Abstract
Reports have suggested that the use of a dangerously tainted form of marijuana, referred to in the vernacular as “wet” or “fry,” has increased. Marijuana cigarettes are dipped into or laced with other substances, typically formaldehyde, phencyclidine, or both. Inhaling smoke from these cigarettes can cause lung injuries.
We report the cases of 2 young adults who presented at our hospital with respiratory failure soon after they had smoked “wet” marijuana cigarettes. In both patients, progressive hypoxemic respiratory failure necessitated rescue therapy with extracorporeal membrane oxygenation. After lengthy hospitalizations, both patients recovered with only mild pulmonary function abnormalities.
To our knowledge, this is the first 2-patient report of severe respiratory failure and rescue therapy with extracorporeal oxygenation after the smoking of marijuana cigarettes thus tainted. We believe that, in young adults with an unexplained presentation of severe respiratory failure, the possibility of exposure to tainted marijuana cigarettes should be considered.
Key words: Formaldehyde/ adverse effects/toxicity; lung diseases/chemically induced; marijuana smoking/adverse effects/complications/epidemiology; phencyclidine abuse; respiration, artificial/methods; respiratory distress syndrome, adult/etiology/therapy; respiratory insufficiency/chemically induced; street drugs/adverse effects; substance abuse detection; substance-related disorders/complications/diagnosis/prevention & control
Numerous reports on alternative forms of tetrahydrocannabinol (THC) can be found in multiple media forums.1–5 Several reports indicate the increased use of marijuana cigarettes, the ingredients of which have been tainted in a potentially harmful fashion.1–4 This altered form of marijuana, referred to in the vernacular as “wet,” “illy,” or “fry,” was first reported in the 1970s and can now be procured rather readily. “Wet” cigarettes are conventional marijuana cigarettes that have been dipped into various fluids or laced with additional substances. The precise ingredients involved in this augmentation process may or may not be known by the end user. The most frequently reported method involves the dipping of marijuana into embalming fluid or formaldehyde that has been mixed with phencyclidine (PCP).3
The exact origin of tainted marijuana cigarettes is unknown. The “wet” cigarettes reported on in the 1970s were probably laced with PCP. At that time, PCP was referred to by marijuana users and dealers as “embalming fluid.” It is postulated that drug dealers subsequently and mistakenly began using genuine embalming fluid to augment marijuana cigarettes, and that this has led to the current formulation with embalming fluid, PCP, or both.3
Cannabis is not typically considered to be a drug that causes respiratory failure. However, exposure to tainted marijuana cigarettes potentially precipitates organ failure, including respiratory failure. Exposure to PCP can increase the prevalence of life-threatening events.3,5 Other reports about tainted marijuana cigarettes chiefly discuss their impact on the central nervous system. The effects include hallucinations, disorientation, impaired coordination, paranoia, sexual disinhibition, and visual disturbances.3–5 We present the cases of 2 young adults who presented with severe respiratory failure—thought to be related to “wet cigarette” exposure—that necessitated therapy with extracorporeal membrane oxygenation (ECMO).
Case Reports
Patient 1
A 27-year-old woman presented at another hospital with respiratory failure and seizures. Her medical history included chronic depression and alcohol and marijuana abuse, but no prior seizures. After transfer to our hospital, she was placed on mechanical ventilation at a low tidal volume, in accordance with the Acute Respiratory Distress Network (ARDSNet) protocol.6 Drug-screening tests were positive for THC and PCP. Chest radiographs revealed bilateral, diffuse pulmonary infiltrates. Computed tomograms showed areas of diffuse consolidation as well as ground-glass attenuation with superimposed inter- and intralobular septal thickening. Empiric antibiotic therapy for presumed pneumonia was started. However, investigations for infectious and noninfectious causes, including a bronchoalveolar lavage, yielded negative results. Echocardiograms showed normal cardiac function and structure. During the next 10 days, progressive respiratory failure with persistent bilateral, diffuse pulmonary infiltrates developed (Fig. 1) despite attempted rescue therapies, including neuromuscular blockade, open lung ventilation, inhaled prostacyclin, and high-frequency-oscillation ventilation. Refractory hypoxemic and hypercapnic respiratory failure (Murray Lung Score,7 4/4/2/3 = 3.25), along with evidence of distributive shock, prompted the implementation of venoarterial ECMO. The patient's tidal volumes were 4 cc/kg with plateau pressures ranging from 40 to 45 cm H2O just before ECMO was initiated. Despite the low tidal volume and ECMO support, the patient's course was complicated by recurrent pneumothorax and by a hemothorax that necessitated thoracotomy. After 35 days, she was weaned from ECMO support and was again placed on conventional mechanical ventilation. Tracheostomy enabled the patient to breathe room air, and she was discharged to an inpatient rehabilitation unit 65 days after her initial hospital admission. After being discharged from the rehabilitation unit, she was able to resume all activities of daily life.
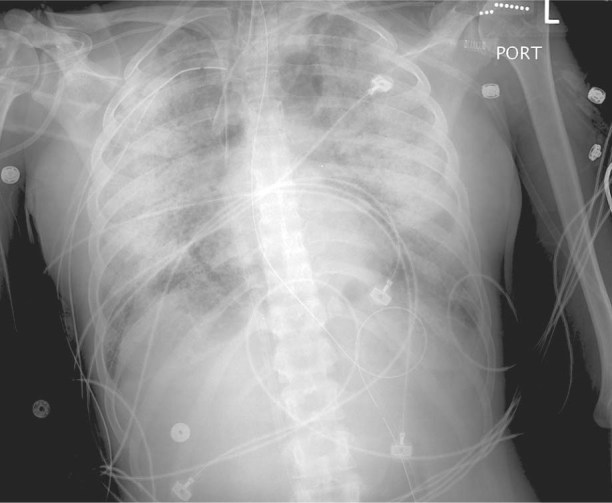
Fig. 1 Patient 1. Chest radiograph at the time of ECMO cannulation shows diffuse pulmonary infiltrates bilaterally.
Six months after the patient's initial hospitalization, she underwent pulmonary-function testing to evaluate her severe acute respiratory distress syndrome (ARDS). Spirometry revealed mild deficiencies in forced expiratory volume in 1 second (FEV1) (60%), in total lung capacity (TLC) (62%), and in diffusing capacity of carbon monoxide (DLCO) (70%). A chest radiograph revealed unilateral basilar scarring, consistent with the location of her recurrent pneumothoraces and hemothorax.
It was learned that the patient had been in her usual state of health before the initial hospital admission. On the night before admission, she had smoked marijuana cigarettes that had been dipped in PCP and embalming fluid.
Patient 2
A 20-year-old man with no past medical problems presented at another hospital with disorientation and hypoxemic respiratory failure. He was intubated and hemodynamically stable upon his transfer to our hospital. Chest radiographs revealed bilateral, diffuse pulmonary infiltrates. Echocardiograms showed normal cardiac function and structure. Drug-screening tests were positive for THC. Investigations for infectious and noninfectious causes yielded negative results. Bronchoscopic evaluation showed mildly edematous airways and yielded a neutrophil-predominant lavage. Ventilation at low tidal volume was used, in accordance with the ARDSNet protocol.6 During the next 11 days, progressive hypoxemic respiratory failure (Murray Lung Score,7 3/4/3/4 = 3.5) and persistent bilateral, diffuse pulmonary infiltrates developed (Fig. 2) despite neuromuscular blockade, inhaled prostacyclin therapy, open lung ventilation, and recruitment maneuvers. The patient's tidal volumes were 5 cc/kg, with plateau pressures ranging from 30 to 35 cm H2O just before venovenous ECMO support was initiated. After 10 days, the patient was placed on conventional mechanical ventilation. He was transferred to an inpatient rehabilitation unit 35 days after his hospital admission.
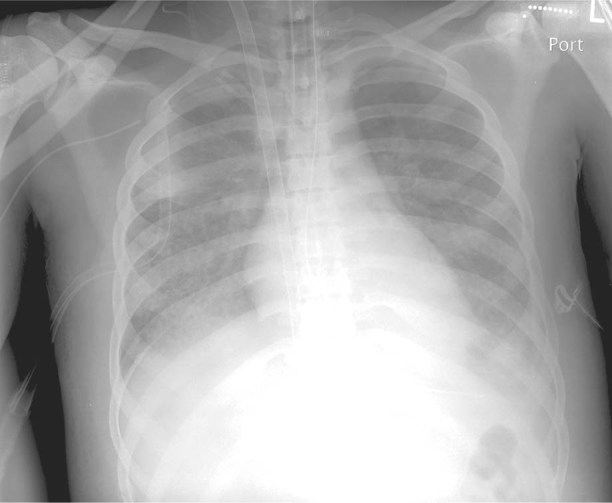
Fig. 2 Patient 2. Chest radiograph at the time of ECMO cannulation shows diffuse pulmonary infiltrates bilaterally.
To follow up on the patient's severe ARDS, his pulmonary function was tested 3 months after his discharge from the hospital. Spirometry revealed a mildly abnormal FEV1 (73%), normal TLC (84%), and normal DLCO (81%). A chest radiograph showed no evidence of parenchymal lung disease. Further information confirmed the patient's history of marijuana abuse and his having smoked tainted marijuana cigarettes just before his initial hospitalization.
Discussion
To our knowledge, these are the first reported cases of severe respiratory failure and the necessity of ECMO use in relation to the smoking of “wet” marijuana cigarettes.
Inhalation Toxicity of Tainted Marijuana Cigarettes
We think that inhalation exposure was the chief culprit in our patients' respiratory failure, given the temporal relationship of their use of tainted marijuana and their similar clinical presentations. Both presented with progressive, severe ARDS without any obvious inciting event. Although respiratory failure relating to smoking tainted marijuana cigarettes has not been previously described, some medical literature supports the adverse effects of the typical ingredients on the respiratory system.
Marijuana use by itself has not been linked to respiratory failure; however, it has been associated with chronic respiratory problems, such as bronchitis, obstructive lung disease, and histopathologic airway changes.8–10 The inhalation of embalming fluid has been linked to bronchitis, lung damage, and airway ulcerations. Pulmonary complications have rarely been reported in association with PCP use.11
The most commonly reported pulmonary symptoms from formaldehyde exposure are acute bronchospasm and occupational asthma.12–14 We found only one report of formaldehyde exposure's causing respiratory insufficiency: Dr. John Porter described his own experience and hospital course after prolonged exposure to formaldehyde.15 While preparing an anatomic specimen with formaldehyde, he developed progressive chest tightness and dyspnea that necessitated hospitalization and oxygen supplementation. Chest radiographs showed interstitial markings that were interpreted to be pulmonary edema. He slowly recovered with corticosteroid therapy and was without subjective symptoms 5 weeks after his hospital admission.15
Formaldehyde exposure has toxic effects at the cellular level. Inhalation exposure results in impairment of self-repair mechanisms16; in rats, varying degrees of respiratory epithelium hyperplasia and metaplasia have occurred, along with focal necrosis and epithelial thickening.13,14,17 It is hypothesized that inhalation of formaldehyde promotes mast-cell degranulation and disrupts nitric oxide regulation.16 This disruption may cause an alteration in both airway and vascular-tone homeostasis. The varying amounts of formaldehyde in embalming fluid, along with the varying degrees and areas of injury, might explain the range in clinical symptoms from bronchial hyperreactivity to noncardiogenic pulmonary edema.18
Specific interactions between formaldehyde and the ingredients in marijuana smoke might also warrant consideration in the pathogenesis of combined exposure. Chronic marijuana inhalation has been identified as a promoter of airway inflammation in human beings.9,10 This underlying chronic inflammation and epithelial disruption could predispose marijuana users to further airway injury from irritants such as formaldehyde; however, the literature describing such a phenomenon is sparse.
Extracorporeal Oxygenation in Severe Respiratory Failure
Our case reports yield evidence of the value of ECMO support in severe respiratory failure. We propose that patients can recover from severe lung injury after inhaling smoke from tainted marijuana cigarettes, and we recommend fairly aggressive therapy—which might include ECMO—in patients who present with single-organ failure and potentially surmountable lung injury.
Long-term pulmonary outcomes in ARDS survivors have often included abnormal pulmonary function test results, such as mild diffusion and restriction limitations. In our female patient, pulmonary tests 6 months after her hospital discharge disclosed only mild abnormalities. In our male patient, tests 3 months after his hospital discharge revealed relatively normal lung function, except for a mild decrease in spirometric values. These results appear similar to or even better than other long-term pulmonary function follow-up data in ARDS patients.
Conclusion
Our patients had similar presentations after similar temporal exposure to tainted marijuana cigarettes. We believe that smoke from tainted marijuana cigarettes could be an unrecognized cause of respiratory failure in young adults who present with an otherwise unclear origin of respiratory failure and ARDS. According to the available literature, the varying quantity and quality of ingredients in marijuana cigarettes can lead to presentations that range from cough and bronchospasm to severe respiratory failure. We recommend that the inhalation of smoke from tainted marijuana cigarettes be considered as the cause of ARDS in young adults, when the clinical context suggests it.
Footnotes
Address for reprints: Christopher R. Gilbert, DO, Pulmonary, Allergy, & Critical Care Medicine, Penn State Milton S. Hershey Medical Center, 500 University Dr., MCH041, Hershey, PA 17033
E-mail: cgilbert1@hmc.psu.edu
References
- 1.Moriarty AL. What's “new” in street drugs: “illy”. J Pediatr Health Care 1996;10(1):41–3. [DOI] [PubMed]
- 2.Peters RJ Jr, Williams M, Ross MW, Atkinson J, McCurdy SA. The use of fry (embalming fluid and PCP-laced cigarettes or marijuana sticks) among crack cocaine smokers. J Drug Educ 2008;38(3):285–95. [DOI] [PMC free article] [PubMed]
- 3.Elwood WN. TCADA research brief: “Fry”: a study of adolescents' use of embalming fluid with marijuana and tobacco. Austin (TX): Texas Commission on Alcohol and Drug Abuse; 1998.
- 4.Nelson LS, Holland JA, Ravikumar PR. Dangerous form of marijuana. Ann Emerg Med 1999;34(1):115–6. [DOI] [PubMed]
- 5.D'Onofrio G, McCausland JB, Tarabar AF, Degutis LC. Illy: clinical and public health implications of a street drug. Subst Abus 2006;27(4):45–51. [DOI] [PubMed]
- 6.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342(18):1301–8. [DOI] [PubMed]
- 7.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome [published erratum appears in Am Rev Respir Dis 1989;139(4): 1065]. Am Rev Respir Dis 1988;138(3):720–3. [DOI] [PubMed]
- 8.Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst 1998;90(16):1198–205. [DOI] [PubMed]
- 9.Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med 1998;157(3 Pt 1):928–37. [DOI] [PubMed]
- 10.Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest 1997; 112(2):319–26. [DOI] [PubMed]
- 11.Tashkin DP. Airway effects of marijuana, cocaine, and other inhaled illicit agents. Curr Opin Pulm Med 2001;7(2):43–61. [DOI] [PubMed]
- 12.Burge PS, Harries MG, Lam WK, O'Brien IM, Patchett PA. Occupational asthma due to formaldehyde. Thorax 1985;40 (4):255–60. [DOI] [PMC free article] [PubMed]
- 13.Appelman LM, Woutersen RA, Zwart A, Falke HE, Feron VJ. One-year inhalation toxicity study of formaldehyde in male rats with a damaged or undamaged nasal mucosa. J Appl Toxicol 1988;8(2):85–90. [DOI] [PubMed]
- 14.Woutersen RA, Appelman LM, Wilmer JW, Falke HE, Feron VJ. Subchronic (13 week) inhalation toxicity study of formaldehyde in rats. J Appl Toxicol 1987;7(1):43–9. [DOI] [PubMed]
- 15.Porter JA. Letter: acute respiratory distress following formalin inhalation. Lancet 1975;2(7935):603–4. [DOI] [PubMed]
- 16.Lino dos Santos Franco A, Damazo AS, Beraldo de Souza HR, Domingos HV, Oliveira-Filho RM, Oliani SM, et al. Pulmonary neutrophil recruitment and bronchial reactivity in formaldehyde-exposed rats are modulated by mast cells and differentially by neuropeptides and nitric oxide. Toxicol Appl Pharmacol 2006;214(1):35–42. [DOI] [PubMed]
- 17.Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundam Appl Toxicol 1996;29(2):208–18. [DOI] [PubMed]
- 18.Salmon AG, Winder B, Brown JP, Riveles K. Technical support document for the derivation of noncancer reference exposure levels. Appendix D.1. Formaldehyde reference exposure levels. Oakland (CA): Air Toxicology and Epidemiology Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. p. 128–69.


