Abstract
The bacterium Alcaligenes xylosoxidans is known to cause several nosocomial infections; however, it rarely causes endocarditis, which has a very high mortality rate. Early isolation of the infection source and prompt identification of the patient's antibiotic sensitivities are paramount if the infection is to be treated adequately.
We present what is apparently only the second documented case of the successful eradication of bioprosthetic valve endocarditis that was caused by pacemaker lead infection with Alcaligenes xylosoxidans. A 62-year-old woman with multiple comorbidities presented with endocarditis of a recently placed bioprosthetic aortic valve. The infection was secondary to pacemaker lead infection. She underwent antibiotic therapy, but an unusual pattern of antibiotic resistance developed. Despite initially adequate therapy, the infection recurred because of virulence induced by antibiotic resistance. Emergent, high-risk surgical treatment involved excising the infected valve and removing the source of the infection (the pacemaker leads). The patient eventually recovered after prolonged antibiotic therapy and close vigilance for recurrent infection. In addition to the patient's case, we discuss the features of this bacteremia and the challenges in its diagnosis.
Key words: Alcaligenes/drug effects; anti-bacterial agents/therapeutic use; bacteremia/complications/diagnosis/drug therapy/etiology; endocarditis, bacterial/diagnosis/ultrasonography; gram-negative bacterial infections/complications/etiology; heart valve prosthesis; opportunistic infections/epidemiology; pacemaker, artificial/microbiology; treatment outcome
Alcaligenes xylosoxidans is an aerobic gram-negative bacterium. It has reportedly caused catheter-related infections, pneumonia, peritonitis, biliary tract infection, meningitis, eye infections, and (rarely) native or prosthetic valve endocarditis, this chiefly in immune-compromised individuals.1–3 This opportunistic human pathogen is often difficult to eradicate because of its unusual antibiotic-susceptibility profile. The organism is frequently found in aqueous environments, and infections can develop consequent to cross-contamination from poor hygienic practices. When the usual diagnostic criteria are used, the clinical identification of infective endocarditis caused by A. xylosoxidans is difficult and is often missed, resulting in high mortality rates. We report a case of a woman who had prosthetic aortic valve endocarditis as a complication of A. xylosoxidans bacteremia that was secondary to pacemaker lead infection. We also discuss the features of this bacteremia and the challenges in its diagnosis.
Case Report
In April 2011, a 62-year-old woman underwent bioprosthetic aortic valve replacement for severe aortic stenosis and placement of a single-chamber pacemaker to treat bradycardia. Her medical history included atrial fibrillation, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, and bilateral lower-extremity ulcers. Three months after this surgery, she was readmitted to the hospital because of cellulitis. She was given intravenous vancomycin and piperacillin/tazobactam for 4 days. The initial blood cultures were positive for A. xylosoxidans. The bacterium was resistant to all aminoglycosides but was susceptible with minimum inhibitory concentration (MIC) to ceftazidime ≤8 μ/mL, piperacillin/tazobactam ≤16 μ/mL, meropenem ≤4 μ/mL, and trimethoprim/sulfamethoxole ≤40 μ/mL. On the basis of these sensitivities, the patient was given 4.5 g of intravenous piperacillin/tazobactam every 6 hours. Repeat blood cultures 5 days later were again positive for the same bacterium, but this time it was resistant to piperacillin/tazobactam with MIC ≥128 μ/mL (Table I).
TABLE I. Antibiotic Sensitivity Pattern of Alcaligenes xylosoxidans at Initial Presentation and at 5 Days
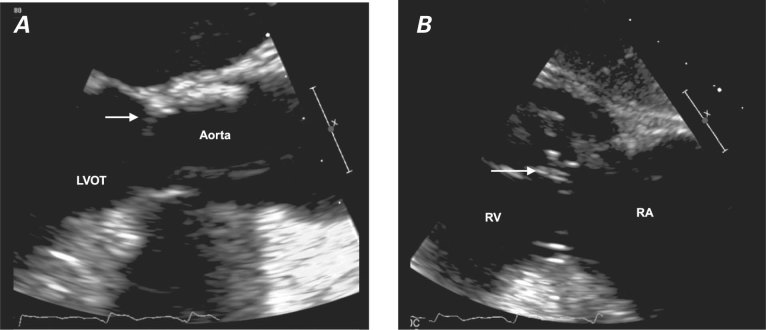
The persistent bacteremia raised a strong suspicion of endocarditis. A transesophageal echocardiogram (TEE) showed a 3 × 4-mm mass on the ventricular side of the aortic valve; the mass also appeared to be attached to the valve ring or strut (Fig. 1A). In addition, an 11 × 12-mm mass was attached to the right ventricular pacemaker lead, consistent with vegetation (Fig. 1B). The patient's antibiotic regimen was changed to 1 g of intravenous meropenem every 8 hours and double-strength oral trimethoprim/sulfamethoxole every 6 hours. Subsequent blood cultures were negative after 1 week and 4 weeks of this 6-week course. Because of the patient's multiple comorbidities, she was not a good candidate for replacement of the bioprosthetic valve. Blood cultures remained sterile 2 weeks after therapy ended, and a transthoracic echocardiogram 2 weeks thereafter revealed no vegetation on the bioprosthetic aortic valve or the pacemaker leads. The patient declined TEE.
Fig. 1 Transesophageal echocardiograms show vegetation on A) the bioprosthetic valve (arrow) and B) the pacemaker lead (arrow).
LVOT = left ventricular outflow tract; RA = right atrium; RV = right ventricle
Almost 5 months after the patient's discharge from the hospital, she presented with hypotension and septic shock that required support with pressors. Blood cultures grew A. xylosoxidans. Urgent TEE revealed a 2.5 × 1.8-cm mobile vegetation attached to the aortic valve prosthesis and extending into the aortic–mitral intervalvular fibrosa as an abscess (Fig. 2A). No dehiscence or periprosthetic regurgitation was evident. Examination of the mitral valve revealed a 1.5 × 1.5-cm vegetation attached to the lateral trigone near the aortic–mitral intervalvular fibrosa, extending directly from the infected aortic valve (Fig. 2B). Moderate mitral regurgitation was present without evidence of mitral stenosis. The patient was started on meropenem, rifampin, and amikacin and continued to require inotropic pressor support. Septic shock with eventual respiratory failure developed, necessitating mechanical ventilation, and worsening heart failure necessitated the placement of an intra-aortic balloon pump. She was scheduled for emergent cardiac surgery, despite the high surgical risk.
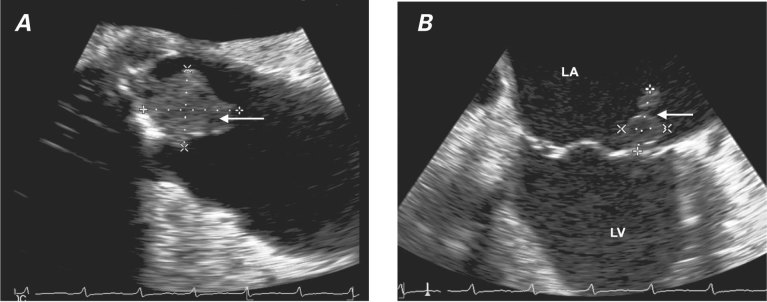
Fig. 2 Transesophageal echocardiograms show A) a 2.5 × 1.8-cm mobile vegetation attached to the aortic valve prosthesis (arrow) and B) a 1.5 × 1.5-cm vegetation on the lateral trigone near the aortic–mitral intervalvular fibrosa (arrow).
LA = left atrium; LV = left ventricle
At surgery, the orifice of the aortic bioprosthesis was found to be completely obliterated by vegetation (Fig. 3), and the infection extended through the aorto–mitral continuity; the lateral margin of the mitral valve had been replaced with vegetation. Wide débridement of the aortic root and anterior mitral leaflet was necessary. Reconstruction of the aorto–mitral continuum was achieved by means of a complex double-patch technique, including aortic root reconstruction with a 23-mm homograft valve conduit (CryoLife Inc.; Kennesaw, Ga) and mitral valve replacement with a 29-mm Epic™ porcine heart valve (St. Jude Medical, Inc.; St. Paul, Minn). The surgical specimen grew A. xylosoxidans that was sensitive to meropenem.
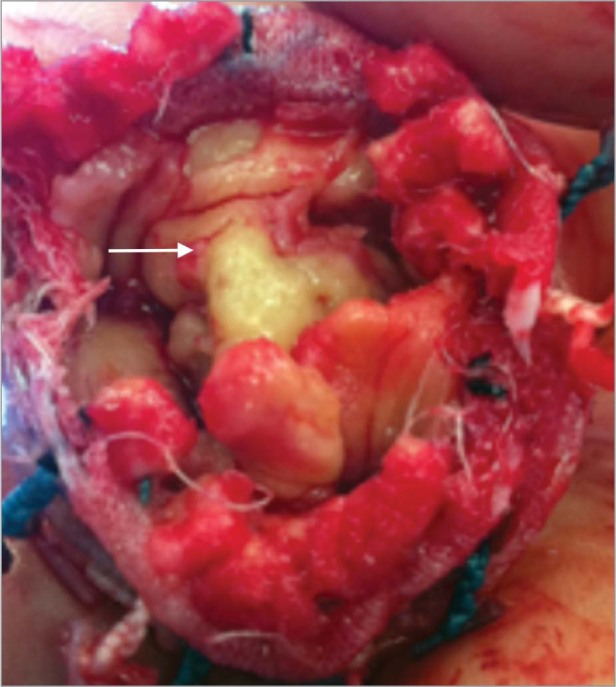
Fig. 3 Intraoperative photograph shows the aortic valve vegetation (arrow).
The patient's postoperative course was complicated by Clostridium difficile colitis; therapy with oral metronidazole yielded clinical resolution of the symptoms. The infected pacemaker leads were explanted; after 1 week of sterile blood cultures, a new dual-chamber pacemaker was inserted with no complications. Repeat blood cultures were sterile. The patient recovered well and underwent intravenous meropenem therapy for 6 more weeks. She was discharged from the hospital after several weeks of rehabilitation. At 2 follow-up examinations, 6 weeks and 3 months afterwards, blood cultures and TEE showed no recurrence of infection.
Discussion
Bergey's Manual of Determinative Bacteriology classifies our patient's bacterium as Alcaligenes xylosoxidans subsp xylosoxidans.1 Yabuuchi and Oyama first identified it in 1971, in purulent ear discharge.2 Alcaligenes xylosoxidans has been cultured from the gastrointestinal tract in human beings; it is commonly found environmentally in aqueous reservoirs, and there have been case reports of transmission from contaminated tap water and the unhygienic hands of healthcare workers.3,4 The bacterium has been cultured from indwelling catheters and from respiratory secretions in endotracheal tubing, notably in patients with cystic fibrosis.5 The incidence of morbidity and death from this infection is higher in immunocompromised patients.6,7 Rare cases of meningitis have occurred secondary to infection of shunt tubing or after vertebral laminectomy.8 Our patient could have acquired the infection from an aqueous source after exposing her leg ulcers to water outdoors, or from postoperative chest tube placement for her high-output heart failure. However, the presence of vegetations on the patient's pacemaker leads suggests that the leads were the source of the bacteremia. To our knowledge, infective endocarditis secondary to pacemaker lead infection by A. xylosoxidans has been reported only once before.9
In a retrospective study of 77 cases of A. xylosoxidans bacteremia,10 70% of the cases had a nosocomial source of infection, and the mortality rate was significantly higher (65%) in patients with endocarditis. Case fatality rates among neonates were the highest, at 80%. In another study, patients with high Acute Physiology and Chronic Health Evaluation (APACHE) II scores and the presence of sepsis syndrome had a higher probability of death because of hematogenous Alcaligenes infection.11 Our patient's infection became resistant to piperacillin/tazobactam, which necessitated a change in therapy to another broad-spectrum bactericidal agent (meropenem) in addition to trimethoprim/sulfamethoxazole.
Also important is the challenge of identifying A. xylosoxidans-related endocarditis, especially in immunocompromised patients. Our patient lacked radiographic, immunologic, and clinical evidence of bacterial endocarditis and met only one major and one minor criterion among the modified Duke clinical criteria,12 which made diagnosis difficult. This difficulty might have been compounded by the patient's recent surgery, recurrent hospitalizations, and chronic kidney disease, which could have led to her immunocompromised state.
Drug resistance in A. xylosoxidans appears to be mediated secondary to plasmids produced by the bacterium; however, other mechanisms of resistance have been observed.13 Uniform resistance has been observed among aminoglycosides and fluoroquinolone antibiotics secondary to alteration of the DNA gyrase or defective transport of the agents through the cell envelope; accordingly, monotherapy with these drugs is not recommended. The rapid shift in antibiotic resistance in our patient appears to have been consistent with the above mechanisms. Piperacillin, ticarcillin/clavulanicacid, ceftazidime, imipenem, and trimethoprim/sulfamethoxazole are among the most active agents, as shown in many studies.14 Penicillin allergy can be a major issue: Ahmed and colleagues15 reported that a patient died of A. xylosoxidans-related endocarditis secondary to penicillin allergy, which precluded the use of bactericidal agents such as piperacillin. Because there are few antibiotics with bactericidal activity against A. xylosoxidans, penicillin-allergic patients who develop infective endocarditis are at high risk of treatment failure. Our patient's blood cultures remained positive in spite of treatment with piperacillin, so combinations of bactericidal agents—such as meropenem and trimethoprim/sulfamethoxazole—were used to effect her full recovery after the replacement of the infected valves and the explantation of the infected pacemaker leads. Given the high mortality rate of endocarditis, the early isolation of infection sources and the prompt identification of antibiotic sensitivities is paramount.
Footnotes
Address for reprints: Abhishek C. Sawant, MD, MPH, 1180 E. Shaw Ave., Suite 101, Fresno, CA 93710
E-mail: acsawant@gmail.com
References
- 1.Bergey DH, Holt JG. Bergey's manual of determinative bacteriology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 75.
- 2.Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol 1971;15(5):477–81. [DOI] [PubMed]
- 3.Kim MJ, Bancroft E, Lehnkering E, Donlan RM, Mascola L. Alcaligenes xylosoxidans bloodstream infections in outpatient oncology office. Emerg Infect Dis 2008;14(7):1046–52.
- 4.Reverdy ME, Freney J, Fleurette J, Coulet M, Surgot M, Marmet D, Ploton C. Nosocomial colonization and infection by Achromobacter xylosoxidans. J Clin Microbiol 1984;19 (2):140–3. [DOI] [PMC free article] [PubMed]
- 5.Beringer PM, Appleman MD. Unusual respiratory bacterial flora in cystic fibrosis: microbiologic and clinical features. Curr Opin Pulm Med 2000;6(6):545–50. [DOI] [PubMed]
- 6.Manfredi R, Nanetti A, Ferri M, Chiodo F. Bacteremia and respiratory involvement by Alcaligenes xylosoxidans in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 1997;16(12):933–8. [DOI] [PubMed]
- 7.Gomez-Cerezo J, Suarez I, Rios JJ, Pena P, Garcia de Miguel MJ, de Jose M, et al. Achromobacter xylosoxidans bacteremia: a 10-year analysis of 54 cases. Eur J Clin Microbiol Infect Dis 2003;22(6):360–3. [DOI] [PubMed]
- 8.Ramos JM, Fernandez-Roblas R, Garcia-Ruiz P, Soriano F. Meningitis caused by Alcaligenes (Achromobacter) xylosoxidans associated with epidural catheter. Infection 1995;23(6): 395–6. [DOI] [PubMed]
- 9.Ahn Y, Kim NH, Shin DH, Park OY, Kim W, Jeong MH, et al. Pacemaker lead endocarditis caused by Achromobacter xylosoxidans. J Korean Med Sci 2004;19(2):291–3. [DOI] [PMC free article] [PubMed]
- 10.Duggan JM, Goldstein SJ, Chenoweth CE, Kauffman CA, Bradley SF. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin Infect Dis 1996; 23(3):569–76. [DOI] [PubMed]
- 11.Aisenberg G, Rolston KV, Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989–2003). Cancer 2004;101(9):2134–40. [DOI] [PubMed]
- 12.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30(4):633–8. [DOI] [PubMed]
- 13.Arroyo JC, Jordan W, Lema MW, Brown A. Diversity of plasmids in Achromobacter xylosoxidans isolates responsible for a seemingly common-source nosocomial outbreak. J Clin Microbiol 1987;25(10):1952–5. [DOI] [PMC free article] [PubMed]
- 14.Glupczynski Y, Hansen W, Freney J, Yourassowsky E. In vitro susceptibility of Alcaligenes denitrificans subsp. xylosoxidans to 24 antimicrobial agents. Antimicrob Agents Chemother 1988;32(2):276–8. [DOI] [PMC free article] [PubMed]
- 15.Ahmed MS, Nistal C, Jayan R, Kuduvalli M, Anijeet HK. Achromobacter xylosoxidans, an emerging pathogen in catheter-related infection in dialysis population causing prosthetic valve endocarditis: a case report and review of literature. Clin Nephrol 2009;71(3):350–4. [DOI] [PubMed]