Abstract
Chromosome structure and function are influenced by transposable elements, which are mobile DNA segments that can move from place to place. hAT elements are a superfamily of DNA cut and paste elements that move by excision and integration. We have characterized two hAT elements, TcBuster and Space Invaders (SPIN), that are members of a recently described subfamily of hAT elements called Buster elements. We show that TcBuster, from the red flour beetle Tribolium castaneum, is highly active in human cells. SPIN elements are currently inactive elements that were recently highly active in multiple vertebrate genomes, and the high level of sequence similarity across widely diverged species and patchy phylogenetic distribution suggest that they may have moved between genomes by horizontal transfer. We have generated an intact version of this element, SPINON, which is highly active in human cells. In vitro analysis of TcBuster and SPINON shows that no proteins other than transposase are essential for recombination, a property that may contribute to the ability of SPIN to successfully invade multiple organisms. We also analyze the target site preferences of de novo insertions in the human genome of TcBuster and SPINON and compare them with the preferences of Sleeping Beauty and piggyBac, showing that each superfamily has a distinctive pattern of insertion. The high-frequency transposition of both TcBuster and SPINON suggests that these transposon systems offer powerful tools for genome engineering. Finally, we describe a Saccharomyces cerevisiae assay for TcBuster that will provide a means for isolation of hyperactive and other interesting classes of transposase mutants.
Keywords: hAT element, target site selection, gene therapy, insertional mutagenesis, transgenesis
Transposable elements are discrete DNAs that can move within and occasionally, between genomes. A revelation of the genomic age is that a considerable fraction of many genomes derives from these elements (1), including about one-half of our own genome (2) and >85% of the maize genome (3). Transposable elements can have considerable impact on genome function because of their ability to move and thus, rearrange DNA; additionally, they often contain regulatory elements such as enhancers, promoters, or terminators. Thus, they are potent agents of natural genome engineering. Transposable elements have also been harnessed by researchers and used in many model organisms for insertional mutagenesis and transgenesis, including gene therapy (4). The most commonly exploited transposable elements are cut and paste DNA transposons, which are mobilized through excision and reinsertion of a DNA intermediate (5). The most popular systems currently used in vertebrate cells are derived from three different DNA transposon superfamilies: Sleeping Beauty, a Tc1/mariner element resurrected from fish (6), Tol2, a fish member of the hAT superfamily (7), and piggyBac, an insect member of the piggyBac superfamily (8). Important considerations in using these elements as tools are their transposition frequency and target site selection. Having elements from distinct families that are not cross-mobilizable is advantageous, because it allows for the use of multiple systems in the same cell.
The effects of transposable elements on genomes are not restricted to cells that are related by vertical descent (i.e., from mother to daughter). Transposable elements can also spread between the genomes of different cells. Bacteriophage μ and retroviruses, which integrate into their host's genomes by transposition, are elements that naturally move between cells. Horizontal transfer between species is very common in bacteria (9, 10), but it is much less frequently observed in eukaryotes and particularly, metazoans, where the sequestration of the germ line provides a significant barrier to horizontal transmission (11). Multiple examples of horizontal transmission have been suggested, however, by the observation of nearly identical transposons, which display a patchy taxonomic distribution and phylogenies that are incongruent with those phylogenies established from the analysis of chromosomal genes (11). There have been several recent descriptions of how currently inactive DNA transposons have likely undergone horizontal transmission between tetrapods. The impressive amplification of these elements in some species indicates that they were highly active at some point. The work by Pace et al. (12) recently described a currently inactive family of hAT transposable elements called Space Invaders (SPIN) that has recently (15–40 Mya) successfully invaded a number of tetrapod genomes. More recently, SPIN was identified in invertebrates as well (13) and also, reptiles and lizards (14, 15). Two properties of SPIN suggest that it might be harnessed as a powerful tool for genome engineering. First, these elements have been able to invade a very broad range of animals, including an insect, a snail, a frog, numerous squamates (lizards and snakes), and several mammalian species (12–15). Second, SPIN has achieved enormous copy numbers in some of these species, averaging many thousands in vertebrates (up to ∼100,000 copies per haploid genome in the tenrec, an afrotherian mammal) (12), making it the most successful DNA transposon family ever reported. These characteristics suggested that SPIN might have an intrinsically high level of activity in a broad range of species, including mammals.
Bioinformatic analysis of hAT elements leads us to divide the hAT superfamily into several major subfamilies (16), two of which are the Ac subfamily and the Buster subfamily, which are named for transposase-related Buster proteins and include the now-inactive Charlie elements identified in the human genome in the work by Smit (17). We have recently described two hAT elements from insects, AeBuster1 from the mosquito Aedes aegypti and TcBuster from the red flour beetle Tribolium castaneum, and we have shown that both are active elements using transposition in Drosphila melanogaster embryos as an assay (16). SPIN elements are also members of the Buster subfamily of hAT transposons. The sequences of these elements are aligned in Fig. S1.
We show here that a consensus derivative of currently inactive mammalian SPIN elements and the currently active insect TcBuster are both highly active in human cells. That SPIN can be resurrected, as has Sleeping Beauty, is significant, because SPINs derive from tetrapods (including some mammalian species) (12), whereas Sleeping Beauty was derived from inactive versions of fish transposons (18). SPIN elements have now been identified in other insects, squamate reptiles, snails, and planaria (14) and should actually be considered an animal transposon; it is possibly the most successful and widespread DNA transposon.
The high activity of TcBuster and SPIN elements will provide useful tools for mammalian genome engineering. We also show that these Buster elements have different target site selection patterns with respect to mammalian genome features than piggyBac and Sleeping Beauty, another attribute that will contribute to their usefulness for genome engineering.
Finally, we describe both integration and excision assays for TcBuster in Saccharomyces cerevisiae that will be useful for isolation of hyperactive mutants for genome engineering and structure function analysis of this interesting class of transposable elements. These assays, combined with the activity of these elements in mammalian cells, establish an efficient pipeline for the screening of hyperactive transposase mutants.
Results
Resurrection of a SPIN Transposon.
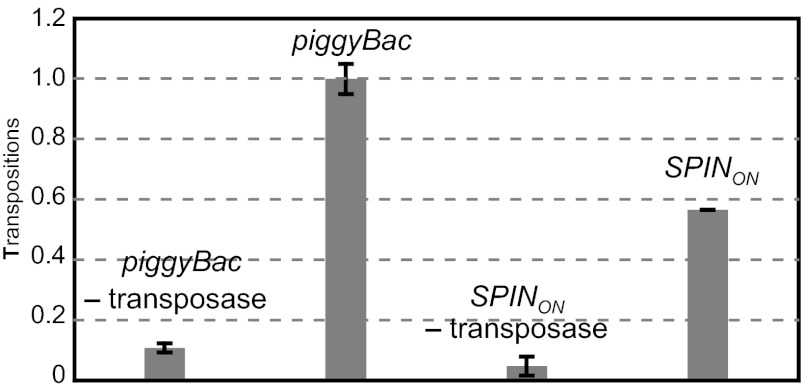
A consensus of SPIN sequences from mammals, including tenrec, bat, and opossum, and another tetrapod, frog, did not yield an intact ORF, because several nucleotide positions remained unspecified. However, by considering the superconsensus and the DNA sequences of individual SPIN elements determined in the work by Pace et al. (12), we generated several intact SPIN ORFs (Tables S1 and S2); expression of these ORFs was not toxic in human cells. We assayed integration promoted by these transposases in human cells by cotransfection of a transposon donor plasmid containing a mini SPIN element in which 250 bp of the L end and 250 bp of the R end flank a drug resistance gene and a helper plasmid expressing the SPIN transposase under control of the CMV promoter. After transfection, cells were grown nonselectively for 2 d, and then, transposon integration was selected for by application of the appropriate antibiotic followed by counting of vital cells stained with methylene blue. Some of the SPIN transposases were active, whereas others were inactive (Fig. S2). The SPIN derivative SPINON was highly active (Fig. 1), and its integration frequency is about 60% of the highly active piggyBac element. The SPINON transposon is an active DNA transposon generated from sequences found in mammals, and therefore, its behavior in mammalian cells is of particular interest, especially given its ability to successfully invade many different organisms.
Fig. 1.
SPINON transposition in mammalian cells. The transposition of piggyBac and SPIN elements containing blasticidin resistance genes from transfected plasmids to the HeLa genome was measured in the presence and absence of transposase expressed from a CMV promoter by selection for blasticidin-resistant cells. The frequency of SPIN transposition compared with the frequency of piggyBac transposition is set as 1.0.
TcBuster is Highly Active in Mammalian Cells.
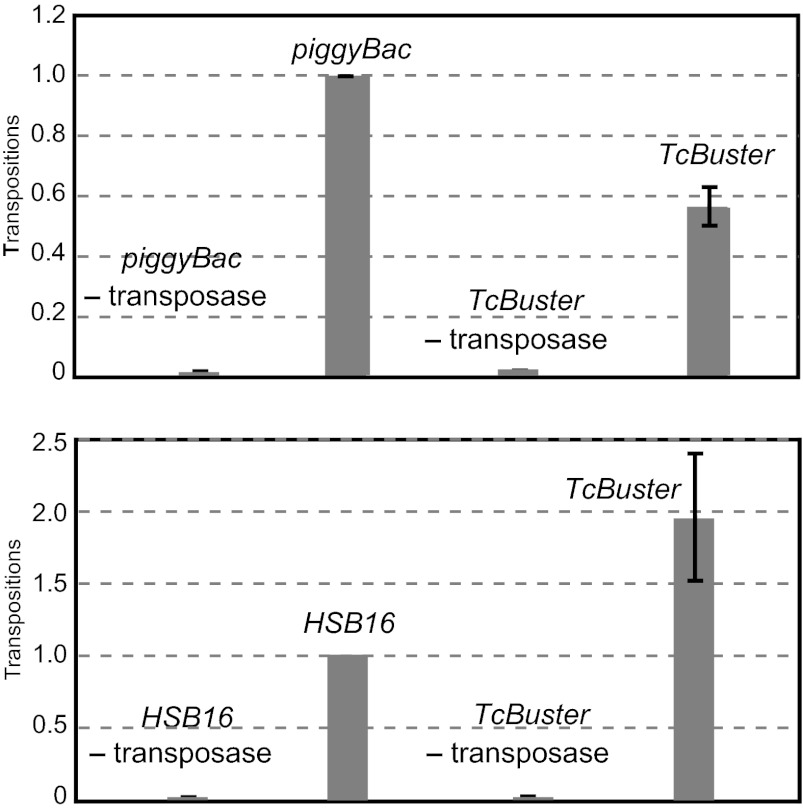
We previously showed that the insect element TcBuster is active in D. melanogaster and Ae. aegypti embryos (16). We now report that it can transpose at relatively high frequency in HeLa cells (Fig. 2, Upper). We assayed chromosomal integration of a mini TcBuster element containing 328 bp of the L end and 145 bp of the R end flanking a drug resistance gene from a donor plasmid DNA after cotransfection of HeLa cells with a helper plasmid expressing the TcBuster transposase, which was not toxic to human cells, using the selection strategy described above for SPINON. Notably, the frequency of TcBuster transposition is greater than the frequency of a hyperactive version of Sleeping Beauty, HyperSB16 (HSB) (19) (Fig. 2, Lower), and it is ∼60% of the frequency of piggyBac, which has been found to be highly active in multiple cell types (8). Thus, both SPINON and TcBuster are highly active, transposing at frequencies close to the frequency of piggyBac.
Fig. 2.
TcBuster transposition in mammalian cells. The transposition of TcBuster, hyperactive Sleeping Beauty, and piggyBac elements containing antibiotic resistance genes from transfected plasmids to the HeLa genome was measured in the presence and absence of transposase by selection for antibiotic-resistant cells; the frequency of transposition of TcBuster transposition is compared with the frequency of a hyperactive Sleeping Beauty (HSB16) (19) and piggyBac.
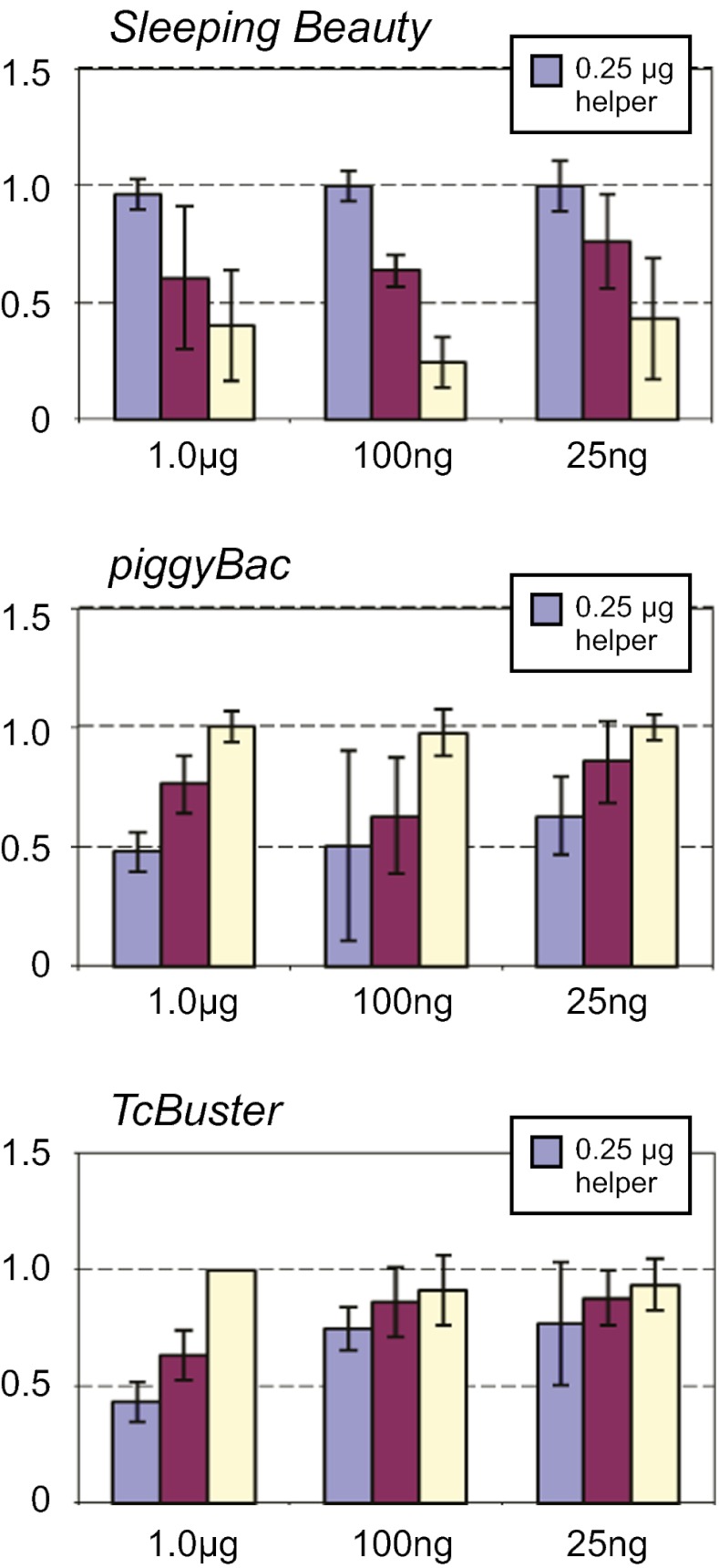
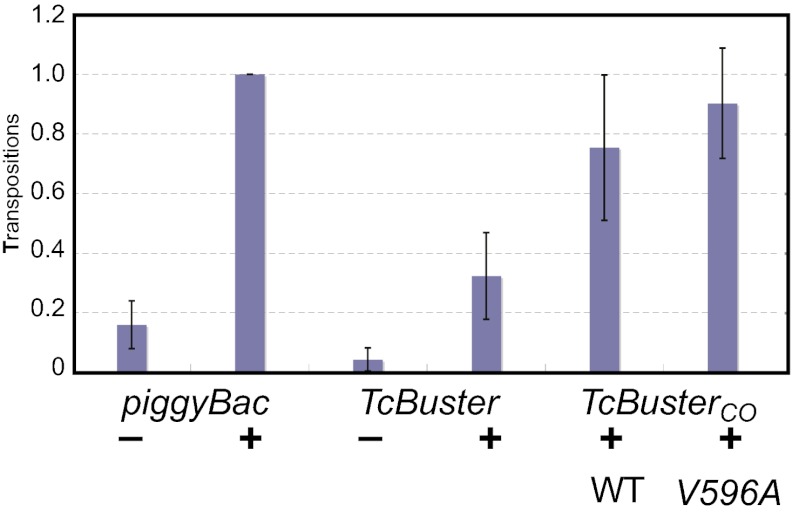
The transposition frequency of Sleeping Beauty in human cells is limited by the phenomenon of overproduction inhibition (that is, transposition frequency actually decreases when transposase expression is increased excessively) (20). To explore overproduction inhibition, we tested Sleeping Beauty, piggyBac, and TcBuster under the same ranges of donor and helper plasmid concentrations and found that, like piggyBac, TcBuster does not exhibit overproduction inhibition under the conditions tested (Fig. 3). This inhibition increases the use of TcBuster as a gene vector, because increased integration frequencies can be achieved simply by increasing the amount of transposase supplied. We also tested whether TcBuster transposition could be increased by using a transposase gene codon-optimized mammalian expression, TcBusterCO (Table S1), compared with the natural insect gene, and we found that transposition in human cells was increased about twofold (Fig. 4). The hyperactive mutant TcBusterCO V596A is considered in more detail below.
Fig. 3.
TcBuster transposition in mammalian cells. The transposition of TcBuster, hyperactive Sleeping Beauty, and piggyBac elements containing antibiotic resistance genes from transfected plasmids to the HeLa genome was measured by selection for antibiotic-resistant cells; the frequency of transposition of TcBuster transposition was compared with the frequency of a hyperactive Sleeping Beauty (HSB16) and piggyBac using different amounts of transposase expression plasmid and the transposon-containing donor plasmid.
Fig. 4.
Transposition of TcBuster variants. The frequency of transposition of several forms of TcBuster is compared with the frequency of piggyBac. TcBuster is the ORF from Tribolium, TcBusterCO is a version codon-optimized for expression in mammalian cells, and TcBusterCO V596A contains an amino acid change based on a hyperactive mutant of the closely related AeBuster1.
SPINON and TcBusterCO Prefer Similar Target Sites in Particular Chromosomal Regions.
To characterize SPINON and TcBusterCO target site selection with respect to multiple genomic features and epigenetic marks, we isolated and sequenced large numbers of de novo transposon-end human genome junctions. After selection for insertions in HeLa cells, we used ligation-mediated PCR to capture transposon–chromosomal junctions from TcBusterCO and SPINON integrants, sequenced them using the 454 (Roche) platform, and determined their position in the human genome. For comparison, we also analyzed Sleeping Beauty and piggyBac insertions (Table S3). We analyzed 4,490 Sleeping Beauty insertion sites, 13,494 piggyBac insertion sites, 6,390 TcBusterCO insertion sites, and 8,333 SPINON insertion sites (Table S4). The numbers of insertion sites analyzed here are larger than in previous studies of transposon integration in human cells (21–25).
We used these large collections of insertion junctions to characterize the sequences in and immediately around the element target sites duplications more fully. Fig. S3A shows web logos generated by each transposon, and the percentages of insertions at particular kinds of target sites are shown in Fig. S3B; the actual number of insertions at particular sites is shown in Table S4. The target site for Sleeping Beauty insertion is TA, and we found that 98.4% of the Sleeping Beauty insertions occurred into a TA. Insertions into CA and TG accounted for 0.5% of the insertions. We found a modest preference for sequences outside the target site duplication (A at −4 and T at +4) (Fig. S3A).
The target site for piggyBac is TTAA. We found that 97.6% of the target sequences used were TTAA. The majority of the non-TTAA target sites are pairs of symmetrically related sequences (CTAA and TTAG and ATAA and TTAT) (Fig. S3B) in which the nonconsensus positions are where the 3′OH transposon end attacks the target DNA on insertion (Fig. S3C). There is little local sequence selectivity beyond the TTAA piggyBac target site duplication (Fig. S3A).
With the hAT Buster elements, there is a strong preference for TA in the middle of the 8-bp target site duplication rather than adjacent to the positions of breakage and joining (Fig. S3C) (16); we find here that 93.6% of TcBusterCO insertions and 95.3% SPIN insertions contain this TA. For both elements, TG and CA in the middle of the 8-bp target site duplication are the other major type of insertion site (Fig. S3B). With SPINON, there is also some preference for TC and GA flanking this central TA; this preference is much less obvious with TcBusterCO. With both SPINON and TcBusterCO, there are modest preferences for particular sequences flanking the target site duplication, the most significant being a preference for A at −7 and T at +7 (Fig. S3A).
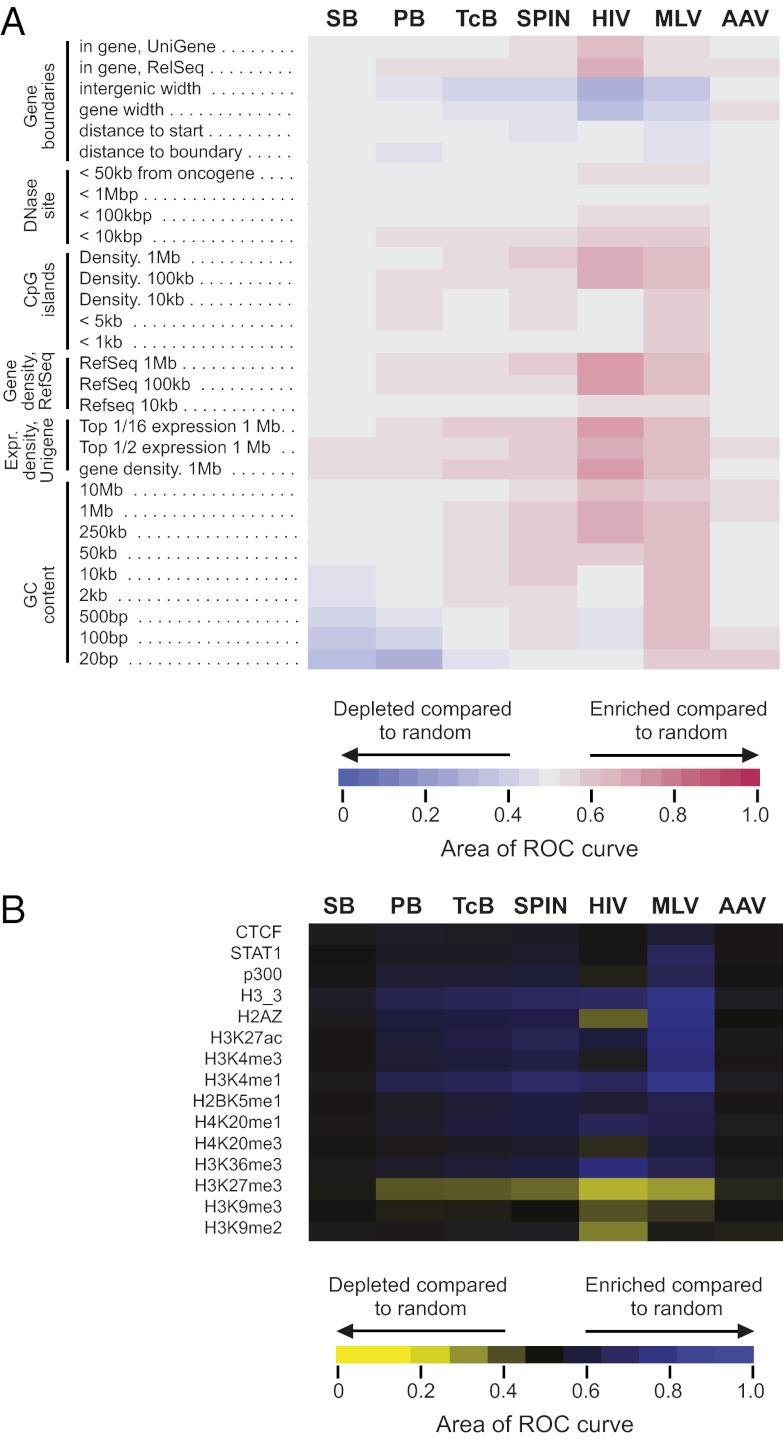
We have also analyzed the genome-wide insertion distributions of TcBusterCO, SPINON, Sleeping Beauty, and piggyBac insertions, comparing them with genomic features such as annotated transcription units and epigenetic marks (Fig. 5). For comparison, we also generated in silico a set of matched random control sites, which are random positions in the genome but matched to integration sites for the proximity to restriction enzyme recognition sites used in recovery of the experimental sites. Use of the matching procedure reduces biases in the analysis arising because of use of restriction enzymes cleavage of genomic DNA in the recovery process. Favoring or disfavoring of transposon integration relative to genomic features is summarized in heat maps using the receiver operator characteristic (ROC) area method for comparison (Fig. 5) (26). For comparison, these heat maps also summarize data on previously obtained patterns for HIV, MLV, and adeno-associated virus (AAV) insertion in HeLa cells (27–29).
Fig. 5.
Distribution of Sleeping Beauty, piggyBac, TcBusterCO, and SPINON insertions on the human genome with respect to the indicated genomic features. (A) Integration frequency near selected genomic features. (B) Integration frequency near bound proteins and modified histones mapped using the ChIP-Seq method. In both A and B, integration site datasets for Sleeping Beauty (SB), piggyBac (PB), TcBusterCO (TCB), and SPINON (SPIN) are indicated by the columns, and genomic features or ChIP-Seq datasets are indicated by the rows (the latter were calculated over 10-kb windows). The departure from random distribution is indicated by colored tiles (key at bottom), and differences from random placement were scored using the ROC area method described in the work by Berry et al. (26). In A, blue indicates that insertions are depleted compared with random. Red indicates features where insertions are enriched compared with random. Gray indicates that the distribution is random. In B, yellow and blue are used to indicate depletion or enrichment. A detailed explanation of the variables studied can be found in the work by Ocwieja et al. (58) or at http://microb230.med.upenn.edu/assets/doc/HeatMapGuide_v12_formatted.doc.
The most striking overall feature of the TcBusterCO, SPINON, Sleeping Beauty, and piggyBac insertion profiles is that they show little insertion bias compared with HIV and MLV. In the human genome, many types of features are correlated with each other (for example, transcription units are located in G/C-rich regions that are also rich in CpG islands and favored sites for cleavage by DNase I). TcBusterCO, SPINON, and piggyBac all showed weak favoring of integration in genomic regions enriched in transcription units, CpG islands, and preferential cleavage sites for DNase I. Sleeping Beauty showed a different pattern, with weaker favoring of integration near these features. All four transposons showed favored integration near genes that were active in HeLa cells based on comparison with transcriptional profiling data for HeLa cells (Fig. 5A, labeled Expr. Density), a pattern that is quite pronounced for HIV (30).
piggyBac and Sleeping Beauty favored integration in short chromosomal regions rich in A/T (Fig. 5A). This trend was most notable at short interval sizes (20 bp surrounding the target site) and for the piggyBac transposon, which targets TTAA at the point of joining, potentially explaining the bias. Sleeping Beauty also showed a strong target sequence preference for integration in A/T-rich regions, but the interval size was larger, suggesting possible contribution of additional factors such as nucleosome wrapping (31).
We have also analyzed the distribution of hAT Buster elements, Sleeping Beauty and piggyBac insertions with respect to epigenetic marks such as histone modification, and the binding of certain regulatory proteins mapped previously in HeLa cells (32) (Fig. 5B). Sleeping Beauty displays no significant difference from random insertion for the large number of epigenetic features that we have evaluated, resembling the pattern for AAV but differing from the other transposons, HIV, and MLV. piggyBac, TcBusterCO, and SPINON showed preferences for integration near chromatin marks characteristic of active transcription units (e.g., H3K27 acetylation and H3K4 monomethylation) and disfavored integration near a mark characteristic of inactive chromatin (H3K27 trimethylation).
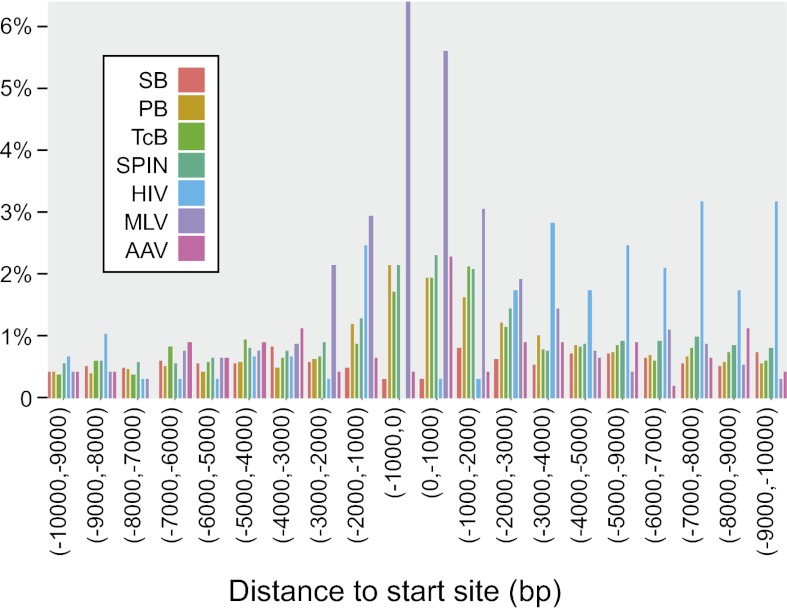
MLV strongly favors integration near gene 5′ ends (28) (Fig. 5A, distance to start; note that the tile is blue on the heat map, because favored distances are shorter than random), and the heat maps in Fig. 5A suggested that this finding is true of TcBusterCO, SPINON, and piggyBac as well. To investigate, integration site frequency was plotted surrounding transcription start sites (Fig. 6). TcBusterCO, SPINON, and piggyBac each showed increased transposition frequency near transcription start sites, and this frequency achieved significance (P = 0.007 and P = 0.0003, respectively, for comparison with matched random controls) for SPINON and piggyBac, although the magnitude of the effect was lower than for MLV. HIV disfavored integration at transcription start sites, which has been reported previously, and unexpectedly, Sleeping Beauty also did.
Fig. 6.
Distribution of Sleeping Beauty, piggyBac, TcBusterCO, and SPINON insertions on the human genome with respect to transcription start sites. Integration sites near transcription start sites were compiled onto a common transcription start site, and the proportions were mapped. The x axis shows the distance from the transcription start site, and the y axis shows the percentage of integration sites in each interval.
TcBusterCO Can Transpose in S. cerevisiae.
We, and others, have shown that Hermes (33) and Ac (34), another hAT Ac subfamily element, can transpose in the yeast S. cerevisiae. We have found that TcBusterCO can also both excise from a donor site and integrate into a new target site in yeast. Expression of TcBusterCO in yeast is not toxic but we were unable, however, to establish a SPINON system in yeast because of lethality in the presence of SPINON transposase and a SPIN transposon (see below).
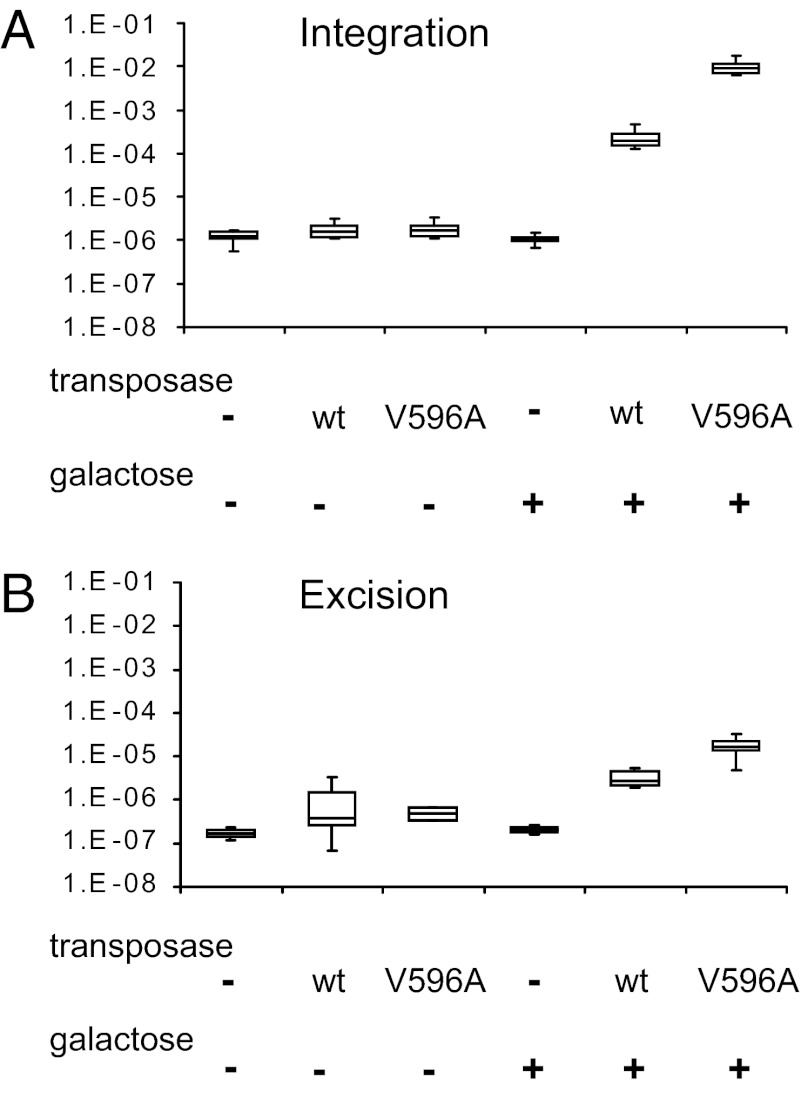
To analyze TcBusterCO integration, we used a two-plasmid system (Fig. S4A). One plasmid expressed the TcBusterCO transposase under the control of the GALS promoter. The other donor plasmid contained a mini TcBuster–ClonNAT element containing 328 bp of the L end and 145 bp of the R end flanking a NatMx cassette that provides resistance to ClonNAT and a separate WT URA3 gene on the plasmid backbone. Plasmid-free cells can be selected for by treatment with fluoroortic acid (5-FOA), because a toxic metabolite is produced in URA3 cells. Thus, chromosomal integration of the mini TcBusterCO element can be assayed by selection for 5-FOAR plasmid-free cells that are also ClonNATR. In particular, we measured the number of 5-FOAR ClonNATR and total cells in colonies grown in the presence or absence of galactose, which induces expression of the transposase. After growth on medium containing galactose to induce transposase expression, we observe a mini TcBusterCO integration frequency of about 5 × 10−4 integrations/cell compared with background frequencies that are about 100-fold lower (i.e., 6.9 × 10−6 in the presence of glucose and 1.3 × 10−6 in the absence of transposase) (Fig. 7). We evaluated the chromosomal location of 12 insertions by Southern blotting and sequencing, finding only a single insertion per cell. All were in different chromosomal positions, and 12 of 12 integrants had the TcBuster consensus nnnTAnnn target site duplication (Table S5).
Fig. 7.
TcBusterCO transposition in yeast. (A) Integration assays that measure the transposition of a TcBuster transposon from a plasmid into the host genome by assaying plasmid-free cells for the presence of a transposon-encoded marker are shown. The transposase is expressed from a plasmid GALS promoter and thus, is induced in the presence of galactose. TcBusterCO V596A has an amino acid change comparable with the hyperactive AeBuster1 V597A mutant. (B) Excision assays that measure the excision of a TcBuster transposon from a donor site in a plasmid URA3 gene by following reversion from Ura− to Ura+ are shown. The transposase is expressed from a plasmid GALS promoter and thus, is induced in the presence of galactose. TcBusterCO V596A has an amino acid change comparable with the hyperactive AeBuster1 V597A mutant.
To measure TcBusterCO excision, we used another two-plasmid system (Fig. S4B). Again, one plasmid expressed the TcBusterCO transposase under the control of the GALS promoter. The other donor plasmid contained a mini TcBuster element inside a URA3 derivative such that reversion from Ura− to Ura+ can be used to measure transposon excision as we have previously described with piggyBac (Fig. S4B) (35). The plasmid URA3 gene contained the yeast actin intron. The actin intron can be readily spliced from the URA3::actin intron mRNA such that cells that are deleted for chromosomal URA3 but contain URA3::actin intron do not require supplementation with uracil for growth (36). When a several-kilobase mini TcBuster transposon containing 328 bp of the L end and 145 bp of the R end flanking a ClonNATR cassette is introduced into the actin intron of URA3::actin intron, however, the large actin mini TcBuster intron cannot be spliced from the mRNA, and cell growth requires supplementation with uracil. Excision of the transposon restores the small actin intron such that cells do not require supplementation with uracil. Thus, measuring the number of Ura+ cells compared with the number of total cells is a measure of transposon excision. We measured Ura+ and total cells in colonies grown in the presence or absence of galactose, which induces expression of the transposase. After growth on galactose, the frequency of TcBusterCO Ura+ revertants is about 2.7 × 10−6, significantly higher than the background level observed after growth in glucose (2.6 × 10−7) or in the absence of transposase (1.2 × 10−7) (Fig. 7B).
Notably, the observed frequency of TcBusterCO integration of 5 × 10−4 is about 200-fold higher than the observed frequency of excision (2.7 × 10−6), likely because of inefficient repair of the hairpins formed in the flanking donor DNA on element excision (34, 37, 38).
We also analyzed the donor site sequence in multiple Ura+ revertants. In the actin intron, the mini TcBuster element is flanked by imperfect target site duplications (CTTTAGGC and CTTTATAC derived from the T. castaneum genome), which are, in turn, flanked by XhoI sites derived from the actin intron. DNA sequence analysis of the donor site in Ura+ revertants revealed that, in 16 of 20 cases, a single XhoI site was present, reconstituting the actin intron sequence. This repair could result from either homology-dependent repair using the chromosomal actin intron in ACT1 as a template or end joining after resection of the flanking T. castaneum genome donor DNA. In the other four cases, a portion of the L-terminal inverted repeat was still present; this finding could reflect incomplete repair or illegitimate recombination that deleted most of the element, which we have seen in other assays lacking a hAT transposase.
We also asked if SPINON could transpose in yeast using both integration and excision assays but found that no cells survived galactose induction in the presence of both the transposase and the mini SPIN transposon element. Cell growth was not affected by the expression of SPINON transposase alone, indicating that lethality is directly related to transposition, perhaps from failure to repair the gaps that flank the newly inserted element or repair the gapped donor site. SPINON induction was also lethal in cells containing a mini TcBuster transposon; the sequence does have some similarity to the SPIN ends, but no lethality or transposition was observed with TcBusterCO transposase and a mini SPINON element.
TcBusterCO Transposase Mutation Results in Increased Transposition in Yeast and Mammalian Cells.
A yeast assay system for transposition is particularly useful, because it provides powerful genetic tools to look for interesting transposase mutants (for example, hyperactive transposase mutants. In other work, we have used the yeast URA3::actin intron excision assay to isolate hyperactive mutants of TcBuster's close relative AeBuster1, finding that AeBuster1 V597A increases AeBuster1 integration about 200-fold and excision about 30-fold. TcBusterCO V596 is equivalent to AeBuster1 V597, and we have examined the effect of TcBusterCO V596A on transposition in both yeast and mammalian cells. In yeast, TcBusterCO V596A increases integration about 50-fold and excision about fivefold (Fig. 7). We also observe increased integration with TcBusterCO V596A in mammalian cells (Fig. 4). Thus, isolation of hyperactive transposases in yeast can provide improved tools for genome engineering in mammalian cells.
We sequenced 12 insertions in the S. cerevisiae genome generated with TcBusterCO V596A, and we found that all inserted into different sites and made 8-bp target site duplications (Table S5).
Purified TcBusterCO and SPINON Transposases Can Promote Transposition in Vitro.
We have also examined the activity of purified TcBusterCO and SPINON transposases in vitro. We tagged the SPINON and TcBusterCO ORFs with affinity tags, expressed them in S. cerevisiae, and purified them by affinity chromatography, finding that both are active in vitro in the absence of other proteins, which may account for their apparently high activity in multiple organisms.
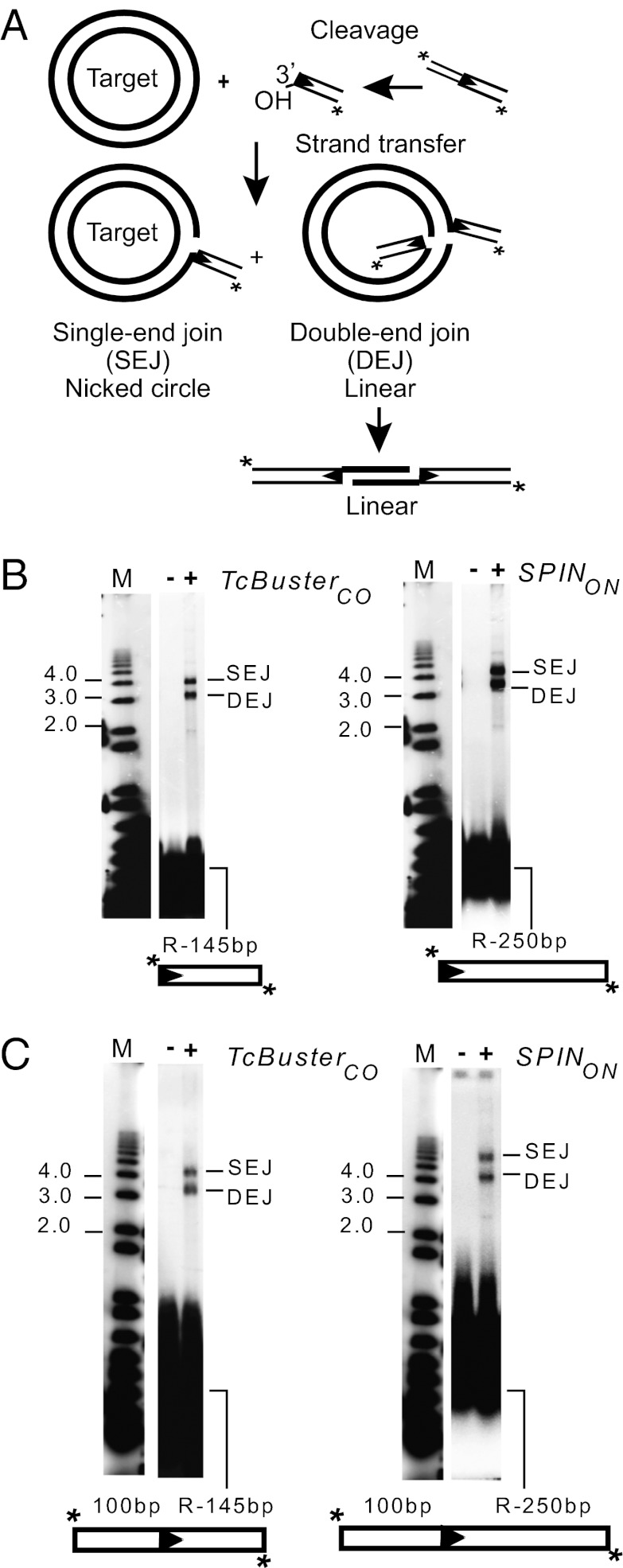
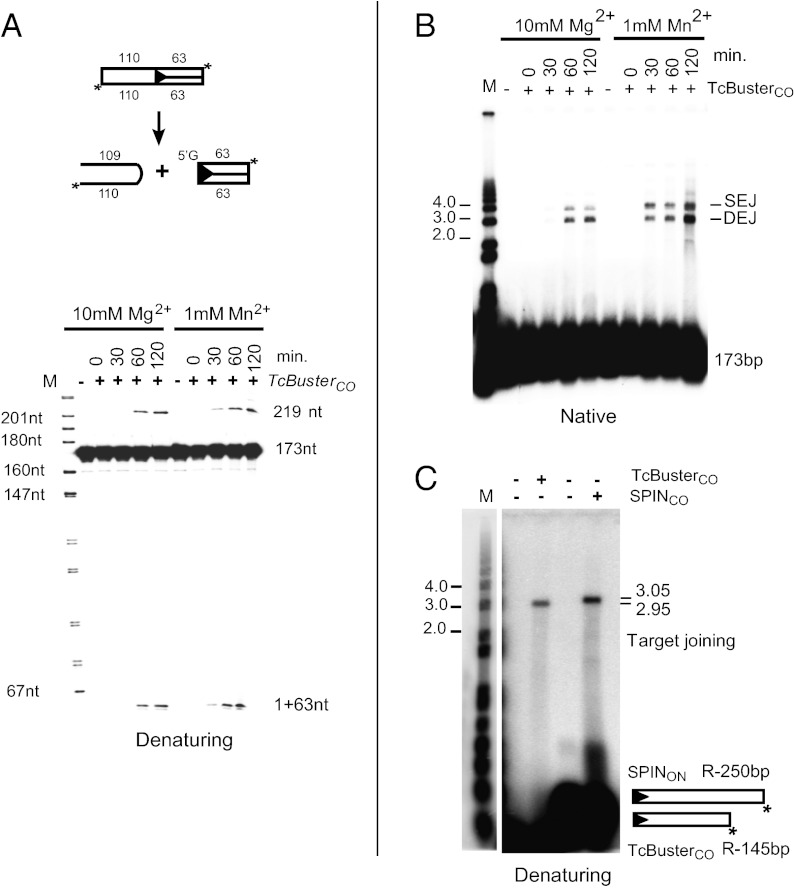
We used two assays to evaluate transposition in vitro (Fig. 8A). In one assay, we used a precleaved transposon-end oligonucleotide in which the 3′OH transposon end was already exposed to evaluate the target joining. In the other assay, we used a transposon-end oligonucleotide in which the end was flanked by donor sequences, allowing us to characterize both donor excision and target joining. Two products are observed in these reactions: one product is a nicked circle form of the target plasmid DNA in which a single transposon-end oligonucleotide is covalently linked to one strand of the target DNA, generating a nicked circle species called a single end join (SEJ), and the other product is a linear plasmid form in which two transposon-end oligoucleotides were joined to the target DNA at the same position to form a double end join (DEJ). In the presence of Mn2+, both purified TcBusterCO and SPINON transposases can promote target joining using a precleaved substrate (Fig. 8B) and can also cleave flanking donor DNA from the transposon end, which can then join to target DNA (Fig. 8C). TcBusterCO can also use Mg2+ as a metal cofactor (Fig. S5).
Fig. 8.
hAT Buster element transposition in vitro. The products of in vitro transposition reactions using end-labeled oligonucleotides and circular plasmid targets are displayed on agarose gels. (A) Substrates, steps, and products of transposition. In SEJs, a single transposon end oligonucleotide joins to the target plasmid, giving a nicked circle; in DEJs, two transposon end oligonucleotides have joined to the target plasmid, giving a linear species. (B) Target joining and coupled cleavage using TcBusterCO transposase and end oligonucleotides as indicated are shown. (C) Target joining and coupled cleavage SPINON and end oligonucleotides as indicated are shown.
Buster Transposons Excise from the Donor Through a Hairpin Intermediate, and 3′OH Transposon Ends Join to the Target DNA.
We have previously shown (39) that the hAT element Hermes excises from the donor DNA through a hairpin reaction that begins with a nick 1 nt into the donor DNA adjacent to the 5′ transposon end, exposing a 3′OH in the top strand of the donor DNA. This 3′OH then attacks its complementary strand, generating a hairpin on the flanking donor DNA and releasing the transposon end with an exposed 3′OH. This 3′OH then directly attacks the target DNA as in other reactions mediated by RNase H-family transposases (40, 41).
To evaluate the Buster element double-strand cleavage reaction that disconnects the transposon end from the flanking donor DNA and target joining, we incubated TcBusterCO transposase with an oligonucleotide substrate in which a transposon end was flanked by nontransposon sequences and examined recombination over time using both Mg2+ and Mn2+ as cofactors (Fig. 9). When the reactions were displayed on a native agarose gel, we observe increasing amounts of both SEJ and DEJ products over time with both cofactors. We also analyzed these reactions by displaying them on denaturing gels, and we observed the formation of a 64-nt transposon top strand resulting from the initial nicking reaction and a 219-bp nucleotide hairpin species (Fig. 9A).
Fig. 9.
Mechanism of Buster element transposition. (A) TcBuster transposition occurs through a hairpin intermediate. The products of transposition reactions over time use TcBusterCO transposase, a 3′ end-labeled oligo in which the transposon end is flanked by donor DNA and divalent metal are displayed (A) on a denaturing acrylamide gel, (B) a native agarose gel, and (C) a denaturing agarose gel. SEJ and DEJ products as described in Fig. 8 are observed on the native gel. On the denaturing gel, a 64-bp fragment containing the top strand of the transposon (63 nt) and 1 nt from the flanking donor DNA (reflecting that the initiating nick occurs 1 nt from the 5′ transposon end into the flanking donor DNA) and flanking donor 219-nt DNA hairpin species containing 110 nt of the bottom strand and 109 nt of top strand increase over time. (B) Coupled cleavage and strand transfer. The products of the reactions in A are displayed on a native agarose gel. (C) The 3′ end of the transposon is attached covalently to the target DNA. The products of reactions using either TcBusterCO or SPINON transposases as indicated and their cognate transposon ends labeled on the 5′ end of the bottom strand are displayed on a denaturing agarose gel. The covalent linkage of the labeled transposon end and target DNA in the reaction product shows that the 3′OH transposon end joined to the target DNA.
To verify that the terminal 3′OH end of the Buster transposons was covalently linked to the substrate DNA, we used TcBusterCO transposase and a precleaved transposon-end oligonucleotide that was labeled at the 5′ end of the strand containing the terminal 3′OH as a substrate; we displayed the reaction products on a denaturing agarose gel, observing a single species the length of the plasmid plus the length of the transposon, consistent with 3′ transposon-end joining to a single target strand (Fig. 9C).
Thus, TcBusterCO transposase from the Buster subfamily of transposons breaks and joins DNA as does Hermes, the Ac subfamily hAT transposase, using the same sorts of phosphoryl transfer reactions as other RNase H-family transposases and required no additional proteins as obligate cofactors.
Discussion
The experiments described here provide detailed analysis of two members of the newly bioinformatically defined Buster subfamily of hAT transposable elements (16) (Fig. S1). One of these elements, TcBuster from the red flour beetle T. castaneum, is likely currently active in this host; we show here that it is active in mammalian cells, yeast, and in vitro, and we have shown previously that it can be active in Drosophila (16). By contrast, there are no known currently active SPIN elements; most of the currently identified elements contain stop codons in the transposase ORF. SPIN elements were identified in the work by Pace et al. (12) in a number of tetrapod genomes, including those genomes of several mammals and more recently, in two invertebrates (13, 15). These elements were highly active in these genomes ∼20–40 Mya, amplifying to high copy number. Intriguingly, the patchy phylogenetic distribution of closely related SPIN elements, combined with their extreme level of sequence similarity, suggests that they entered these different hosts by horizontal transmission. We have made an active SPIN element that we have called SPINON by synthesizing a consensus version.
We have shown that both TcBuster and SPINON are highly active in mammalian cells, and we have compared them to two other transposons that are widely used in mammalian cell genome engineering: Sleeping Beauty, which has been resurrected from bony fish, and piggyBac, which was discovered in a baculovirus of the cabbage looper moth (18, 42). Both SPINON and TcBusterCO have transposition frequencies in HeLa cells higher than the hyperactive HSB16 mutant of the Sleeping Beauty transposase (19), and they approach the activity of the piggyBac transposase in these cells (Figs. 1–4). We did not see signs of overproduction inhibition for the TcBuster transposase or piggyBac transposase under our experimental conditions. Future work will be required to compare TcBuster and SPINON with the 100× Sleeping Beauty transposase and the hyperactive piggyBac transposase in different cell types (43).
Target Site Selection at the Nucleotide Level.
We have analyzed hAT Buster element target site selection in mammalian cells and for comparison, target selection by Sleeping Beauty and piggyBac. For all elements, we used ligation-mediated PCR to recover large numbers of de novo mammalian insertion sites and analyzed them by next generation sequencing; the number of target sites ranged from 4,500 to 13,500 among the elements, which is larger than other transposon datasets from mammalian cells studied to date.
A previous study indicated that nnnTAnnn is the preferred target site of Buster elements (16). We have found that the requirement for the central TA dinucleotide is, in fact, quite stringent, being present in 94.6% of the 6,390 TcBuster insertion sites and 95.3% of 8,333 SPINON mammalian insertions (Fig. S3 and Table S4). The preference for this Buster target feature is very similar to the TA (98.4%) and TTAA (97.6%) target site requirements that we have found for the Sleeping Beauty and piggyBac elements, respectively (44, 45). The Buster target site duplication TAs are in the center of the 8-bp target site duplication (nnnTAnnn), whereas the Mariner/Tc1 TA (Sleeping Beauty) and piggyBac TTAA comprise the entire target site duplication. We know of no other element having such a stringent requirement for a particular sequence in an internal portion of the target site duplication (Fig. S3). The central TA is a defining feature of elements of the Buster subfamily of elements; most members of the hAT Ac subfamily prefer to insert into nTnnnnAn sites (16). The stringency of the Buster elements for nnnTAnnn provides an excellent system to study the mechanism of target site selection at the molecular level. Our other work on the Ac subfamily element Hermes, including the establishment of an in vitro transposition system (39) and analysis of Hermes structure at the crystallographic level (41), will provide useful complementary tools for future mechanistic studies of the hAT Buster elements.
Our analysis of large numbers of de novo insertion sites for piggyBac, TcBuster, and SPIN also revealed preferred secondary insertion sites. With piggyBac, the most common secondary sites contained a C or A at the position of transposon-end joining as opposed to the standard T in a TTAA target site. With the Buster elements, secondary sites had TG or CA changes at the central TA of the nnnTAnnn target site duplication rather than changes at the positions of end joining (Fig. S3 and Table S4). It will be interesting to analyze the effects of these changes on transposon excision.
Target Site Selection at the Genome-Wide Level.
We have also analyzed the pattern of Sleeping Beauty, piggyBac, TcBusterCO, and SPINON insertions with respect to genomic-wide features, such as genes and gene regulatory sites, and epigenetic features, such as posttranslationally modified histones (Fig. 5). Three of the transposons show a weak favoring of integration in gene-rich, transcriptionally active regions (piggyBac, TcBusterCO, and SPINON). Genomic features associated with active genes, and gene-rich regions were also associated, including CpG islands, DNase I cleavage sites, histone acetylation sites, and activating histone methylation marks. The H3K27 trimethylation mark, which is negatively associated with transcription, was negatively associated with integration of these three transposons. Sleeping Beauty was the most divergent, showing little preference for or against integration near these genomic features, which was seen in a previous study (26). Sleeping Beauty most resembled AAV, which becomes integrated at DNA double-strand breaks (46). Evidently, the placement of spontaneous DNA double-strand breaks is relatively insensitive to the chromosomal features studied here, although why Sleeping Beauty integration should show a similar distribution is an interesting topic for additional study.
The three transposons that favored integration in gene-rich regions (piggyBac, TcBusterCO, and SPINON) also tended to favor integration near gene 5′ ends, paralleling MLV. Sleeping Beauty, in contrast, resembled HIV in disfavoring integration near transcription start sites. For HIV, integration targeting is known to be largely due to tethering by binding to the cellular LEDGF/p75 protein (38, 47, 48), and therefore, the lack of integration near transcription start sites may be caused by the lack of the tethering factor. Whether MLV binds a positively acting tethering factor at transcription start sites is unknown but is, at present, a leading model. It may be that piggyBac, TcBusterCO, and SPINON bind to cellular factors at transcription start sites that promote nearby integration. Our previous studies of the distribution of Hermes insertions in the S. cerevisiae genome suggest that a major mechanism for targeting these insertions to transcription start sites is the lack of nucleosome binding in these regions (33).
TcBusterCO and SPINON Are Promising Tools for Genome Engineering.
In addition to being interesting in their own right because of their important roles in genome structure and function and as elaborate protein–DNA machines, DNA transposons have been used in many organisms as important tools for genome engineering, including insertional mutagenesis and transgenesis. Notably, they have been used for the identification of genes involved in human disease and in the treatment of some of these genetic diseases through gene therapy (5, 6, 49). For example, their ability to move within genomes and act as mutagens in model systems has been elegantly used to identify genes potentially involved in oncogenesis in mice, leading to the identification of their human orthologs as possible candidates for additional investigation (50–52). The two transposons used for these studies were Sleeping Beauty and piggyBac, each being members of different superfamilies of transposons and having different target site preferences. Their ability to carry genes into genomes has led to the development of these transposons and the Tol2 element from the medaka fish as gene vectors for use in human cells (4, 53). Tol2 is a member of the hAT superfamily and therefore, has a target site preference different from the preferences of piggyBac and Sleeping Beauty (54). Having transposons that have different target site preferences and differ in their mechanisms of excision and integration during transposition is important to provide broad genome coverage in mutagenesis studies, and it also allows the possibility of introducing different transposons with different cargoes into genomes, which may be especially useful with human genome engineering, gene vectors for use in human gene therapy, regenerative medicine, and rodent genetics.
The development of the TcBuster yeast system provides a powerful means not only of studying transposition (for example, identifying host proteins involved in transposition by analyzing transposition in the yeast deletion strain collection) but importantly, for isolating hyperactive versions of the TcBuster transposase that will be hyperactive in mammalian cells. We have already used a similar yeast excision assay to identify hyperactive piggyBacs that are also hyperactive in mammalian cells (21) and isolate hyperactives of AeBuster1, which is closely related to TcBuster. We have shown here that changes in similar amino acids in TcBusterCO can lead to increased transposition. A hyperactive 100× Sleeping Beauty mutant (55, 56) has been successfully isolated by site-directed mutagenesis, and is hyperactive in HeLa cells as well as murine embryonic and adult stem cells. Such hyperactive transposases facilitate transposon use as vectors in human gene therapy, regenerative medicine, and rodent genetics (44, 55). As a consequence, the use of these hAT transposons should accelerate the ability to find genes involved in disease in rodent models and therefore, identify the relevant human orthologs.
The generation and testing of mutants of at least TcBuster will be accelerated by our ability to determine their function both in vitro and in vivo in yeast before placing into mammalian systems. This pipeline, which should be able to be extended to the SPINON tranposases by making replacements, should generate a suite of transposases that will further expand the growing toolkit of transposons that can be used in mammalian systems.
Methods
Transposition in Mammalian Cells.
Transposition was measured in HeLa cells by cotransfecting a donor plasmid containing mini transposon-containing segments from the ends of the element flanking an antibiotic resistance gene or other marker and a helper plasmid expressing a transposase under a human CMV promoter, which was followed by selection for antibiotic-resistant cells.
Integration Site Recovery, 454 Sequencing, and Analysis.
Mammalian integration sites were recovered as described (31). Briefly, genomic DNA was extracted from an integration transfection library using the DNeasy tissue kit (Qiagen); 2 μg genomic DNA were digested overnight with ApoI or BstYI, and then ligated to linkers overnight at 16 °C. Nested PCR was then carried out under stringent conditions using transposon end-specific primers complementary to transposon sequences and linker-specific primers complementary to the DNA linker. DNA barcodes were included in the second-round PCR primers to track sample origin. The PCR products were gel-purified, pooled, and sequenced using 454 sequencing platform. Only sequences that uniquely aligned to the human genome by BLAT (hg18, version 36.1; >98% match score) and began within 3 bp of the LTR end were used in downstream analyses.
Bioinformatic Analysis of Target Site Duplications and Other Genomic Features.
Detailed bioinformatic methods for analysis of association with chromosomal features are described in the work by Berry et al. (26). The methods for generating heat maps based in receiver operating characteristic curves (ROC) are as described in the work by Berry et al. (26). A detailed description of the methods used to generate the genomic features heat map in Fig. 5A can be found at http://microb230.med.upenn.edu/assets/doc/HeatMapGuide_v12_formatted.doc.
TcBuster Integration and Excision Assays in S. cerevisiae.
Integration assays were performed in BY4727 (MAT alpha his3∆200 leu2∆0 lys2∆0 met150 trp1∆63 ura3∆0) (57) after transformation with the Trp+ pGALS TcBuster transposase helper plasmid and the pRS416 URA3 mini TcBuster-ClonNAT donor plasmid. Integration was measured by selection for 5-FOA–resistant plasmid-free cells containing the ClonNAT-resistant mini TcBuster element. Excision assays were performed in BY4727 transformed with the TRP+ pGALS TcBusterCO transposase helper plasmid and the HIS+ URA3::actin intron::TcBuster-ClonNat excision donor plasmid. Excision was measured by selection for cells that reverted from Ura− to Ura+.
TcBusterCO and SPINON Transposase Expression and Purification from Yeast.
Strep-HA tags (IBA) were added to the N termini of the TcBusterCO and SPINON transposases, allowing for purification from yeast extracts by affinity chromatography on a Strep Tactin (IBA) column. More than 60% of transposase protein was recovered and 90% pure as judged from protein gels.
In Vitro Transposition Assays.
One hundred fifty nanomolar transposase was incubated with 1.5 nM radiolabeled transposon end and 10 nM pUC19 plasmid as the target DNA in 25 mM MOPS, pH 7.0, 25 mM Tris⋅HCl, pH 8.0, 37.5 mM NaCl, 1 mM MnCl2, 5 mM DTT, 20% (vol/vol) glycerol or DMSO, and 100 μg/mL BSA in a final volume of 20 μL at 37 °C in 1 h. Transposon end segments were either flanked by donor DNA or had exposed 3′OH transposon termini; end fragments were generated by PCR.
Supplementary Material
Acknowledgments
We thank Dr. Sue Brown (Kansas State University, Manhattan, KS), Dr. David O'Brochta (University of Maryland, College Park, MD), and Dr. Bradley Fletcher (University of Florida, Gainesville, FL) for providing T. castaneum genomic DNA, piggyBac plasmids, and a Sleeping Beauty plasmid, respectively. We thank Kefang Xie for identifying the hyperactive AeBuster1 hyperactive mutant in a screen in yeast. We also thank Patti Kodeck for her assistance with the manuscript and Helen McComas for her assistance with the figures. This work was supported by National Institutes of Health Grants GM077582 (to C.F.), AI52845 (to F.D.B.), AI45741 (to P.W.A. and N.L.C.), and GM076425 (to N.L.C.). The work was also supported by a grant from the Maryland Stem Cell Research Fund (to N.L.C.), and N.L.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession nos. JS684848–JS799249).
See Author Summary on page 1988 (volume 110, number 6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121543109/-/DCSupplemental.
References
- 1.Biémont C. A brief history of the status of transposable elements: From junk DNA to major players in evolution. Genetics. 2010;186:1085–1093. doi: 10.1534/genetics.110.124180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Schnable PS, et al. The B73 maize genome: Complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 4.Izsvák Z, Hackett PB, Cooper LJ, Ivics Z. Translating Sleeping Beauty transposition into cellular therapies: Victories and challenges. Bioessays. 2010;32:756–767. doi: 10.1002/bies.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claeys Bouuaert C, Chalmers RM. Gene therapy vectors: The prospects and potentials of the cut-and-paste transposons. Genetica. 2010;138:473–484. doi: 10.1007/s10709-009-9391-x. [DOI] [PubMed] [Google Scholar]
- 6.Ivics Z, Izsvak Z. The expanding universe of transposon technologies for gene and cell engineering. Mob DNA. 2010;1:25. doi: 10.1186/1759-8753-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007;8(Suppl 1):S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim A, Pyykko I. Size matters: Versatile use of PiggyBac transposons as a genetic manipulation tool. Mol Cell Biochem. 2011;354:301–309. doi: 10.1007/s11010-011-0832-3. [DOI] [PubMed] [Google Scholar]
- 9.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 10.Gogarten JPTJ, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol. 2005;3:679–687. doi: 10.1038/nrmicro1204. [DOI] [PubMed] [Google Scholar]
- 11.Schaack S, Gilbert C, Feschotte C. Promiscuous DNA: Horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol. 2010;25:537–546. doi: 10.1016/j.tree.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pace JK, 2nd, Gilbert C, Clark MS, Feschotte C. Repeated horizontal transfer of a DNA transposon in mammals and other tetrapods. Proc Natl Acad Sci USA. 2008;105:17023–17028. doi: 10.1073/pnas.0806548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert C, Schaack S, Pace JK, 2nd, Brindley PJ, Feschotte C. A role for host-parasite interactions in the horizontal transfer of transposons across phyla. Nature. 2010;464:1347–1350. doi: 10.1038/nature08939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert C, Hernandez SS, Flores-Benabib J, Smith EN, Feschotte C. Rampant horizontal transfer of SPIN transposons in squamate reptiles. Mol Biol Evol. 2012;29(2):503–515. doi: 10.1093/molbev/msr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novick P, Smith J, Ray D, Boissinot S. Independent and parallel lateral transfer of DNA transposons in tetrapod genomes. Gene. 2010;449:85–94. doi: 10.1016/j.gene.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Arensburger P, et al. Phylogenetic and functional characterization of the hAT transposon superfamily. Genetics. 2011;188:45–57. doi: 10.1534/genetics.111.126813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smit AF. Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr Opin Genet Dev. 1999;9:657–663. doi: 10.1016/s0959-437x(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 18.Ivics Z, Hackett PB, Plasterk RH, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 19.Baus J, Liu L, Heggestad AD, Sanz S, Fletcher BS. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol Ther. 2005;12:1148–1156. doi: 10.1016/j.ymthe.2005.06.484. [DOI] [PubMed] [Google Scholar]
- 20.Hartl DL, Lozovskaya ER, Nurminsky DI, Lohe AR. What restricts the activity of mariner-like transposable elements. Trends Genet. 1997;13:197–201. doi: 10.1016/s0168-9525(97)01087-1. [DOI] [PubMed] [Google Scholar]
- 21.Yusa K, Zhou L, Li MA, Bradley A, Craig NL. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci USA. 2011;108:1531–1536. doi: 10.1073/pnas.1008322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, et al. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Mol Ther. 2010;18:1803–1813. doi: 10.1038/mt.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvan DL, et al. Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J Immunother. 2009;32:837–844. doi: 10.1097/CJI.0b013e3181b2914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yant SR, et al. High-resolution genome-wide mapping of transposon integration in mammals. Mol Cell Biol. 2005;25:2085–2094. doi: 10.1128/MCB.25.6.2085-2094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigdal TJ, Kaufman CD, Izsvák Z, Voytas DF, Ivics Z. Common physical properties of DNA affecting target site selection of sleeping beauty and other Tc1/mariner transposable elements. J Mol Biol. 2002;323:441–452. doi: 10.1016/s0022-2836(02)00991-9. [DOI] [PubMed] [Google Scholar]
- 26.Berry C, Hannenhalli S, Leipzig J, Bushman FD. Selection of target sites for mobile DNA integration in the human genome. PLoS Comput Biol. 2006;2:e157. doi: 10.1371/journal.pcbi.0020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewinski MK, et al. Retroviral DNA integration: Viral and cellular determinants of target-site selection. PLoS Pathog. 2006;2:e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 29.Miller DG, et al. Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol. 2005;79:11434–11442. doi: 10.1128/JVI.79.17.11434-11442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schröder AR, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang GP, Ciuffi A, Leipzig J, Berry CC, Bushman FD. HIV integration site selection: Analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res. 2007;17:1186–1194. doi: 10.1101/gr.6286907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meylan S, et al. A gene-rich, transcriptionally active environment and the pre-deposition of repressive marks are predictive of susceptibility to KRAB/KAP1-mediated silencing. BMC Genomics. 2011;12:378. doi: 10.1186/1471-2164-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gangadharan S, Mularoni L, Fain-Thornton J, Wheelan SJ, Craig NL. DNA transposon Hermes inserts into DNA in nucleosome-free regions in vivo. Proc Natl Acad Sci USA. 2010;107:21966–21972. doi: 10.1073/pnas.1016382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weil CF, Kunze R. Transposition of maize Ac/Ds transposable elements in the yeast Saccharomyces cerevisiae. Nat Genet. 2000;26:187–190. doi: 10.1038/82827. [DOI] [PubMed] [Google Scholar]
- 35.Mitra R, Fain-Thornton J, Craig NL. piggyBac can bypass DNA synthesis during cut and paste transposition. EMBO J. 2008;27:1097–1109. doi: 10.1038/emboj.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Gabriel A. Patching broken chromosomes with extranuclear cellular DNA. Mol Cell. 1999;4:873–881. doi: 10.1016/s1097-2765(00)80397-4. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Marshall K, Yamaguchi M, Haber JE, Weil CF. Microhomology-dependent end joining and repair of transposon-induced DNA hairpins by host factors in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:1351–1364. doi: 10.1128/MCB.24.3.1351-1364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall HM, et al. Role of PSIP1/LEDGF/p75 in lentiviral infectivity and integration targeting. PLoS ONE. 2007;2:e1340. doi: 10.1371/journal.pone.0001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L, et al. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature. 2004;432:995–1001. doi: 10.1038/nature03157. [DOI] [PubMed] [Google Scholar]
- 40.Craig N. In: Mobile DNA II. Craig N, Craigie R, Gellert M, Lambowitz A, editors. Washington: ASM Press; 2002. pp. 423–456. [Google Scholar]
- 41.Hickman AB, Chandler M, Dyda F. Integrating prokaryotes and eukaryotes: DNA transposases in light of structure. Crit Rev Biochem Mol Biol. 2010;45:50–69. doi: 10.3109/10409230903505596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cary LC, et al. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- 43.Grabundzija I, et al. Comparative analysis of transposable element vector systems in human cells. Mol Ther. 2010;18:1200–1209. doi: 10.1038/mt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elick TA, Bauser CA, Principe NM, Fraser MJ., Jr PCR analysis of insertion site specificity, transcription, and structural uniformity of the Lepidopteran transposable element IFP2 in the TN-368 cell genome. Genetica. 1996;97:127–139. doi: 10.1007/BF00054620. [DOI] [PubMed] [Google Scholar]
- 45.Liu CL, et al. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 2005;3:e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller DG, Petek LM, Russell DW. Adeno-associated virus vectors integrate at chromosome breakage sites. Nat Genet. 2004;36:767–773. doi: 10.1038/ng1380. [DOI] [PubMed] [Google Scholar]
- 47.Ciuffi A, et al. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11:1287–1289. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- 48.Shun MC, et al. LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration. Genes Dev. 2007;21:1767–1778. doi: 10.1101/gad.1565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupuy A. Splendours and miseries of the benefit-risk ratio. Ann Dermatol Venereol. 2010;137:267–268. doi: 10.1016/j.annder.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221–226. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- 52.Dupuy AJ, Jenkins NA, Copeland NG. Sleeping beauty: A novel cancer gene discovery tool. Hum Mol Genet. 2006;15:R75–R79. doi: 10.1093/hmg/ddl061. [DOI] [PubMed] [Google Scholar]
- 53.Ivics Z, Izsvák Z. Transposons for gene therapy! Curr Gene Ther. 2006;6:593–607. doi: 10.2174/156652306778520647. [DOI] [PubMed] [Google Scholar]
- 54.Hori H, Suzuki M, Inagaki H, Oshima T, Koga A. An active Ac-like transposable element in teleost fish. J Mar Biotechnol. 1998;6:206–207. [PubMed] [Google Scholar]
- 55.Belay E, et al. Novel hyperactive transposons for genetic modification of induced pluripotent and adult stem cells: A nonviral paradigm for coaxed differentiation. Stem Cells. 2010;28:1760–1771. doi: 10.1002/stem.501. [DOI] [PubMed] [Google Scholar]
- 56.Xue XHX, et al. Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood. 2009;114:1319–1330. doi: 10.1182/blood-2009-03-210005. [DOI] [PubMed] [Google Scholar]
- 57.Brachmann CB, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 58.Ocwieja KE, et al. HIV integration targeting: A pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog. 2011;7:e1001313. doi: 10.1371/journal.ppat.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]