Abstract
We sought to determine whether preoperative statin treatment is more effective in reducing, after cardiac surgery with cardiopulmonary bypass, systemic inflammatory response and myocardial damage markers in patients who have elevated preoperative interleukin-6 levels than in patients who have normal preoperative interleukin-6 levels.
The study involved a prospective cohort of 164 patients who underwent coronary and valvular surgery with cardiopulmonary bypass. There were 2 study groups: group A (n = 60), patients with elevated preoperative interleukin-6 levels; and group B (n = 104), patients with normal preoperative interleukin-6 levels. Each group was subdivided according to whether patients were (group 1) or were not (group 2) treated preoperatively with statins. Accordingly, the subdivided study groups were A1 (n = 40), A2 (n = 20), B1 (n = 56), and B2 (n = 48). The plasma levels of proinflammatory interleukin-6 were measured 1, 6, 24, and >72 hours after surgery.
The baseline, operative, and postoperative morbidity and mortality characteristics were similar in all groups. Group A1 had significantly lower levels of interleukin-6 and troponin I than did group A2 at all postoperative time points. Group B1 had significantly lower levels of interleukin-6 than did group B2 postoperatively. There were no significant differences in troponin I levels between groups B1 and B2.
We conclude that, in patients with preoperative activation of the inflammatory system, preoperative treatment with statins is associated with lower postoperative interleukin-6 and troponin I levels after cardiac surgery with cardiopulmonary bypass.
Key words: Anticholesteremic agents/therapeutic use, biological markers, coronary artery bypass/adverse effects, inflammation mediators, interleukin-6/blood, postoperative complications/blood, systemic inflammatory response syndrome/prevention & control, statin treatment, troponin/blood
Some sequelae to cardiac surgery with cardiopulmonary bypass (CPB) appear to be related to an excessive systemic inflammatory response and to the cardiac biomarkers released in reaction to CPB and surgical trauma.1,2 As a result of systemic inflammatory response, the plasma levels of some factors such as tumor necrosis factor (TNF-α), and interleukin (IL)-6 and IL-8 are elevated in patients who undergo cardiac interventions.3,4 Further, there is a correlation between elevated IL levels (mainly elevated IL-6) and some postoperative complications.5–10 Currently, no drugs or techniques have been shown to reduce the severity or incidence of systemic inflammatory response.
Statins, which are 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors used as primary and secondary prevention measures, are effective hypolipidemic agents that have shown efficacy in reducing cardiovascular events.11–14 The mechanism of action of statins, which act as anti-inflammatory agents, has been elucidated in several in vitro studies. The pleiotropic effects of statins might, in part, explain the clinical benefits of statins that cannot be attributed to their hypolipidemic properties.15 Statins also have reduced the morbidity and mortality rates associated with cardiovascular surgery, and their long-term use has improved bypass graft patency and long-term mortality rates in patients undergoing coronary artery bypass grafting (CABG).16,17 The benefits of statins for reducing perioperative death and morbidity in cardiovascular surgery, mainly in valvular surgery, are more controversial, although some studies have shown the benefits of statins on perioperative death and morbidity related to atrial fibrillation and stroke rates in patients undergoing cardiovascular surgery.11,18–22 Statins also reduce inflammatory markers. The preoperative administration of statins decreases proinflammatory cytokines, mainly IL-6, in the first hours after cardiac surgery.23–26 It would be very helpful to determine which group of patients would benefit most from statin use.
We hypothesized that the preoperative administration of statins would more strongly affect patients with preoperative activation of the inflammatory system (that is, patients with high preoperative levels of IL-6) than it would affect patients with normal preoperative levels of IL-6; and we hypothesized that this response would manifest itself as a more substantial reduction of postoperative levels of IL-6 and myocardial injury biomarkers. Before this study, there were no prospective studies that tested this hypothesis. On the basis of preoperative statin treatment and inflammation state, we prospectively analyzed the reduction of postoperative IL-6 and troponin I levels that rose as a result of systemic inflammatory response and complications after cardiac surgery with CPB.
Patients and Methods
This prospective study included 164 consecutive patients who underwent cardiac surgery with CPB from November 2008 through November 2009 at the Hospital Clinico Universitario of Santiago de Compostela (Spain). We included all patients who underwent coronary surgery (isolated coronary surgery or coronary surgery plus valvular surgery) and noncoronary surgery (isolated valvular surgery) during this time period. We excluded patients who had undergone any type of transplantation procedure; patients who were being treated with corticosteroids or immunosuppressants; patients who had infections, active tumors, autoimmune diseases, or acute renal failure; and patients who were undergoing surgery on an emergency basis.
The 164 patients included in the study were divided into 2 study groups: group A (n = 60) included patients with elevated preoperative levels of IL-6; and group B (n = 104) included patients with normal preoperative IL-6 levels. Each group was subdivided according to whether patients were (group 1) or were not (group 2) treated preoperatively with statins. Thus, the study groups were as follows: group A1 (n = 40), group A2 (n = 20), group B1 (n = 56), and group B2 (n = 48).
All group 1 patients (those in groups A1 and B1) had dyslipidemia and were treated with statins for a minimum of 3 weeks before surgery. In this group, statins were administered in accordance with routine protocols, and, as usual, were administered the night before surgery. After surgery, statin treatment continued throughout the entire hospitalization period. In both groups, antiplatelet agents were discontinued 10 days before surgery. Other medications were not administered on the morning of surgery. The study protocol was approved by the local ethics committee. Informed consent was obtained from each of the patients included in the study.
Surgical Procedure and Postoperative Care
Anesthesia induction and maintenance were similar for all patients and consisted of weight-appropriate doses of midazolam, vecuronium bromide, remifentanil, and sevoflurane. During the surgical procedure, the following values were monitored using a Datex-Ohmeda Aestiva 3000® standard anesthesia device with an AS/3 monitor® (Datex-Ohmeda Instrumentarium Corp.; Helsinki, Finland): systemic and pulmonary blood pressure, central venous pressure, esophageal and urethral temperature, and ventilation capacity. Pulse oximetry and 5-lead electrocardiography were also used throughout the procedure. All patients underwent valvular surgery or CABG with CPB, with induction of mild hypothermia (between 32 and 33 °C) and electromechanical arrest of the heart by aortic clamping and the intermittent administration (every 20 minutes) of cold-blood cardioplegic solution.
We used a Stöckert SIII® extracorporeal circulation machine (Stöckert Instrumente GmbH; Munich, Germany) that was equipped with an SCP Revolution® centrifugal pump (Stöckert) and an attached SIII® heat exchanger (Stöckert). Before connection to the extracorporeal circulation, the patients were given 3 mg/kg of sodium heparin; an activated coagulation time of above 300 seconds was maintained during the entire procedure. Additional doses of heparin were used as necessary. A Dideco D903 Avant® membrane oxygenator (Dideco S.p.A.; Modena, Italy) was used; the circuit was primed with a base of 1,000 mL of lactated Ringer's crystalloid solution, 0.5 mg/kg of mannitol, 100 mL of 1 M sodium bicarbonate, and 0.5 mg/kg of sodium heparin.
The surgical approach was always performed through a standard median sternotomy. In patients with ischemic heart disease, venous or arterial grafts (or both) were used for coronary revascularization. The ascending aorta and the right atrium were cannulated in the usual manner. Two cannulae were used in the right atrium for mitral- and tricuspid-valve disease interventions, as well as a single cavoatrial cannula for coronary and aortic procedures.
At the end of the surgical procedures, the patients were transferred to the recovery unit, where they remained sedated with propofol, morphine, and midazolam (administered as needed). The patients remained intubated and connected to a mechanical ventilator set in synchronized intermittent mandatory ventilation mode between 6 and 10 mL/kg at a respiratory rate of 12 breaths/min; the positive end-expiratory pressure set between 5 and 7 cmH2O, with a trigger between –1 and 1 cmH2O; and the fraction of inspired oxygen adjusted according to arterial blood gas analysis. The minimum mechanical ventilation time was 6 hours; after 6 hours, patients with suitably stable hemodynamic, ventilation, oxygenation, and temperature conditions were extubated. Hemodynamic monitoring was maintained for at least the first 12 hours of the postoperative period.
Blood Sample Collection, Storage, and Analysis
The following biochemical values were measured at baseline (before the surgical intervention) and after surgery once the patients were stabilized: erythrocyte sedimentation rate, iron, transferrin, ferritin, thyroid-stimulating hormone, total bilirubin, serum oxaloacetic transaminase, serum glutamic pyruvic transaminase, gamma-glutamyl transferase, alkaline phosphatase, hematocrit, creatinine, and blood urea nitrogen.
Interleukin-6 serum levels were determined in venous blood samples at the following time points: before anesthesia induction; at the end of the CPB surgery; 6 and 24 hours after surgery, and before discharge from the hospital, when the patient was in stable condition.
The samples of blood for IL-6 determination were collected via venipuncture in tubes with ethylenediaminetetraacetic acid (EDTA) and immediately centrifuged at 4 °C and stored at −20 °C until analysis. The IL-6 was measured using the IMMULITE® DCP® system (Siemens AG; Erlangen, Germany), which is an enzyme immunometric assay that uses solid-phase chemiluminescence in conjunction with the IMMULITE® DCP® automatic analyzer and is designed for the quantitative measurement of interleukins.
Statistical Analysis
The qualitative data are expressed in absolute numbers (percentages). The quantitative data are presented as the average values ± SD; the median value (interquartile range) is given as well (or instead), when the distribution of the variable values is markedly outside of the normal distribution as shown by the Kolmogorov-Smirnoff and Shapiro-Wilks tests. Univariate analyses were performed to compare the variables obtained in the patient groups that had received (groups A1 and B1) or had not received (groups A2 and B2) preoperative statins. For comparison of quantitative data, Student's t test (parametric) or the Mann-Whitney U test (nonparametric) was used as appropriate. Comparison of the qualitative data was performed using the χ2 test, applying the Yates correction to obtain the most conservative results.
The data were analyzed with the SPSS statistical package for Windows, version 15.0 (IBM Corporation; Armonk, NY). P values of less than 0.05 were considered statistically significant.
Results
Baseline Patient Characteristics and Postoperative Clinical Evolution
The clinical and operative characteristics, as well as the clinical course in the postoperative period, were not significantly different between patients with elevated preoperative levels of IL-6 (group A) and patients with normal preoperative levels of IL-6 (group B). Rather, the characteristics and postoperative courses seemed to depend on whether the patients were treated preoperatively with statins (Tables I, II, III and IV). Atorvastatin was the most-used statin (59.7% in group A1; 55.2% in group B1), followed by simvastatin (24.5% in group A1; 28.5% in group B1), pravastatin (13.1% in group A1; 16.3% in group B1), and lovastatin (2.7% in group A1). The most common dosage was 20 mg per day for atorvastatin, simvastatin, and lovastatin, and 40 mg per day for pravastatin. None of the patients showed side effects attributable to statins.
TABLE I. Baseline Clinical, Operative, and Postoperative Characteristics of Patients with Preoperative Interleukin-6 >5 pg/mL
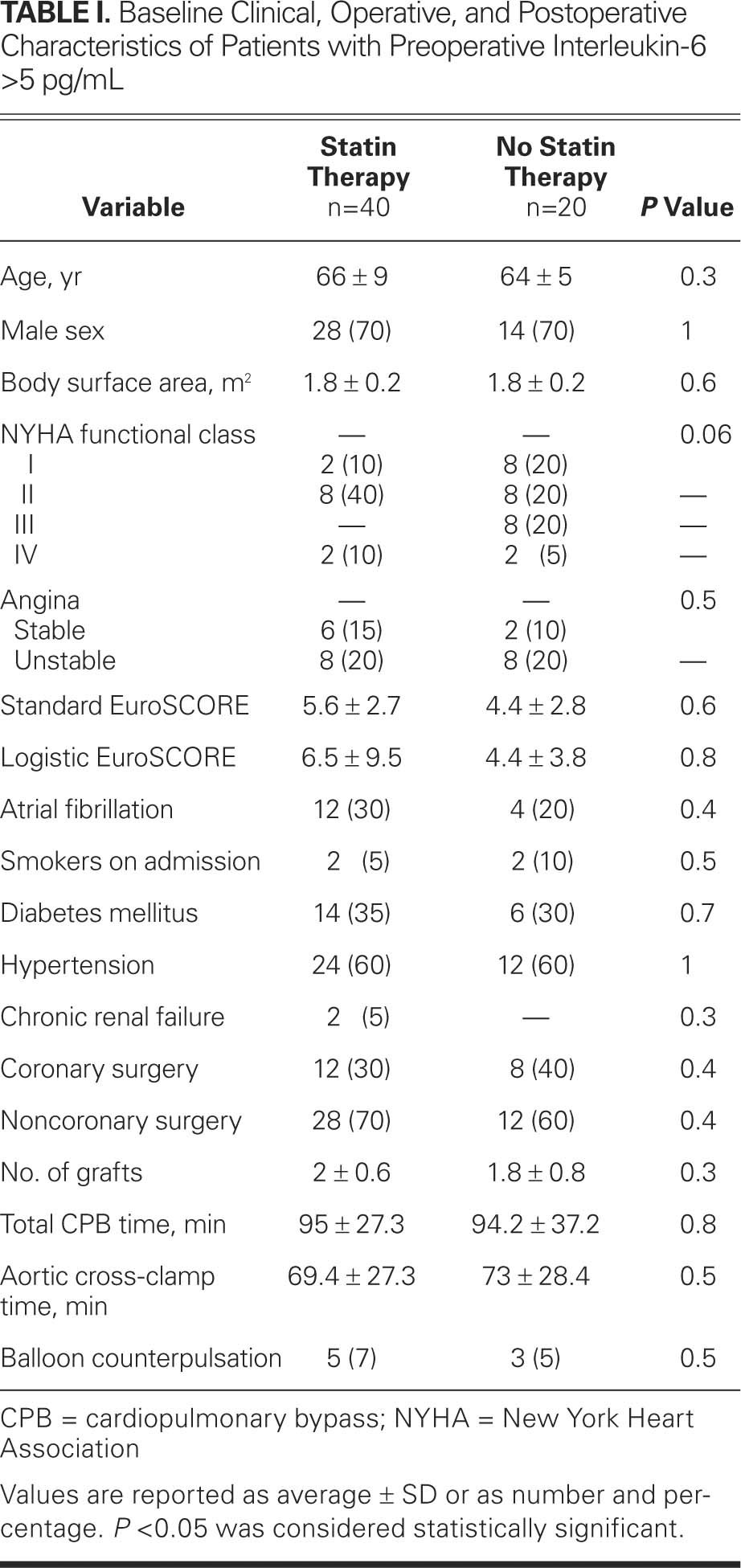
TABLE II. Postoperative Course of Patients with Preoperative Interleukin-6 >5 pg/mL
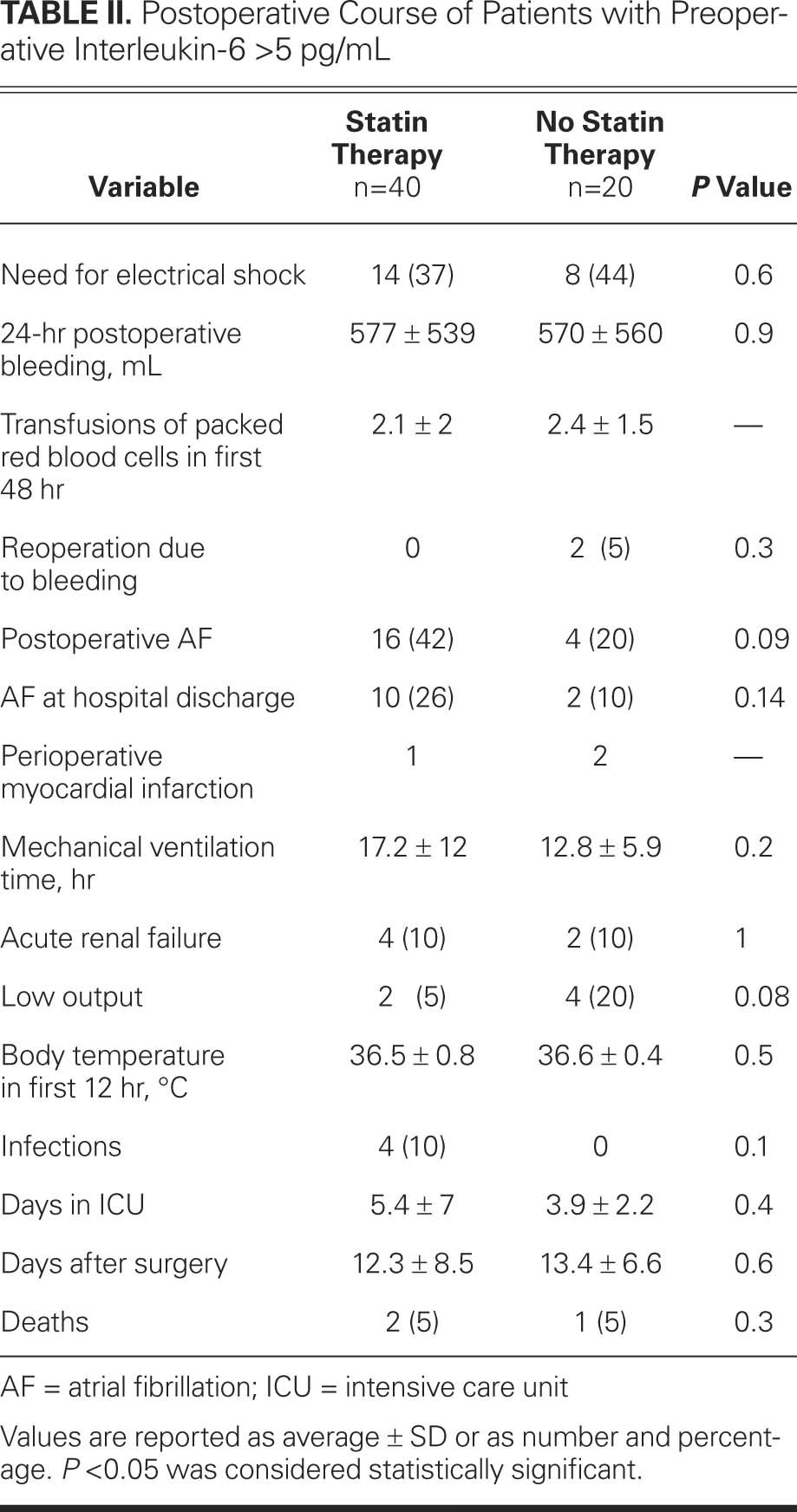
TABLE III. Baseline Clinical, Operative, and Postoperative Characteristics of Patients with Preoperative Interleukin-6 <5 pg/mL
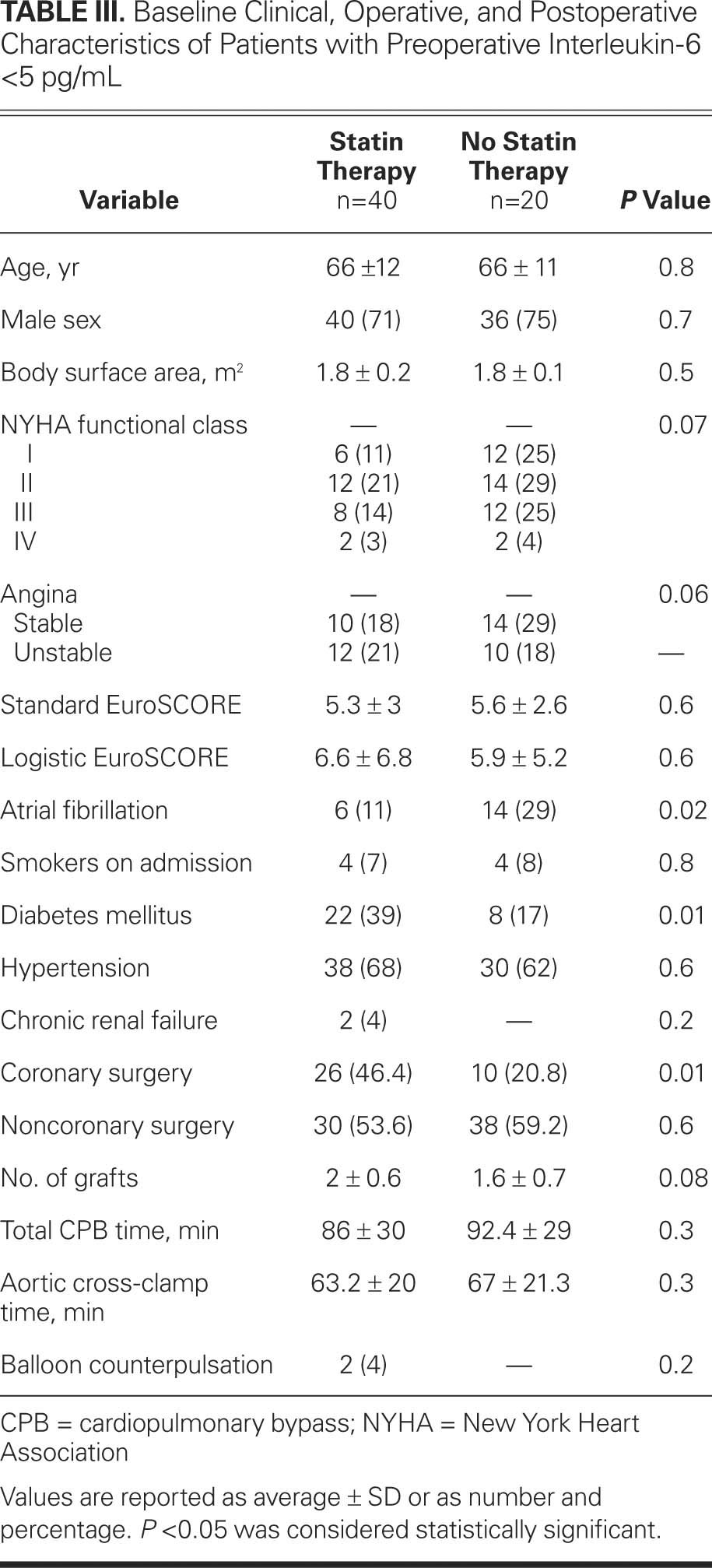
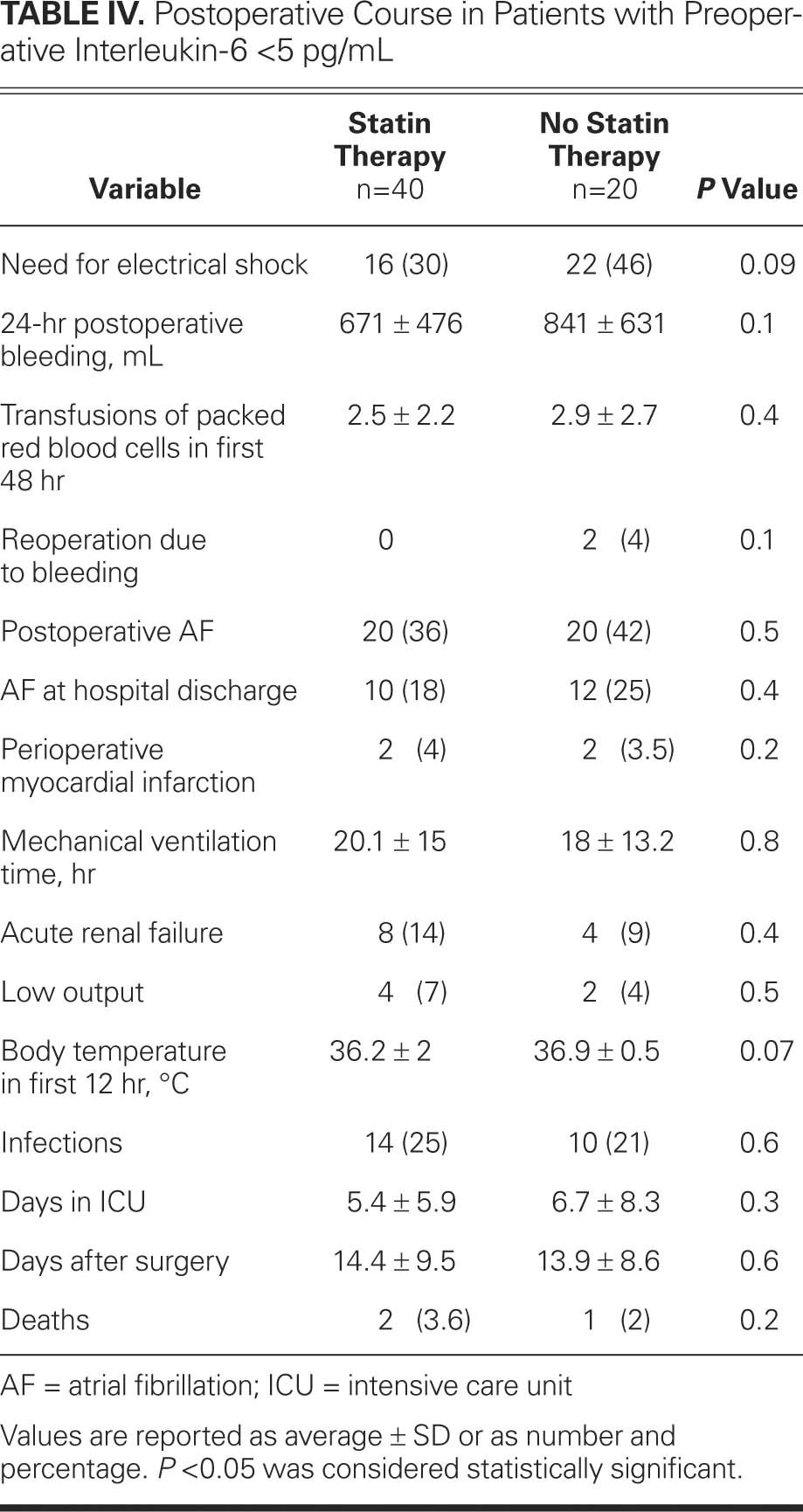
TABLE IV. Postoperative Course in Patients with Preoperative Interleukin-6 <5 pg/mL
Baseline and Postoperative Patient Characteristics
The baseline and postoperative characteristics were similar in groups A1 and B1 (statins) and in groups A2 and B2 (no statins). Only the preoperative total cholesterol and low-density-lipoprotein cholesterol (LDL-C) levels were significantly lower in group A1 when compared with those of group A2 (total cholesterol, 159.67 ± 41.3 vs 181.79 ± 49.5 mg/dL, P = 0.005; LDL-C, 92.36 ± 32.1 vs 117.89 ± 27 mg/dL, P = 0.001), and significantly lower in group B1 when compared with those of group B2 (total cholesterol, 159.34 ± 38.7 vs 183.86 ± 49.6 mg/dL, P = 0.003; LDL-C, 92.22 ± 33.6 vs 117.93 ± 39 mg/dL, P = 0.001). The levels of preoperative high-density-lipoprotein cholesterol (HDL-C) and triglycerides were very similar in groups A and B. Similarly, the postoperative total-cholesterol, LDL-C, HDL-C, and triglyceride levels did not differ significantly between the 2 study groups.
Interleukin-6 Levels Over Time
For the group of patients with elevated preoperative levels of IL-6 (group A), Figure 1A shows the IL-6 levels over time. The postoperative IL-6 levels were significantly lower in patients treated with statins than in patients not being treated with statins: 1 hour after surgery, 91.6 ± 66 pg/mL in group A1 versus 159.3 ± 149.4 pg/mL in group A2, P = 0.03; at 6 hours, 70.2 ± 30.5 pg/mL in group A1 versus 155.7 ± 67.7 pg/mL in group A2, P = 0.005; at 24 hours, 64.3 ± 47.6 pg/mL in group A1 versus 106 ± 114.5 pg/mL in group A2, P = 0.02; and before discharge from the hospital, 22.1 ± 10.3 pg/mL in group A1 versus 38.9 ± 25.6 pg/mL in group A2, P = 0.003. We also determined the average levels of IL-6 in the first 24 hours after surgery (adjusted according to the number of 6-hour periods included in each pair of measurements). Figure 2A shows that the IL-6 levels were significantly lower in patients treated with statins than in those not treated with statins: 72.6 ± 34.7 pg/mL in group A1 versus 131.8 ± 104.3 pg/mL in group A2, P = 0.004.
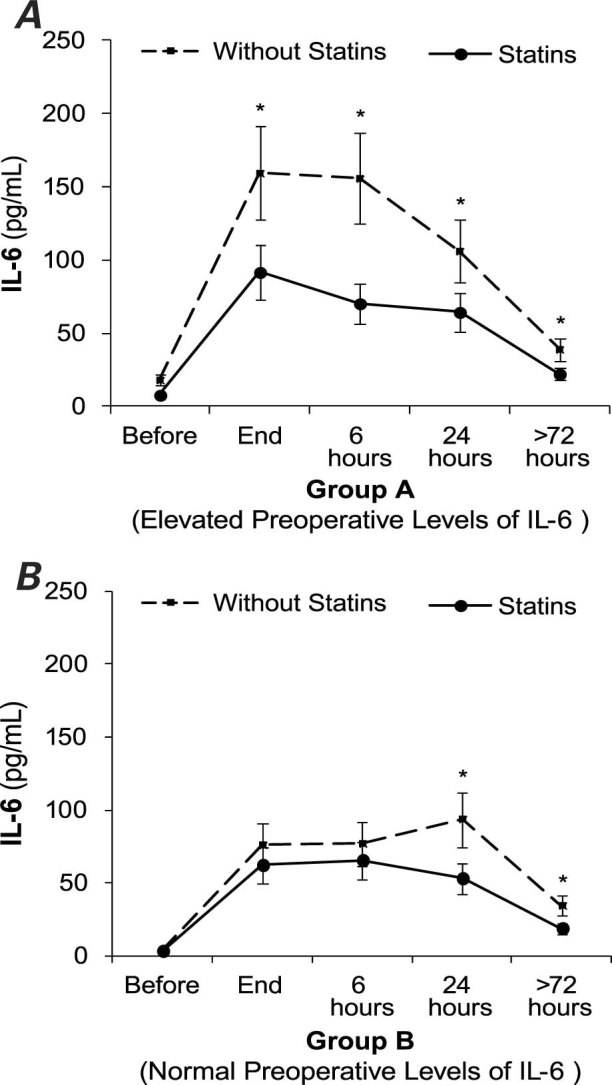
Fig. 1 Interleukin-6 (IL-6) levels over time, before and after cardiopulmonary bypass surgery in patients treated preoperatively with statins and in patients not treated with statins. A) Elevated preoperative levels of IL-6. B) Normal preoperative levels of IL-6. Error bars indicate the standard error of the mean.
* P < 0.05
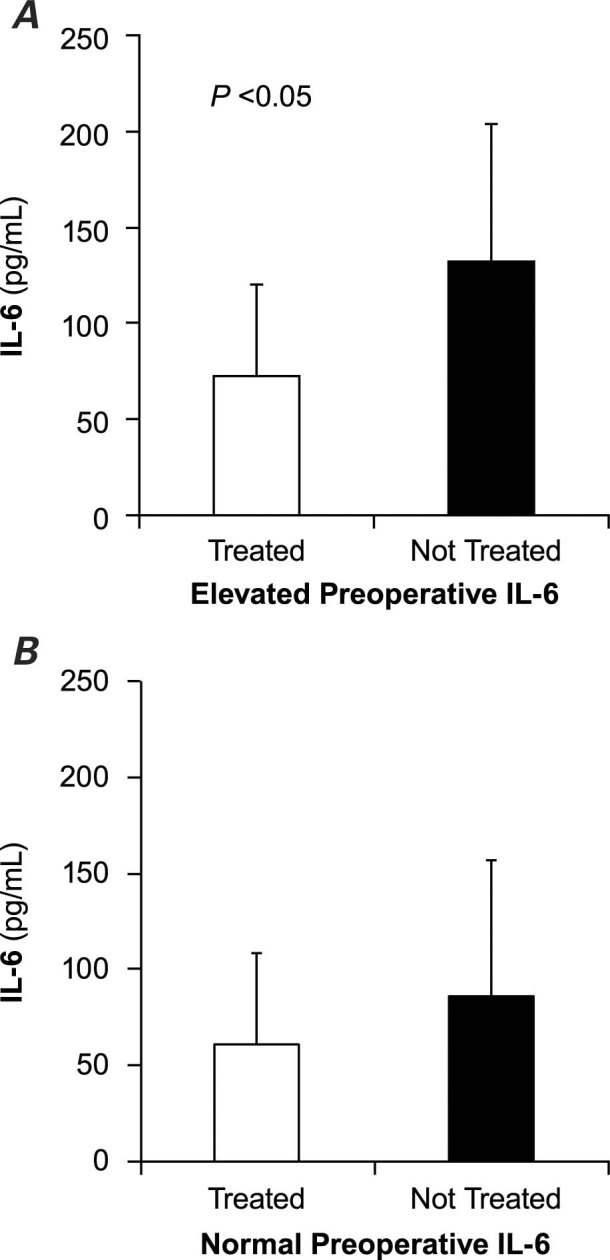
Fig. 2 Levels of interleukin-6 (IL-6) in the first 24 hours (average ± SD) in patients treated preoperatively with statins and in patients not treated with statins. A) Elevated preoperative levels of IL-6. B) Normal preoperative levels of IL-6.
Figure 1B shows the chronological evolution of IL-6 levels in the group with normal preoperative levels of IL-6 (group B). Patients treated with statins (group B1) had lower levels of IL-6 at every time-point than did patients not treated with statins (group B2), but the difference was statistically significant only at 24 hours (53 ± 32.9 pg/mL in group B1 vs 93.5 ± 49.9 pg/mL in group B2, P = 0.001) and at discharge from the hospital (18.7 ± 12.5 pg/mL in group B1 vs 34.2 ± 28.7 pg/mL in group B2, P = 0.001). The average levels of IL-6 in the first postoperative 24 hours were lower in patients treated with statins, but the difference was not statistically significant (Fig. 2B).
Troponin I Levels Over Time
Levels of troponin I were elevated in both groups A and B once the patients arrived at the postsurgery unit (Fig. 3). Group A1, the patients with elevated preoperative levels of IL-6 (Fig. 3A) who were taking statins, had significantly lower levels of troponin I at the 3 time points than did patients in group A2 (patients with elevated preoperative levels of IL-6 who were not on statin therapy): 1 hour after surgery, 2.3 ± 1.5 versus 4.8 ± 5.8 ng/mL, P = 0.02; at 6 hours, 4.1 ± 3.1 versus 6.3 ± 4.4 ng/mL, P = 0.03; and at 24 hours, 3.6 ± 2.1 versus 5.8 ± 4 ng/mL, P = 0.03. For patients with normal preoperative levels of IL-6 (group B), postsurgical IL-6 levels were not significantly different, regardless of the presence or absence of statin therapy (Fig. 3B).
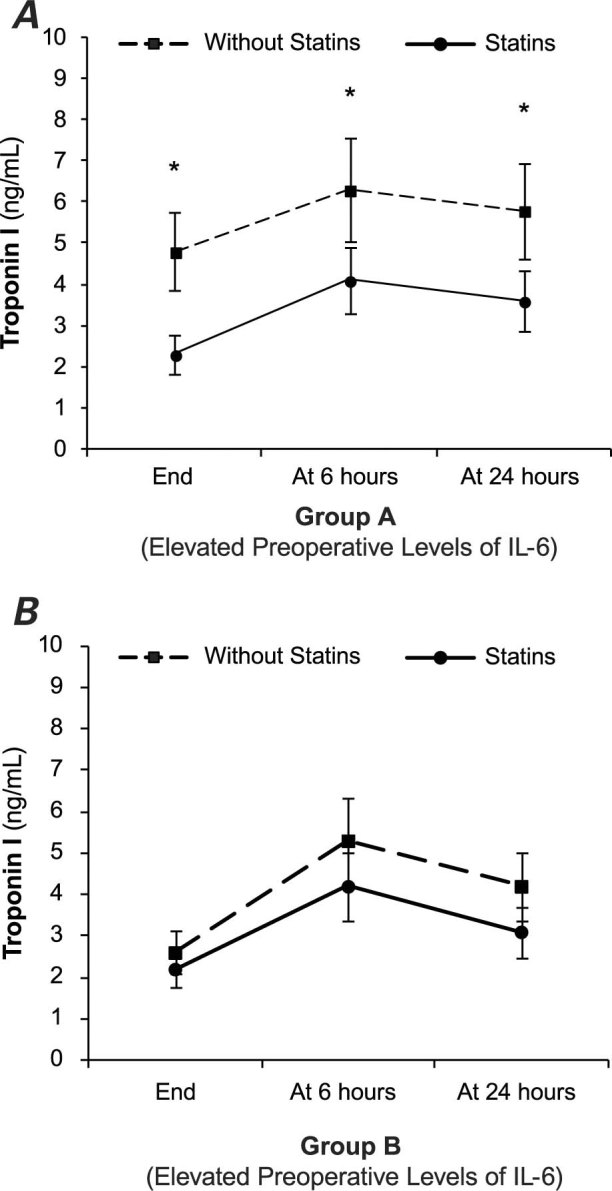
Fig. 3 Troponin I levels (average ± SD) over time, after cardiopulmonary bypass surgery in patients treated preoperatively with statins and in patients not treated with statins. A) Elevated preoperative levels of interleukin-6 (IL-6). B) Normal preoperative levels of IL-6.
* P < 0.05
Discussion
Because of systemic inflammatory response, the plasma levels of some factors, such as TNF-α, IL-8, and particularly IL-6, are elevated in patients undergoing cardiac surgery interventions.3,4 The most important function of IL-6 is coordination of the acute phase of inflammation. Interleukin-6 regulates inflammation, induces B-cell differentiation, activates T cells, induces T-cell cytotoxicity, and controls the differentiation of monocytes into macrophages. Therefore, preoperative elevation of IL-6 might indicate an underlying inflammatory state. In addition, elevated serum levels of IL-6 after cardiovascular surgery appear to be partly responsible for morbidity associated with the inflammatory response to CPB: after all, IL-6 levels can predict the likelihood of infection in patients with abnormal ventricular function,8 are correlated with mortality rates in pediatric cardiac surgery,5 and can aid in the early identification of acute kidney injury.6,7,10 Our previous study showed that preoperative statin therapy decreases proinflammatory cytokines, mainly IL-6, in the first hours after cardiovascular surgery.23–26
In the present study, we found elevated postoperative IL-6 levels in both study groups (A and B), with significantly lower IL-6 levels in the groups treated with statins (groups A1 and B1). This reduction in IL-6 was greater and statistically significant at all time-points in patients with elevated preoperative levels of IL-6 who were being treated with statins (group A1), compared with patients with normal preoperative levels of IL-6 treated with statins (group B1). Similarly, group A1 had lower average postoperative levels of IL-6 in the first 24 hours after surgery, compared with group B1. This study recorded IL-6 levels at 5 time-points and calculated the average postoperative IL-6 levels.
This is the first study to show that statin therapy before cardiac surgery with CPB in patients who have previously elevated IL-6 significantly reduces postoperative IL-6 levels, compared with patients who have normal preoperative IL-6 levels.
Mannacio and colleagues27 published the first randomized study to show that pretreatment with rosuvastatin decreases the incidence of myocardial damage in patients undergoing coronary surgery. We showed the same result in a previous study,26 regardless of whether the procedure was CABG or valvular surgery. Notably, in CABG without myocardial infarction, the amount of released cardiac biomarkers seems to be associated with an increased risk of death and late cardiac events.2 Accordingly, an absolute reduction in myocardial biomarker release, as we observed in this study, could forecast a reduction of early and late adverse events.
In common with other studies of similar design,23–26 our study found no difference in postsurgical morbidity and death that corresponded with the preoperative administration of statin therapy. Only studies with larger patient populations found that preoperative statin therapy helped reduce death and morbidity after cardiovascular surgery.16–20,28
Statins' anti-inflammatory properties, pleiotropic effects, and ability to reduce myocardial biomarkers account for their beneficial effects on cardiac interventions. Theoretically, high preoperative levels of IL-6 indicate activation of the inflammatory system. Accordingly, patients with high preoperative levels of IL-6 might be the best candidates for the preemptive administration of statins as anti-inflammatory drugs and myocardial protectors in cardiac surgery with CPB. This approach is supported by our findings that such patients had significantly lower postoperative levels of IL-6 and troponin I. It would be very helpful to determine which patients have high preoperative levels of IL-6, because they would benefit most from statins' reduction of death and morbidity.
This study has some limitations. It was a nonrandomized, single-center study involving a rather small cohort of patients. Several statins were used at different dosages and for different periods of time.
Conclusions
In cardiac-surgery patients who showed preoperative evidence of an activated inflammatory system, preoperative therapy with statins was associated with more effective reduction both of myocardial injury biomarkers and of biochemical indicators of postoperative systemic inflammatory response. Larger randomized studies are needed to confirm and extend these findings, as well as to elucidate the clinical implications.
Acknowledgments
The authors thank the anesthesiology and nursing teams of University Hospital Santiago de Compostela for their invaluable technical assistance in collecting blood samples.
Footnotes
Address for reprints: José Rubio Alvarez, MD, Department of Cardiovascular Surgery, University Hospital Santiago de Compostela, La Choupana sn., 15706 Santiago de Compostela, Spain
E-mail: framan1@hotmail.com
References
- 1.McGuinness J, Bouchier-Hayes D, Redmond JM. Understanding the inflammatory response to cardiac surgery. Surgeon 2008;6(3):162–71. [DOI] [PubMed]
- 2.Petaja L, Salmenpera M, Pulkki K, Pettila V. Biochemical injury markers and mortality after coronary artery bypass grafting: a systematic review. Ann Thorac Surg 2009;87(6): 1981–92. [DOI] [PubMed]
- 3.Karu I, Taal G, Zilmer K, Pruunsild C, Starkopf J, Zilmer M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand Cardiovasc J 2010;44 (2):119–24. [DOI] [PubMed]
- 4.Halter J, Steinberg J, Fink G, Lutz C, Picone A, Maybury R, et al. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol 2005; 37(3):272–7. [PMC free article] [PubMed]
- 5.Millar AB, Armstrong L, van der Linden J, Moat N, Ekroth R, Westwick J, et al. Cytokine production and hemofiltration in children undergoing cardiopulmonary bypass. Ann Thorac Surg 1993;56(6):1499–502. [DOI] [PubMed]
- 6.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 2009;13(4): R104. [DOI] [PMC free article] [PubMed]
- 7.Musleh GS, Datta SS, Yonan NN, Grotte GJ, Prendergast BA, Hasan RI, Deyrania AK. Association of IL6 and IL10 with renal dysfunction and the use of haemofiltration during cardiopulmonary bypass. Eur J Cardiothorac Surg 2009;35 (3):511–4. [DOI] [PubMed]
- 8.Sander M, von Heymann C, von Dossow V, Spaethe C, Konertz WF, Jain U, Spies CD. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg 2006;102(6): 1623–9. [DOI] [PubMed]
- 9.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J 2004;45(2):183–93. [DOI] [PubMed]
- 10.Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, et al. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care 2010;14(5):R181. [DOI] [PMC free article] [PubMed]
- 11.Dembowski E, Davidson MH. A review of lipid management in primary and secondary prevention. J Cardiopulm Rehabil Prev 2009;29(1):2–12. [DOI] [PubMed]
- 12.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285(13): 1711–8. [DOI] [PubMed]
- 13.Simes RJ, Marschner IC, Hunt D, Colquhoun D, Sullivan D, Stewart RA, et al. Relationship between lipid levels and clinical outcomes in the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Trial: to what extent is the reduction in coronary events with pravastatin explained by on-study lipid levels? Circulation 2002;105(10):1162–9. [DOI] [PubMed]
- 14.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7–22. [DOI] [PubMed]
- 15.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004;109(23 Suppl 1):III39–43. [DOI] [PubMed]
- 16.Clark LL, Ikonomidis JS, Crawford FA Jr, Crumbley A 3rd, Kratz JM, Stroud MR, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg 2006;131(3):679–85. [DOI] [PubMed]
- 17.Kulik A, Ruel M. Statins and coronary artery bypass graft surgery: preoperative and postoperative efficacy and safety. Expert Opin Drug Saf 2009;8(5):559–71. [DOI] [PubMed]
- 18.Collard CD, Body SC, Shernan SK, Wang S, Mangano DT; Multicenter Study of Perioperative Ischemia (MCSPI) Research Group, Inc.; Ischemia Research and Education Foundation (IREF) Investigators. Preoperative statin therapy is associated with reduced cardiac mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2006;132 (2):392–400. [DOI] [PubMed]
- 19.Fedoruk LM, Wang H, Conaway MR, Kron IL, Johnston KC. Statin therapy improves outcomes after valvular heart surgery. Ann Thorac Surg 2008;85(5):1521–6. [DOI] [PMC free article] [PubMed]
- 20.Virani SS, Nambi V, Lee VV, Elayda M, Reul RM, Wilson JM, Ballantyne CM. Does preoperative statin therapy improve outcomes in patients undergoing isolated cardiac valve surgery? Am J Cardiol 2008;102(9):1235–9. [DOI] [PubMed]
- 21.Anselmi A, Possati G, Gaudino M. Postoperative inflammatory reaction and atrial fibrillation: simple correlation or causation? Ann Thorac Surg 2009;88(1):326–33. [DOI] [PubMed]
- 22.Liakopoulos OJ, Choi YH, Kuhn EW, Wittwer T, Borys M, Madershahian N, et al. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg 2009;138(3):678–686.e1. [DOI] [PubMed]
- 23.Chello M, Patti G, Candura D, Mastrobuoni S, Di Sciascio G, Agro F, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med 2006;34(3):660–7. [DOI] [PubMed]
- 24.Brull DJ, Sanders J, Rumley A, Lowe GD, Humphries SE, Montgomery HE. Statin therapy and the acute inflammatory response after coronary artery bypass grafting. Am J Cardiol 2001;88(4):431–3. [DOI] [PubMed]
- 25.Liakopoulos OJ, Dorge H, Schmitto JD, Nagorsnik U, Grabedunkel J, Schoendube FA. Effects of preoperative statin therapy on cytokines after cardiac surgery. Thorac Cardiovasc Surg 2006;54(4):250–4. [DOI] [PubMed]
- 26.Martinez-Comendador JM, Alvarez JR, Mosquera I, Sierra J, Adrio B, Carro JG, et al. Preoperative statin treatment reduces systemic inflammatory response and myocardial damage in cardiac surgery. Eur J Cardiothorac Surg 2009;36(6):998–1005. [DOI] [PubMed]
- 27.Mannacio VA, Iorio D, De Amicis V, Di Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. J Thorac Cardiovasc Surg 2008;136(6):1541–8. [DOI] [PubMed]
- 28.Vaduganathan M, Stone NJ, Lee R, McGee EC Jr, Malaisrie SC, Silverberg RA, et al. Perioperative statin therapy reduces mortality in normolipidemic patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2010;140(5):1018–27. [DOI] [PubMed]


