Significance
HSV expresses numerous functions to suppress the host and replicate at body orifices and yet establishes silent, latent infections in sensory neurons. One hypothesis that addresses the apparent contradiction is that peripheral ganglia serve as barriers to the spread of viruses via neurons in the CNS and that HSV usurps these functions to establish a latent state. This report examines the role of the corepressor element-1 silencing transcription factor (CoREST)/REST repressor complex in establishment of latency and reactivation. Mirroring earlier studies showing that expression of dominant-negative REST suppresses latency and increases virulence, a WT REST inserted into the viral genome enables latency but blocks reactivation.
Keywords: NGF, protein synthesis
Abstract
HSVs transit from vigorous replication at the portal of entry into the body to a latent state in sensory neurons in which only noncoding (e.g., latency-associated transcript) and micro-RNAs are expressed. In productive infection, viral genes must be sequentially derepressed at two checkpoints. A leading role in the repression of viral genes is carried out by histone deacetylase (HDAC)/corepressor element-1 silencing transcription factor (CoREST)/lysinespecific demethylase1(LSD1)/RE1-silencing transcription factor (REST) repressor complex (HCLR). Previously, we reported that to define the role of the components of the HCLR complex in the establishment of latency, we constructed recombinant virus (R112) carrying a dominant-negative REST that bound response elements in DNA but could not recruit repressive proteins. This recombinant virus was unable to establish latency. In the current studies, we constructed a virus (R111) carrying WT REST with a WT genome. We report the following findings: (a) R111 readily established latent infection in trigeminal ganglia; however, although the amounts of viral DNAs in latently infected neurons were similar to those of WT virus, the levels of latency-associated transcript and micro-RNAs were 50- to 100-fold lower; (b) R111 did not spontaneously reactivate in ganglionic organ cultures; however, viral genes were expressed if the synthesis of REST was blocked by cycloheximide; and (c) histone deacetylase inhibitors reactivated the WT parent but not the R111 recombinant virus. The results suggest that REST plays a transient role in the establishment of latency but not in reactivation and suggest the existence of at least two phases at both establishment and reactivation.
The primary objective of this report is to extend the studies on the role of repressor element-1 silencing transcription factor (REST), a component of the histone deacetylase (HDAC) 1 or 2/CoREST/lysine-specific demethylase 1 (LSD1)/REST (HCLR) repressor complex, in the establishment of latent infection in sensory neurons by HSV-1 (1–3) To this end, we are reporting the results of comparisons of a WT HSV-1 and a mutant carrying within its WT DNA the gene encoding human REST with respect to their ability to establish latent infections and reactivate from latency. The background and our expectations in undertaking this study are as follows.
HSV-1 is transmitted by physical contact between infected and uninfected tissues. On entry into the body, or with the onset of replication and spread, the virus infects nerve endings and is transported to the nuclei of sensory or autonomic ganglia innervating that site. In mice and other animal model systems, the virus establishes latency in some neurons but multiplies in others (4). Ultimately, in mice, after an interval of 4 wk, only latent virus can be detected in the peripheral ganglia (5). Neurons harboring latent virus contain, in addition to viral DNA, a long noncoding transcript in the form of an unresolved lariat that is designated latency-associated transcript (LAT) (6–8), micro-RNAs (miRNAs) (9–13), and small RNAs (14). The prevailing thought is that these viral gene products enable the maintenance of HSV in a latent state and, by extension, the viability of the neuron. The fundamental question is the identity of the mechanism by which a vigorously replicating virus, on entry into the body, is silenced in neurons harboring latent virus.
A clue into the suppression of viral DNA expression in ganglia emerged from a closer examination of the events in productively infected cells. In brief, in productively infected cells, viral gene expression results from sequential derepression of groups of viral genes at three checkpoints (5). Thus, VP16, a virion protein brought into the cells during infection, recruits several cellular proteins, including LSD1, to derepress α gene promoters. One α gene product, infected cell protein 0 (ICP0), derepresses β and γ1 genes. Ultimately, the onset of viral DNA synthesis enables the expression of very late, or γ2, genes (4). To derepress β and γ genes, ICP0 performs two functions. First, it degrades in conjunction with the UbcH5A ubiquitin conjugating enzyme the promyelocytic leukemia protein (PML) and SP100 (15), two components of ND10 nuclear bodies (16). ICP0 also binds to CoREST, a component of the HCLR repressor complex, and dislodges HDACs from the CoREST/REST complex (17–19). The significance of the latter function of ICP0 emerged from a study showing that a dominant-negative CoREST that retained the capacity to bind REST but was unable to bind HDACs complemented, in part, the replication of an ICP0 null mutant (20).
Sequential transition of viral gene expression poses advantages to virus in that it makes available effectors, enzymes, and structural protein in an orderly fashion and in appropriate quantities (4). The HCLR complex is a repressor of neuron-specific genes in nonneuronal cells (21–25). REST has been reported to be present mainly in nonneuronal cells (21, 26) and, rarely, in neurons (27–29). Its response element, RE1, although well-defined, is long and somewhat degenerate (30–33). Because HSV-1 must have evolved REST response elements in its DNA to be regulated by the HCLR complex, the question arose as to whether the potential subjugation of HSV-1 to the HCLR repressor complex conferred on HSV-1 an advantage in its transition from robust replication at the portal of entry to a dormant state in the neurons of peripheral ganglia. To test this hypothesis, we constructed a virus in which a mutated REST lacking its terminal repressor binding site and driven by the SV40 early promoter was inserted into the WT genome (1). If the HCLR complex played a role in the establishment of latent virus, the defective dominant-negative REST would compete with WT REST for binding to DNA but would not silence its gene. Indeed, the recombinant virus replicated significantly better, yielded fewer latently infected neurons, and was more virulent than the WT virus (1).
In this report, we focus on the properties of a recombinant virus (R111) carrying a human REST gene within a WT viral genome, as tested in a ganglionic organ culture model system (34). In this model system, trigeminal ganglia are excised from mice inoculated by a corneal route 30 d earlier and are incubated intact in medium containing anti-NGF antibody. In WT virus-infected ganglia, the virus reactivates within 24 h after excision, which is well within the time frame of a single cycle of viral replication. The significant finding is that the R111 recombinant virus does not express any viral genes, except low levels of human REST, on incubation of excised ganglia in medium containing anti-NGF antibody. Viral genes are expressed if the ganglia are incubated in the same medium but also containing inhibitory concentrations of cycloheximide. More importantly, the results enable us to discern a short, less than 5-h interval in which the decision to reactivate is made. This interval follows excision of ganglia and precedes the activation phase during which viral gene products accumulate and LAT and miRNAs decrease.
Results
Experimental Design.
All the studies described in this report are based on analyses of trigeminal ganglia immediately after excision from mice infected by the corneal route or maintained in organ cultures. In all experiments, the mice were administered 105 plaque-forming units per cornea by procedures approved by the institutional animal use committee. The administered viruses were the WT HSV-1(F) isolate or the recombinant virus R111 carrying within its WT genome the gene encoding human REST driven by the SV40 early promoter (1). As described elsewhere (34), the trigeminal ganglia were incubated immediately after excision in medium containing anti-NGF antibody to accelerate reactivation or NGF and EGF to retard reactivation. All data points are geometrical mean averages from six randomized ganglia.
Pattern of Expression of WT and R111 Recombinant Viruses During the Establishment of Latency.
In this series of experiments, trigeminal ganglia were excised on days 1, 3, 7, 14, and 30 after inoculation; extracted; and analyzed by means of quantitative (q) PCR or qRT-PCR with respect to viral DNA, mRNAs encoded by genes representative of the three major kinetic classes (α, β, and γ), LAT, and representative viral miRNAs (miR-H3, mir-H5, and miR-H6). The results were as follows.
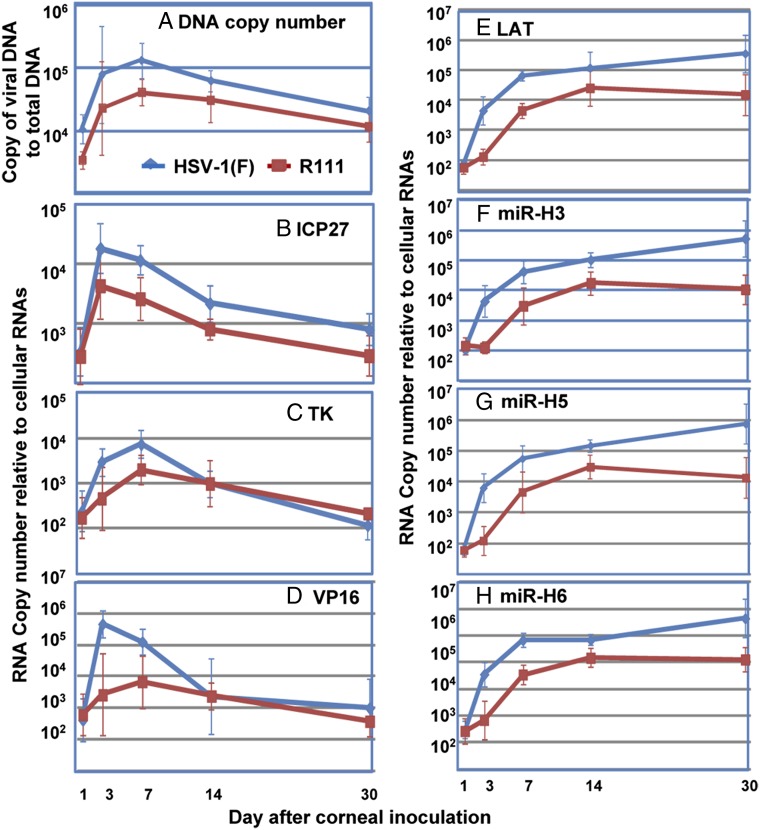
Earlier studies have shown that following corneal inoculation, the virus is transported to the trigeminal ganglion in less than 24 h and that two parallel events take place. In some neurons, the virus establishes a latent, silent state. In other neurons, the virus replicates, and it is most likely that the virus in these neurons is transmitted and replicates in other ganglionic cells. The peak virus yields were detected between the third and 10th days after infection. The results obtained in the current study with HSV-1(F) virus (Fig. 1A) are consistent with these results. Thus, HSV-1(F) DNA reached peak levels between days 3 and 14 after infection. The peak rates of R111 DNA accumulation followed a similar pattern but were threefold lower than those of the WT parent. At the end of the 30-d interval from the time of inoculation, WT virus DNA declined nearly 10-fold from peak levels. R111 DNA levels were only twofold lower than those of the WT parent virus.
Fig. 1.
DNA replication and viral gene expression in murine trigeminal ganglia after infection. On the indicated days after inoculation, mouse trigeminal ganglia were removed and extracted. (A) DNA copy numbers normalized with respect to 50 ng of cellular DNA were plotted over days postinoculation. Copies of ICP27 (B), TK (C), VP16 (D), and LAT (E) normalized to 50 ng of cellular RNA and mirH3 (F), mirH5 (G), and mirH6 (H) normalized to 108 copies of cellular miRNA by let-7a were plotted over days postinoculation. The numbers shown hereafter are geometrical means ± SE based on assays of six trigeminal ganglia per group.
The patterns of viral mRNA (Fig. 1 B–D) accumulation followed the patterns of accumulation of viral DNA with some exceptions. Thus, peak levels of viral mRNAs were detected primarily on days 3 and 7 after infection. The levels of R111 mRNA ranged from three- to 10-fold lower than those of WT virus at those times. On day 30, the levels of HSV-1(F) mRNAs decreased as much as 100-fold relative to the levels of mRNAs at peak levels (days 3–7). The levels of R111 mRNAs were within a twofold range lower than those observed in ganglia infected with WT virus.
The patterns of accumulation of LAT and miRNAs (Fig. 1 E–H) were generally similar for each virus. In each case, the rates of accumulation of LAT and miRNAs were steepest between days 1 and 7. They continued to accumulate at a slower rate between days 7 and 30 in ganglia infected with WT virus but leveled off in ganglia infected with R111 recombinant virus. Overall, the levels of R111 LAT and miRNAs were at least 10-fold lower than those in ganglia infected with WT virus on day 7 and as much as 100-fold lower on day 30.
Trigeminal Ganglia Containing Latent R111 Mutant Virus Do Not Reactivate on Incubation in Medium Containing Anti-NGF Antibody.
In this series of experiments, excised ganglia from mice infected with the WT HSV-1(F) parent virus or the recombinant virus R111 were incubated in medium containing anti-NGF antibody. This medium was selected to accelerate the reactivation of latent virus. At the time of excision (0 h) and at 5, 11, 19, or 24 h after excision, the ganglia in groups of six were individually analyzed with respect to expression of viral genes, LAT, or select viral mRNAs. The results were as follows:
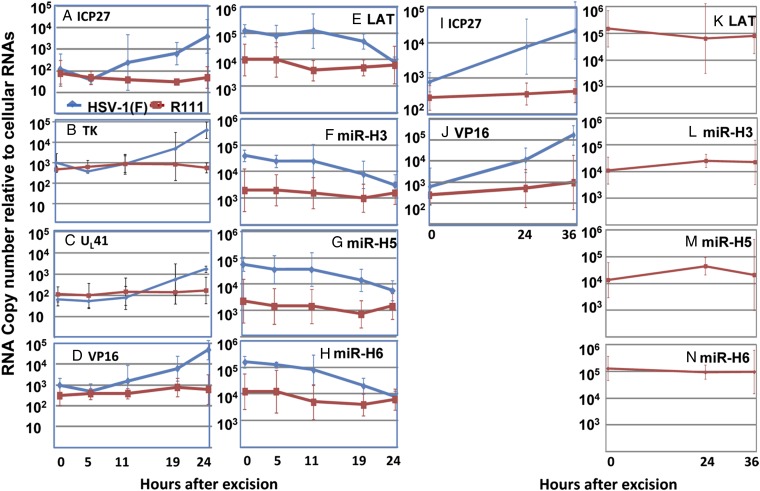
i) As previously reported, HSV-1(F) mRNAs began to accumulate 5 h after excision and increased in amount by ∼50- to 100-fold by 24 h after excision (Fig. 2 A–D). Concomitantly, as reported previously, LAT and viral miRNAs decreased ∼10-fold (Fig. 2 E–H).
ii) In contrast to HSV-1(F)–infected ganglia, there was no increase in the accumulation of viral mRNA (Fig. 2 A–D) or decrease in the accumulation of LAT or miRNAs (Fig. 2 E–H) in ganglia infected with R111 recombinant virus.
iii) In a second experiment, the incubation period was extended to 36 h postexcision. Again, we detected no significant accumulation of R111 ICP27 or VP16 mRNA (Fig. 2 I and J, respectively) or decrease in LAT or miRNAs (Fig. 2 K–N).
Fig. 2.
Viral gene, miRNA, and LAT expression during virus reactivation from latently infected ganglia induced by NGF depletion. On 30 d postinoculation, trigeminal ganglia were excised from WT parent or R111 virus-infected mice, incubated in 199V containing anti-NGF (1 μg/mL) for the indicated time (hours), and then extracted. Normalized copies of ICP27, TK, VP16, and UL41 mRNAs and of LAT, mirH3, mirH5, and mirH6 were plotted as a function of time after excision.
R111 Viral Genes Are Expressed in Ganglia Incubated After Excision in Medium Containing Anti-NGF Antibody and Cycloheximide.
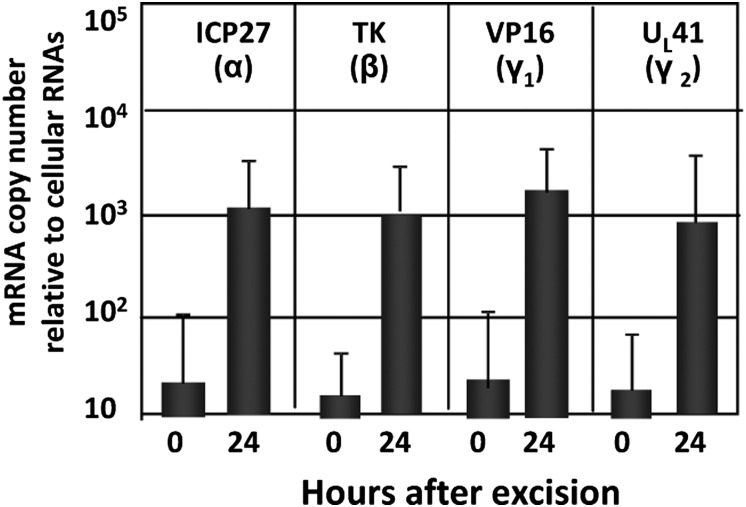
One hypothesis that could explain the results reported in the preceding section is that human REST expressed by the R111 recombinant virus repressed the expression of viral genes. A simple test of this hypothesis is to incubate the ganglia after excision in medium containing anti-NGF antibody and cycloheximide to block the synthesis of proteins, including the human REST expressed by the gene embedded in the recombinant virus. As shown in Fig. 3, representatives of all kinetic classes of viral genes were expressed in medium containing anti-NGF and cycloheximide.
Fig. 3.
Viral gene expression during virus reactivation from R111 latently infected ganglia incubated in anti-NGF and cycloheximide. On 30 d postinoculation, murine trigeminal ganglia were removed from R111 latently infected mice and incubated in 199V containing anti-NGF with cycloheximide (150 μg/mL) for 24 h. At 0 h (columns 1, 3, 5, and 7) or 24 h (columns 2, 4, 6, and 8), copies of ICP27, TK, VP16, and UL41 mRNAs were quantified and normalized to 50 ng of cellular RNAs.
The results of these studies indicate the following: (a) R111 recombinant is not reactivation-defective because it is able to reactivate in the presence of inhibitors of protein synthesis in the same manner as the WT parent virus, and (b) because the only significant difference in the WT and R111 viruses is the presence of the REST gene in the latter virus, the data suggest that expression of this gene blocks reactivation and that suppression of protein synthesis, including that of REST, enables reactivation.
Expression of Human REST in Trigeminal Ganglia of Mice Infected with the R111 Recombinant Virus.
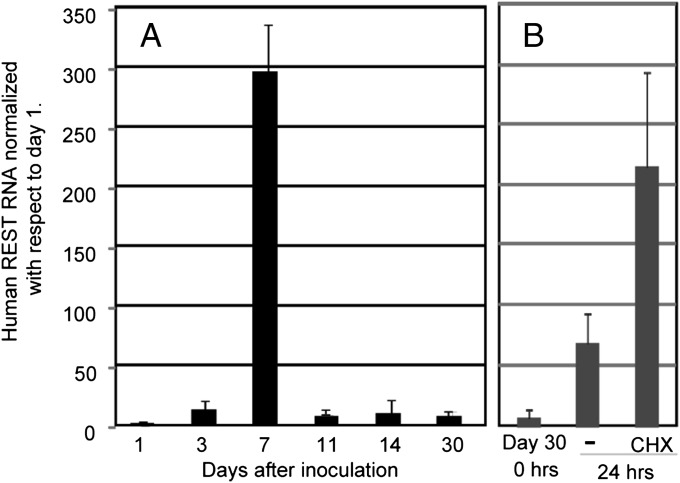
The experiments described above implicated the human REST gene embedded in the genome of a WT virus as a suppressor of reactivation of latent virus. It was of interest therefore to determine the pattern of expression of the human REST in the infected trigeminal ganglia. In these experiments, we used human REST-specific primers listed in Materials and Methods to measure by qRT-PCR the accumulation of REST mRNA during the 30-d interval after corneal inoculation and following excision. Specifically, in this series of experiments, trigeminal ganglia inoculated with R111 recombinant virus were excised at 1, 3, 7, 11, 14, and 30 d after corneal inoculation. In addition, on day 30, a set of ganglia samples was excised and incubated either in medium containing anti-NGF antibody or in medium containing both anti-NGF antibody and cycloheximide. The results shown in Fig. 4 were as follows. We detected very little human REST on day 1. The amounts of human REST increased 13-fold on day 3, increased 300-fold on day 7, and then decreased to a steady state of ∼10-fold higher levels on days 11 through 30. After excision and incubation, the levels of REST mRNA increased eightfold in the absence of cycloheximide and nearly 25-fold in the presence of cycloheximide. The results suggest that the pattern of expression of human REST in mice during the 30-d period after corneal inoculation is similar to the pattern of accumulation of R111 viral DNA and viral mRNAs as shown in Fig. 1 A–D. The key features of the results were the following: (i) In ganglionic organ culture, the levels of human REST increased eightfold in the absence of cycloheximide, and this increase was not matched by a similar increase in viral mRNAs (Fig. 2), and (ii) in the presence of cycloheximide designed to block the synthesis of REST protein, both human REST and viral mRNAs increased in tandem (Figs. 2 and 4).
Fig. 4.
Expression of human REST in trigeminal ganglia of mice infected with the R111 recombinant virus. (A) Mouse trigeminal ganglia were excised at 1, 3, 7, 11, 14, and 30 d after corneal inoculation and then subjected to analysis of human REST mRNA expression. (B) On day 30, mice ganglia removed were extracted immediately or after 24 h of incubation in medium containing anti-NGF with or without cycloheximide (CHX; 150 μg/mL) and subjected to human REST mRNA expression assay. Copies of human REST detected by primers specific to the human REST gene were normalized to 50 ng of cellular RNA.
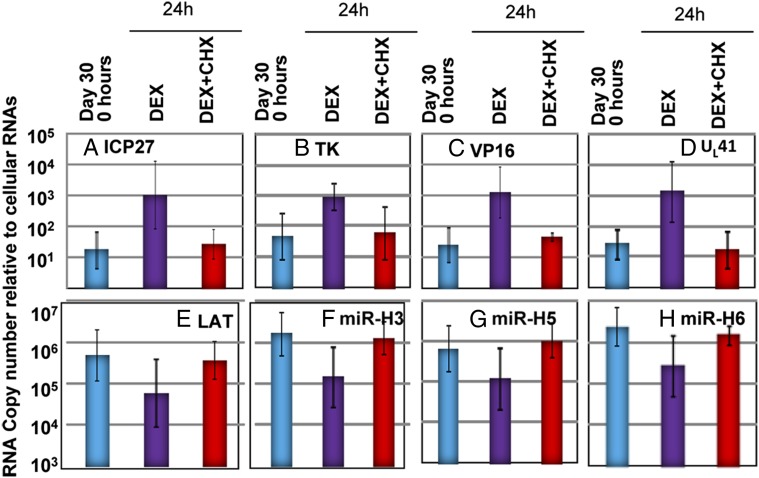
Cycloheximide Blocks Dexamethasone-Dependent Activation of Latent Virus and the Decrease in LAT and Viral miRNA.
In studies published elsewhere, we showed that activation of viral genes representing all kinetic classes in ganglionic organ cultures does not require prior protein synthesis. In that experiment, excised ganglia were incubated in medium containing cycloheximide. In Fig. 3, we show that viral genes are expressed in ganglia infected with R111 virus on incubation in medium containing cycloheximide. The results presented in Fig. 5 are designed to show that cycloheximide is not a universal activator of viral genes embedded in latent virus in ganglia. Specifically, we reported previously that dexamethasone activates viral gene expression concurrently with induction of apoptosis in trigeminal ganglia incubated after excision in medium containing NGF and EGF. In the experiment shown in Fig. 5, trigeminal ganglia were excised and incubated in medium containing NGF plus EGF alone with dexamethasone or dexamethasone and cycloheximide, as described in the figure legend. The results show that dexamethasone induced a 50- to 100-fold increase in the accumulation of viral mRNAs (Fig. 5 A–D) and, concurrently, a 10-fold decrease in the accumulation of LAT and miRNAs (Fig. 5 E–H). Addition of cycloheximide to the medium blocked both the increase in accumulation of viral mRNAs and the decrease in LAT and miRNAs. We conclude that (a) activation of viral gene expression by dexamethasone requires prior protein synthesis and (b) cycloheximide is not a universal activator of expression of viral gene in neurons harboring latent virus.
Fig. 5.
Viral gene expression in HSV-1(F) latently infected trigeminal ganglia incubated in medium containing dexamethasone (DEX) and cycloheximide (CHX). Trigeminal ganglia excised 30 d after inoculation were processed immediately after excision (blue) or after 24 h of incubation in medium containing NGF plus EGF with dexamethasone (50 μM, purple) or NGF plus EGF with both dexamethasone (50 μM, red) and cycloheximide (100 μg/mL, red). Copies of ICP27, TK, VP16, UL41, LAT, mirH3, mirH5, and mirH6 were normalized to cellular RNAs.
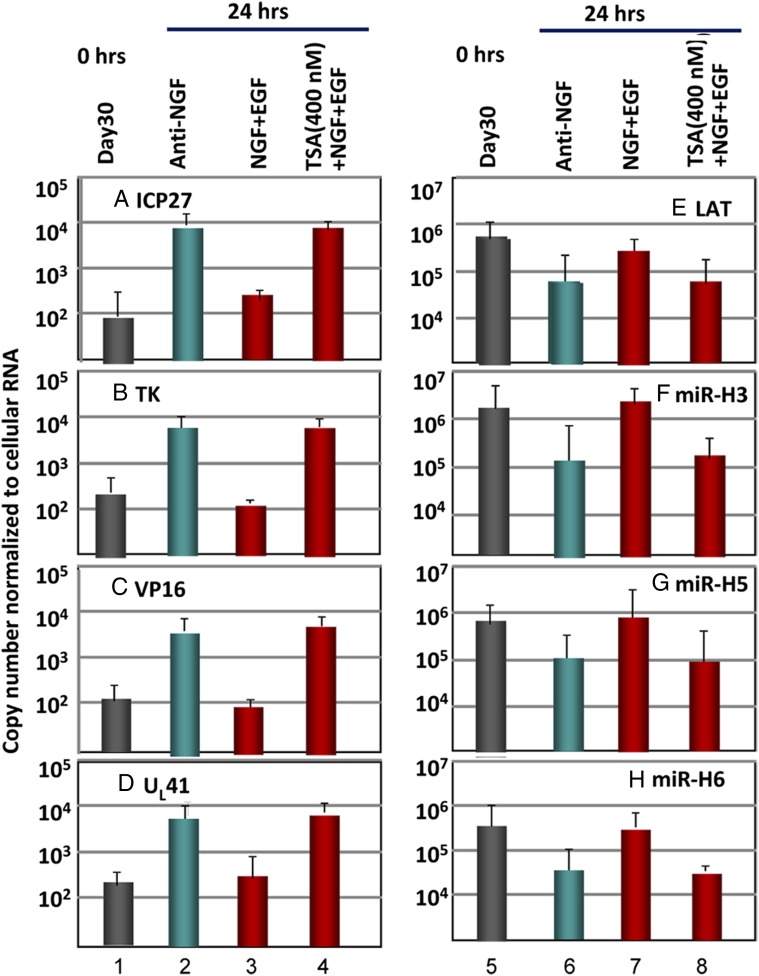
Trichostatin A Induced the Reaction of WT Virus but Not the R111 Recombinant Virus in Trigeminal Ganglion Organ Cultures.
We report two series of experiments. In the first, trigeminal ganglia excised 30 d after infection with WT HSV-1(F) parent viruses were subjected to immediate analyses (Fig. 6, columns 1 and 5) or were incubated in medium containing anti-NGF antibody (Fig. 6, columns 2 and 6); in medium containing both NGF and EGF (Fig. 6, columns 3 and 7); or in medium containing NGF, EGF, and trichostatin A (TSA; an HDAC inhibitor) (Fig. 6, columns 4 and 8). The results were as follows.
Fig. 6.
Effect of the HDAC inhibitor TSA on reactivation of HSV-1(F). Trigeminal ganglia excised 30 d after inoculation of HSV-1(F) were processed immediately after excision (columns 1 and 5) or after 24 h of incubation in medium containing anti-NGF antibody (columns 2 and 6), NGF plus EGF (columns 3 and 7), or NGF plus EGF plus TSA (400 nM) (columns 4 and 8). The figure shows the geometrical mean amounts of viral mRNAs or viral miRNAs and LAT normalized with respect to cellular RNAs.
Compared with day 30 (0 h), in ganglia maintained in medium containing anti-NGF antibody, there was a 50- to 70-fold increase in the amounts of viral mRNAs and a 10-fold decrease in LAT and miRNA levels at 24 h after excision (Fig. 6, compare columns 1 and 4 with columns 2 and 5). There was no substantive increase in the levels of viral RNAs or decrease in LAT and miRNAs in medium containing NGF and EGF (Fig. 6, compare columns 1 and 4 with columns 3 and 7, respectively). Finally, the levels of viral mRNAs increased, and there was a decrease in the amounts of LAT and miRNAs in trigeminal ganglia incubated in medium containing NGF, EGF, and TSA (Fig. 6, compare columns 3 and 7 with columns 4 and 8). We conclude that the HDAC inhibitor TSA reactivated trigeminal ganglia incubated in medium containing NGF plus EGF.
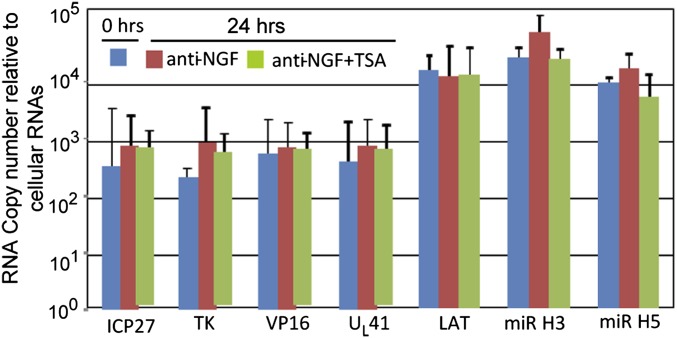
In the second series of experiments, we tested the effect of TSA on reactivation of R111 recombinant virus from trigeminal ganglia excised 30 d after corneal inoculation. As noted above, incubation of trigeminal ganglia in medium containing anti-NGF antibody induces virus reactivation in trigeminal ganglia containing WT virus but not the R111 recombinant virus. To test the effects of TSA under the most favorable conditions, the experiment was performed on trigeminal ganglia incubated in medium containing anti-NGF antibody. The results shown in Fig. 7 indicate that the levels of viral mRNAs, LAT, or miRNAs did not change significantly from the time of excision of the ganglia to 24 h of incubation. The results indicate that TSA did not reactivate R111 recombinant virus even under conditions that enable spontaneous reactivation of WT virus.
Fig. 7.
Effect of the HDAC inhibitor TSA on the reactivation of R111 recombinant virus. Trigeminal ganglia excised 30 d after inoculation of R111 were processed immediately after excision (blue) or after 24 h of incubation in medium containing anti-NGF antibody (red) or anti-NGF plus TSA (400 nM, green). The figure shows the geometrical mean amounts of viral mRNAs or viral miRNAs and LATs normalized with respect to cellular RNAs.
Discussion
This report deals with a comparison of WT parent HSV-1 and the R111 recombinant virus in which the human REST gene, driven by the SV40 early promoter, was inserted into a neutral noninterfering site within a WT virus genome. The salient features of the results are as follows:
i) Our studies have shown that WT HSV replicates in trigeminal ganglion organ cultures for at least 2 wk after corneal inoculation (34). By day 30, the virus is silenced and present in a latent state only. The results of the studies presented here indicate that R111 DNA accumulates in ganglia at a rate threefold lower by day 7 and decreases to a level approximately twofold lower than that of WT viral DNA. The patterns of accumulation of α (ICP27), β [thymidine kinase (TK)], and γ (VP16) mRNAs of the two viruses are essentially similar. They decay more rapidly than viral DNA, and by day 30, they decline to a similar level. In contrast to viral DNA and RNA, the amounts of R111 LAT and mRNAs are 50- to 100-fold lower than those of WT parent virus.
ii) R111 recombinant virus does not reactivate in infected ganglionic organ cultures. Reactivation was observed on cultivation of the ganglia in the presence of cycloheximide. Previously, we have shown that representatives of all kinetic classes of viral genes reactivate at once in the absence of prior protein synthesis by incubating the ganglia in medium containing cycloheximide (34). In support of the conclusion that cycloheximide does not activate viral gene expression in a nonspecific manner, we show that induction of reactivation of latent WT virus by exposure of ganglia to dexamethasone can be blocked by incubation of the ganglia in medium containing cycloheximide. Interestingly, HDAC inhibitor that induces reactivation of WT virus failed to do so in organ cultures of ganglia harboring latent R111 recombinant virus.
iii) Finally, human REST expressed by the R111 recombinant virus followed a pattern of accumulation similar to that of viral mRNAs during the first 30 d after corneal inoculations. In ganglionic organ cultures, human REST mRNA increased eightfold in the absence of cycloheximide and more than 20-fold in the presence of the drug. In contrast, as noted above, viral mRNA did not increase from its base level during incubation in the absence of the drug.
It is convenient to discuss the significance of the results of this and several related previous reports in the context of the fundamental hypothesis of HSV latency. In brief, peripheral ganglia may be viewed as barriers to the spread of viruses via neurons from the peripheral tissues of the CNS. The hypothesis predicts that neurons, like other cells in the body, possess the innate immune mechanisms to respond to the presence of viruses in ways designed to block their replication (35–38). The hypothesis envisions that one such mechanism is the expression of HCLR repressor complex (3, 21, 22, 24, 25, 39–41). Specifically, a stress response generated by virus entry recruits or activates REST to enable the assembly of the HCLR complex. Stress responses have been postulated to activate REST in neurons afflicted by Huntington disease (42–45). The second component of the hypothesis is that HSV takes advantage of the neuronal stress response to enter into a silent, latent state. To assist in the execution of this plan, HSV evolved a DNA sequence that allows itself to be suppressed in neurons and a mechanism to maintain an equilibrium between total suppression and potential to exit from the latent state. An oversimplification of the hypothesis is that if the stress is inadequate or thwarted by viral gene products, the virus multiplies. If the stress response is appropriate, the virus is committed to a latent state.
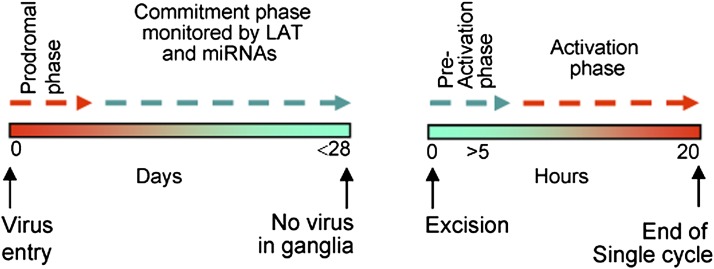
To apply available data as a test of the hypothesis, it is convenient for heuristic reasons to discuss establishment and reactivation separately and to divide the interval from entry into the cell to establishment of the latent state into two time segments, the prodromal phase and the commitment phase, as illustrated in the model presented in Fig. 8. The results may be summarized as follows:
i) Activation of the HCLR complex is one of the stress responses to infection. This conclusion is based on the observation that insertion of a dominant-negative REST into a WT viral genome driven by an SV40 early promoter blocks the establishment of latency and exhibits a level of virulence superior to that of the WT parent virus (1). Because REST has been reported to be present mainly in nonneuronal cells and rarely in neurons (21, 26–29), we may infer that it is either made de novo or translocated from satellite cells. The duration of the prodromal phase is unknown but is unlikely to be very long, because long-term expression of REST is likely to impair the viability of stressed neurons significantly. A short duration of the prodromal phase may also be inferred from the data presented in this report. As shown in Fig. 4, REST mRNA decreased more than 30-fold between days 7 and 11 after inoculation of mice with the R111 recombinant virus.
ii) During the commitment phase, the viral chromosome is converted into facultative chromatin (46). The duration of this conversion is unclear, in large part, because viral replication in murine trigeminal ganglia reaches peak levels at 7 d after infection and continues for at least another week. Hence, infection of new neurons and commitments to latency could occur at any time during this interval. Several reports indicate that various factors are recruited to the viral genome in the course of the commitment phase (47–54).
iii) Several potentially important questions arise from the observations that in ganglia harboring latent R111 recombinant virus at the end of the commitment phase (between 14 and 30 d after corneal inoculation), there was a less than twofold decrease in viral DNA but a 10- to 100-fold decrease in LAT and miRNAs relative to the levels detected in ganglia harboring WT virus. At early stages of R111 recombinant virus replication (3–14 d after infection), there was a threefold decrease in viral DNA accumulation and a three- to 10-fold decrease in the accumulation of viral mRNAs. These decreases could reflect a decrease in the number of cells in which the virus replicated, and perhaps an increase in the number of cells committed to latency. These differences were maintained or, in some cases (e.g., total viral DNA), reduced by day 30 after infection. It is more difficult to explain the large differences in the amounts of LAT and miRNAs in ganglia harboring the R111 mutant compared with those infected with the HSV-1(F) WT parent. It is noteworthy that the accumulations of LAT and miRNAs in ganglia infected with the R111 recombinant lag behind those in WT virus-infected neurons starting virtually from day 1 after infection (Fig. 1 E–H). These results suggest that the HCLR complex, enriched by the accumulation of human REST, repressed not only viral mRNAs but LAT and, by extension, miRNAs. The disparity between the levels of LAT and miRNAs and those of viral mRNAs may reflect the possibility that the repressive effects of the HCLR complex were not overcome even after the levels of human REST had decreased between days 7 and 30 after infection.
Fig. 8.
Model of temporal phases in the establishment and reactivation of latent virus. The model postulates phases both at the establishment of latency (Left) and at reactivation (Right), at which time the decision to commit to latency or commit to reactivation take place.
The second component of the data presented here concerns reactivation from the latent state. In the murine trigeminal ganglion organ culture model, the stimulus for reactivation is excision of the ganglion (34, 55). Incubation in medium devoid of NGF accelerates reactivation, whereas incubation in medium containing NGF and EGF delays viral gene expression (34). In medium containing antibody to NGF, viral gene expression can be detected after 5 h of incubation. Between 5 and 24 h after excision, mRNAs representative of all viral gene kinetic groups increase 100-fold in amount. Viral DNA also increases in amount, indicating that viral proteins are made. At the same time, viral LAT and miRNA concentrations decrease at least 10-fold (34). It is convenient to define the initial phase lasting no more than 5 h as the preactivation phase and the remaining time interval as the activation phase.
The characteristics of the preactivation phase are as follows: (a) Preactivation does not require de novo protein synthesis because mRNA representative of all kinetic classes of viral genes is made and accumulates at identical rates in ganglionic organ cultures incubated in medium containing anti-NGF antibody and cycloheximide (34), and (b) proapoptotic drugs accelerate the reactivation in ganglionic organ cultures maintained in medium containing NGF plus EGF (56). As illustrated in Fig. 5, protein synthesis is required during the preactivation phase to enable at least one proapoptotic drug, dexamethasone, to reactivate the virus in medium containing NGF and EGF.
The studies on the recombinant virus R111 suggest that the HCLR repressor complex does not play a role in the reactivation of HSV in sharp contrast to the role of the repressor complex in the establishment of latency. This conclusion is based largely on the observation that the R111 recombinant virus did not reactivate in ganglionic organ cultures incubated in medium containing anti-NGF antibody. In these ganglia, the amounts of human REST mRNA increased eightfold. The virus did reactivate in medium containing anti-NGF antibody and cycloheximide (Fig. 3). In ganglia incubated in medium containing cycloheximide, human REST mRNA increased >30-fold. The results suggest that (a) inclusion of the REST gene did not alter the genomic structure, such that it could not reactivate because viral genes were expressed in the presence of cycloheximide, and (b) because the expression of REST was lower in the absence of cycloheximide, the data could be interpreted to indicate that REST repressed both viral genes and itself. Finally, in contrast to the events following entry of virus after retrograde transport from the periphery, the data suggest that reactivation does not trigger a stress response that leads to activation of REST.
In these and other published studies, HDAC inhibitors induced reactivation of WT virus (57–59). The same concentration of HDAC inhibitor was ineffective in inducing the reactivation of R111 recombinant virus in ganglionic organ cultures maintained in medium containing anti-NGF antibody. These results suggest that the repressive effect of REST in ganglionic organ cultures primed for reactivation is not mediated by HDAC. Indeed, at its termini, REST binds numerous repressors that may act independent of HDACs.
Finally, throughout these studies, the simultaneous expression of representative genes of the various kinetic classes of HSV DNA was in sharp contrast to the ordered derepression of viral genes in productively infected cells following exposure to virus. A simple explanation of these observations is that in productively infected cells, the mission of the virus is to multiply to the highest possible titer to enable efficient transmission. Ordered delivery of viral proteins in specific amounts and with specific timing ensures optimal yields. In contrast, simultaneous expression of all viral genes during reactivation from latency is likely to minimize yield, but the mission of the virus is to assemble enough viruses to reach the portal of egress from the body (e.g., mouth, genitals) rather than to overwhelm the host with infectious virus.
Materials and Methods
Viruses.
HSV-1(F) is a limited-passage prototype strain used in this laboratory (60). The construction and properties of the recombinant virus R111 were described elsewhere (1). In this recombinant virus, the gene encoding human REST was inserted between the genes encoding UL3 and UL4. The recombinant virus replicates to WT virus levels in all cell lines tested to date. All viruses were titered in Vero cells obtained originally from the American Type Culture Collection.
Murine Model of Virus Infection.
Four-week-old inbred female CBA/J mice (Jackson Laboratories) received unrestricted access to food and water. All animal studies were done according to protocols approved by the Institutional Animal Care and Use Committee. Following light scarification of the cornea, 1 × 105 plaque-forming units of virus were applied in a drop-wise manner in a volume of 5 μL to each cornea of the mice. Trigeminal ganglia were excised on the indicated days and subjected to DNA replication and viral gene expressions assays.
Murine Model of Virus Reactivation.
Trigeminal ganglia were removed 30 d after infection and incubated at 37 °C, in medium 199V, plus 5% CO2, supplemented with 1 μg/mL anti-NGF antibody (Abcam) for 24 h. To block virus reactivation temporarily, trigeminal ganglia were incubated in medium containing 300 ng/mL NGF plus EGF (Invitrogen). The HDAC inhibitors TSA and dexamethasone were purchased from Sigma and dissolved in DMSO, with final applied concentrations of 400 nM and 50 μM, respectively.
DNA Copy Number Assays.
Total DNA was extracted from murine trigeminal ganglia as reported previously (1). The quantification of viral DNA copy numbers in trigeminal ganglia was performed by SYBR green real-time PCR technology (StepOnePlus system; Applied Biosystems) using viral TK gene primers and murine adipsin gene primers as an internal control (1).
RNA Isolation and Assays.
RNAs depleted and enriched of small RNAs (<200 nt) were extracted by means of a mirVana miRNA isolation kit (Ambion) according to the manufacturer’s instructions. RNA was transcribed as described previously (34). Viral gene RNAs and miRNAs (mir-H3, mir-H5, and mir-H6) were quantified by Taqman qRT-PCR assays with respect to mRNA encoding the neuron-specific MAP2 gene. The sequences of primers and probes were reported elsewhere (34). Expressions of human REST were quantified by SYBR green real-time PCR technology, with the following primers: forward, 5′ GGCACGGAAGGAGCAAGT 3′, and reverse, 5′ GGTGAGAGATCCTCTGTGC 3′.
Acknowledgments
These studies were supported by National Cancer Institute Grant 5R37CA078766.
Footnotes
The authors declare no conflict of interest.
References
- 1.Du T, Zhou G, Khan S, Gu H, Roizman B. Disruption of HDAC/CoREST/REST repressor by dnREST reduces genome silencing and increases virulence of herpes simplex virus. Proc Natl Acad Sci USA. 2010;107(36):15904–15909. doi: 10.1073/pnas.1010741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roizman B, Zhou G, Du T. Checkpoints in productive and latent infections with herpes simplex virus 1: Conceptualization of the issues. J Neurovirol. 2011;17(6):512–517. doi: 10.1007/s13365-011-0058-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou G, Te D, Roizman B. The CoREST/REST repressor is both necessary and inimical for expression of herpes simplex virus genes. MBio. 2011;2(1):e00313–e10. doi: 10.1128/mBio.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 5.Roizman B. The checkpoints of viral gene expression in productive and latent infection: The role of the HDAC/CoREST/LSD1/REST repressor complex. J Virol. 2011;85(15):7474–7482. doi: 10.1128/JVI.00180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88(3):790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spivack JG, Woods GM, Fraser NW. Identification of a novel latency-specific splice donor signal within the herpes simplex virus type 1 2.0-kilobase latency-associated transcript (LAT): Translation inhibition of LAT open reading frames by the intron within the 2.0-kilobase LAT. J Virol. 1991;65(12):6800–6810. doi: 10.1128/jvi.65.12.6800-6810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell W, Lirette R, Fraser N. Mapping of low abundance latency-associated RNA in trigeminal ganglia of mice latently infected with herpes virus simplex virus type 1. J Gen Virol. 1990;71:125–132. doi: 10.1099/0022-1317-71-1-125. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 10.Deatly AM, Spivack JG, Lavi E, Fraser NW. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci USA. 1987;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umbach JL, et al. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454(7205):780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR. Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol. 2009;83(20):10677–10683. doi: 10.1128/JVI.01185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW. 2006. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature 442(7098):82–85., and retraction (2008) 451(7178):600.
- 14.Shen W, et al. Two small RNAs encoded within the first 1.5 kilobases of the herpes simplex virus type 1 latency-associated transcript can inhibit productive infection and cooperate to inhibit apoptosis. J Virol. 2009;83(18):9131–9139. doi: 10.1128/JVI.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett RD, et al. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72(8):6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu H, Roizman B. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc Natl Acad Sci USA. 2003;100(15):8963–8968. doi: 10.1073/pnas.1533420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc Natl Acad Sci USA. 2005;102(21):7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu H, Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc Natl Acad Sci USA. 2007;104(43):17134–17139. doi: 10.1073/pnas.0707266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu H, Roizman B. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J Virol. 2009;83(9):4376–4385. doi: 10.1128/JVI.02515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu H, Roizman B. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J Virol. 2009;83(1):181–187. doi: 10.1128/JVI.01940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15(5):500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Gopalakrishnan V. REST and the RESTless: In stem cells and beyond. Future Neurol. 2009;4(3):317–329. doi: 10.2217/fnl.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger E, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 24.Shi YJ, et al. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, et al. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol Cell. 2006;23(3):377–387. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121(4):645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Griffith EC, Cowan CW, Greenberg ME. REST acts through multiple deacetylase complexes. Neuron. 2001;31(3):339–340. doi: 10.1016/s0896-6273(01)00386-5. [DOI] [PubMed] [Google Scholar]
- 28.Koenigsberger C, Chicca JJ, 2nd, Amoureux MC, Edelman GM, Jones FS. Differential regulation by multiple promoters of the gene encoding the neuron-restrictive silencer factor. Proc Natl Acad Sci USA. 2000;97(5):2291–2296. doi: 10.1073/pnas.050578797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimojo M, Hersh LB. Regulation of the cholinergic gene locus by the repressor element-1 silencing transcription factor/neuron restrictive silencer factor (REST/NRSF) Life Sci. 2004;74(18):2213–2225. doi: 10.1016/j.lfs.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316(5830):1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 31.Jothi R, Cuddapah S, Barski A, Cui K, Zhao K. Genome-wide identification of in vivo protein-DNA binding sites from ChIP-Seq data. Nucleic Acids Res. 2008;36(16):5221–5231. doi: 10.1093/nar/gkn488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otto SJ, et al. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27(25):6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai MC, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du T, Zhou G, Roizman B. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci USA. 2011;108(46):18820–18824. doi: 10.1073/pnas.1117203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Dujaili LJ, et al. Ocular herpes simplex virus: How are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 2011;6(8):877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr DJJ, Härle P, Gebhardt BM. The immune response to ocular herpes simplex virus type 1 infection. Exp Biol Med (Maywood) 2001;226(5):353–366. doi: 10.1177/153537020122600501. [DOI] [PubMed] [Google Scholar]
- 37.Divito S, Cherpes TL, Hendricks RL. A triple entente: Virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36(1-3):119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 38.Toma HS, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 39.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437(7057):432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 40.Tapia-Ramírez J, Eggen BJ, Peral-Rubio MJ, Toledo-Aral JJ, Mandel G. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc Natl Acad Sci USA. 1997;94(4):1177–1182. doi: 10.1073/pnas.94.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Vogel JL, Narayanan A, Peng H, Kristie TM. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat Med. 2009;15(11):1312–1317. doi: 10.1038/nm.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martí E, et al. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010;38(20):7219–7235. doi: 10.1093/nar/gkq575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson R, et al. The Human Accelerated Region 1 noncoding RNA is repressed by REST in Huntington’s disease. Physiol Genomics. 2010;41:269–274. doi: 10.1152/physiolgenomics.00019.2010. [DOI] [PubMed] [Google Scholar]
- 44.Shimojo M. Huntingtin regulates RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and dynactin p150 Glued. J Biol Chem. 2008;283(50):34880–34886. doi: 10.1074/jbc.M804183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuccato C, et al. Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington’s disease. J Neurosci. 2007;27(26):6972–6983. doi: 10.1523/JNEUROSCI.4278-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knipe DM, et al. Snapshots: Chromatin control of viral infection. Virology. 2013;435(1):141–156. doi: 10.1016/j.virol.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799(3-4):246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6(3):211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 49.Nicoll MP, Proença JT, Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol Rev. 2012;36(3):684–705. doi: 10.1111/j.1574-6976.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh J, Fraser NW. Temporal association of the herpes simplex virus genome with histone proteins during a lytic infection. J Virol. 2008;82(7):3530–3537. doi: 10.1128/JVI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva L, Cliffe A, Chang L, Knipe DM. Role for A-type lamins in herpesviral DNA targeting and heterochromatin modulation. PLoS Pathog. 2008;4(5):e1000071. doi: 10.1371/journal.ppat.1000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bryant KF, Colgrove RC, Knipe DM. Cellular SNF2H chromatin-remodeling factor promotes herpes simplex virus 1 immediate-early gene expression and replication. MBio. 2011;2(1):e00330–e10. doi: 10.1128/mBio.00330-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang QY, et al. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc Natl Acad Sci USA. 2005;102(44):16055–16059. doi: 10.1073/pnas.0505850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwiatkowski DL, Thompson HW, Bloom DC. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. J Virol. 2009;83(16):8173–8181. doi: 10.1128/JVI.00686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kriesel JD, Ricigliano J, Spruance SL, Garza HH, Jr, Hill JM. Neuronal reactivation of herpes simplex virus may involve interleukin-6. J Neurovirol. 1997;3(6):441–448. doi: 10.3109/13550289709031190. [DOI] [PubMed] [Google Scholar]
- 56.Du T, Zhou G, Roizman B. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci USA. 2012;109(36):14616–14621. doi: 10.1073/pnas.1212661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neumann DM, Bhattacharjee PS, Giordani NV, Bloom DC, Hill JM. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. J Virol. 2007;81(23):13248–13253. doi: 10.1128/JVI.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neumann DM, Bhattacharjee PS, Hill JM. Sodium butyrate: A chemical inducer of in vivo reactivation of herpes simplex virus type 1 in the ocular mouse model. J Virol. 2007;81(11):6106–6110. doi: 10.1128/JVI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danaher RJ, et al. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript (LAT)-independent manner in neuronal cells. J Neurovirol. 2006;11:306–317. doi: 10.1080/13550280590952817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]