity to controlled lipid homeostasis. Certainly, several of the novel molecular genetic tools available now, compared to 40 years ago, will be helpful to gain new insights in BAT controlled energy homeostasis and promises new approaches to pharmacologically control body weight.
KEY POINTS.
Brown adipose tissue (BAT) is existent in human adults, it is able to dissipate excess energy by generating heat and it might be involved in human body weight control
Environmental factors such as cold, diet, physical activity as well as ageing are tightly linked to BAT activity
Neuronal and peripheral circuits control BAT-mediated thermogenesis
Development of brownish adipocytes (so called beige or brite) in white adipose tissue (WAT)
The activation of BAT and/or browning of WAT are promising targets to treat metabolic diseases such as diabetes and hyperlipidemia
There is an urgent need for prospective studies to unravel the potential use of BAT activation and/or browning for human health
Acknowledgments
Funding sources: Dr Heeren: This work was financially supported by the State Excellence Cluster NAME.
Dr Münzberg: This work was financially supported by the National Institute of Health R01-DK092587, P20-RR021945, P30-DK072476
Footnotes
Conflict of interest
Dr Heeren: no conflict of interest
Dr Münzberg: no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 4.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ. BAT: a new target for human obesity? Trends Pharmacol Sci. 2009;30:387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 9.Aquila H, Link TA, Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 1985;4:2369–2376. doi: 10.1002/j.1460-2075.1985.tb03941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton GM, Wagenvoord RJ, Kemp A, Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978;82:515–521. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls DG, Rial E. A history of the first uncoupling protein, UCP1. J Bioenerg Biomembr. 1999;31:399–406. doi: 10.1023/a:1005436121005. [DOI] [PubMed] [Google Scholar]
- 12.Himms-Hagen J, Cui J, Danforth E, Jr, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 13.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34 (Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 15.Prusiner SB, Cannon B, Lindberg O. Oxidative metabolism in cells isolated from brown adipose tissue. 1. Catecholamine and fatty acid stimulation of respiration. Eur J Biochem. 1968;6:15–22. doi: 10.1111/j.1432-1033.1968.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Heldmaier G, Klaus S, Wiesinger H, Friedrichs U, Wenzel M. Cold acclimation and thermogenesis. 1998:347–358. [Google Scholar]
- 17.Griggio MA. Thermogenic mechanisms in cold-acclimated animals. Braz J Med Biol Res. 1988;21:171–176. [PubMed] [Google Scholar]
- 18.Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 20.Clapham JC, Arch JR. Targeting thermogenesis and related pathways in anti-obesity drug discovery. Pharmacol Ther. 2011;131:295–308. doi: 10.1016/j.pharmthera.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, Dimicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 29.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 30.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers RC, Barnes MJ, Hermann GE. Leptin “gates” thermogenic action of thyrotropin-releasing hormone in the hindbrain. Brain Res. 2009;1295:135–141. doi: 10.1016/j.brainres.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T, Bray GA. Catecholamine turnover in rats with ventromedial hypothalamic lesions. Am J Physiol. 1984;246:R558–R565. doi: 10.1152/ajpregu.1984.246.4.R558. [DOI] [PubMed] [Google Scholar]
- 33.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- 34.Kelly L, Bielajew C. Ventromedial hypothalamic regulation of brown adipose tissue. Neuroreport. 1991;2:41–44. doi: 10.1097/00001756-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Dimicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 36.Samuels BC, Zaretsky DV, Dimicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 38.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012:3. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 40.Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- 41.Griffin JD, Boulant JA. Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J Physiol. 1995;488 ( Pt 2):407–418. doi: 10.1113/jphysiol.1995.sp020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin JD, Kaple ML, Chow AR, Boulant JA. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J Physiol. 1996;492 ( Pt 1):231–242. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelso SR, Boulant JA. Effect of synaptic blockade on thermosensitive neurons in hypothalamic tissue slices. Am J Physiol. 1982;243:R480–R490. doi: 10.1152/ajpregu.1982.243.5.R480. [DOI] [PubMed] [Google Scholar]
- 44.Boulant J. Handbook of Physiology - Hypothalamic Neurons Controlling Body Temperature. Academic Press; 1996. [Google Scholar]
- 45.Dean JB, Boulant JA. Effects of synaptic blockade on thermosensitive neurons in rat diencephalon in vitro. Am J Physiol. 1989;257:R65–R73. doi: 10.1152/ajpregu.1989.257.1.R65. [DOI] [PubMed] [Google Scholar]
- 46.Kelso SR, Perlmutter MN, Boulant JA. Thermosensitive single-unit activity of in vitro hypothalamic slices. Am J Physiol. 1982;242:R77–R84. doi: 10.1152/ajpregu.1982.242.1.R77. [DOI] [PubMed] [Google Scholar]
- 47.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 48.Boulant JA, Chow AR, Griffin JD. Determinants of hypothalamic neuronal thermosensitivity. Ann N Y Acad Sci. 1997;813:133–138. doi: 10.1111/j.1749-6632.1997.tb51684.x. [DOI] [PubMed] [Google Scholar]
- 49.Hunt JL, Zaretsky DV, Sarkar S, Dimicco JA. Dorsomedial hypothalamus mediates autonomic, neuroendocrine, and locomotor responses evoked from the medial preoptic area. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00574.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, Dimicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008;1200C:39–50. doi: 10.1016/j.brainres.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaretskaia MV, Zaretsky DV, Dimicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 53.Bagnol D, Lu XY, Kaelin CB, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voss-Andreae A, Murphy JG, Ellacott KL, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 55.Rossi J, Balthasar N, Olson D, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 57.van deWall E, Leshan R, Xu AW, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 59.DAVIS TR, MAYER J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am J Physiol. 1954;177:222–226. doi: 10.1152/ajplegacy.1954.177.2.222. [DOI] [PubMed] [Google Scholar]
- 60.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 61.Trayhurn P, Thurlby PL, James WP. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings] Proc Nutr Soc. 1976;35:133A. [PubMed] [Google Scholar]
- 62.Joosten HF, van der Kroon PH. Role of the thyroid in the development of the obese-hyperglycemic syndrome in mice (ob ob) Metabolism. 1974;23:425–436. doi: 10.1016/0026-0495(74)90090-0. [DOI] [PubMed] [Google Scholar]
- 63.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 64.Himms-Hagen J. Defective brown adipose tissue thermogenesis in obese mice. Int J Obes. 1985;9 (Suppl 2):17–24. [PubMed] [Google Scholar]
- 65.Rafael J, Herling AW. Leptin effect in ob/ob mice under thermoneutral conditions depends not necessarily on central satiation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R790–R795. doi: 10.1152/ajpregu.2000.278.3.R790. [DOI] [PubMed] [Google Scholar]
- 66.Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependent mechanism in brown adipose tissue. Am J Physiol Endocrinol Metab. 2001;280:E372–E377. doi: 10.1152/ajpendo.2001.280.2.E372. [DOI] [PubMed] [Google Scholar]
- 67.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 68.Pulinilkunnil T, He H, Kong D, et al. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 70.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Kerman IA, Laque A, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res. 2006;1117:118–124. doi: 10.1016/j.brainres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 77.Rogers RC, McDougal DH, Hermann GE. Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study. Brain Res. 2011;1385:47–55. doi: 10.1016/j.brainres.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coppola A, Liu ZW, Andrews ZB, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez M, Varela L, Vazquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klaus S, Keipert S, Rossmeisl M, Kopecky J. Augmenting energy expenditure by mitochondrial uncoupling: a role of AMP-activated protein kinase. Genes Nutr. 2012;7:369–386. doi: 10.1007/s12263-011-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 83.Li C, Chen P, Smith MS. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology. 1998;139:1645–1652. doi: 10.1210/endo.139.4.5905. [DOI] [PubMed] [Google Scholar]
- 84.Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- 85.Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- 86.Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- 87.Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 88.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cinti S. The adipose organ at a glance. Dis Model Mech. 2012;5:588–594. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci. 2011;16:1233–1260. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 92.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulz TJ, Huang TL, Tran TT, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stephens M, Ludgate M, Rees DA. Brown fat and obesity: the next big thing? Clin Endocrinol (Oxf) 2011;74:661–670. doi: 10.1111/j.1365-2265.2011.04018.x. [DOI] [PubMed] [Google Scholar]
- 97.Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5--connecting nutrition and metabolism. Thyroid. 2008;18:167–174. doi: 10.1089/thy.2007.0255. [DOI] [PubMed] [Google Scholar]
- 98.Silvestri C, Ligresti A, Di MV. Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord. 2011;12:153–162. doi: 10.1007/s11154-011-9167-3. [DOI] [PubMed] [Google Scholar]
- 99.Vegiopoulos A, Muller-Decker K, Strzoda D, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 100.Madsen L, Pedersen LM, Lillefosse HH, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Mejhert N, Galitzky J, Pettersson AT, et al. Mapping of the fibroblast growth factors in human white adipose tissue. J Clin Endocrinol Metab. 2010;95:2451–2457. doi: 10.1210/jc.2009-2049. [DOI] [PubMed] [Google Scholar]
- 104.Jonker JW, Suh JM, Atkins AR, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canto C, Auwerx J. Cell biology. FGF21 takes a fat bite. Science. 2012;336:675–676. doi: 10.1126/science.1222646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fisher FM, Kleiner S, Douris N, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei W, Dutchak PA, Wang X, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 112.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed) 2010;2:495–503. doi: 10.2741/e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sengenes C, Berlan M, De GI, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 115.Haas B, Mayer P, Jennissen K, et al. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal. 2009;2:ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- 116.Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nilsson SK, Heeren J, Olivecrona G, Merkel M. Apolipoprotein A-V; a potent triglyceride reducer. Atherosclerosis. 2011;219:15–21. doi: 10.1016/j.atherosclerosis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 119.Davies BS, Beigneux AP, Fong LG, Young SG. New wrinkles in lipoprotein lipase biology. Curr Opin Lipidol. 2012;23:35–42. doi: 10.1097/MOL.0b013e32834d0b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heeren J, Niemeier A, Merkel M, Beisiegel U. Endothelial-derived lipoprotein lipase is bound to postprandial triglyceride-rich lipoproteins and mediates their hepatic clearance in vivo. J Mol Med (Berl) 2002;80:576–584. doi: 10.1007/s00109-002-0351-5. [DOI] [PubMed] [Google Scholar]
- 121.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 122.Bartelt A, Merkel M, Heeren J. A new, powerful player in lipoprotein metabolism: brown adipose tissue. J Mol Med (Berl) 2012;90:887–893. doi: 10.1007/s00109-012-0858-3. [DOI] [PubMed] [Google Scholar]
- 123.Williams KJ, Fisher EA. Globular warming: how fat gets to the furnace. Nat Med. 2011;17:157–159. doi: 10.1038/nm0211-157. [DOI] [PubMed] [Google Scholar]
- 124.Bartelt A, Heeren J. The holy grail of metabolic disease: brown adipose tissue. Curr Opin Lipidol. 2012;23:190–195. doi: 10.1097/MOL.0b013e328352dcef. [DOI] [PubMed] [Google Scholar]
- 125.Zechner R, Zimmermann R, Eichmann TO, et al. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 128.Hondares E, Rosell M, Diaz-Delfin J, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13:238–240. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 130.Enerback S, Jacobsson A, Simpson EM, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 131.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem. 2006;281:31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 132.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 133.Yamada T, Katagiri H, Ishigaki Y, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–229. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 134.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 135.van Marken Lichtenbelt WD. Brown adipose tissue and the regulation of non-shivering thermogenesis. Curr Opin Clin Nutr Metab Care. 2012;15:000. doi: 10.1097/MCO.0b013e3283599184. [DOI] [PubMed] [Google Scholar]
- 136.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010;1212:E20–E36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 137.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 139.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 140.Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 141.Ouellet V, Labbe SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 143.Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]