Abstract
Ectodermal dysplasia is a group of congenital syndromes affecting a variety of ectodermal derivatives. Among them, ectrodactyly, ectodermal dysplasia, and cleft lip/palate (EEC) syndrome is caused by single point mutations in the p63 gene, which controls epidermal development and homeostasis. Phenotypic defects of the EEC syndrome include skin defects and limbal stem-cell deficiency. In this study, we designed a unique cellular model that recapitulated major embryonic defects related to EEC. Fibroblasts from healthy donors and EEC patients carrying two different point mutations in the DNA binding domain of p63 were reprogrammed into induced pluripotent stem cell (iPSC) lines. EEC-iPSC from both patients showed early ectodermal commitment into K18+ cells but failed to further differentiate into K14+ cells (epidermis/limbus) or K3/K12+ cells (corneal epithelium). APR-246 (PRIMA-1MET), a small compound that restores functionality of mutant p53 in human tumor cells, could revert corneal epithelial lineage commitment and reinstate a normal p63-related signaling pathway. This study illustrates the relevance of iPSC for p63 related disorders and paves the way for future therapy of EEC.
Keywords: TRP63, rare disease, cornea
Ectodermal dysplasia are rare syndromes characterized by abnormal development of the skin and ectodermal derivatives, like teeth, hair, cornea, and nails. Among these syndromes, some are related to mutations on the transcription factor p63 (TP63) and represent a group of autosomal dominant ectodermal dysplasia associated with orofacial clefting and limb abnormalities. The severe phenotype of p63-null mice highlighted the major role of p63 in embryonic development, and particularly in the development of ectodermal lineages (1, 2). Five syndromes in which p63 mutations have been detected include ectrodactyly, ectodermal dysplasia, and cleft lip/palate syndrome (EEC), ankyblepharon, ectodermal dysplasia, and cleft lip/palate syndrome (AEC), limb mammary syndrome, acro-dermato-ungual-lacrimal-tooth syndrome, and Rapp-Hodgkin syndrome. EEC mutations are clustered in the DNA-binding domain and AEC mutations are found in the sterile α-motif or transactivation inhibitory domain (3). Although there are some clinical similarities and overlap between the syndromes, the specific location of p63 mutations in the different domains of the gene shows a strong genotype-phenotype correlation, and thus different molecular mechanisms behind the various p63-associated syndromes. Clinical and penetrance variability are observed for the same mutation, suggesting that the number of p63 patients could be underestimated (3, 4). Moreover, variability of phenotype among syndromes could be a result of specific functional consequences of a single mutation. For example, two hot-spot mutants, R304W and R204W, both located in the DNA binding domain of the p63 gene, represent EEC syndrome but, based on their analogy with p53 mutations, may act differently because R304W interferes with DNA binding and R204W with global protein structure/stability of p63, influencing the transcription of target genes differently (5, 6).
In addition to skin defects, EEC patients suffer from visual morbidity with progressive limbal stem-cell deficiency that leads to severe visual impairments and blindness (7, 8). Therefore, modeling of these diseases is essential to identify abnormalities in molecular processes involving p63, their effects on cell growth and skin development, and for drug screening. In vitro cellular models of rare skin and corneal diseases are obtained by the use of patient-derived primary epidermal cells. Given that p63 is a master regulator of embryonic steps of epithelial development, cellular models that could recapitulate the main steps of skin and corneal epithelial development in vitro are necessary. The recently discovered capacity of human somatic cells to be relatively easily reprogrammed into embryonic stem cell-like pluripotent stem cells (iPSC) offers numerous perspectives in therapies by providing patient-specific differentiated cells on demand and novel cellular models for specific pathologies. iPSC technology provide pluripotent stem cells carrying genetic characteristics of patients. These cells have the remarkable ability to recapitulate in vitro the main steps of human embryonic development, and they provide urgently needed tools to generate patient-specific, organotypic disease models. These cellular models may be used for the discovery of novel drugs both in a flexible and highly specific manner, because they facilitate high-throughput compound screening and toxicity assays. Finally, unlike patient primary cells, iPSC derived from patients’ cells provide researchers with cells with unlimited proliferation capacity. The clinical penetrance of the p63 gene is highly variable, apparently because of other genetic and epigenetic factors (9, 10). Therefore, animal models in which a single EEC mutation is inserted by knock-in may not reproduce the human pathology. Here, we derived iPSC lines from healthy control and EEC patients and evaluated their ability to differentiate into epidermal and corneal epithelial cells. Our study demonstrated that they displayed impaired epithelial commitment that could be partially rescued by a small therapeutic compound.
Results
Derivation of iPSC Lines from WT and EEC Fibroblasts.
iPSC lines were obtained by lentiviral infection of primary dermal fibroblasts isolated from one healthy individual and two EEC patients carrying single point mutations R304W or R204W in the p63 gene. These two mutations located in the DNA binding domain are among the five hotspots accounting for 90% of EEC. Several clones with typical iPSC morphology (Fig. S1A) were expanded and two to three lines for each donor were used for subsequent characterization and experiments (WT or +/+, R204W/+ and R304W/+). Pluripotency of iPSC was confirmed by the expression of various markers such as octamer-binding transcription factor 4 (OCT4), TRA-1-80, and alkaline phosphatase by staining (Fig. S1A) and OCT4, sex-determining region Y box-2 (SOX2), DNA methyltransferase 3b (DNMT3b), and NANOG by quantitative PCR (qRT-PCR) (Fig. S1B). We have previously developed a purely data-driven approach, termed PluriTest, toward empirically defining the human pluripotent state, because the gold standard germ-line transmission is impossible for human cells (11). Briefly, the PluriTest data model was derived via interrogation of large-scale datasets of genome-wide somatic and pluripotent expression profiles and can be used to rapidly and confidently assess the pluripotency of human cells through bioinformatic analysis of microarray data from new stem-cell preparations without the sacrifice of laboratory animals for Teratoma assays (11). All iPSC lines displayed high pluripotency scores, similarly to human embryonic stem cells (hESC) and other fibroblast-derived iPSC (Fig. S1C). Finally, iPSCWT and iPSCEEC lines were able to form embryoid bodies in suspension and differentiate into cell types belonging to the three germ layers upon adhesion (Fig. S1D). This finding was confirmed by qRT-PCR analysis on gene expression specific for neuroectodermal (NCAM1), endodermal (AFP), and mesodermal (CD31) fates. In addition, EEC-iPSC were able to differentiate into trophoectoderm, as shown by CDX2 expression after 6 d with bone morphogenetic protein-4 (BMP-4) (Fig. S1E). Taken together, these data confirmed that we obtained pluripotent iPSC lines and we next explored their potential to model molecular characteristics of EEC syndrome.
Impaired Epidermal Differentiation of EEC-iPSC Lines.
EEC patients suffer from impaired skin development. To define whether EEC-iPSC lines could mimic these defects, we optimized protocols to differentiate human iPSC into epidermal cells. Epidermal commitment of embryonic stem cells can be induced by BMP-4, which inhibits neural differentiation and promotes epidermal fate of neuroectodermal cells (12–14). Prolonged treatment with BMP-4 and ascorbic acid combined with keratinocyte growth conditions have been shown to promote efficiently hESC differentiation into mature keratinocytes (14). However, applying this protocol to iPSC lines appeared inefficient because most of the cells differentiate in a heterogenous manner, with few ectodermal-like cells and with large cystic-like structures (Fig. S2A). We found that extraembryonic cells expressing CDX2 and CGHα are produced from iPSC in response to a high dose of BMP-4 (Fig. S2B), suggesting that iPSC, contrary to hESC, do not efficiently differentiate into ectodermal lineage because they failed to first spontaneously commit into neuroectoderm (15). Because inhibition of the TGF-β/nodal pathway promotes lost of pluripotency and neuroectodermal commitment (16), we tested the effect of a TGF-β inhibitor, SB431542, on the epidermal differentiation of iPSC. Ectodermal differentiation of iPSC was dramatically improved in presence of SB431542. Morphologically, the colonies lost their pluripotent aspect faster and acquired a homogenous ectodermal phenotype within 7 d (Fig. S2A). Pluripotency markers (Oct4 and Dnmt3b) and trophoectoderm markers (CDX2 and CGHα) were reduced but the keratinocyte marker K14 was increased (Fig. S2B). Differentiation of iPSC treated with BMP-4, AA, and SB431542 for 30 d led to mature keratinocytes (Fig. S2Ci), as demonstrated by expression of K14 and K10 (Fig. S2C ii and iii), signs of spontaneous stratification (Fig. S2C iv and v), and ability to form typical keratinocyte colonies upon splitting (Fig. S2Cvi). This process demontrates that TGF-β inhibition significantly improved the production of epidermal cells from iPSC lines.
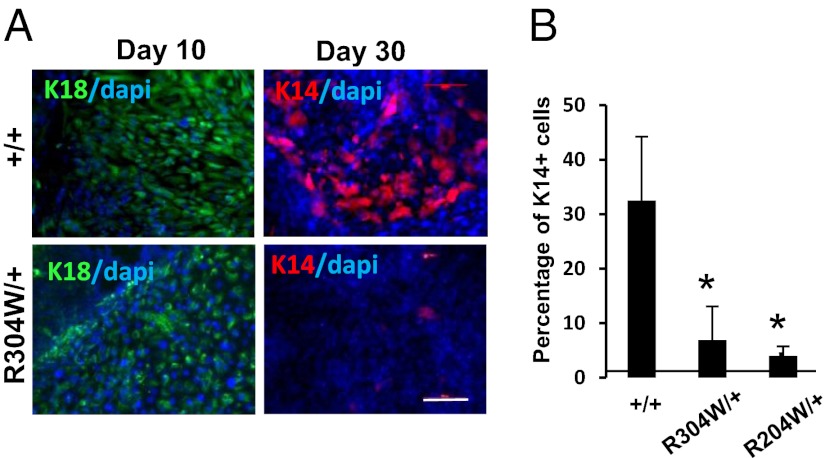
iPSCEEC were subjected to epidermal commitment according to the above protocol. In response to SB431542 and BMP-4 during the first 10 d, iPSCEEC initiated ectodermal commitment, as illustrated by typical ectodermal cell morphology similarly to iPSCWT cells (Fig. S2A). However, although iPSCWT cells underwent epidermal transition from days 13–15 for the production of keratinocyte-like areas proliferating during the next 10 d, iPSCEEC continued to display the same ectodermal morphology (Fig. S3A). Only a small number of keratinocyte-like cells could be noticed occasionally. Immunofluorescence staining (Fig. 1A) and quantification by flow cytometry (Fig. S3B) confirmed that both iPSCR304W/+ and iPSCR204W/+ could produce K18+ ectodermal progenitors to a similar extent as iPSCWT (42.5% and 46%, respectively). However, iPSCR304W/+ failed to further differentiate into K14+ epidermal cells (2.2%), compared with iPSCWT (27.5%) (Fig. 1 and Fig. S2B). Similar results have been obtained for iPSCR204W/+ (Fig. 1B). Of note, expression of p63 was up-regulated to the same extent during epidermal commitment of both WT and mutated iPSC (Fig. S3C).
Fig. 1.
Impaired epidermal differentiation of EEC-iPSC. iPSC+/+, iPSCR204W/+, and iPSCR304W/+ cells were subjected to epidermal differentiation protocol in presence of BMP-4 and SB431542, and immunofluorescence for K18 and K14 after 15 d of differentiation (A) or analyzed by flow cytometry analysis for K18 and K14 after 25 d of differentiation (B). The data are an average of the percentage of K14+ cells ± SE from two independent iPSC clones. *P < 0.001. (Scale bar: 20 μm.)
Impaired Corneal Differentiation of EEC-iPSC Is Rescued by APR-246/PRIMA-1MET.
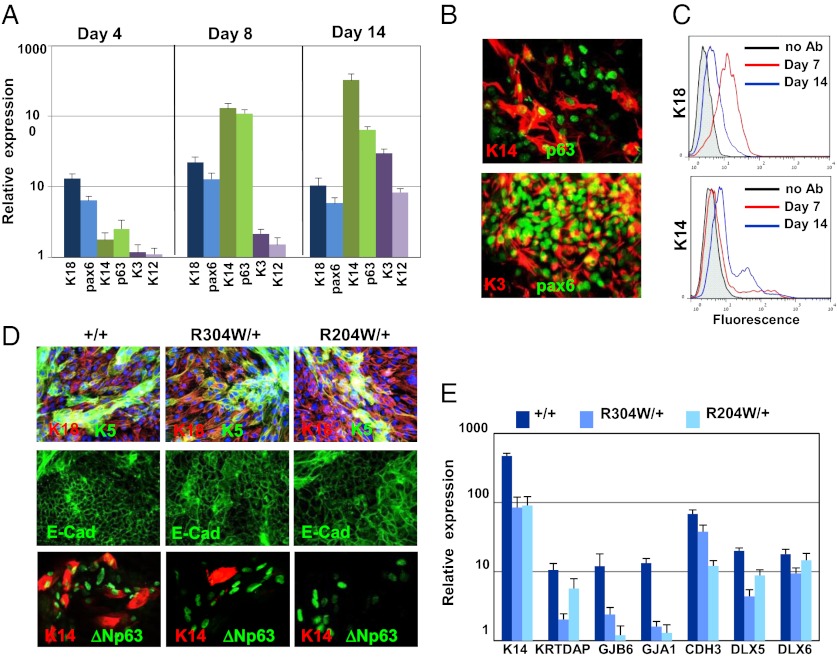
EEC patients suffer from visual morbidity because of impaired cornea associated with limbal stem-cell deficiency (7). iPSC lines were induced to corneal fate using a slight modification of a protocol designed by Lako and colleagues for hESC (17). In brief, iPSC lines were seeded on collagen IV in the presence of medium conditioned by human corneal fibroblasts (COF) and treated with BMP-4 between days 0 and 3. As illustrated by real-time qRT-PCR analysis, human iPSC lines underwent sequential differentiation into ectodermal precursors (K18+/Pax6+) at day 4, markers of corneal progenitors (K14+/p63+/pax6+) appeared at day 8, and markers of terminally differentiated corneal epithelial (Pax-6+/K3+/K12+) cells were expressed at day 14 (Fig. 2A). Remarkably, at day 14, most of the cells became corneal epithelial cells, as detected by immunofluorescence staining (Fig. 2B) and FACS analysis (Fig. 2C). p63 is a putative marker of corneal stem cells, which are located in the limbus, a defined region at the corneal periphery (18). We next challenged iPSCEEC for their ability to undergo proper corneal epithelial commitment compared with the iPSCWT. Similar production of ectodermal progenitors (K18+/E-cadherin+) was observed at day 10 for iPSCWT, iPSCR204W/+, and iPSCR304W/+ (Fig. 2D). However, K14 and K3 staining revealed the inability of iPSCEEC to undergo further commitment for the production of limbal cells and corneal cells, respectively, compared with iPSCWT. In parallel, expression of several p63-dependent genes was evaluated on day 10 of commitment by qRT-PCR (Fig. 2E). These genes include genes that are enhanced at embryonic day (E) 14.5 only in the presence of p63 (GJA1, GJB6, KRTDAP, and KRT14) (19) and p63-target genes, of which deregulation during epithelial development is associated with ectodermal dysplasia [DLX5, DLX6, and CDH3 (P-Cadherin)] (20, 21). Interestingly, most of these genes were significantly less expressed in mutated cells compared with control cells (Fig. 2E).
Fig. 2.
Impaired corneal epithelial commitment of iPSC lines. iPSC+/+ were seeded on collagen IV-coated dishes in corneal fibroblast-conditioned medium that was supplemented with BMP-4 for the first 3 d of differentiation. Cells were harvested at the indicated time points and subjected to real-time PCR analysis of ectodermal markers (pax6 and K18), corneal epithelial progenitor markers (K14 and p63), and markers of terminally differentiated corneal-epithelial cells (K3 and K12) (A). Cells were collected at day 14 of differentiation and subjected to coimmunofluorescent staining of p63 and K14 or pax6 and K3 (B). Flow cytometry analysis of iPSC+/+ that were harvested at days 7 and 14 of differentiation and stained with K18 or K14 antibodies is shown in C. iPSC+/+ and iPSCEEC lines were differentiated into corneal epithelial fate for 10 d (E). Immunostaining followed by fluorescent microscopy was performed for determining the expression of the indicated proteins (D), and real-time PCR analysis was performed for determining the relative expression of the indicated epithelial transcripts (E). (Magnification: B, 40×; D, 20×.)
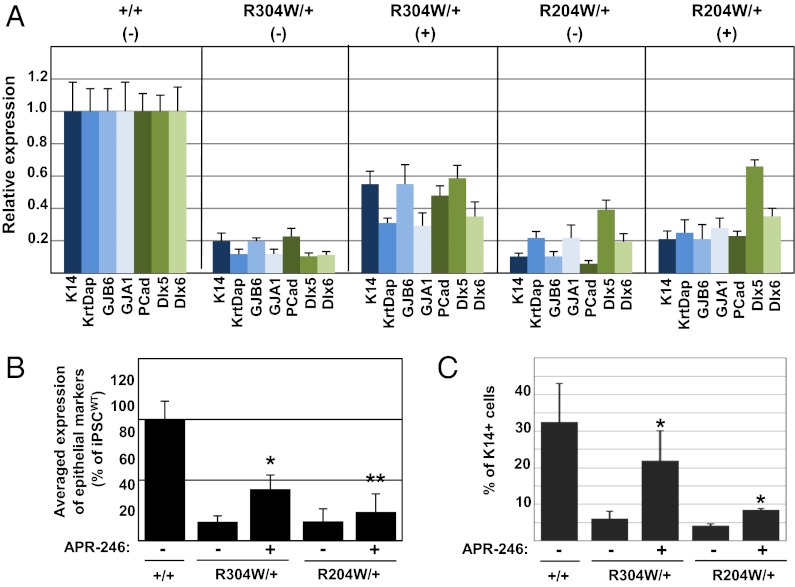
Because corneal epithelial commitment of iPSC appeared more efficient and much faster than epidermal fate, we employed this system for testing whether the small compound APR-246/PRIMA-1MET, that was recently shown to restore p63-induced apoptosis in human tumor cells (22), could rescue corneal epithelial commitment of iPSCEEC. Thus, iPSCWT, iPSCR204W/+, and iPSCR304W/+ cells were treated with APR-246 (20 μM) from day 3 to day 14 and corneal commitment was monitored at day 14. APR-246/PRIMA-1MET had no significant effect on differentiation of iPSCWT cells (Fig. S4). Notably, a partial restoration in expression of epithelial markers by iPSCEEC was observed in the presence of APR-246 (Fig. 3A). The average expression of these factors by iPSCEEC was ∼15% compared with iPSCWT. However, a significant increase was demonstrated in the presence of APR-246, to 43 ± 12% in iPSCR304W/+ and to 24 ± 15% and iPSCR204W/+, compared with iPSCWT (Fig. 3B). Similarly, the expression of K14 protein was significantly elevated by APR-246 treatment, as shown by flow cytometry analysis (Fig. 3C). APR-246/PRIMA-1MET had no significant effect on cell proliferation (Fig. S5), excluding the possibility that this effect was a result of enhanced cell proliferation of iPSCEEC cells. Of note, the rescue effect was systematically stronger for the mutant R304W. We thus concluded that patient iPSC cells are able to recapitulate in vitro EEC major molecular defects, which could be potentially restored by APR-246 treatment.
Fig. 3.
Rescue of corneal differentiation of EEC-iPSC by APR-246. The indicated iPSC lines were differentiated into corneal epithelial cells for 14 d in the presence (+) or absence (−) of APR-246 treatment (as detailed in Materials and Methods). (A) Real-time PCR analysis showing the relative expression of the indicated epithelial transcripts. (B) Average of gene-expression data presented in A. Results show data obtained from two independent experiments ± SD. (C) Flow cytometry analysis for K14 expression. Results show average data obtained from three independent experiments ± SD. Asterisks indicate for statistical significance (*P < 0.01; **P < 0.05) between APR-246 treated samples compared with control (untreated cells).
Discussion
Congenital diseases, like ectodermal dysplasia syndromes, lack experimental models that could recapitulate embryonic events in vitro. Attempts have been made to express exogenously mutated forms of p63 in mouse embryonic stem cells that provided interesting insights on molecular signaling pathways (23). However, this approach does not reproduce pathophysiological conditions of equimolarity between mutated and wild-type alleles. Here, we reprogrammed fibroblasts isolated from EEC patients into pluripotent iPSC lines and stimulated them to undergo in vitro major steps of epidermal and corneal-epithelial development. iPSCEEC recapitulated impaired epithelial commitment, demonstrating the utility of this model for congenital skin or corneal diseases. We observed that p63-mutated pluripotent cells failed to make the transition from K8/K18 ectodermal precursors to K14 epidermal cells, confirming the key role of p63 in controlling the early embryonic epidermal switch, as already suggested by the hESC model (23, 24) and by p63-deficient mice (19).
BMP-4–based protocols have been established to obtain keratinocytes from pluripotent cells (12, 14, 25) and very recently, Itoh et al. used BMP-4 combined with retinoic acid for a limited time to generate keratinocytes from iPSC from dystrophic epidermolysis bullosa patients reaching 30–40% of K14+ cells that could be enriched only after passaging or cell sorting (26). Despite possible enrichment and satisfactory 3D potential (14, 26), improved protocols for highly homogenous production of keratinocytes from pluripotent cells are still needed to consider cell therapy. We observed that BMP-4–induced epidermal commitment of iPSC was significantly low compared with what was reported with hESC (14), apparently because of limited spontaneous neuroectoderm commitment that led to the generation of BMP-4–induced trophoectoderm. We found that TGF-β inhibition efficiently promoted neuroectoderm engagement and that concomitant addition of BMP-4 increased the production of K14+ cells. These findings may be useful for optimizing the production of keratinocytes from human iPSC for future cell therapy.
In contrast, corneal epithelial commitment appeared much more efficient and homogenous (27). For that reason, the effect of APR-246 was tested on corneal fate. The small molecule APR-246, also known as PRIMA-1MET (p53-dependent reactivation and induction of massive apoptosis) can restore p53-induced apoptosis in several types of cancer cells (28). APR-246 was recently tested in a phase I/II clinical trial. Furthermore, APR-246 has been shown to also target mutant forms of p73 and p63, an effect that is presumably caused by highly homologous structural elements among the three p53 family member proteins (22). Our study is unique in showing that APR-246 can partially restore the molecular circuitry downstream of p63, which was affected in embryonic lineage commitment of EEC patient-derived cells. Four genes that were previously shown to be elevated in WT mice but not in p63-null mice at E14.5 (GJA1, GJ6B, KRT14, KRTDAP) (19) were rescued by APR-246. Moreover, the level of expression of three p63-target genes (DLX5, DLX6, and CDH3/P-Cadherin) that are known to be associated with EEC syndrome (20, 21) was significantly enhanced by APR-246. Interestingly, we found that APR-246 acted differently on the R204W and R304W mutants. Whereas both mutations are found within the DNA-binding domain, R304W is located in the Zn-binding pocket that is thought to affect the direct binding of p63 to the DNA phosphate moiety, but R204W possibly causes more drastic conformational changes and misfolding of the p63 protein (5). As a matter of fact, Rökaeus et al., have also reported differential effects of APR-246 on the TAp63γR204W and TAp63γR304W mutants: APR-246 caused cell death in the presence of TAp63γR304W but induced mainly growth arrest in cells with TAp63γR204W (22). It would be interesting to investigate whether there is a difference between the binding of APR-246 to the two mutated p63 forms by in vitro assays. One could hypothesize that a putative binding site for APR-246 is less exposed in the p63R204W protein comparing to p63R304W, and that would explain the milder restoration of p63R204W activity, or that R204W-induced conformational changes are less sensitive to APR-246 binding or APR-246-mediated refolding. Animal models are useful to confirm therapeutic function before translating to patients. However, knock-in mice for EEC mutations are not yet available and may not reproduce faithfully the human pathology. Therefore, our study paves the way for future therapy of p63-related diseases that could be developed relatively fast, as APR-246 has already been tested in clinical trials in patients with hematological malignancies or prostate cancer.
Materials and Methods
Derivation of iPSC.
Dermal fibroblasts were isolated and amplified from skin biopsy obtained after informed consent from one healthy individual and two EEC patients (carrying p63R304W or p63R204W). Fibroblasts were reprogrammed using a lentival polycistronic cassette expressing OCT4, SOX2, KLF4, and C-MYC or OCT4, SOX2, and KLF4 only (29), as previously described (30).
Pluripotency Characterization.
Pluripotency of iPSC was determined by immunofluorescence, real-time qRT-PCR and PluriTest analysis as described in detail in SI Materials and Methods and in ref. 30.
In Vitro Differentiation Protocols.
iPSC were differentiated in vitro as described in detail in SI Materials and Methods.
Epidermal Differentiation.
iPSC were mechanically detached in small clumps (two to four colonies/six-well) and seeded on mitomycin-treated 3T3-G2 feeders in medium [DMEM/F12 supplemented with 15% (vol/wt) knockout serum replacement (Invitrogen), 1 mM Glutamine, 100 μM β-Mercaptoethanol, 100 μM nonessential amino acids]. Two days later, medium was switched to Green medium (30). BMP-4 (25 ng/mL; Peprotech) and ascorbic acid (0.3 mM; Sigma), and SB431542 (10 μM; Tocris) were added from day 0 for up to 30 d.
Corneal Epithelial Differentiation.
COFs were grown in fibroblast medium [DMEM (Invitrogen) and FCS (Invitrogen] and arrested by mitomycin treatment (8 μg/mL; Sigma) for 3 h followed by washing with medium and incubation overnight. The next day, COFs were incubated with Green medium (detailed above) and conditioned media was collected every day for up to 10 d and was stored at −20 °C. To induce corneal epithelial differentiation, iPSC were seeded on collagen IV-coated dishes (0.5 mg/mL; Sigma) in the presence of conditioned media supplemented with 0.5 nM BMP-4 for the first 3 d. APR-246 treatment (20 μM) was initiated at day 3 to minimize toxic effects.
qRT-PCR, Immunofluorescence, and Flow Cytometry.
Detailed procedures are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work has been partially supported by the European Union 6th Framework Integrated Project Grant LSHB-CT-2005-019067 (to D.A.); Agence Nationale de Recherche ANR-08-GENOPAT-024-03 and ANR-erare2-SkinDev (to D.A.); Israel Ministry of Science and Technology Grant MOST3-6494 (to D.A.); “Poste Vert” fellowship of Institut National de la Santé et de la Recherche Médicale, “Chateaubriand” fellowship of the Embassy of France in Israel and European Molecular Biology Organization short-term fellowship (to R.S.-F.); a PhD fellowship from the Israeli ministry of integration (to L.S.); and an Else-Kröner Fresenius Stiftung fellowship (to F.-J.M.).
Footnotes
Conflict of interest statement: K.G.W. is cofounder, shareholder, and member of the board of Aprea AB, a company that develops p53-based cancer therapy including the compound APR-246.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201753109/-/DCSupplemental.
References
- 1.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 2.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 3.Rinne T, Brunner HG, van Bokhoven H. p63-associated disorders. Cell Cycle. 2007;6:262–268. doi: 10.4161/cc.6.3.3796. [DOI] [PubMed] [Google Scholar]
- 4.Itin PH. Rationale and background as basis for a new classification of the ectodermal dysplasias. Am J Med Genet A. 2009;149A:1973–1976. doi: 10.1002/ajmg.a.32739. [DOI] [PubMed] [Google Scholar]
- 5.Celli J, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- 6.Browne G, et al. Differential altered stability and transcriptional activity of ΔNp63 mutants in distinct ectodermal dysplasias. J Cell Sci. 2011;124:2200–2207. doi: 10.1242/jcs.079327. [DOI] [PubMed] [Google Scholar]
- 7.Di Iorio E, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology. 2012;119(1):74–83. doi: 10.1016/j.ophtha.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 8.McNab AA, Potts MJ, Welham RA. The EEC syndrome and its ocular manifestations. Br J Ophthalmol. 1989;73:261–264. doi: 10.1136/bjo.73.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanbokhoven H, Melino G, Candi E, Declercq W. p63, a story of mice and men. J Invest Dermatol. 2011;131:1196–1207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- 10.Rinne T, Hamel B, van Bokhoven H, Brunner HG. Pattern of p63 mutations and their phenotypes—Update. Am J Med Genet A. 2006;140:1396–1406. doi: 10.1002/ajmg.a.31271. [DOI] [PubMed] [Google Scholar]
- 11.Williams R, Schuldt B, Müller FJ. A guide to stem cell identification: Progress and challenges in system-wide predictive testing with complex biomarkers. Bioessays. 2011;33:880–890. doi: 10.1002/bies.201100073. [DOI] [PubMed] [Google Scholar]
- 12.Aberdam E, et al. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells. 2008;26:440–444. doi: 10.1634/stemcells.2007-0588. [DOI] [PubMed] [Google Scholar]
- 13.Metallo CM, Ji L, de Pablo JJ, Palecek SP. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2008;26:372–380. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- 14.Guenou H, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet. 2009;374:1745–1753. doi: 10.1016/S0140-6736(09)61496-3. [DOI] [PubMed] [Google Scholar]
- 15.Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 16.Smith JR, et al. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini G, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalom-Feuerstein R, et al. ΔNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ. 2011;18:887–896. doi: 10.1038/cdd.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Iacono N, et al. Regulation of Dlx5 and Dlx6 gene expression by p63 is involved in EEC and SHFM congenital limb defects. Development. 2008;135:1377–1388. doi: 10.1242/dev.011759. [DOI] [PubMed] [Google Scholar]
- 21.Shimomura Y, Wajid M, Shapiro L, Christiano AM. P-cadherin is a p63 target gene with a crucial role in the developing human limb bud and hair follicle. Development. 2008;135:743–753. doi: 10.1242/dev.006718. [DOI] [PubMed] [Google Scholar]
- 22.Rökaeus N, et al. PRIMA-1(MET)/APR-246 targets mutant forms of p53 family members p63 and p73. Oncogene. 2010;29:6442–6451. doi: 10.1038/onc.2010.382. [DOI] [PubMed] [Google Scholar]
- 23.Rostagno P, et al. Embryonic stem cells as an ectodermal cellular model of human p63-related dysplasia syndromes. Biochem Biophys Res Commun. 2010;395:131–135. doi: 10.1016/j.bbrc.2010.03.154. [DOI] [PubMed] [Google Scholar]
- 24.Medawar A, et al. DeltaNp63 is essential for epidermal commitment of embryonic stem cells. PLoS ONE. 2008;3:e3441. doi: 10.1371/journal.pone.0003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coraux C, et al. Reconstituted skin from murine embryonic stem cells. Curr Biol. 2003;13:849–853. doi: 10.1016/s0960-9822(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2011;108:8797–8802. doi: 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalom-Feuerstein R, et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells. 2012;30:898–909. doi: 10.1002/stem.1068. [DOI] [PubMed] [Google Scholar]
- 28.Bykov VJ, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 29.Somers A, et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petit I, et al. Induced pluripotent stem cells from hair follicles as a cellular model for neurodevelopmental disorders. Stem Cell Res (Amst) 2012;8:134–140. doi: 10.1016/j.scr.2011.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.