Abstract
Basidiobolomycosis is a chronic subcutaneous infection of the trunk and limbs due to Basidiobolus ranarum. The disease is well known in tropical areas, although recent cases of gastrointestinal basidiobolomycosis have also been reported in Arizona. We describe a young immunocompetent women who had presented with eosinophilia and lung infiltrates. She subsequently died, and diagnosis of disseminated basidiobolomycosis was made on the basis of histological features at autopsy.
CASE REPORT
A nonsmoking, 40-year-old housewife was admitted to the hospital with sudden acute dyspnea and hypotension; she died a few minutes after admission to the emergency room due to cardiorespiratory arrest.
Three days before, the patient noted the appearance of shortness of breath; hard subcutaneous and painless swellings localized to the back of the shoulders and arms were also noted. For 4 months she had been monitored as an outpatient with low-grade fever and increasing eosinophilia (white cell counts were 13.800 and then 28.280; eosinophils, 11% and then 30%), an elevated erythrocyte sedimentation rate (43 mm/h) and C-reactive protein (3.9 mg/dl), and increased immunoglobulin E (2,583 kU/liter). At that time, pulmonary function tests documented airflow obstruction and chest radiographs revealed a right upper lobe infiltrate. The oxygen saturation was 98% while on room air. Laboratory tests, including percentage of lymphocytes and total count of peripheral blood, hepatitis B virus and hepatitis C virus serological markers, and human immunodeficiency virus status, didn't reveal any form of immunosuppression. She did not suffer from diabetes mellitus or peptic ulcer disease. Symptoms persisted despite antibiotics (macrolide, penicillin-cephalosporin), inhaled corticosteroids, and long-acting β2 agonist treatment.
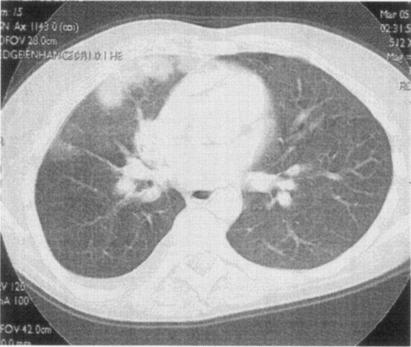
A high-resolution computed tomography showed patchy subpleural infiltrates with a halo sign in the right lung (Fig. 1). Enlarged mediastinal lymph nodes, right pleural effusion, and two cysts in the liver were also detected. Bronchoscopy documented a nodular submucosal infiltration involving the distal trachea and proximal right bronchial system.
FIG. 1.
High-resolution computed tomography scan at the hilar level showing patchy subpleural infiltrates with a halo sign in the upper right lobe.
Mucosal biopsies from the right main bronchus showed only eosinophil infiltration and necrosis. There were no organisms, granulomas, or evidence of neoplasm. Subsequently a surgical lung biopsy was taken from the middle right lobe. The histologic findings were similar to those of the transbronchial biopsy: eosinophil infiltration and necrosis with no organism identified. Skin biopsy from subcutaneous nodules, performed 1 day before death, showed eosinophilic panniculitis, in which scattered hyphal elements were present.
Autopsy report.
The macroscopic examination revealed necrotic small white-yellow opacities (3 to 4 mm in diameter) in the pericardium, parietal pleura of the right hemithorax, visceral surface of the right lung, liver, spleen, kidneys, adrenal glands, pancreas, uterus and peritoneum; the stomach presented fungating necrotic mucosal lesions. Small yellowish nonulcerated nodules were also detected in the distal portion of the trachea and in the right bronchial tree. A thrombus in the pulmonary vein supplying the right upper lobe extending to and partially occluding the right atrium was also documented.
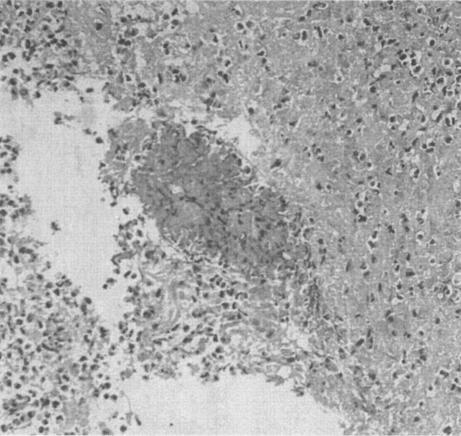
The microscopic examination of the lesions identified at autopsy showed extensive necrosis and a marked eosinophil infiltrate involving numerous organs (brain, pancreas, kidney, stomach, lung spleen); within the necrosis hollow round or cylindrical structures 15 to 20 μm in diameter were seen. These resembled fungal hyphae and had a surrounding Splendori-Hoeppli phenomenon (radiating, intensely eosinophilic granular material surrounding empty structures) (Fig. 2).
FIG. 2.
Hematoxylin and eosin-stained section of the lung showing intensily eosinophilic granular material embedded in an eosinophilic rich inflammatory infiltrate.
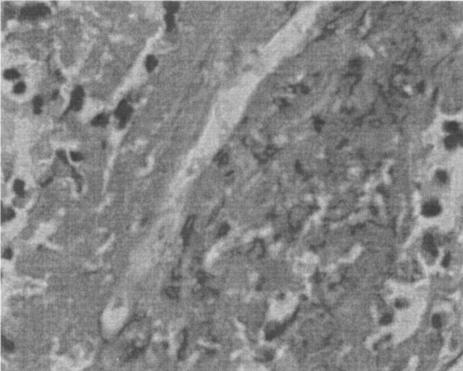
The apparent hyphal elements (Fig. 3) on hematoxylin and eosin-stained section were also identified on periodic acid-Schiff stain (PAS) and silver impregnation-stained (Gomori methenamine silver and Grocott) section and appeared as broad, pleomorphic, sparsely septated hyphae.
FIG. 3.
Hematoxylin and eosin-stained section of lung showing a broad optically empty hyphal structure surrounded by necrotic and eosinophilic infiltrate. Magnification, ×400.
A morphological diagnosis of disseminated basidiobolomycosis was rendered based on the distinctive morphology.
Cultures and serologic tests for Basidiobolus ranarum or other Entomophthorales agents had not been done because of the clinical suspicion of neoplasm or vasculitis. No serum was saved.
Discussion.
Basidiobolomycosis is a rare fungal infection due to B. ranarum, an environmental saprophyte found worldwide (13). It is a member of the order Entomophthorales, of the class Zygomycetes (6), which has been isolated from decaying vegetation, foodstuffs, fruits, and soil and from the gastrointestinal tracts of reptiles, amphibians, fish, and insectivorous bats (10). On microscopic examination of the culture material, it can be identified by characteristically beaked zygospores.
It can be isolated from surgical specimens, and it should be inoculated soon after resection because does not survive at 4°C. Sabouraud agar is an adequate medium, and visible growth is usually present 2 to 3 days after incubation at 25 to 30°C. Colonies appear white or pale grey and have radial folds. The fungal elements appear as broad, pleomorphic, sparsely septated hyphae, which stain faintly with fungal stains (Grocott, Gomori methenamine silver, PAS). Definitive diagnosis requires culture of the organism. Serodiagnosis with immunodiffusion can be employed as an adjunctive diagnostic method; the test appears to be very specific for B. ranarum with no cross-reactivity with other species of the order Entomophthorales, but its sensitivity has not been determined (9, 10, 12, 22).
In the case presented, the diagnosis of disseminated basidiobolomycosis was based on the distinctive morphology (see Acknowledgments, review of R. Naefie and the Infection Disease Branch of the Armed Forces Institutes of Pathology in Washington, D.C.). The diagnosis was made on the basis of the autopsy findings; there were silver-positive and PAS-positive hyphal structures within associated Splendore-Hoeppli phenomena and marked eosinophil infiltration with necrosis, histological findings nearly unique for B. ranarum in human tissue (7, 13, 14). The only organisms in the histologic differential are Conidiobolus coronatus, Conidiobolus incongruus, and Pythium insidiosum, organisms that are associated with head and neck disease in humans or pleuropericarditis in immunosuppressed patients; however, these microorganisms would be equally rare as a cause of disseminated disease in immunocompetent subjects (4, 8, 15, 19).
The mode of acquisition of the disease remains poorly understood. The portal of entry is believed to be the skin, after insect bites, scratches, and minor cuts. It is most common in young children as a disease of the skin and subcutaneous tissues, involving the thighs and buttocks. Most cases have been reported in tropical and south-tropical climates, mainly in Indonesia and East and West Africa (1, 2, 5, 6, 18).
Gastrointestinal basidiobolomycosis is exceedingly rare; only 15 cases have been reported worldwide (2 cases from Nigeria, 4 cases from Brazil, 1 case from Kuwait, and 7 cases from the United States, all from the state of Arizona so far) (3, 11, 14, 17, 20). Here we report the first case of disseminated basidiobolomycosis in an immunocompetent woman in which the first clinical manifestations were related to lung involvement associated with eosinophilia.
We hypothesize that the patient described in this report initially had a gastrointestinal infection, since involvement of the stomach was confirmed at autopsy, and gastrointestinal involvement, including gastric involvement, has been previously documented in the literature (3, 11, 14, 20, 22). The involvement of the tracheobronchial tree could have been caused by gastroesophageal reflux. The reason for dissemination of the disease in the previously healthy individual is unclear (21). The clinical differential diagnosis at presentation included the following: neoplasm (especially lung cancer or lymphoma), more common infections (especially tuberculosis), pulmonary infiltrates with eosinophilia and allergic bronchopulmonary fungal disease, and vasculitic syndromes (e.g., Churg-Strauss disease and Wegener's granulomatosis). None of these syndromes fit the unusual clinical aspects of this case well, and none were supported by the histopathologic findings.
Based on the observations of Marshall Lyon et al. (14), visceral involvement in basidiobolomycosis is being increasingly recognized in industrialized countries, and the reasons for this are unclear. Probably this peculiar geographic distribution should be related to a specific style of life and dietetic behavior. Case patients in Arizona had amphibians or reptiles outside their homes (five patients), fewer case patients washed vegetables before eating them (four patients) and camped near a lake or river during the previous year (three patients); however, the patients had a history of diabetes mellitus (in three patients), peptic ulcer disease (in two), or pica (in one) (16). The patient presented had nothing in her history to suggest any predilection for infection by this very unusual organism. The common denominator for this case and the series reported from Arizona appears to be involvement of the gastrointestinal tract.
Acknowledgments
We gratefully acknowledge the review of the autopsy histologic sections by Ron Naefie at the Infectious Disease Branch at the Armed Forces Institute of Pathology in Washington, D.C. The features were considered characteristic of infection by B. ranarum.
REFERENCES
- 1.Antonelli, M., P. Vignetti, M. Dahir, M. S. Mohamed, and A. A. Favah. 1987. Entomophthoromycosis due to Basidiobolus in Somalia. Trans. R. Soc. Trop. Med. Hyg. 81:186-187. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt, A. L., S. M. Arruda, J. A. de Andreade, and E. M. Carvalho. 1991. Basidiobolomycosis: a case report. Pediatr. Dermatol. 8:325-328. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1999. Gastrointestinal basidiobolomycosis: Arizona. Morb. Mortal. Wkly. Rep. 48:710-714. [PubMed] [Google Scholar]
- 4.Chandler, F. W., and J. C. Watts. 1997. Zygomycosis, p. 1113-1120. In D. H. Connor, F. W. Chandler, D. A. Schwartz, H. J. Manz, E. E. Lack, J. K. Baird, and J. P. Utz (ed.), Pathology of infectious diseases. Appleton & Lange, Stamford, Conn.
- 5.de Leon-Bojorge, B., R. Ruiz-Maldonado, and R. Lopez-Martinez. 1988. Subcutaneous phycomycosis caused by Basidiobolus haptosporus: a clinicopathologic and mycologic study in a child. Pediatr. Dermatol. 5:33-36. [DOI] [PubMed] [Google Scholar]
- 6.Dromer, F., and M. R. McGinnis. 2002. Zygomycosis, p. 297-308. In E. Anaissie, M. R. McGinnis, and M. A. Pfaller (ed.), Clinical Mycology. Churchill Livingstone, New York, N.Y.
- 7.Gugnani, H. C. 1999. A review of zygomycosis due to Basidiobolus ranarum. Eur. J. Epidemiol. 15:923-929. [DOI] [PubMed] [Google Scholar]
- 8.Heath, J. A., T. E. Kiehn, A. E. Brown, M. P. LaQuaglia, L. J. Steinherz, G. Bearman, M. Wong, and P. G. Steinherz. 2002. Pythium insidiosum pleuropericarditis complicating pneumonia in a child with leukemia. Clin. Infect. Dis. 35:E60-E64. [DOI] [PubMed] [Google Scholar]
- 9.Imwidthaya, P., and S. Srimuang. 1992. Immunodiffusion test for diagnosing basidiobolomycosis. Mycopathologia 118:127-131. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman, L., L. Mendoza, and P. G. Standard. 1990. Immunodiffusion test for serodiagnosing subcutaneous zygomycosis. J. Clin. Microbiol. 28:1887-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan, Z. U., M. Khoursheed, R. Makar, S. Al-Waheeb, I. Al-Bader, A. Al-Muzaini, R. Chandy, and A. S. Mustafa. 2001. Basidiobolus ranarum as an etiologic agent of gastrointestinal zygomycosis. J. Clin. Microbiol. 39:2360-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshi, G., T. Kurien, D. Sudarsanam, A. J. Selvapandian, and K. E. Mammen. 1972. Subcutaneous phycomycosis caused by Basidiobolus: a report of three cases. Sabouraudia 10:237-243. [PubMed] [Google Scholar]
- 13.Kwon-Chung, K. J., and J. E. Bennet. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 14.Marshall Lyon, G., J. D. Smilack, K. K. Komatsu, T. M. Pasha, J. A. Leighton, J. Guarner, T. V. Colby, M. D. Lindsley, M. Phelan, D. W. Warnock, and R. A. Hajjeh. 2001. Gastrointestinal basidiobolomycosis in Arizona: clinical and epidemiological characteristics and review of the literature. Clin. Infect. Dis. 32:1448-1455. [DOI] [PubMed] [Google Scholar]
- 15.Mukhpadhyay, D., L. M. Ghosh, A. Thammayya, and M. Sanyal. 1995. Entomophthoromycosis caused by Conidiobolus coronatus: clinicomycological study of a case. Auris Nasus Larynx 22:139-142. [DOI] [PubMed] [Google Scholar]
- 16.Pasha, T., J. Leighton, J. Smilack, K. Komutsu, and C. Kioski. 1999. Gastrointestinal basidiobolomycosis—Arizona, 1994-1999. Morb. Mortal. Wkly. Rep. 48:710-713. [PubMed] [Google Scholar]
- 17.Pasha, T. M., J. A. Leighton, J. D. Smilack, J. Heppell, T. V. Colby, and L. Kaufman. 1997. Basidiobolomycosis: an unusual fungal infection mimicking inflammatory bowel disease. Gastroenterology 112:250-254. [DOI] [PubMed] [Google Scholar]
- 18.Scholtens, R. E., and S. M. Harrison. 1994. Subcutaneous phycomycosis. Trop. Geogr. Med. 46:371-373. [PubMed] [Google Scholar]
- 19.Sharma, N. L., V. K. Mahajan, and P. Singh. 2003. Orofacial conidiobolomycosis due to Conidiobolus incongruous. Mycoses 46:137-140. [DOI] [PubMed] [Google Scholar]
- 20.Yousef, O. M., J. D. Smilack, D. M. Kerr, R. Ramsey, L. Rosati, and T. V. Colby. 1999. Gastrointestinal basidiobolomycosis. Morphologic findings in a cluster of six cases. Am. J. Clin. Pathol. 112:610-616. [DOI] [PubMed] [Google Scholar]
- 21.Wasim, Y., H. M. Assaf, and N. A. Rotowa. 2003. Invasive gastrointestinal Basidiobolus ranarum infection in an immunocompetent child. Pediatr. Infect. Dis. J. 22:281-282. [PubMed] [Google Scholar]
- 22.Zavasky, D., T. Loftus, H. Segal, and K. Carrol. 1999. Gastrointestinal zygomatic infection caused by Basidiobolus ranarum: case report and review. Clin. Infect. Dis. 28:1244-1248. [DOI] [PubMed] [Google Scholar]