Abstract
Myeloid differentiation primary response protein 88 (MyD88) is classically known as an adaptor, linking TLR and IL-1R to downstream signaling pathways in the innate immune system. In addition to its role in innate immune cells, MyD88 has been shown to play an important role in T cells. How MyD88 regulates helper T-cell differentiation remains largely unknown, however. Here we demonstrate that MyD88 is an important regulator of IL-17-producing CD4+ T helper cells (Th17) cell proliferation. MyD88-deficient CD4+ T cells showed a defect in Th17 cell differentiation, but not in Th1 cell or Th2 cell differentiation. The impaired IL-17 production from MyD88-deficient CD4+ T cells is not a result of defective RAR-related orphan receptor γt (RORγt) expression. Instead, MyD88 is essential for sustaining the mammalian target of rapamycin (mTOR) activation necessary to promote Th17 cell proliferation by linking IL-1 and IL-23 signaling. MyD88-deficient CD4+ T cells showed impaired mTOR activation and, consequently, reduced Th17 cell proliferation. Importantly, the absence of MyD88 in T cells ameliorated disease in the experimental autoimmune encephalomyelitis model. Taken together, our results demonstrate that MyD88 has a dual function in Th17 cells by delivering IL-1 signaling during the early differentiation stage and integrating IL-23 signaling to the mTOR complex to expand committed Th17 cells.
Myeloid differentiation primary response protein 88 (MyD88) was originally isolated as a cloned cDNA that was induced in M1 myeloblastic leukemia cells on activation with IL-6 (1). The function of MyD88 was then uncovered, because the C-terminal portion of MyD88 was found to be similar to the Drosophila Toll receptor and the mammalian IL-1 receptor (IL-1R) (2). This conserved region in the cytoplasmic tails of IL-1R and Toll-like receptor (TLR) is referred to as the Toll/IL-1R (TIR) domain. MyD88 is now known to play an essential role in the innate immune response by linking members of the TLR and IL-1R superfamily to the downstream activation of NF-κB and MAPKs (3).
Among cytokines produced by activated innate immune cells, IL-23 has been shown to promote production of the proinflammatory cytokine IL-17 in activated T cells (4). IL-17–producing CD4+ T helper (Th17) cells were identified after the discovery that IL-23 is linked to traditionally Th1-associated autoimmune disorders, such as experimental autoimmune encephalitis (EAE). Il23a−/− mice, but not Il12a−/− mice, were shown to be autoimmune-resistant (5). IL-23 is required for Th17 cell-mediated autoimmune disorders in vivo, but the role of IL-23 in Th17 cell differentiation remains controversial (6). Previous studies support the idea that IL-23 helps expand or maintain Th17 cells (7–9). In addition, a recent study reemphasized the importance of IL-23 in the generation of pathogenic Th17 cells, showing that they can be generated with IL-23, IL-6, and IL-1β (10).
In addition to IL-23, other cytokines, including TGF-β, IL-6, and IL-1, play roles in Th17 cell development. TGF-β with proinflammatory cytokines was shown to be critical in the support of Th17 cell differentiation (8, 11, 12). In particular, an inflammatory cytokine, IL-6, favors Th17 cell development by inhibiting regulatory T cells (13). The role of IL-1 in Th17 cell differentiation has been investigated as well. IL-1 receptor type 1-deficient (Il1r1−/−) mice showed a lower incidence of EAE and severe defects in the induction of IL-17–producing T cells (14). IL-1 signaling in T cells was further shown to be involved in early Th17 cell differentiation by regulating IFN regulatory factor 4 and RAR-related orphan receptor γt (RORγt) (15).
Although roles for MyD88 in the innate immune system are well established, little is known about their potential function in the adaptive immune system. Several studies have demonstrated important roles of MyD88 in T cells. For instance, T-cell expression of MyD88 is required for resistance to Toxoplasma gondii (16); the MyD88-dependent signaling pathway in CD4+ T cells has been shown to enhance proliferation and augment humoral immune responses (17); and MyD88 is required for T-cell effector function in the development of inflammatory bowel disease (18). Interestingly, although Myd88−/− T cells were found to exhibit decreased IL-17 production (18), how T-cell differentiation could be regulated by MyD88 was not clear in that study.
Here we investigated the molecular mechanism by which MyD88 regulates CD4+ T-cell differentiation. Our results demonstrate that MyD88 contributes to Th17 cell differentiation, but not to Th1 or Th2 cell differentiation. Both IL-1 and IL-23 signaling depend on MyD88 and result in up-regulation of IL-23R. MyD88-deficient Th17 cells show reduced IL-23R expression and mTOR activation, leading to impaired Th17 cell proliferation. Furthermore, MyD88 is crucial for proper Th17 cell differentiation in vivo. Thus, our findings reveal a unique role for the innate adaptor MyD88 in the regulation of Th17 cell differentiation.
Results
Impaired IL-17A Production in MyD88-Deficient CD4+ T Cells.
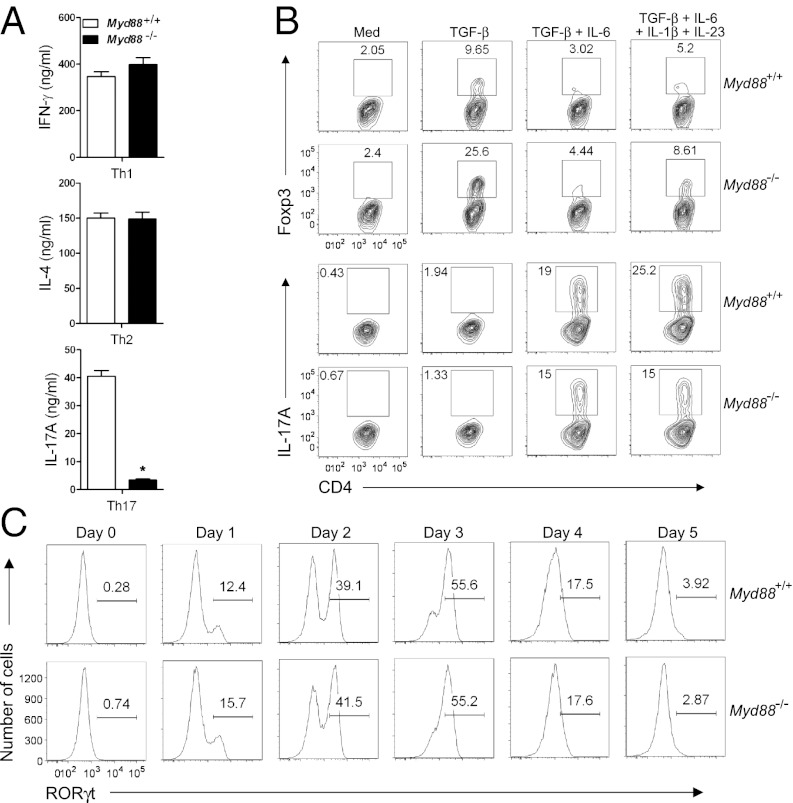
MyD88-deficient (Myd88−/−) T cells have been shown to exhibit decreased IL-17 production (18), but the underlying mechanism has been unclear. To study how MyD88 regulates Th17 cell differentiation, we enriched CD4+ T cells from MyD88-sufficient (i.e., WT) and Myd88−/− mice and performed in vitro T-cell differentiation. The Myd88−/− CD4+ T cells showed a substantial defect in IL-17A production under Th17 cell-polarizing conditions (Fig. 1A). MyD88 deficiency did not affect IFN-γ production under Th1 cell-polarizing conditions or IL-4 production under Th2 cell-polarizing conditions (Fig. 1A).
Fig. 1.
Impaired production of IL-17A in MyD88-deficient CD4+ T cells is independent of RORγt. (A) Purified Myd88−/− or WT CD4+ T cells were cultured under Th1-, Th2-, or Th17-polarizing conditions for 5 d, followed by restimulation with plate-bound anti-CD3 for 24 h, as described in Materials and Methods. ELISA was performed to detect the amounts of IFN-γ, IL-4, and IL-17A in culture supernatants. *P < 0.05. (B) Purified CD4+ cells were cultured in the presence of the indicated cytokines for 3 d, and Foxp3 and IL-17A expression was analyzed by flow cytometry. (C) RORγt protein levels were analyzed by flow cytometry during Th17 differentiation. Results are representative of three experiments (A and B) or two experiments (C).
Given our observation of impaired IL-17A production after T-cell restimulation, we next asked whether the defect started during T-cell differentiation. Myd88−/− CD4+ T cells already showed a lower frequency of IL-17A+ cells after 3 d of Th17 cell differentiation (Fig. S1A), although the defect was less dramatic than that observed after restimulation. Again, Myd88−/− CD4+ T cells showed no defect in IFN-γ expression under Th1 cell-polarizing conditions (Fig. S1A). In addition, the induction of Il17a mRNA was poorer in Myd88−/− CD4+ T cells compared with WT under Th17 cell-polarizing conditions; however, Ifng mRNA levels were comparable in WT and Myd88−/− CD4+ T cells (Fig. S1B). Along with IL-17A, the production of IL-17F, another IL-17 cytokine family member, was severely impaired from Myd88−/− CD4+ T cells after restimulation, and the defect in Il17f mRNA level was detected based on the early Th17 cell differentiation (Fig. S1C).
RORγt Is Expressed Normally in Myd88−/− Th17 Cells.
IL-2 or IL-10 signaling has been shown to modulate Th17 cell generation (19, 20); however, in the present study, impaired IL-17 production in Myd88−/− CD4+ T cells was not related to increased IL-2 or IL-10 production (Fig. S2). Given that Myd88−/− CD4+ T cells are defective in IL-17 production under Th17 cell-polarizing conditions but not in IFN-γ or IL-4 production under Th1 or Th2 cell-polarizing conditions, respectively, we speculated that the defect might result from cytokine signaling specific to Th17 cell differentiation. Among Th17-skewing cytokines, including TGF-β, IL-6, IL-1β, and IL-23, we first focused on IL-1β because of the well-established role of MyD88 as an adaptor in IL-1R signaling (3). Indeed, the defect in IL-17A production from Myd88−/− CD4+ T cells was less marked in the absence of IL-1β (Fig. S3A). However, the defect could not be explained solely by the lack of IL-1 signaling in Myd88−/− CD4+ T cells, because they still exhibited impaired IL-17A production without IL-1β (Fig. S3A). A similar defect in Il17a mRNA expression from Myd88−/− CD4+ T cells was observed in the absence of IL-1β (Fig. S3B).
We then investigated whether TGF-β signaling was altered in Myd88−/− CD4+ T cells by assessing Foxp3 expression as a readout. Compared with WT, Myd88−/− CD4+ T cells expressed more Foxp3 after treatment with TGF-β alone (Fig. 1B, Upper). TGF-β–induced Foxp3 has been shown to inhibit Th17 cell differentiation by antagonizing RORγt (21). Thus, we evaluated whether enhanced TGF-β–induced Foxp3 expression in Myd88−/− CD4+ T cells results in reduced RORγt-directed IL-17 expression. After cells were cultured with various cytokines, both WT and Myd88−/− CD4+ T cells showed greatly reduced Foxp3 expression under IL-17–producing conditions (TGF-β + IL-6 or TGF-β + IL-6 + IL-1β + IL-23) (Fig. 1B). Importantly, under Th17-polarizing conditions, Myd88−/− CD4+ T cells showed no significant decrease in Rorc mRNA levels (Fig. S4) or RORγt expression (Fig. 1C). These data suggest that impaired IL-17 production from Myd88−/− CD4+ T cells is not related to reduced RORγt expression.
IL-1β and IL-23 Contribute Synergistically to IL-17 Production.
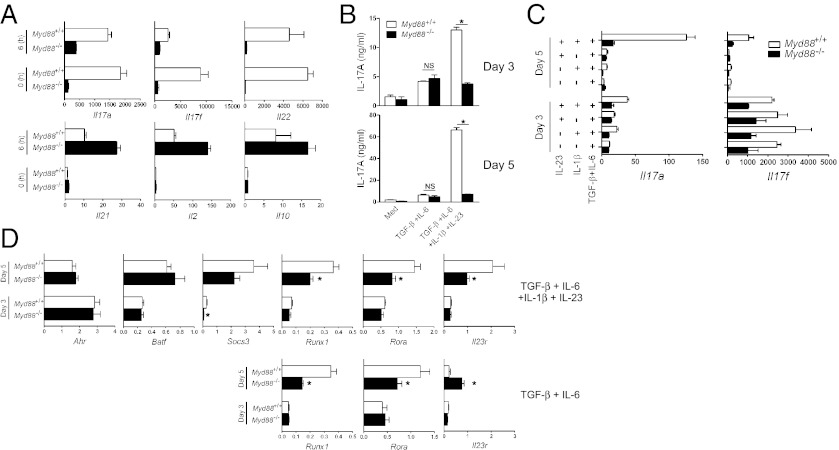
Because we observed the greatest impairment in IL-17 production from Myd88−/− CD4+ T cells after anti-CD3 restimulation (Fig. 1A), we investigated T-cell responses during the later differentiation stage. At 5 d after Th17 cell-polarizing cultures, cells were restimulated with anti-CD3 for 6 h to assess mRNA levels of cytokines. Consistent with our results presented above, genes for IL-17 cytokine family members IL-17A, IL-17F, and IL-22 were poorly expressed from Myd88−/− CD4+ T cells after anti-CD3 restimulation (Fig. 2A). Importantly, Myd88−/− CD4+ T cells did not exhibit a global defect in cytokine expression; IL-21, IL-2, and IL-10 expression was actually enhanced (Fig. 2A). Of note, on day 5 (0 h), mRNA levels of IL-17A, IL-17F, and IL-22 were increased in WT, but not in Myd88−/−, CD4+ T cells (Fig. 2A). This finding prompted us to examine the later stage of Th17 cell differentiation in depth.
Fig. 2.
IL-1β and IL-23 contribute synergistically to IL-17 production. (A) Purified CD4+ T cells were cultured under Th17-polarizing conditions for 5 d. Cells were harvested before restimulation (0 h) or after 6 h with plate-bound anti-CD3. The amounts of Il17a, Il17f, Il22, Il21, Il2, and Il10 mRNA were quantified by qRT-PCR. (B) CD4+ T cells were cultured with the indicated cytokines or media (Med) for 3 or 5 d. Culture supernatants were collected, and IL-17A production was assessed by ELISA. *P < 0.05. NS, not significant. (C and D) Cells were cultured with the indicated cytokines for 3 or 5 d. qRT-PCR was performed to measure the levels of Il17a and Il17f (C) and of Ahr, Batf, Socs3, Runx1, Rora, and Il23r (D). *P < 0.05, WT vs. Myd88−/− cells. After normalization to Actb, mRNA levels in fresh isolated CD4+ T cells (day 0) were used as a control to compare expression levels. Results are representative of three independent experiments.
IL-17A production was further increased at day 5 in WT, but not in Myd88−/− CD4+ T cells, when the differentiation was driven by IL-6, TGF-β, IL-1β, and IL-23 (Fig. 2B). The increase in IL-17A production during day 3–5 in WT was not observed without IL-1β and IL-23, suggesting a critical role of IL-1β– and IL-23–mediated signaling. At day 5, the amount of Il17a mRNA in WT was increased when both IL-1β and IL-23 were added together with TGF-β and IL-6 in the culture (Fig. 2C). Similarly (albeit with different kinetics), Il17f gene expression was maintained in the presence of IL-1β and IL-23 together with TGF-β and IL-6 (Fig. 2C).
Along with Rorc, several transcription factors or molecules, including Ahr (22), Batf (23), Socs3 (24), Runx1 (25), Rora (26), and Il23r (13), are known to be involved in Th17 cell differentiation. We tested whether the impairment of IL-17 expression in Myd88−/− CD4+ T cells by IL-1β and IL-23 is caused by differential expression of these factors. The expression of Ahr, Batf, and Socs3 mRNA was comparable in WT and Myd88−/− CD4+ T cells (Fig. 2D). The expression of Runx1 and Rora mRNA did not differ with or without IL-1β and IL-23; however, Il23r expression was greatly increased at day 5 in WT, but not in Myd88−/−, CD4+ T cells by IL-1β and IL-23 (Fig. 2D).
MyD88-Dependent IL-23R Expression Enhances IL-17A Production.
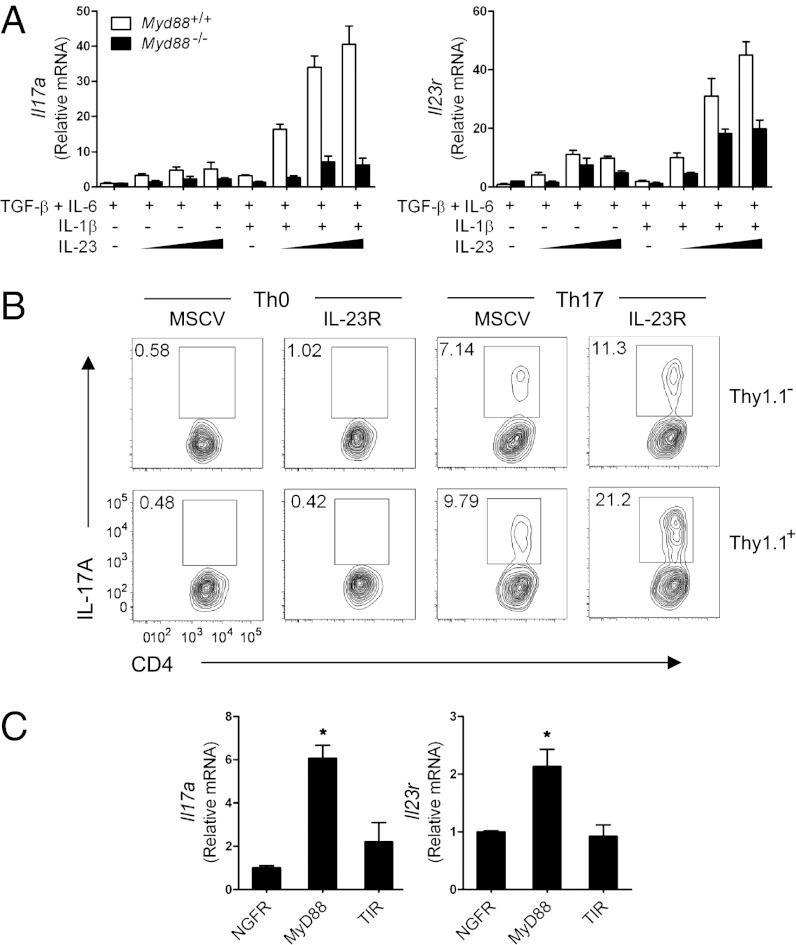
We then examined whether IL-23R–mediated signaling requires MyD88 for Th17 cell differentiation. Unlike WT CD4+ T cells, which showed a synergistic effect on Il17a expression by IL-1β and IL-23, Myd88−/− CD4+ T cells failed to demonstrate the same effect even with various combinations of IL-1β and IL-23 (Fig. 3A, Left). In parallel studies, Il23r expression was lower from Myd88−/− CD4+ T cells compared with WT, suggesting a potential role of MyD88 in the induction of IL-23R (Fig. 3A, Right).
Fig. 3.
MyD88-dependent IL-23R expression contributes to IL-17A production. (A) Purified CD4+ T cells were activated and cultured in the presence of the indicated cytokines for 5 d. 5, 20, or 100 ng/mL of IL-23 was added to the culture. Il17a and Il23r mRNA expression was quantified by qRT-PCR. After normalization to Actb, mRNA levels of CD4+ T cells treated with TGF-β + IL-6 were used as a control. (B) Myd88−/− CD4+ T cells were transduced with retroviral vectors encoding IRES-Thy1.1 (murine stem cell virus) or IL-23R-IRES-Thy1.1 (IL-23R) and cultured for 5 d under Th0 or Th17 conditions. IL-17A production was analyzed by flow cytometry. (C) Myd88−/− CD4+ T cells were transduced with retroviruses expressing NGFR, WT MyD88-NGFR (MyD88), or mutant MyD88 (truncated TIR domain)-NGFR (TIR) and cultured under Th17 conditions for 5 d. NGFR+CD4+ T cells were sorted, and mRNA expression of Il17a and Il23r was quantified by qRT-PCR. After normalization to Actb, mRNA levels of NGFR-transduced CD4+ T cells were used as a control. *P < 0.05. Results are representative of two independent experiments (A and C) or three independent experiments (B).
If poor induction of IL-23R expression in Myd88−/− CD4+ T cells contributes to low IL-17 production, then overexpression of IL-23R should restore IL-17 in Myd88−/− CD4+ T cells. To test this, we infected Myd88−/− CD4+ T cells with retrovirus expressing IL-23R. IL-23R overexpression had little effect on IL-17A expression under Th0 cell-polarizing conditions, but increased the frequency of IL-17A+ cells under Th17 cell-polarizing conditions (Fig. 3B). These findings indicate that impaired IL-17 expression from Myd88−/− CD4+ T cells can be restored, at least in part, by IL-23R expression.
To further test the role of MyD88-dependent IL-23R expression in IL-17 production, we retrovirally transduced Myd88−/− CD4+ T cells with an empty vector, WT MyD88, or TIR. The TIR construct lacks the death domain of MyD88, and thus its downstream signaling, including IL receptor-associated kinase (IRAK) interaction and NF-κB activation, is impaired (17). Transduced cells (CD4+ NGFR+) were sorted, and Il17a and Il23r gene expression was assessed. We found that WT MyD88, but not TIR, induced the expression of Il17a and Il23r in Myd88−/− CD4+ T cells (Fig. 3C), suggesting that MyD88 is essential for inducing the IL-23R expression necessary for IL-17 production.
IL-23–Mediated Signaling Promotes Th17 Cell Expansion by Activating mTOR.
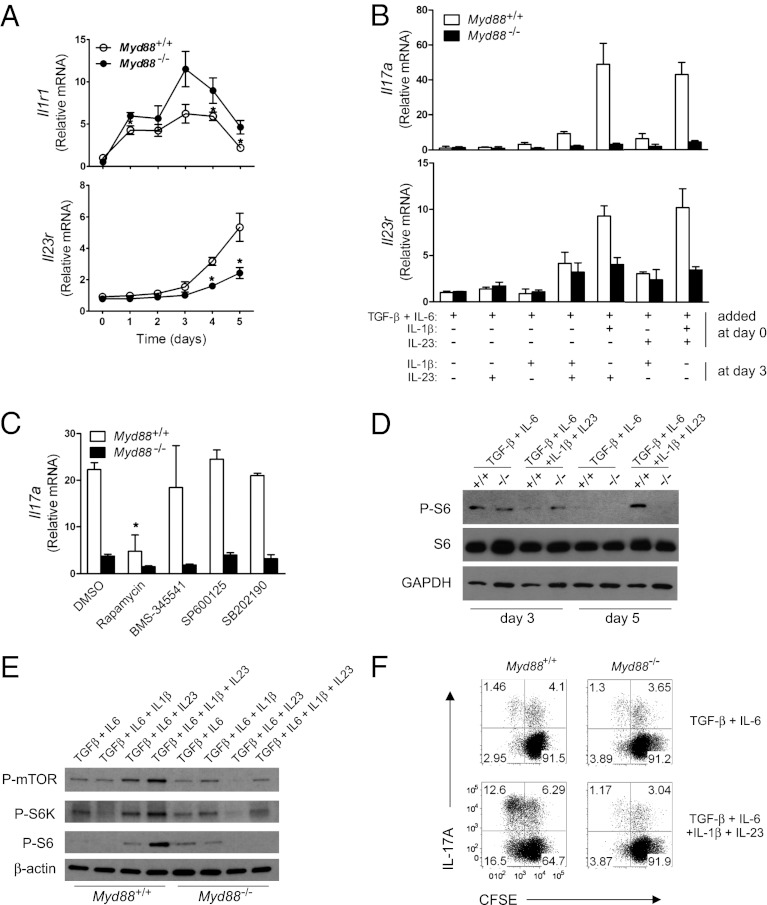
To understand how IL-1 and IL-23 signaling collaborates during Th17 cell differentiation, we first analyzed the temporal expression of their receptors. In line with the observation that IL-1 signaling is critical during early Th17 cell development (15), Il1r1 expression was increased during early Th17 cell differentiation and diminished after 3 d of culture (Fig. 4A and Fig. S5). Myd88−/− CD4+ T cells exhibited no defect in Il1r1 expression; in contrast, Il23r expression was low until day 3 and increased during later Th17 cell differentiation, which was not observed in Myd88−/− CD4+ T cells (Fig. 4A). Because these two receptors were expressed during different time periods, we asked whether IL-1 signaling and IL-23 signaling are sequentially involved in Th17 cell differentiation. To test this, we added IL-1β or IL-23 to the cultures at different time points. When IL-1 signaling was absent during early Th17 cell development, Il17a and Il23r expression was poorly induced (Fig. 4B). Notably, when cells were initially cultured with TGF-β, IL-6, and IL-1β, adding IL-23 at day 3 potently induced Il17a and Il23r expression, similar to when all cytokines were added at the beginning (Fig. 4B; compare the fifth and seventh groups). Taken together, these results indicate that IL-1 signaling is essential during early Th17 cell development, and that IL-23 is required mainly for later Th17 cell differentiation.
Fig. 4.
IL-1 and IL-23 signaling sequentially promotes Th17 cell proliferation. (A) The amounts of Il1r1 and Il23r mRNA during Th17 cell differentiation (with TGF-β + IL-6 + IL-1β + IL-23) were quantified by qRT-PCR. *P < 0.05. (B) CD4+ T cells were activated and cultured with the indicated cytokines for 3 d. At the end of day 3, additional cytokines were added to the indicated cultures. On day 5, qRT-PCR was performed to measure the levels of Il17a and Il23r mRNA. (C) Cells were cultured under Th17-polarizing conditions for 3 d. The indicated inhibitors ( 0.1 μM rapamycin, 5 μM BMS-345541, 10 μM SP600125, and 5 μM SB202190) or DMSO was added at the end of day 3. On day 5, mRNA expression of Il17a was quantified by qRT-PCR. *P < 0.05, DMSO vs. inhibitors treatment in WT. (D) CD4+ T cells from WT (designated +/+) or Myd88−/− (designated −/−) mice were activated and cultured with indicated cytokines. The whole-cell lysates were prepared on day 3 or day 5. P-S6, S6, and GAPDH were detected by immunoblot analysis. (E) Cells were activated and cultured with the indicated cytokines. At day 5, the whole-cell lysates were prepared. P-mTOR, P-S6K, P-S6, and β-actin were detected by immunoblot analysis. (F) Cells were activated and cultured with the indicated cytokines for 3 d. At the end of day 3, cells were washed, labeled with carboxyfluorescein diacetate succinimidyl ester, and cultured in T-cell medium for additional 3 d in the presence of same cytokines. Carboxyfluorescein diacetate succinimidyl ester dilution and IL-17A expression were analyzed by flow cytometry. Results are representative of two independent experiments (A and E) or three independent experiments (B, C, D, and F).
We next asked which signaling molecules are involved in the later Th17 cell differentiation. To test this, we added specific inhibitors for mTOR, IKK, JNK, and p38 at day 3 of Th17 cultures and assessed Il17a expression at day 5. Our results show that blocking NF-κB or MAPK signaling had little effect on Il17a expression (Fig. 4C). However, rapamycin treatment reduced Il17a expression, suggesting that the mammalian target of rapamycin (mTOR)-mediated signaling pathway is critical for the later Th17 cell differentiation. Moreover, Il23r expression was reduced by rapamycin treatment (Fig. S6). In fact, phosphorylation of S6 ribosomal protein was sustained in WT at day 5 when IL-1β and IL-23 were present together with IL-6 and TGF-β (Fig. 4D). Furthermore, both IL-1β and IL-23 are required for activation of the mTOR signaling pathway (Fig. 4E). Because mTOR activation is linked to cell proliferation, we assessed whether IL-1 and IL-23 signaling promotes cell proliferation. WT and Myd88−/− CD4+ T cells proliferated at a similar rate during early stages of Th0 or Th17 cell differentiation (Fig. S7). However, during later Th17 cell differentiation (day 3–5), MyD88-dependent IL-1β and IL-23 signaling was required for the cell proliferation (Fig. 4F). Taken together, these findings indicate that MyD88 plays a critical role in integrating IL-1 and IL-23 signaling for Th17 cell proliferation and expansion.
MyD88 Is Required for IL-23 Signaling During Later Th17 Cell Differentiation.
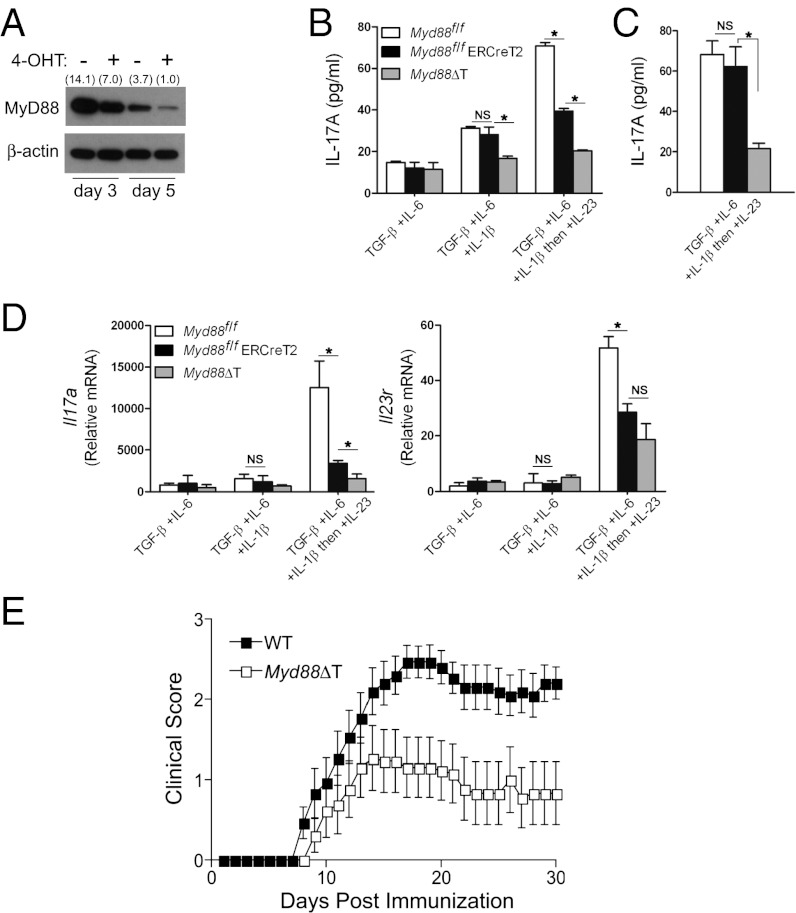
Although both IL-1β and IL-23 have been shown to be important for MyD88-mediated Th17 cell proliferation, whether MyD88 is required for IL-23 signaling is unclear. Because early IL-1 signaling is essential for MyD88-mediated Th17 cell differentiation, we addressed this question using inducible deletion of Myd88 during later Th17 cell differentiation. As shown in Fig. 5A, treatment of cells from Myd88fl/fl ERCreT2 mice with 4-hydroxytamoxifen (4-OHT) (27) reduced the level of MyD88. To test whether MyD88 is required for IL-23 signaling during later Th17 cell differentiation, CD4+ T cells from WT (Myd88fl/fl), Myd88fl/fl ERCreT2, or Myd88fl/fl CD4-Cre (Myd88ΔT) mice (27) were cultured initially with TGF-β, IL-6, and IL-1β. To avoid hindrance of early IL-1 signaling, 4-OHT was added at day 2, followed by IL-23 at day 3. As shown in previously (Fig. 4B), adding IL-23 to the cultures increased IL-17A production in WT, but not in Myd88−/−, CD4+ T cells (Fig. 5B). Importantly, in the presence of 4-OHT, IL-17A production from Myd88fl/fl ERCreT2 CD4+ T cells was significantly reduced compared with WT (Fig. 5 B and C). mRNA expression of Il17a and Il23r genes was also poorly induced in Myd88fl/fl ERCreT2 CD4+ T cells compared with WT (Fig. 5D). In addition, MyD88-dependent IL-23 signaling was required for activation of mTOR signaling pathway (Fig. S8). Taken together, these results indicate that MyD88 is an important regulator not only of early IL-1, but also of later IL-23 signaling for Th17 cell proliferation.
Fig. 5.
MyD88 is required for IL-23 signaling and Th17 cell differentiation in vivo. (A) CD4+ T cells from Myd88fl/fl ERCreT2 mice were cultured and treated at day 1 with 4-OHT (1 μM) or untreated. MyD88 and β-actin were detected by immunoblot analysis from the whole-cell lysates prepared at day 3 or 5. Densitometry values normalized to β-actin are shown in parentheses. (B and D) CD4+ T cells were activated and cultured initially with TGF-β + IL-6 or TGF-β + IL-6 + IL-1β. 4-OHT (1 μM) was added to all of the cultures at day 2, and IL-23 (20 ng/mL) was added to the indicated culture at day 3. On day 5, IL-17A production in culture supernatants was assessed by ELISA (B), or qRT-PCR was performed to measure the levels of Il17a and Il23r (D). *P < 0.05. NS, not significant. (C) CD4+ T cells were cultured as indicated in B without 4-OHT. IL-17A production was measured by ELISA on day 5. *P < 0.05. NS, not significant. Results are representative of three independent experiments (A) or two independent experiments (B–D). (E) WT or Myd88ΔT mice were immunized with 100 µg of MOG peptide in complete Freund’s adjuvant and given 100 ng of pertussis toxin IV on day 0 and 2 postimmunization. Mice were scored (0–5) daily for evidence of clinical disease (n = 16 for WT; n = 13 for Myd88ΔT). Shown are the combined data of three independent experiments, all of which yielded similar results. Error bars represent SEM.
MyD88 Is Crucial for Th17 Cell Differentiation in Vivo.
To determine whether the proposed role for MyD88 in Th17 cell differentiation is relevant in vivo, EAE was induced in WT and Myd88ΔT mice. All WT mice (16 of 16) developed EAE after immunization with myelin oligodendrocyte glycoprotein (MOG), compared with only 6 of 13 Myd88ΔT mice (Table S1). EAE was less prevalent in Myd88ΔT mice compared with WT mice (Fig. 5E); however, the disease was of similar onset and severity in the two groups (Table S1). Taken together, these data demonstrate that MyD88 has a crucial role for proper Th17 cell differentiation in vivo.
Discussion
Many molecules classically known to be critical for the innate immune system also have important functions in adaptive immune responses (28, 29). The present study shows that MyD88 plays an important role in Th17 cell differentiation. In addition, it demonstrates the underlying molecular mechanism by which MyD88-mediated IL-1 signaling sequentially synergizes with IL-23 to promote Th17 cell proliferation.
IL-23 has a unique function in the maintenance of a T-cell subset producing IL-17 (6). IL-23R was shown to be essential for the terminal differentiation of Th17 cells in vivo (9); however, the molecular mechanism governing expression of the IL-23R remains unclear. IL-23R expression was abolished in the absence of Th17-inducing proinflammatory cytokines such as IL-6 (13). Importantly, it has been suggested that IL-23 induces IL-23R expression via positive feedback regulation (30, 31). Our results further show that MyD88-mediated IL-1 signaling in CD4+ T cells is a prerequisite for the up-regulation of IL-23R by IL-23. IL-1 and IL-23 are produced mainly by activated antigen-presenting cells. Of interest, IL-1 also up-regulates IL-23 subunit p19 gene expression in antigen-presenting cells (32). Thus, IL-1 and IL-23 signaling seems to be closely regulated through MyD88 in both innate and adaptive immune cells.
IL-1 signaling on T cells is known to be important (14, 15). The incidence of EAE is significantly lower in Il1r1−/− mice compared with WT mice, correlated with a failure to induce Th17 cells (14). The molecular mechanism by which IL-1R contributes to Th17 cell differentiation remains unclear, however. Chung et al. (15) reported that IL-1 signaling is required for early Th17 cell differentiation by up-regulating RORγt and IFN regulatory factor 4 expression; however, as shown in the present study, RORγt expression is not altered in Myd88−/− CD4+ T cells. This discrepancy might be related to the fact that MyD88 is an adaptor not only for IL-1R, but also for TLR. Chung et al. (15) used a coculture system in which dendritic cells and T cells were cultured in the presence of LPS. Several previous studies have uncovered the critical role of TLR in the adaptive immune system (17, 33, 34). Of note, Reynolds et al. (35) recently showed that TLR2 signaling in CD4+ T cells promotes Th17 responses. Thus, in the experimental setup of Chung et al. (15), the MyD88-dependent signaling pathway was likely activated by TLR even in the absence of IL-1 signaling on T cells. Whether RORγt functions, such as transcriptional activity, are altered in Myd88−/− CD4+ T cells remains elusive.
SIGIRR, a negative regulator for TLR and IL-1R signaling, has been shown to suppress Th17 cell proliferation (36). Importantly, this study demonstrated that Th17 cell effector function is linked to mTOR-mediated cell proliferation. When fully differentiated WT and Sigirr−/− Th17 cells were treated with IL-1, IL-1–induced mTOR kinase activation was increased in Sigirr−/− Th17 cells compared with WT cells (36). In line with this finding, our results show that MyD88-dependent IL-1 and IL-23 signaling is required for the later stage of Th17 cell proliferation by activating the mTOR signaling pathway.
There is growing interest in the interplay between host metabolism and the immune system. In particular, mTOR plays an essential role in integrating environmental cues. Several previous studies have shown that mTOR critically regulates the differentiation of T cells (37–40). mTOR in T cells acts to integrate signal 1 (antigen recognition through T-cell receptor) and signal 2, the integrated sum of environmental cues, including CD28, IL-2R, amino acids. and skewing cytokines (41). In the present study, Myd88−/− CD4+ T cells demonstrated no global defect in mTOR activation; however, MyD88 deficiency in T cells could not integrate IL-1 and IL-23 signaling (signal 2) and failed to generate sustained mTOR activation. This may explain why MyD88 deficiency affects only IL-17 expression, even though mTORC1 was shown to be involved in both Th1 and Th17 responses (40).
In conclusion, our findings show that MyD88 has a dual function in Th17 cell differentiation. MyD88 delivers IL-1 signaling during the initial commitment stage and links it to IL-23 signaling, which expands committed Th17 cells via mTOR activation. Given that MyD88 also plays an important role in activating innate immune responses, MyD88 serves as a good example linking two arms of the immune systems. Our results have important implications for targeting MyD88-dependent signaling in both innate and adaptive immune cells to treat Th17 cell-associated diseases.
Materials and Methods
Myd88-deficient, Myd88flox/flox, Myd88flox/flox CD4-Cre, Myd88flox/flox CreT2, and WT mice were used for the experiments. Purified CD4+ T cells were activated by plate-bound anti-CD3 and soluble anti-CD28. Tamoxifen-induced MyD88 deletion was monitored by immunoblot analysis after treating the cells with 4-OHT at different time points. Mice were injected with MOG peptide and also pertussis toxin on days 0 and 2 after immunization. Full materials and methods with associated references, eight figures and one table can be found at the extended online supporting information.
Supplementary Material
Acknowledgments
We thank Sang-Won V. Kim and Dan R. Littman (New York University, New York, NY) for the IL-23R retroviral vectors, Sei Yoshida and Ken Inoki (University of Michigan, Ann Arbor, MI) for helpful comments on mTOR signaling, and Ryan H. Newton (Beth Israel Deaconess Medical Center) for a critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI062789 (to L.A.T.) and AI073677 (to C.-H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206048110/-/DCSupplemental.
References
- 1.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5(7):1095–1097. [PubMed] [Google Scholar]
- 2.Hultmark D. Macrophage differentiation marker MyD88 is a member of the Toll/IL-1 receptor family. Biochem Biophys Res Commun. 1994;199(1):144–146. doi: 10.1006/bbrc.1994.1206. [DOI] [PubMed] [Google Scholar]
- 3.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R–mediated signaling. Trends Biochem Sci. 2002;27(9):474–482. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 5.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Cua DJ. Th17 cell differentiation: The long and winding road. Immunity. 2008;28(4):445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaRosa DF, et al. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci USA. 2008;105(10):3855–3860. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman AE, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25(5):783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukata M, et al. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol. 2008;180(3):1886–1894. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38(7):1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 23.Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat, and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman AH, et al. Antiviral memory CD8 T-cell differentiation, maintenance, and secondary expansion occur independently of MyD88. Blood. 2011;117(11):3123–3130. doi: 10.1182/blood-2010-11-318485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto K, et al. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464(7293):1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 29.Shaw MH, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10(12):1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Che Mat NF, Zhang X, Guzzo C, Gee K. Interleukin-23-induced interleukin-23 receptor subunit expression is mediated by the Janus kinase/signal transducer and activation of transcription pathway in human CD4 T cells. J Interferon Cytokine Res. 2011;31(4):363–371. doi: 10.1089/jir.2010.0083. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56(9):2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu FL, et al. Interleukin (IL)-23 p19 expression induced by IL-1beta in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-kappaB and AP-1 dependent pathway. Rheumatology (Oxford) 2007;46(8):1266–1273. doi: 10.1093/rheumatology/kem055. [DOI] [PubMed] [Google Scholar]
- 33.Caramalho I, et al. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197(4):403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bessa J, Kopf M, Bachmann MF. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 2010;184(9):4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds JM, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32(5):692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulen MF, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32(1):54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell JD, Delgoffe GM. The mammalian target of rapamycin: Linking T cell differentiation, function, and metabolism. Immunity. 2010;33(3):301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci USA. 2009;106(43):18327–18332. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.