Abstract
We have prospectively analyzed three antigens for serodiagnosis of tuberculosis (TB). These antigens were tuberculous glycolipid antigen, lypoarabinomannan polysaccharide antigen, and antigen 60 (A60), which was derived from purified protein derivatives. Of the 131 patients with active pulmonary TB, 57 were both smear and culture negative and 14 had chronic active pulmonary TB that remained smear positive for >12 months of chemotherapy. One hundred twenty healthy adults were controls. The percentages of patients positive in all three tests were 58.8% for smear-positive active pulmonary TB and 71.4% for chronic active pulmonary TB. When the results of the three serodiagnostic tests were evaluated in combination, the sensitivity increased to 91.5% in patients with active pulmonary TB and to 86.0% in smear- and culture-negative patients. The false-positive rate of the three-test combination was 12.5% in the healthy control groups. In conclusion, it was not possible to detect all of the antibodies against antigenic substances in the cell walls of the tuberculous bacilli in the sera of all TB patients by using available serodiagnostic tests. However, the combined use of tests with three separate antigens maximizes the effectiveness of serodiagnosis.
Arloing described the first serodiagnostic test for tuberculosis (TB), which used hemagglutination, in 1898 (2), but since then progress in serodiagnosis has been slow. In the last decade, studies of new assays that use various antigens (7, 10, 11, 12, 18, 20) for measurement of serum antibodies to Mycobacterium tuberculosis in patients with TB have been reported. Enzyme-linked immunosorbent assay (ELISA)-based serological tests to detect antibodies to M. tuberculosis are simple and inexpensive and are a potentially practical tool for the diagnosis of active pulmonary TB. However, almost all of the assays are limited by sensitivity, especially in smear-negative TB patients. An additional limitation is the variability in sensitivity, depending on both the investigator and the geographic origin of the survey participants (5, 15). However, the reported specificity of >90% is acceptable for a serodiagnostic test (7, 10, 11, 12, 18, 20).
Previously, the development of a rapid diagnostic ELISA for TB that is specific for antibodies to antituberculous glycolipid (anti-TBGL) was reported (17). The combined use of trehalose-dimycolate and minor glycolipids in the TBGL assay, rather than purified trehalose-dimycolate alone, results in increased diagnostic sensitivity for TB (13). The cell wall antigen composition of each patient isolate of tuberculous bacilli differs, resulting in antibodies with different specificities among patients (4, 9). We hypothesized that the TB patient does not produce antibodies against all antigenic substances in the cell wall of the tuberculous bacilli and that the specificities of the antibodies differ among patients. Consequently, the use of more than a single antigen would improve the sensitivity of serodiagnosis for active pulmonary TB. In order to test these hypotheses, we conducted a prospective clinical trial with three serodiagnostic tests that used antigens with different immunological specificities. The antigens were the glycolipid antigen TBGL, the well-known lypoarabinomannan (LAM) polysaccharide antigen, and the best known, antigen 60 (A60), which is derived from purified protein derivatives. We also evaluated the usefulness of these tests, both alone and in combination, for the diagnosis of active pulmonary TB.
MATERIALS AND METHODS
Study subjects.
We prospectively studied 138 patients who were diagnosed as having active pulmonary TB by both clinical symptoms and chest X-ray findings. The patients were enrolled between April 2000 and March 2001 at the time of their first visit. Patients who had positive smear tests documented in their medical records, which had been sent by local physicians, were not enrolled in the study because serodiagnosis of TB was not necessary for them. As positive controls, we chose 14 patients with chronic active pulmonary TB who were smear positive on bacteriological examination and resistant to rifampin and isoniazid for >12 months of chemotherapy.
Other respiratory diseases, such as lung cancer, infectious lung disease, or interstitial pneumonia, were diagnosed by chest X ray, consistent with the working diagnosis, physician examination, and other relevant clinical information. One hundred eleven patients were included in the other-respiratory-disease group. This group was comprised of 67 patients with chronic obstructive pulmonary disease, 20 patients with lung cancer, 19 patients with idiopathic pulmonary disease, 4 patients with bacterial pneumonia, and 1 patient with sarcoidosis. Acid-fast bacilli had not been detected in any patients in this group. The 120 healthy subjects with normal chest radiograms and no respiratory symptoms were enrolled from the primary health care office. One hundred and nine of these healthy subjects had positive tuberculin skin tests.
All subjects were negative for human immunodeficiency virus infection. The subjects gave informed consent to participate in this study, which was conducted according to our institutional guidelines.
Diagnostic criteria.
Active pulmonary TB was confirmed if smear and/or culture of sputum specimens was positive for M. tuberculosis. When smear and culture tests were negative, active pulmonary TB was confirmed by positive tuberculin test results and abnormal chest X-ray findings, which improved after treatment for 3 months with three or four antituberculosis drugs (rifampin, ethambutol, isoniazid, streptomycin, or pyrazinamide). Improvement was confirmed by unanimous agreement of three independent specialists after review of the chest X-ray. Clinical active pulmonary TB is class 3 according to the American Thoracic Society TB classification (3, 8). Patients whose chest X-ray evaluation indicated no change in condition after 3 months of treatment were not included in the study. Patients were also excluded from the study if nontuberculous mycobacteriosis (NTM) was confirmed by isolation of potentially pathogenic nontuberculous mycobacteria from sputum samples.
Specimens.
Sputum specimens for ordinary examination by smear staining and cultivation were obtained for three consecutive days, using previously described methods (16). The sputum specimens were digested and decontaminated with a solution of 2% sodium hydroxide (NaOH) and N-acetyl-l-cysteine and then centrifuged for 15 min at 3,000 × g. The supernatant was removed, and the remaining sediment was mixed in a 1:10 dilution with sterile water. The processed sample was stained with auramine O fluorochrome and examined by fluorescent microscopy. Ziehl-Nielson acid-fast staining was used to confirm the presence of acid-fast bacilli. Mycobacterial cultures were performed by inoculating 0.1 ml of the processed sample onto tubes of Ogawa medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) (11) and into the liquid media of the Mycobacteria Growth Indicator Tube system (Becton Dickinson) according to the manufacturer's instructions. Identification of M. tuberculosis and NTM was determined by a DNA-DNA hybridization method with a DDH Mycobacteria kit (Kyokuto Pharmaceuticals, Tokyo, Japan).
Sputum specimens for nucleic acid amplification testing (NAT) were collected separately in special containers from every enrolled TB patient on the first study day. The NAT was performed using the PCR method (Amplicor MTB; Roche Molecular Systems, Inc., Pleasanton, Calif.) according to the manufacturer's instructions.
Sera were obtained on the first day before chemotherapy and stored at −20°C.
Assay method.
The TBGL antibody was measured by ELISA with a Determiner TBGL kit (Kyowa Medex Co., Ltd., Tokyo, Japan). The LAM antibody was detected with the MycoDot kit (Mossmann Associates, Blackstone, Mass.). Detection of antibody was colorimetric, and the intensities of the test reactions were compared to those on the reference comb. The A60 antibody was measured by ELISA with an Anda-TB kit (ANDA Biologicals S.A., Strasbourg, France), which detects anti-A60 immunoglobulin G (IgG), IgA, and IgM as separate responses. The kits for LAM and TBGL antibody testing were specific for IgG responses. The manufacturer's instructions for test performance were followed for all commercial assays used in the study. For all ELISAs, absorbance at 450 nm was measured with an MTP-120 plate reader (Corona Electric Co., Ltd., Tokyo, Japan). Serum specimens were assayed without prior knowledge of the clinical status.
The quantitative results of the TBGL test were expressed as units per milliliter, and A60 test results were expressed as units. The LAM test data were expressed using a five-level scale. The cutoff points were 2 U/ml for TBGL antibody, 225 U for A60 antibody, and 1+ for LAM antibody, according to the standards set by the manufacturer of each test.
Statistical analyses.
Statistical analyses were performed using Statcel computer software (OMS, Ltd., Saitama, Japan). The values were evaluated either by Student's t test or a multiple-comparison test (Scheffe's F test) for a single factor. The results are expressed as means ± 1 standard deviation.
RESULTS
Diagnostic performance of single tests for active TB.
In this study, 138 patients were enrolled at the time of their first visit, and 117 cases were diagnosed as active pulmonary TB. Seven cases of cured TB, six cases of NTM, and eight cases of other respiratory diseases were excluded from the study. At least one of the serodiagnostic tests was positive for five patients with NTM. Among patients with cured TB, there were two cases in which all serodiagnostic tests were positive.
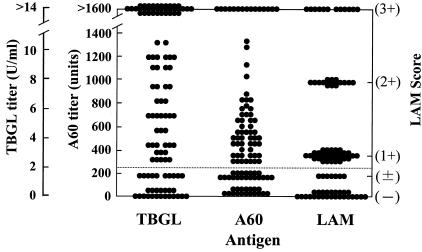
Figure 1 shows the antibody titer distribution in patients with active pulmonary TB with the three serodiagnostic tests. The rates of positivity of the three serodiagnostic tests are summarized by patient group in Table 1. In the active pulmonary TB patient group, the test sensitivities of 70.1 (TBGL), 67.5 (LAM), and 70.9% (A60) did not differ significantly. Among the 117 patients with active pulmonary TB, 83 (70.9%) were smear negative and 57 patients (48.7%) were both smear and culture negative. For the 57 patients who were both smear and culture negative, the sensitivities of serodiagnostic tests were 63.2 (TBGL), 66.7 (LAM), and 63.2% (A60). In this subgroup, the sensitivity of NAT (24.6%) was statistically lower than that of each serodiagnostic test (P < 0.001). In the A60 test, the sensitivity increased from 70.9 to 74.4% by detection of anti-A60 IgA and IgM in addition to anti-A60 IgG in 117 patients with active pulmonary TB. In the other-respiratory-disease group, the specificities of the serodiagnostic tests with TBGL, LAM, and A60 were 89.2 (TBGL), 97.3 (LAM), and 91.0% (A60). In the healthy control group, the specificities were 92.5 (TBGL), 97.5 (LAM), and 95.0% (A60).
FIG. 1.
Distribution of antibody titers obtained using three serodiagnostic tests in patients with active pulmonary TB. The cutoff point for each test is indicated by the dotted line.
TABLE 1.
Characteristics of study population and rates of serodiagnostic positivity using assays for antibodies to three distinct antigens of M. tuberculosis
| Patient group and diagnostic results | No. of cases | No. male/ female | Age (yr) (mean ± SD) | % Positivity for antibody to:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| TBGL
|
LAM
|
A60
|
|||||||
| n | % | n | % | n | % | ||||
| Active pulmonary TB | 117 | 98/19 | 53.6 ± 18.6 | 82 | 70.1 | 79 | 67.5 | 83 | 70.9 |
| Smear positive, culture positive | 34 | 27/7 | 55.1 ± 18.0 | 26 | 76.5 | 26 | 76.5 | 28 | 82.4 |
| Smear negative | 83 | 71/12 | 52.8 ± 18.8 | 56 | 67.5 | 53 | 63.9 | 55 | 66.3 |
| Culture positive | 26 | 26/0 | 57.0 ± 16.4 | 20 | 76.9 | 15 | 57.7 | 19 | 73.1 |
| Culture negative | 57 | 45/12 | 50.9 ± 19.6 | 36 | 63.2 | 38 | 66.7 | 36 | 63.2 |
| Chronica and smear positive | 14 | 9/5 | 59.8 ± 13.7 | 11 | 78.6 | 12 | 85.7 | 12 | 85.7 |
| Other respiratory disease | 111 | 66/40 | 62.3 ± 16.4 | 12 | 10.8 | 3 | 2.7 | 10 | 9.0 |
| Healthy control | 120 | 82/38 | 41.9 ± 8.8b | 9 | 7.5 | 3 | 2.5 | 6 | 5.0 |
Chemotherapy for >12 months; smear positive.
Significantly different from the value for active pulmonary TB (P < 0.05).
In both the active pulmonary TB and the healthy control groups, the positive predictive values were 90.1 (TBGL), 96.3 (LAM), and 77.0% (A60). The negative predictive values of three tests were 75.3 (TBGL), 75.0 (LAM), and 77.0% (A60).
Evaluation of results from multiple serodiagnostic tests for diagnosis of active pulmonary TB.
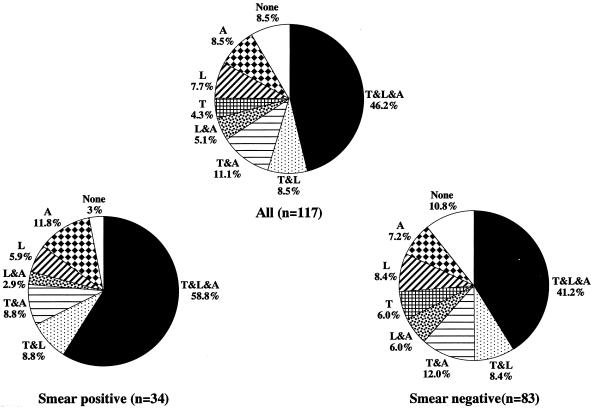
The percentages of positive results for all three tests were 58.8% in patients with smear-positive active pulmonary TB and 71.4% in patients with chronic active pulmonary TB (Fig. 2). The rate was even lower (41.2%) in smear-negative TB patients. When the antigen-antibody reaction time in the LAM test was increased to 30 min, the result became negative in one patient with chronic active pulmonary TB who previously had positive results in the other two tests. When the cutoff values were decreased by 25% in both the TBGL and A60 tests, there were also two patients with negative results in the chronic active pulmonary TB group. These results revealed that not all of the antibodies against antigenic substances in the cell walls of the tuberculous bacilli could be detected, even in the sera of patients with chronic active pulmonary TB. However, these patients were positive in at least one of the three serologic tests.
FIG. 2.
Sensitivities of serodiagnostic tests, using results from all possible combinations of tests, in groups of patients with active pulmonary TB. The assays for antibodies to TBGL (T), LAM (L), and A60 (A) are shown.
The positive rates were calculated using the number of patients who were positive in any combination of tests divided by the number of patients in each group. The positive rates, calculated from the results of serodiagnostic tests in combination, either with or without NAT, are summarized in Table 2. In all subgroups of patients with active TB, the positive results with the combined serodiagnostic tests were significantly higher than when any single assay was used (P < 0.05). By using combined results from three serodiagnostic tests, the sensitivity increased to 91.5% in 117 patients with active pulmonary TB. Even in the subgroup of 57 patients who were both smear and culture negative, the sensitivity increased to 86.0% when the diagnostic criteria included data from three tests. The sensitivity remained at 85.7% in the subgroup of 56 patients who were both smear and NAT negative. Eighteen patients with other respiratory diseases had at least one positive serodiagnostic test, and seven of these were positive in two or three serodiagnostic tests (Table 3). The specificities of the serodiagnostic tests in combination with TBGL, LAM, and A60 were high (83.8%; 93 of 111 cases) in the other-respiratory-disease group. This specificity also remained high (87.5%; 105 of 120 cases) in the healthy control group, even when three cases with two positive serodiagnostic tests were included.
TABLE 2.
Rates of positivity using multiple serodiagnostic antibody assays in patients with different diagnostic results for active pulmonary TB
| Patient group and diagnostic results | No. of cases | % Positivity with combined results from antibody assays toa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T and/or L
|
T and/or A
|
L and/or A
|
T and/or L and/or A
|
T and/or L and/or A and/or N
|
|||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Active pulmonary TB | 117 | 97 | 82.9 | 98 | 83.8 | 102 | 87.2 | 107 | 91.5 | 109 | 93.2 |
| Smear positive, culture positive | 34 | 29 | 85.3 | 31 | 91.2 | 33 | 97.1 | 33 | 97.1 | 34 | 100 |
| Smear negative | 83 | 68 | 81.9 | 67 | 80.7 | 69 | 83.1 | 74 | 89.2 | 75 | 90.4 |
| Culture positive | 26 | 23 | 88.5 | 24 | 92.3 | 23 | 88.5 | 25 | 96.2 | 26 | 100 |
| Culture negative | 57 | 45 | 78.9 | 43 | 75.4 | 46 | 80.7 | 49 | 86.0 | 49 | 86.0 |
| NAT positive | 27 | 21 | 77.8 | 23 | 85.2 | 26 | 96.3 | 26 | 96.3 | ||
| NAT negative | 56 | 45 | 80.4 | 44 | 78.6 | 42 | 75.0 | 48 | 85.7 | ||
| Chronic and smear positive | 14 | 14 | 100 | 14 | 100 | 13 | 92.9 | 14 | 100 | ||
T, TBGL; L, LAM; A, A60; N, NAT.
TABLE 3.
Specificities of multiple serodiagnostic antibody assays in patients with other respiratory diseases and healthy control subjects
| Group | No. of cases | Specificity of combined results from antibody assays toa:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| T and/ or L
|
T and/ or A
|
L and/ or A
|
T and/or L and/or A
|
||||||
| n | % | n | % | n | % | n | % | ||
| Other respiratory disease | 111 | 98 | 88.3 | 93 | 83.8 | 100 | 90.1 | 93 | 83.8 |
| Healthy control | 120 | 109 | 90.8 | 107 | 89.2 | 111 | 92.5 | 105 | 87.5 |
T, TBGL; L, LAM; A, A60.
In both the active pulmonary TB and the healthy control groups, the positive predictive value using results from three serodiagnostic tests in combination was 87.7% and the negative predictive value was increased to 91.3%. The predictive values in the subgroup of smear- and culture-negative patients were 83.1% for positive and 92.1% for negative.
Relationship of serodiagnostic positivity rate and chest X-ray lesions in patients with active pulmonary TB.
The positive rates using results from either single or combined serodiagnostic tests in patients grouped according to chest X-ray findings are summarized in Table 4. The sensitivity of serodiagnosis was higher in patients with cavitary lesions than in those with noncavitary lesions. In patients with minimally advanced lesions, the sensitivities obtained by serodiagnosis were 73.3 (TBGL), 60.0 (LAM), and 53.3% (A60). Combining the results from any two serodiagnostic methods gave significantly increased sensitivity in every combination compared to results with any single assay (P < 0.05). When the results from three serodiagnostic methods were evaluated in combination, the sensitivity increased to 92.2% in patients with noncavitary lesions and 86.7% in patients with minimally advanced lesions.
TABLE 4.
Rates of positivity using multiple serodiagnostic antibody assays in patients with different radiologic profiles of active pulmonary TB
| Patient group and chest X-ray findingsa | n | Positivity with results from either single or combined antibody assays tob:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single
|
Combination
|
||||||||||||||
| TBGL
|
LAM
|
A60
|
T and/or L
|
T and/or A
|
L and/or A
|
T and/or L and/or A
|
|||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Cavitary | 48 | 35 | 72.9 | 35 | 72.9 | 36 | 75.0 | 41 | 85.4 | 40 | 83.3 | 43 | 89.6 | 44 | 91.7 |
| Noncavitary | 64 | 44 | 68.8 | 43 | 67.2 | 46 | 71.9 | 54 | 84.4 | 54 | 84.4 | 55 | 85.9 | 59 | 92.2 |
| Moderately advanced | 49 | 33 | 67.3 | 34 | 69.4 | 38 | 77.6 | 41 | 83.7 | 42 | 85.7 | 44 | 89.8 | 46 | 93.9 |
| Minimally advanced | 15 | 11 | 73.3 | 9 | 60.0 | 8 | 53.3 | 13 | 86.7 | 12 | 80.0 | 11 | 73.3 | 13 | 86.7 |
Five patients with pleural effusion were excluded.
T, TBGL; L, LAM; A, A60.
Effectiveness of serodiagnostic methods with multiple antigens.
The results in Tables 1 and 2 indicated that the lower the sensitivity of a single serodiagnostic method, the greater the improvement in sensitivity when it was used in combination with other serodiagnostic methods. Figure 2 shows the sensitivities of serodiagnostic tests in all combinations arranged according to the category of active pulmonary TB. In 117 patients with active pulmonary TB, the use of the two-test combinations increased the number of detectable cases by 29 (L and A, T and A, and T and L; 6 + 13 + 10 = 29 cases), or 24.8%. Rates of detection with only single assays for antibody were 5 (4.3%) for TBGL, 9 (7.7%) for LAM, and 10 (8.5%) for A60. In total, 91.5% of active pulmonary TB cases could be detected when the supplementary serodiagnostic tests were used.
DISCUSSION
There are several facts that we should recognize in order to establish the serodiagnostic method for tuberculous disease. The cell wall antigen compositions of the tuberculous bacilli differ among isolates (4, 9). The TB patient does not produce antibodies against all antigenic substances in the cell walls of the tuberculous bacilli, and the specificities of the antibodies differ among patients. The diverse antibody response to M. tuberculosis may be governed by HLA types (1). In our study, the percentages of patients who were positive in all three tests were not high, even in patients with smear-positive and chronic active pulmonary TB. The antibody response in these patients, who had experienced long-term and heavy exposure to bacilli, was more diverse than we had expected based on previous reports (4, 20). Furthermore, when the results of a single test were supplemented with results from other tests, the detection rate increased to 91.5% in the active pulmonary TB group. These results indicate that in patients with active TB, the antibody response to the antigens of the bacillus cell walls may be unique to the patient's own isolate.
In a previous study, the use of a combination of tests with several different antigens did not improve the diagnosis (6). The sensitivities of tests using two antigens, other than A60, were low. The additional antigens were secreted proteins with properties similar to those of A60. In another study, a maximum sensitivity of 84% was obtained when results from seven tests were combined, but there was a reciprocal drop in specificity to 55% for controls (19). The appropriate combination of antigens may be important for devising an appropriate assay for an antibody to M. tuberculosis. We selected the best-known antigens (LAM and A60), in addition to TBGL, because the sensitivities for detecting antibodies to these three antigens in patients with active pulmonary TB are high (LAM, 72 to 93%; A60, 83 to 88%; TBGL, 79 to 90%), as reported in many published studies (5, 7, 10, 11, 17, 20, 21). These studies also reported specificities of >90%, indicating the potential diagnostic value of using this combination of antigens.
In this study, the sensitivities of 67.5% for the LAM antibody, 70.9% for the A60 antibody, and 70.1% for the TBGL antibody in patients with active pulmonary TB were lower than those in the series of studies mentioned above that used three antigens. Because TB patients who had previous smear-positive test results were excluded, the smear-positive patients represented only 29.1% of the total number of patients and resulted in a figure lower than those of previous studies. In patients with smear-negative pulmonary TB, the sensitivities of 63.9% for the LAM test, 66.3% for the A60 test, and 67.5% for the TBGL test were similar to those previously reported (5, 7, 10, 11, 17, 20, 21). Our lower sensitivity for detecting antibody may be consistent with the predominantly smear-negative population in our study. Accordingly, the population of our study seemed to be appropriate for examining the usefulness of a serodiagnosis in the case of active pulmonary TB patients, particularly in a smear-negative population.
In all subgroups, the assay using a combination of serodiagnostic tests statistically increased the positivity rates compared with a single assay (P < 0.05) without an adjustment in the cutoff value. It was previously reported that clinical utility was improved by using the TBGL and PCR tests in combination in patients with smear-negative and culture-negative active pulmonary TB (16). However, 86% of patients with smear-negative and culture-negative active pulmonary TB in this study had antibody to at least one of the three antigens, and there was no improvement in sensitivity when NAT was added to the battery of three serodiagnostic tests. The positive predictive value remained at a level of 87.7%, and the negative predictive value was improved to 91.3%. The specificities of the serodiagnostic tests in combination with TBGL, LAM, and A60 remained sufficiently high (87.5%; 105/120 cases) in the control group. These results show that a single serodiagnostic method is not satisfactory, but that the combined results from multiple serodiagnostic tests were very effective, for detecting active pulmonary TB at a high rate, even in noninfectious patients who were smear, culture, and PCR negative. The ability to serologically diagnose active pulmonary TB at an early stage, before tuberculous bacilli can be detected in sputa, may be effective in preventing the spread of the infection.
In a previous report, it was demonstrated that TBGL antibodies were positive in 25.9% of cases of cured TB, in 15% of patients with other respiratory diseases, and in 17.2% of older healthy subjects (17). The number of false positives in serodiagnostic testing might correspond to the extent of tuberculous infections and NTM infections in the other-respiratory-disease and healthy control groups. The patients with NTM had positive results from serodiagnostic tests because trehalose-dimycolate, LAM, and purified protein derivative are present as common cell wall components of all acid-fast organisms, such as mycobacteria and Nocardia. The rates of Mycobacterium avium-M. intracellulare complex infection were 12.5% in patients with chronic obstructive pulmonary disease and 3.8% in healthy controls, as determined by serodiagnosis with specific glycopeptidolipids as antigens (14). Some cross-reactions are inevitable. In this study, the false positives might be a result of either latent TB infection or TB infection in close contact with a bacteriologically positive case of tuberculosis, because Osaka had a high incidence of tuberculosis (67 cases per 100,000 persons) in 2000. Actually, two cases of cured TB were positive in all three serodiagnostic tests in this study. It should be clarified that these cases were suspected of having latent TB infections. It may also be predicted that the patients who had two or three positive serodiagnostic tests in the other-respiratory-disease group had latent TB or NTM infections. This false-positive rate may be lower in the United States or European countries, where the incidence of TB is lower than that in Japan. Consequently, we chose the healthy subjects as a control group when we calculated the positive and negative predictive values. Our future investigations will focus on the problem of the serodiagnosis of latent TB and NTM infections.
While implementing the use of three serodiagnostic tests may be considered cumbersome, there are definite advantages. The tests that we have used are commercially available and are performed using established protocols, making their incorporation into a clinical laboratory fairly simple and straightforward. The reaction times of 20 min for the LAM test, 2 h for the A60 test, and 2 h for the TBGL test are shorter than the time required for NAT. According to figures compiled by the Japanese Health Insurance Institute, the costs of testing for acid-fast bacilli using current methods are ¥380 ($3.20) for smears, ¥1,980 ($16.50) for culture, and ¥4,800 ($40) for NAT versus the ¥1,200 ($10) cost of serodiagnostic testing. The cost of the serodiagnostic test is four times less than that of NAT. When the patients with opacities consistent with a diagnosis of TB by chest X-ray could not be diagnosed by smear, culture, or NAT, bronchoscopic procedures were used in some cases. This procedure is invasive and expensive (¥31,000; $258). The positive rate of serodiagnostic tests was 86% in these patients. Therefore, serodiagnostic testing is useful as a TB diagnostic tool and is a relatively simple and inexpensive method compared to NAT and the bronchoscopic procedure. The assay for the A60 IgG test alone appeared to be adequate for detecting an immune response to M. tuberculosis. While there was no real sensitivity advantage in including additional assays of IgA or IgM, the different times of detection may be useful in some situations. The IgG antibody can be detected in serum after 1 or 2 months of tuberculous infection, and the IgM antibody can be detected in serum earlier than IgG.
Our data indicate that future multicenter and multiarea studies directed at the evaluation of the effectiveness of serodiagnosis with a battery of antigens from M. tuberculosis are warranted. The combination of LAM, A60, and TBGL appears to be the best choice of antigens for the serodiagnosis of TB.
Acknowledgments
We thank Shuuji Kawasumi for technical assistance and Kyowa Medex and Ramco Japan for their donation of serodiagnostic kits.
REFERENCES
- 1.Arend, S. M., A. Geluk, K. E. van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. M. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arloing, S. 1898. Agglutination de bacille de la tuberculose vrate. C. R. Acad. Sci. 126:1398-1400. [Google Scholar]
- 3.Bass, J. B., L. S. Farer, P. C. Hopewell, R. F. Jacos, and D. E. Snider. 1990. Diagnostic standards and classification of tuberculosis. Am. Rev. Respir. Dis. 142:725-735. [DOI] [PubMed] [Google Scholar]
- 4.Chaicumpar, K., and I. Yano. 1997. Studies of polymorphic DNA fingerprinting and lipid pattern of Mycobacterium tuberculosis patient isolate in Japan. Microbiol. Immunol. 41:107-119. [DOI] [PubMed] [Google Scholar]
- 5.Chan, E. D., R. Reves, J. T. Belisle, P. J. Brennan, and W. E. Hahn. 2000. Diagnosis of tuberculosis by a visually detectable immunoassay for lipoarabinomannan. Am. J. Respir. Crit. Care Med. 161:1713-1719. [DOI] [PubMed] [Google Scholar]
- 6.Chiang, I. H., J. Sou, K. J. Bai, T. P. Lin, K. T. Luh, C. J. Yu, and P. C. Yang. 1997. Serodiagnosis of tuberculosis. A study comparing three specific mycobacterial antigens. Am. J. Respir. Crit. Care Med. 156:906-911. [DOI] [PubMed] [Google Scholar]
- 7.Del Prete, R., V. Picca, A. Mosca, M. D'Alagni, and G. Miragliotta. 1998. Detection of anti-lipoarabinomannan antibodies for the diagnosis of active tuberculosis. Int. J. Tuberc. Lung Dis. 2:160-163. [PubMed] [Google Scholar]
- 8.Dunlap, N. E., J. Bass, P. Fujiwara, P. Hopewell, C. R. Horsburgh, M. Salfinger, and P. M. Simon. 1999. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara, N. 1997. Distribution of antigenic glycolipids among Mycobacterium tuberculosis strains and their contribution to virulence. Kekkaku 72:193-205. [PubMed] [Google Scholar]
- 10.Gevaudan, M. J., C. Bollet, D. Charpin, M. N. Mallet, and P. De Micco. 1992. Serological response of TB patients to antigen 60 of BCG. Eur. J. Epidemiol. 8:666-676. [DOI] [PubMed] [Google Scholar]
- 11.Gupta, S., S. Kumari, J. N. Banwalikar, and S. K. Gupta. 1995. Diagnosis utility of the estimation of mycobacterial antigen A60 specific immunoglobulins IgM, IgA and IgG in the sera of cases of adult human tuberculosis. Tuberc. Lung Dis. 76:418-424. [DOI] [PubMed] [Google Scholar]
- 12.Harboe, M., and H. G. Wiker. 1992. The 38-kDa protein of Mycobacterium tuberculosis. Chest 100:1689-1693. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura, M., R. Maekura, I. Yano, and H. Kohno. 1997. Enzyme immunoassay to detect antituberculous glycolipid antigen (anti-TBGL antigen) antibody in serum for diagnosis of tuberculosis. J. Clin. Lab. Anal. 11:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitada, S., R. Maekura, N. Toyoshima, N. Fujiwara, I. Yano, T. Ogura, M. Ito, and K. Kobayashi. 2002. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex with an enzyme immunoassay that uses a mixture of glycopeptidolipid antigens. Clin. Infect. Dis. 35:1328-1335. [DOI] [PubMed] [Google Scholar]
- 15.Lawn, S. D., E. H. Frimpong, and E. Nyarko. 1997. Evaluation of a commercial immunodiagnostic kit incorporating lipoarabinomannan in serodiagnosis of pulmonary tuberculosis in Ghana. Trop. Med. Int. Health 2:978-981. [DOI] [PubMed] [Google Scholar]
- 16.Maekura, R., H. Kohno, A. Hirotani, Y. Okuda, M. Itou, and I. Yano. 2003. A prospective clinical evaluation of the serologic tuberculous glycolipid (TBGL) test for the diagnosis of smear-negative pulmonary tuberculosis used in combination with nucleic acid amplification test. J. Clin. Microbiol. 41:1322-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maekura, R., Y. Okuda, M. Nakagawa, T. Hiraga, S. Yokota, M. Ito, I. Yano, H. Kohno, M. Wada, C. Abe, T. Toyoda, T. Kishimoto, and T. Ogura. 2001. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 39:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maekura, R., M. Nakagawa, Y. Nakamura, T. Hiraga, Y. Yamamura, I. Masami, E. Ueda, S. Yano, S. Oka, and K. Kashima. 1993. Clinical evaluation of rapid serodiagnosis of pulmonary TB by ELISA with cord factor as antigen purified from Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 148:997-1001. [DOI] [PubMed] [Google Scholar]
- 19.Pottumarthy, S., V. C. Well, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sada, E., D. Aguilar, M. Torres, and T. Herrera 1992. Detection of lipoarabinomannan as a diagnostic test for TB. J. Clin. Microbiol. 30:2415-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]