Abstract
Here, we evaluate an alternative approach of preconditioning pancreatic islets before transplantation using a potent agonist of growth-hormone-releasing hormone (GHRH) to promote islet viability and function, and we explore the adrenal gland as an alternative transplantation site for islet engraftment. The endocrine microenvironment of the adrenal represents a promising niche with the unique advantages of exceptional high oxygen tension and local anti-inflammatory and immunosuppressive properties. GHRH agonists have been shown to promote islet graft survival and function, which may help to reduce the islet mass necessary to reverse diabetes. In the present study, the most potent GHRH agonist MR403 was tested on insulinoma cells, isolated rat islets, and adrenal β-cell cocultures in vitro. GHRH receptor is expressed on both adrenal cells and islets. MR403 caused a significant increase in cell viability and proliferation and revealed an antiapoptotic effect on insulinoma cells. Viability of rat islets was increased after treatment with the agonist and in coculture with adrenal cells. Rat islets were transplanted into diabetic mice to the intraadrenal transplant site and compared with the classical transplants underneath the kidney capsule. Graft function and integration were tested by metabolic follow-up and immunohistochemical staining of intraadrenal grafts. A rapid decrease occurred in blood glucose levels in both models, and all animals reached normoglycemia within the first days after transplantation. Our studies demonstrated that the adrenal may be an attractive site for islet transplantation and that GHRH analogs might allow reduction of the islet mass needed to reverse a diabetic status.
Keywords: β-cell replacement, regenerative therapy, type 1 diabetes mellitus
Despite steady improvement in insulin formulations and technically controlled application methods, complete normalization of metabolic control and prevention of blood glucose excursions are achieved only rarely in patients with diabetes mellitus. Patients with insufficient glucose control are at risk for the development of micro- and macrovascular complications (1, 2). Therefore, pursuit of alternative treatment options and the potential for recapitulating endogenous insulin production remain a major research focus. One such potential approach is the transplantation of pancreatic islets for the treatment of type 1 diabetes mellitus. Significant progress has been made over the past decade with the introduction of improved immunosuppressive protocols and with isolation of high-quality human islets for clinical transplantation (3–5). Along with this progress, long-term clinical results have been improved significantly, and clear evidence has been shown for the superiority of islet transplantation over insulin therapy in regard to glycemic control and the development of diabetes-associated complications (6).
However, the requirement for chronic immunosuppression and the progressive loss of islet function after their intraportal infusion are major burdens that restrict this treatment option to the most critically endangered and advanced patients with type 1 diabetes (7). One major limitation of advancements in islet transplantation is the partial or total loss of the islet graft within a few days or weeks after its implantation (8). This high rate of nonfunction or early dysfunction has been attributed, in part, to poorly viable or functional islets, either damaged initially during retrieval and isolation (9, 10) or collapsed due to insufficient vascularization, continuous hypoxia, or lack of regeneration/proliferation post transplantation (11, 12). Currently more than 90% of clinical islet transplantations are performed by infusion into the portal vein with subsequent embolization to the liver. Although the liver site has been extremely well characterized, it remains suboptimal as up to 60% of islets transplanted there die shortly after transplantation (13). A major reason for this massive islet loss is that the hepatic parenchymal oxygen tension is well below that of the pancreas (12). In addition, the frequent changes in blood glucose levels and high glucose concentrations are known to be deleterious to the islets. Therefore, many studies have pursued alternative sites with a more adequate microenvironment for pancreatic islet transplantation, such as organs (renal subcapsule, omentum, peritoneum, subcutaneous site, muscle) (14–16), various vascular sites (celiac artery, vein, spleen, lung) (17, 18), immunoprivileged sites (testis, thymus) (19, 20), or devices and capsules (21–25). These may optimize engraftment, survival, and function as well as decrease immunogenicity and promote proliferation and regeneration.
The retrieval process itself causes significant ischemic and mechanical injury, rendering islets more susceptible to posttransplant stress (26–28). The islets are deprived of their vascular supply and microenvironment. However, to be metabolically active, the islets require access to oxygen, nutrients, and other metabolites at a physiological pH and with exclusion of toxic metabolites and oxidative stress (26, 28, 29). As in the early posttransplant setting, revascularization of transplanted islets has not yet occurred, and the islets are supplied with oxygen by diffusion only; close proximity to a proper vascular system is therefore essential (12, 30). Ideally, islets should be transplanted into a site with high oxygen tension.
As an endocrine tissue, pancreatic islets require additional environmental factors for physiologically sensing and responding to glucose. They require access to representative blood glucose levels, and their product, insulin, must be released through an appropriate vascular route of delivery. From the immunological perspective, the ideal transplantation site should confer immunoprivilege by minimizing early inflammatory reactions, thus limiting β-cell apoptosis. From the surgical standpoint, an ideal transplantation site should allow easy access to minimize procedure-related complications and should offer the possibility of monitoring by visualization, biopsy, or differential sampling.
The adrenal gland may offer the unique features favoring its use as a transplantation site for pancreatic islets because of high oxygen tension through a dense vascularization (30), a high endothelial cell content, and a local anti-inflammatory and immunoprotective environment (31, 32). Furthermore, findings derived from model organisms have shown a direct relationship between the degree of hyperglycemia and adrenal cortical function and steroidogenesis (33). Steroidogenic factor 1 (SF1), the key transcription factor of the steroid hydroxylase genes in the adrenal, is also implicated in pancreatic development. In fact, SF1 participates in the vascular and ductal development of the pancreas in zebrafish, mice, and humans (33). Disruption of SF1 function in the fetus leads to abnormal development of the pancreatic islets due to poor vascularization. This emphasizes the close functional relationship of the two endocrine systems (34, 35).
Despite these potentially highly beneficial characteristics of the adrenal gland as a transplant site for pancreatic islets, the adrenal morphologically allows only for transplantation of a reduced islet mass. Therefore, it is of major importance to transplant high-quality islets with optimized viability and function. Agonists of growth-hormone-releasing hormone (GHRH) (36) have been shown previously to promote islet graft survival and function (24, 37) and to improve cardiac structure and function after myocardial infarction (38, 39). The group of one of us (A.V.S.) has now synthesized even more potent GHRH agonists such as MR403 for preconditioning isolated islets. The exposure of islet culture to MR403 improved islet quality mainly due to preservation of viability. This agonist may thus allow for a reduction in the islet mass needed to reverse diabetes and may promote integration and engraftment of an optimized islet graft in this innovative transplantation site.
The GHRH agonist MR403 was analyzed for effects on viability, proliferation, and apoptosis on insulinoma (INS-1) cells. MR403 was tested on INS-1 cells, isolated rat islets, and adrenal β-cell cocultures in vitro. Rat islets were also transplanted into diabetic mice at the classical model site beneath the kidney capsule and compared with the intraadrenal transplant site. Graft function and integration was tested by metabolic follow-up and immunohistochemical staining of intraadrenal grafts.
Results
GHRH Receptor Expression.
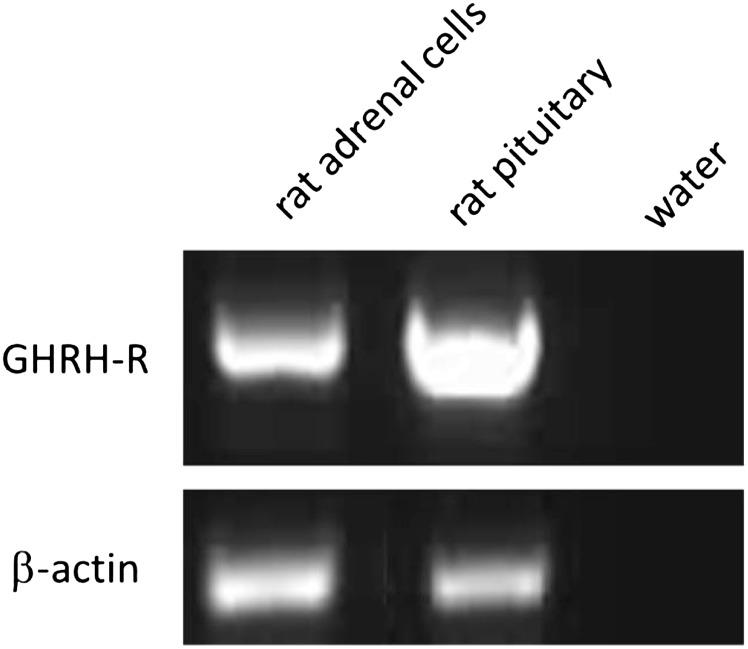
The mRNA expression for GHRH receptor in isolated rat adrenal cells was demonstrated by RT-PCR. Rat pituitary was used as positive control (Fig. 1).
Fig. 1.
Expression of GHRH receptor (GHRH-R) in isolated rat adrenal cells. RT-PCR analysis of GHRH-R in isolated rat adrenal cells. Rat pituitary was used as positive control, water was used as negative control, and β-actin was used as loading control.
Effect of GHRH Analog MR403 on INS-1 cells.
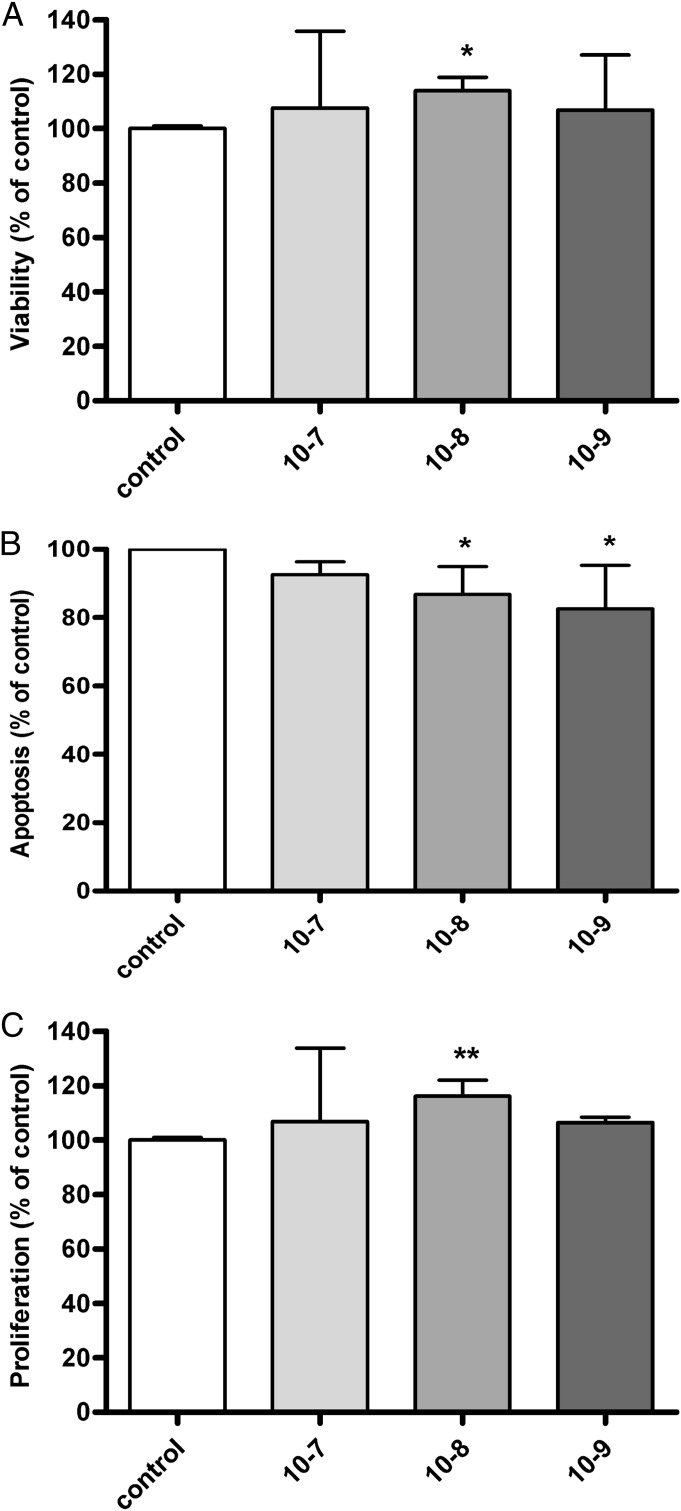
INS-1 cells were treated with the GHRH agonist MR403 at concentrations from 10−7 to 10−9 M for 24 h and were analyzed for viability and apoptosis. Exposure to MR403 resulted in increased viability at all concentrations used, compared with the control dimethyl sulfoxide (DMSO). A significant effect was seen at a concentration of 10−8 M of MR403 with an improvement of 15% in viability, compared with control (Fig. 2A). In addition, treatment of INS-1 cells with MR403 resulted in an overall reduction of apoptosis, as assessed by decreased caspase 3/7 activity. The most effective concentrations of MR403 that provided significantly reduced apoptosis, compared with control, were 10−8 and 10−9 M (Fig. 2B). For determination of proliferation activity, BrdU incorporation was performed in INS-1 cells following exposure to MR403. The maximal enhancement of 16% in proliferation rate compared with control was found at a 10−8 M concentration of MR403 (Fig. 2C). Taken together, with MR403 treatment, there was a maximal effect on all tested parameters at a concentration of 10−8 M. Incubation with 0.1% (vol/vol) DMSO was used as the control in all experiments.
Fig. 2.
In vitro effects of the GHRH agonist MR403 on INS-1 cells. (A) MR403 (10−8 M) significantly improved viability (15% compared with control) after 24 h in culture (n = 3). (B) Apoptosis as indicated by activity of caspases 3/7 was significantly reduced by 14% (10−8 M) or 18% (10−9 M) after treatment with MR403 for 24 h (n = 4). (C) MR403 significantly stimulated cell proliferation (16% versus control) after 24 h in culture (n = 3). Altogether, MR403 pretreatment showed maximum effect on all tested parameters at a concentration of 10−8 M. **P < 0.01; *P < 0.05.
Effect of GHRH Analog MR403 on Rat Islets and Coculture of Islet/Adrenal Cells.
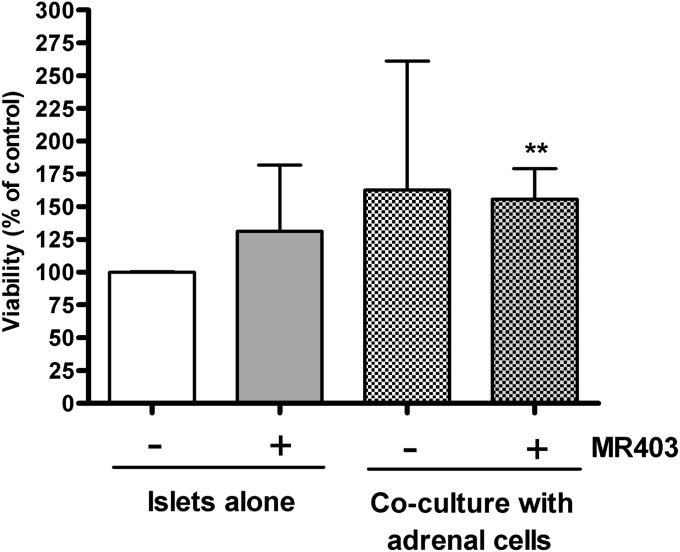
Isolated rat islets and cocultured islets were treated with MR403 at the concentration of 10−8 M, identified as most effective on INS-1 cells, or with 0.1% (vol/vol) DMSO as control for 24 h and then analyzed for viability. Treatment with MR403 induced an increase of islet viability of 31%, compared with control. When cocultured with adrenal cells alone, islets considerably improved in viability; however, a significant improvement occurred when the GHRH agonist MR403 was added to the coculture. With this combination, islet viability improved by 55 ± 23%, compared with control (P < 0.01; Fig. 3).
Fig. 3.
In vitro effect of the GHRH agonist MR403 on islet viability on islets alone and on islets within the islet–adrenal coculture system (n = 4). Islets were cultured either alone or in coculture with adrenal cells and treated with MR403 (10−8 M) for 24 h. Addition of GHRH agonist alone to islets resulted in an increase of viability, and a significant improvement (56% compared with control) was seen in coculture condition and addition of the GHRH agonist. **P < 0.01 compared with islets alone.
Immunohistochemical Analysis of Intraadrenal Islet Grafts.
Morphologic analysis of the retrieved islet containing adrenal glands showed nearly intact adrenal cell composition with islet clusters integrated mostly in the cortex. No leukocyte infiltration, hemorrhage, signs of necrosis, or apoptotic cells were observed. Immunostaining of insulin revealed intense cytosolic staining and intact islet morphology indicating graft viability (Fig. 4).
Fig. 4.
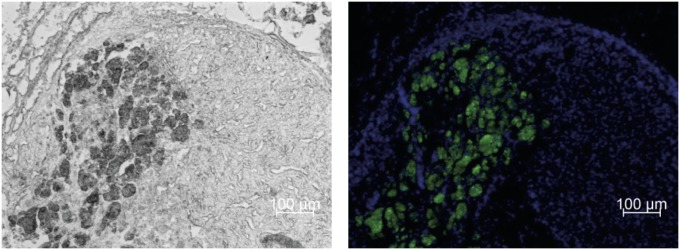
Islet transplantation into the adrenal gland. Serial cryosections of retrieved islet-bearing adrenals were stained for insulin (green) to detect pancreatic islets within the adrenal tissue. Sections were costained with DAPI for visualization of cell nuclei (blue). Representative images are shown by bright field (Left) and fluorescent microscopy (Right).
In Vivo Function of Intraadrenal Islet Grafts.
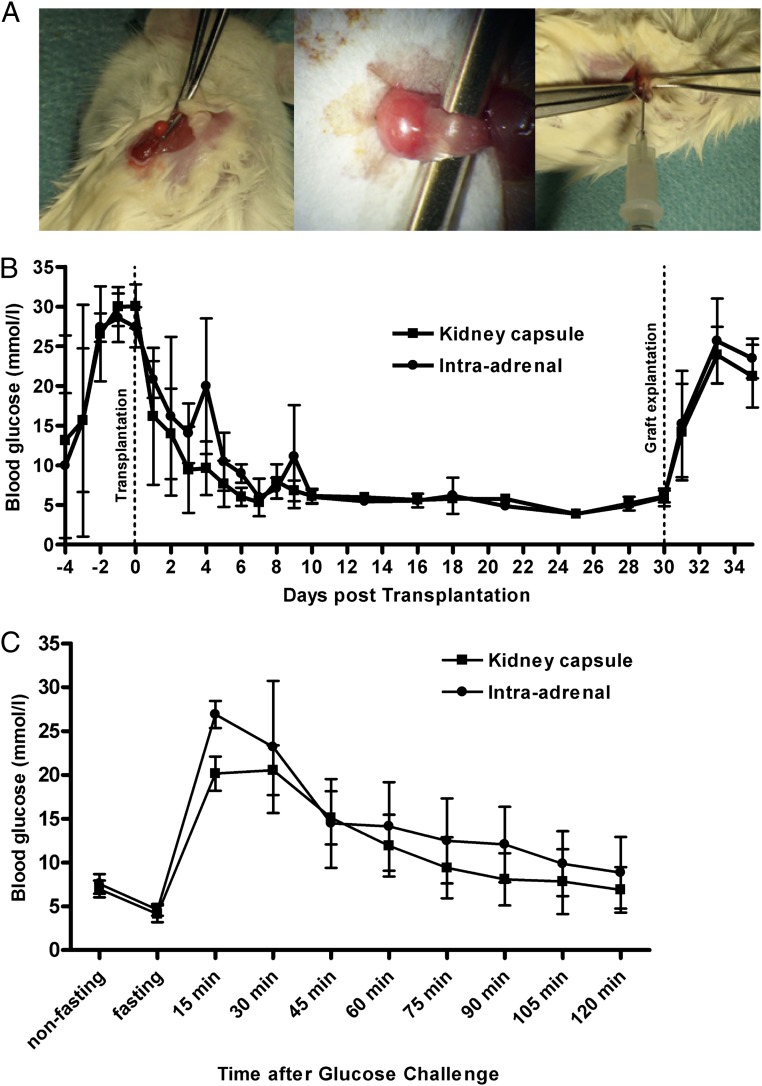
Non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mice were used as islet recipients, and diabetes was induced by a single i.p. injection of streptozotocin (STZ). The procedure of islet transplantation into the adrenal was technically feasible and did not cause any significant bleeding or macroscopically apparent injury of the adrenal tissue (Fig. 5A). During the procedure, the animals did not show any circulatory disturbance due to adrenal manipulation. All animals showed a rapid decrease in blood glucose levels and reached normoglycemia within a few days after transplantation (Fig. 5B) without any difference between the adrenal transplantation site (n = 4) compared with the standard kidney capsule transplantation model (n = 4). After removing the islet grafts by unilateral nephrectomy or adrenalectomy, respectively, the animals showed an immediate recurrence of hyperglycemia. On day 10 post transplantation, an i.p. glucose tolerance test (ipGTT) was performed with 3 g/kg body weight of D-glucose. All animals showed a swift increase in blood glucose followed by a rapid normalization to reach target blood glucose levels after 2 h (Fig. 5C). The intraadrenally transplanted animals were not inferior to the standard model regarding blood glucose kinetics.
Fig. 5.
Islet transplantation (300 islet equivalents) beneath the kidney capsule (standard model) or in the adrenal of streptozotocin-induced diabetic NOD-SCID mice. (A) Intraadrenal transplantation model: the left adrenal was carefully exposed (Left and Center), and islets concentrated to a minimal volume were injected through the upper pole of the gland (Right). (B) All animals of both groups showed a rapid and persistent recovery from diabetes upon transplantation. Retrieval of the graft-bearing kidney or adrenal gland, respectively, induced immediate recurrence of diabetes. (C) On day 10 following islet transplantation, animals were subjected to an i.p. glucose tolerance test. All animals, irrespective of the transplantation site, were able to revert initial hyperglycemia to normal ranges within 2 h.
Discussion
The transplantation of pancreatic islets has become an established treatment option for patients with unstable glycemic control, repeated hypoglycemia, and exhausted insulin therapy. In addition to the risk associated with this procedure, compared with whole-pancreas transplantation, the efficacy of islet transplantation has evolved into a nearly equivalent alternative due to improved cell harvest techniques and immunosuppressive regimens (4). However, progressive loss of intraportally transplanted islets, mainly due to chronic hypoxia, an adverse cytokine/chemokine environment, and local inflammatory activity, are critical issues for maintenance of chronic long-term graft survival and function (13, 26, 27). Therefore, strategies to robustly condition pancreatic islets and identify a more appropriate transplantation site are of particular interest for the further advancement of clinical islet transplantation.
The idea of the adrenal serving as a niche for pancreatic islets is derived from (i) the fact that both the adrenals and the pancreas are endocrine tissues with a similar microenvironment; (ii) the unique feature of extensive vascularization of the adrenal gland, which reduces hypoxic stress; (iii) the hypothesis of antiapoptotic and proproliferative effects of various signaling molecules within the adrenal; and (iv) the unique advantage of a local anti-inflammatory and immunosuppressive microenvironment. In addition, to promote islet quality before transplantation and improve β-cell survival, in the present study the islets were pretreated with a synthetic GHRH agonist that has been proven highly beneficial for improvement of β-cell engraftment and metabolic function after transplantation (37). This combined approach may allow reduction of the islet mass that is currently needed to reverse diabetes, may improve islet graft function by enhancing engraftment, and may minimize islet loss in the early transplant phase by improved vascularization and microenvironmental factors.
The adrenal gland receives 10 times the amount of perfusion than would be suggested by the size of the organ. Each adrenal cell is in direct contact with an endothelial cell. This intimate interaction between endothelial cells and adrenal cells provides crucial trophic signals for cortical cells as well as neuroendocrine chromaffin cells (40). The highly vascularized tissue provides an ideal niche to study β-cell–endothelial cell interactions. Furthermore, this crosstalk between β-cell and endothelial cell can be ideally studied under growth hormone and GHRH stimulation, known to be critical for the trophic effect of adrenal vasculature and β-cell survival. These unique properties make the adrenal gland a suitable transplant site for pancreatic islets. The islet transplantation model presented here is based on a concept that was previously developed in the mouse for analysis of the growth and development of different endocrine cells in the natural environment of this organ (41).
Our studies have demonstrated that intraadrenal islet transplantation is a technically feasible and functionally promising approach to restore normoglycemia in a diabetic mouse model. Further experiments will need to focus on revascularization. Moreover, a more detailed future study of adrenal cell–islet cell interaction under the influence of GHRH is required.
We have shown that GHRH receptors are expressed on rat and human islets (37) as well as in the rat adrenal. Although pretreatment of the pancreatic islet with the GHRH analog MR403 resulted in improved viability, this effect was even more pronounced when the GHRH analog was administered in islet cell–adrenal cell coculture systems. There is an established functional interaction of the GHRH–GH–insulin-like growth factor 1 (IGF1) axis with both islets and adrenal cells that may represent the common denominator for this finding. GHRH peptides, growth hormone, and IGF are thus crucial factors in providing a trophic stimulus on the adrenal cortex and are potent regulators of steroid metabolism both in the adrenal and in the periphery, including islets (42).
These growth factors have been implicated in early formation and development of the adrenal and may be involved in maintaining appropriate adrenal function in the early adrenocorticotrophic hormone (ACTH)-independent phase. Thus, GHRH-receptor agonists may have the potential not only to improve islet function, but also to promote engraftment and integration of islets in the adrenal microenvironment.
The present study on intraadrenal islet transplantation in combination with preconditioning of islet graft with a potent GHRH-receptor agonist links the increasing insights into adrenal nature, endocrine cell interactions, and unique adrenal propensities for a strategy on islet transplantation. The combination of the intraadrenal transplantation approach with administration of GHRH-receptor agonist suggests a unique association of distinct endocrine cell systems under one organ capsule.
Materials and Methods
Peptide Analog Preparation.
GHRH analog MR403 was synthesized in the laboratory of A.V.S. and used at concentrations of 10−7–10−9 M. The analogs were dissolved in DMSO and diluted with medium. The final concentration of DMSO was 0.1% (vol/vol).
INS-1 Cell Line.
Rat insulinoma cells (INS-1) were cultured in RPMI Medium 1640 (PAA cell culture company) containing 2 mM L-glutamine, 10% (vol/vol) FBS, 1 mM Na-pyruvate, 50 μM 2-mercaptoethanol, and 100 U/mL penicillin/streptomycin (Gibco) at 37 °C in a 5% (vol/vol) CO2 humidified incubator. Medium was exchanged every second day, and cells were passaged once per week.
Exposure of INS-1 Cells to GHRH Analogs.
INS-1 cells were grown for 72 h before experimentation. Cells were treated with the agonist MR403 at different concentrations (10−7–10−9 M) or with 0.1% (vol/vol) DMSO as control for 24 h.
Rat Islet Isolation and Culture.
Pancreatic islets were isolated from female Wistar rats as described previously (37) and according to guidelines established by the University of Dresden Institutional Animal Care and Use Committee. Briefly, animals were anesthetized by 3% (vol/vol) isoflurane, and digestion solution (Collagenase V; Sigma-Aldrich) was injected via the pancreatic duct. Islets were isolated by discontinuous Ficoll gradient centrifugation (Sigma-Aldrich). Isolated islets were cultured in RPMI 1640 (PAA) supplemented with 10% (vol/vol) FBS at 37 °C in a 5% (vol/vol) CO2 atmosphere before further experimentation. Yield and purity of islets were determined by microscopic sizing after staining with dithizone (Sigma-Aldrich) as previously described (37).
Adrenal Cell Isolation and Culture.
Female Wistar rats were used as adrenal donors according to guidelines established by the University of Dresden Institutional Animal Care and Use Committee. After euthanasia, the adrenal glands were rapidly removed and kept in PBS at 4 °C. After removal of adipose tissue, the capsule was removed. The tissue was then incubated at 37 °C in PBS containing collagenase type II (2.0 mg/mL; Sigma-Aldrich) and DNase (0.15 mg/mL; Sigma-Aldrich). After incubation for 30 min, the digestion was stopped by adding cold PBS. The dispersed cells were filtered through a 100-µm restrainer and centrifuged at 1,200 × g for 5 min at 4 °C. Erythrocyte lysis buffer (155 mmol/L NH4Cl, 5.7 mmol/L K2HPO4, 0.1 mmol/L EDTA) was added to the cell pellet and incubated for 5 min at room temperature. After two washing steps, cells were collected by centrifugation and resuspended in RPMI 1640 containing 10% (vol/vol) FBS for culture at 37 °C in 5% (vol/vol) CO2 atmosphere.
Coculture System.
Isolated adrenal cells were plated in a 24-well tissue culture plate at a density of 150,000 cells per well. After 2 d, media were exchanged, and MR403 (10−8 M) or DMSO was added to the fresh media. For the coculture, 150 islet equivalents were seeded in inserts (1.0-µm pore size; Greiner Bio One) and treated with MR403 (10−8 M) or 0.1% (vol/vol) DMSO for 24 h before further assessments.
Assessment of Viability, Apoptosis, and Proliferation in INS-1 Cells and Rat Islets.
Viability was assessed using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. Proliferation was measured using BrdU Cell Proliferation Assay (Millipore) following the manufacturer’s description. Apoptosis was assayed by determination of caspase 3/7 activity using Caspase-Glo 3/7 Assay (Promega) according to the manufacturer’s instructions.
In Vivo Islet Functional Assessment.
NOD-SCID mice were used as islet recipients following guidelines established by the University of Dresden Institutional Animal Care and Use Committee. Diabetes was induced by a single i.p. injection of 180 mg/kg STZ (Sigma). Serum glucose was monitored using a commercial glucometer (AccuChek Aviva; Roche). Animals were considered diabetic if nonfasting blood glucose was >25 mmol/L (450 mg/dL) for at least 2 consecutive days. For transplantation, isolated rat islets were cultured for 48 h, and samples of 300 islet equivalents were transplanted underneath the left kidney capsule (n = 4) or into the adrenal gland (n = 4). For intraadrenal transplantation, the left adrenal was exposed, and the islets concentrated in a total volume of 10 µL were injected directly into the adrenal using a microtiter syringe and a blunted needle (Fig. 5A). Blood glucose levels were measured daily throughout the observation period of 35 d. On day 10, an ipGTT was performed to challenge the islet grafts. Blood glucose levels were recorded before injection and 15, 30, 45, 60, 90, and 120 min following glucose injection (3 g/kg). The restoration and maintenance of normoglycemia due to islet graft function was verified by removal of the graft-bearing organ on day 30. Animals were then metabolically followed for another 5 d before euthanasia. The animal experiments and housing were in accordance with University of Dresden Institutional Animal Care and Use Committee.
Fluorescent Immunohistochemistry of Transplanted Organs.
The graft-bearing kidneys and adrenals were explanted on day 30 after transplantation and fixed with 4% (vol/vol) paraformaldehyde for 10 h, stored in 30% (vol/vol) sucrose for 24 h, embedded in tissue-freezing medium, and frozen at −80 °C. Immunohistochemical staining was performed on 10-μm cryosections. As primary antibody, guinea pig anti-insulin at 1:100 (polyclonal, ab7842; Abcam) was used. After washing in PBS with 0.5% (vol/vol) Tween, goat anti-guinea pig (Alexa Fluor488, code 106–545-003; Jackson Laboratories) at a concentration of 1:500 was applied as secondary antibody. Immunofluorescence microscopy was performed on Zeiss Axiovert 200M with AxioCamMRc5.
RT-PCR.
Total RNA was isolated from frozen adrenal cell pellets using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. For RT-PCR, 1 μg RNA from each sample was reverse-transcribed into cDNA by Moloney murine leukemia virus reverse transcriptase using oligo(dT) primers (Promega) in a final volume of 25 μL. One microliter of cDNA was amplified in a 10-μL solution containing 1.5 mM MgCl2, 1× PCR buffer (Invitrogen), 0.25 mM of each deoxynucleotide (Promega), 1 unit of Taq DNA Polymerase (Invitek), and 0.5 μM of each of the different primers. Samples were heated for 5 min at 94 °C and then subjected to 15 s at 94 °C, 15 s at 65 °C, and 15 s at 72 °C for 40 cycles and finally amplified at 72 °C for 1 min. Following agarose gel electrophoresis and ethidium bromide staining, bands were visualized under UV light. Primer sequences (5′–3′) used were rat GHRH receptor (sense: 5′-ccaaaccagctttctggtggc-3′; antisense: 5′-ggcctagcactcagagg-3′) and β-actin (sense: 5′-gtgtgatggtgggtatgggtcagaa-3′; antisense: 5′-accagaggcatacagggacaacaca-3′) primers.
Statistical Analysis.
In all experiments, statistical differences between experimental groups versus appropriate controls were determined using one-way analysis of variance. Data are presented as mean ± SEM. Statistical significance was tested by analysis of variance with Student t test. Differences were considered significant at values of P < 0.05.
Acknowledgments
We thank Trianfyllos Chavakis for help editing the manuscript and Astrid Schröter for help with the manuscript’s preparation . This work was supported by Deutsche Forschungsgemeinschaft Grant KFO 252 “Microenvironment of the Adrenal” (to B.L., H.M., and S.R.B.), Grant TR127 (to B.L. and S.R.B.), and Grant BR1179 (to B.L.); the Centre for Regenerative Therapies Dresden; the Paul Langerhans Institute Dresden; and the German Center for Diabetes Research. Studies in Miami were supported by the Medical Research Service of the Veterans Affairs Department and the University of Miami Miller School of Medicine, Departments of Pathology and Medicine; the W. H. Coulter Foundation (A.V.S.); and the Austin Weeks Family Endowment for Urologic Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Gerstein HC, et al. ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polak JF, et al. DCCT/EDIC Research Group Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60(2):607–613. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CITR Research Group 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR) Cell Transplant. 2009;18(7):753–767. doi: 10.3727/096368909X470874. [DOI] [PubMed] [Google Scholar]
- 4.Barton FB, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 6.Thompson DM, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig B, Ludwig S, Steffen A, Saeger HD, Bornstein SR. Islet versus pancreas transplantation in type 1 diabetes: Competitive or complementary? Curr Diab Rep. 2010;10(6):506–511. doi: 10.1007/s11892-010-0146-y. [DOI] [PubMed] [Google Scholar]
- 8.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–276. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Goto M, et al. Key factors for human islet isolation and clinical transplantation. Transplant Proc. 2005;37(2):1315–1316. doi: 10.1016/j.transproceed.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transpl Int. 2003;16(9):613–632. doi: 10.1007/s00147-003-0651-x. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50(3):489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann R, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56(3):594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 13.Bennet W, et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 14.Espes D, Eriksson O, Lau J, Carlsson PO. Striated muscle as implantation site for transplanted pancreatic islets. J Transplant. 2011;2011:352043. doi: 10.1155/2011/352043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jindal RM, Sidner RA, McDaniel HB, Johnson MS, Fineberg SE. Intraportal vs kidney subcapsular site for human pancreatic islet transplantation. Transplant Proc. 1998;30(2):398–399. doi: 10.1016/s0041-1345(97)01327-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, et al. In situ application of hydrogel-type fibrin-islet composite optimized for rapid glycemic control by subcutaneous xenogeneic porcine islet transplantation. J Control Release. 2012;162(2):382–390. doi: 10.1016/j.jconrel.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Coppens V, et al. Human blood outgrowth endothelial cells improve islet survival and function when co-transplanted in a mouse model of diabetes. Diabetologia. 2012;56(2):382–390. doi: 10.1007/s00125-012-2754-3. [DOI] [PubMed] [Google Scholar]
- 18.Kakabadze Z, et al. An isolated venous sac as a novel site for cell therapy in diabetes mellitus. Transplantation. 2012;94(4):319–324. doi: 10.1097/TP.0b013e31825e4a83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias-Díaz J, et al. CT-guided fine-needle approach for intrathymic islet transplantation in a diabetic patient. Pancreas. 1996;12(1):100–102. doi: 10.1097/00006676-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Nasr IW, et al. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005;174(10):6161–6168. doi: 10.4049/jimmunol.174.10.6161. [DOI] [PubMed] [Google Scholar]
- 21.Barkai U, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2012 doi: 10.3727/096368912X657341. in press. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald P, et al. Feasibility of localized immunosuppression: 1. Exploratory studies with glucocorticoids in a biohybrid device designed for cell transplantation. Pharmazie. 2010;65(6):421–428. [PubMed] [Google Scholar]
- 23.Gibly RF, Zhang X, Lowe WL, Shea LD. Porous scaffolds support extrahepatic human islet transplantation, engraftment and function in mice. Cell Transplant. 2012 doi: 10.3727/096368912X636966. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig B, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109(13):5022–5027. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedraza E, et al. Macroporous three dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2012 doi: 10.3727/096368912X657440. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7(1):38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottino R, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 28.Emamaullee JA, et al. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes. 2008;57(6):1556–1566. doi: 10.2337/db07-1452. [DOI] [PubMed] [Google Scholar]
- 29.Mysore TB, et al. Overexpression of glutathione peroxidase with two isoforms of superoxide dismutase protects mouse islets from oxidative injury and improves islet graft function. Diabetes. 2005;54(7):2109–2116. doi: 10.2337/diabetes.54.7.2109. [DOI] [PubMed] [Google Scholar]
- 30.Ansurudeen I, et al. Vascular-adrenal niche: Endothelial cell-mediated sensitization of human adrenocortical cells to angiotensin II. Horm Metab Res. 2006;38(7):476–480. doi: 10.1055/s-2006-948136. [DOI] [PubMed] [Google Scholar]
- 31.Albertin G, et al. Adrenomedullin and vascular endothelium growth factor genes are overexpressed in the regenerating rat adrenal cortex, and AM and VEGF reciprocally enhance their mRNA expression in cultured rat adrenocortical cells. Int J Mol Med. 2005;16(3):431–435. [PubMed] [Google Scholar]
- 32.Hinson JP, Kapas S. The role of endothelial cell products in the regulation of adrenocortical function: Actions of endothelin, nitric oxide, adrenomedullin and PAMP. Horm Metab Res. 1998;30(6–7):334–340. doi: 10.1055/s-2007-978894. [DOI] [PubMed] [Google Scholar]
- 33.Powers JW, Mazilu JK, Lin S, McCabe ER. The effects of hyperglycemia on adrenal cortex function and steroidogenesis in the zebrafish. Mol Genet Metab. 2010;101(4):421–422. doi: 10.1016/j.ymgme.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Mazilu JK, Powers JW, Lin S, McCabe ER. ff1b, the SF1 ortholog, is important for pancreatic islet cell development in zebrafish. Mol Genet Metab. 2010;101(4):391–394. doi: 10.1016/j.ymgme.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Morales A, et al. Differential expression of steroidogenic factors 1 and 2, cytochrome p450scc, and steroidogenic acute regulatory protein in human pancreas. Pancreas. 2008;37(2):165–169. doi: 10.1097/MPA.0b013e318168dd8c. [DOI] [PubMed] [Google Scholar]
- 36.Izdebski J, et al. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92(11):4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107(28):12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanashiro-Takeuchi RM, et al. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI) Proc Natl Acad Sci USA. 2012;109(2):559–563. doi: 10.1073/pnas.1119203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107(6):2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavakis T, Kanczkowski W, Willenberg HS, Bornstein SR. Endothelial dysfunction: A critical determinant in inflammation-associated adrenal insufficiency? Eur J Clin Invest. 2011;41(8):917–919. doi: 10.1111/j.1365-2362.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso CC, Bornstein SR, Hornsby PJ. Optimizing orthotopic cell transplantation in the mouse adrenal gland. Cell Transplant. 2010;19(5):565–572. doi: 10.3727/096368910X509077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid J, et al. Modulation of pancreatic islets-stress axis by hypothalamic releasing hormones and 11beta-hydroxysteroid dehydrogenase. Proc Natl Acad Sci USA. 2011;108(33):13722–13727. doi: 10.1073/pnas.1110965108. [DOI] [PMC free article] [PubMed] [Google Scholar]