Abstract
Nature and physiological status of antigen-presenting cells, such as dendritic cells DCs, are decisive for the immune reactions elicited. Multiple factors and cell interactions have been described that affect maturation of DCs. Here, we show that DCs arising in the absence of immunoglobulins (Ig) in vivo are impaired in cross-presentation of soluble antigen. This deficiency was due to aberrant cellular targeting of antigen to lysosomes and its rapid degradation. Function of DCs could be restored by transfer of Ig irrespective of antigen specificity and isotype. Modulation of cross-presentation by Ig was inhibited by coapplication of mannan and, thus, likely to be mediated by C-type lectin receptors. This unexpected dependency of splenic DCs on Ig to cross-present antigen provides insights into the interplay between cellular and humoral immunity and the immunomodulatory capacity of Ig.
Dendritic cells (DCs) constitute the subset of professional antigen-presenting cells (APCs) that is most potent in initiating adaptive immune responses. To prime naïve CD4+ helper or CD8+ cytotoxic T cells, DCs process and present antigen in the context of MHC II or MHC I, respectively. MHC II presentation is largely restricted to exogenous antigen taken up via different endocytotic mechanisms. In contrast, MHC I presentation is restricted to endogenous antigen in most cells types. However, DCs are specifically equipped with an alternative pathway for presentation of exogenous antigen via MHC I, referred to as cross-presentation (1–4). Given that many viruses do not directly infect DCs, initiation of most CD8+ T-cell responses requires cross-priming of such cells via cross-presentation.
The molecular mechanisms of cross-presentation remain largely elusive, and multiple pathways of antigen transport, processing, and loading might exist, which are not mutually exclusive.
Ovalbumin (OVA) is one of the best studied model antigens in cross-presentation. Soluble OVA has been proposed to be engulfed via mannose receptor (MR) mediated endocytosis into specialized stable early endosomal compartments. Subsequently, antigen is exported to the cytosol, processed by proteasomal degradation and reimported via transporter associated with antigen processing (TAP) to early endosomes for final trimming by the insulin-regulated aminopeptidase (IRAP) and loaded onto MHC I molecules (3, 5–7). However, different forms of antigen may be cross-presented via different routes (4).
Homeostasis and function of the immune system requires complex interactions between its components. Accordingly, T and B cells influence development, function, and maturation status of DCs. In addition to the well-established role of T cells in shaping DC function (8–10), B cells appear to be able to modulate the functional maturation of DCs (11). Thus, lack of B cells skews the T-cell response toward Th1 by promoting expression of IL-12 by DCs. Such regulatory function is likely to be mediated via secretion of cytokines (11). Immunoglobulins (Ig) constitute the largest fraction of secretory molecules from B cells. They, mostly in the form of immune complexes (ICs) or acting via Fc receptors, have been suggested to influence DC function and, in particular, cross-presentation (12, 13). However, the mechanism and extent how Ig and/or ICs affect DC maturation and antigen presentation remain poorly understood.
Therefore, we tested the hypothesis that development of fully functional DCs depends on the presence of a functional adaptive immune system. We observed that cross-presentation of soluble antigen by splenic conventional DCs (cDCs) generated in lymphopenic mice was severely impaired. This inefficient cross-presentation in the absence of T and B cells was due to aberrant antigen trafficking and rapid degradation of antigen, thus preventing efficient loading and antigen presentation by MHC I. We showed that efficient cross-presentation depended on serum Ig, which presumably acts via C-type lectin receptors (CLRs). Taken together, our results reveal a unique mechanism for regulation of DC development via soluble Ig.
Results
Impaired Cross-Presentation by Splenic cDCs Generated in a Lymphopenic Environment.
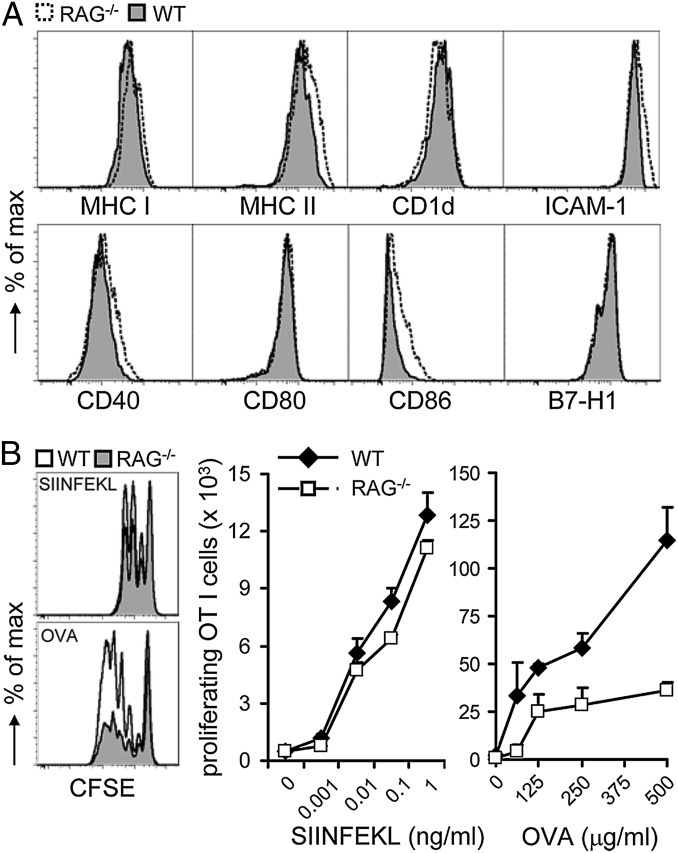
Function of DCs critically depends on their maturation status. Therefore, first, we reassessed how the lack of components of the adaptive immune system affects maturation of splenic DCs to full function. To this end, we examined splenic cDCs from RAG-deficient mice that lack T and B cells and WT mice for their maturation status and capacity to present antigen. No major differences in surface expression of MHC I, MHC II, CD1d, ICAM-1, and other costimulatory molecules were observed (Fig. 1A), indicating an overall similar maturation status of splenic cDCs from RAG−/− and WT mice. Next, we sensitized cDCs from either mouse strain with soluble OVA, which requires cross-presentation, or with cognate peptide (SIINFEKL, OVA257–264), which is independent of cross-presentation. OVA protein-sensitized splenic cDCs isolated from RAG-deficient mice were impaired in priming OT I T cells compared with OVA-loaded WT cDCs (Fig. 1B). In contrast, peptide-pulsed cDCs from RAG-deficient and WT mice primed OT I T cells equally well. Thus, although displaying a comparable maturation status at the cell surface, splenic cDCs from RAG-deficient mice cannot cross-present soluble antigen effectively.
Fig. 1.
Selectively impaired cross-presentation by splenic cDCs generated in a lymphopenic environment. (A) Splenocytes of WT and RAG−/− mice were electronically gated as CD11chiCD11b+/−CD8α+/−B220− population analyzed for expression of the indicated markers. Data are representative of at least four or five mice per group in six independent experiments. (B) Splenic cDCs isolated from WT or RAG−/− mice were loaded with OVA257–264 peptide or OVA protein for 1 h. CFSE-labeled OT I T cells were incubated for 1.5 d (peptide) or 2 d (OVA) with cDCs at a 10:1 ratio. The proliferative response of T cells was enumerated by flow cytometry. Numbers of proliferating cells are shown (mean + SEM). Data are representative of multiple independent experiments with a minimum three mice per group.
Absence of T and B Cells During cDC Development in Vivo Leads to Different Antigen Trafficking and Enhanced Degradation.
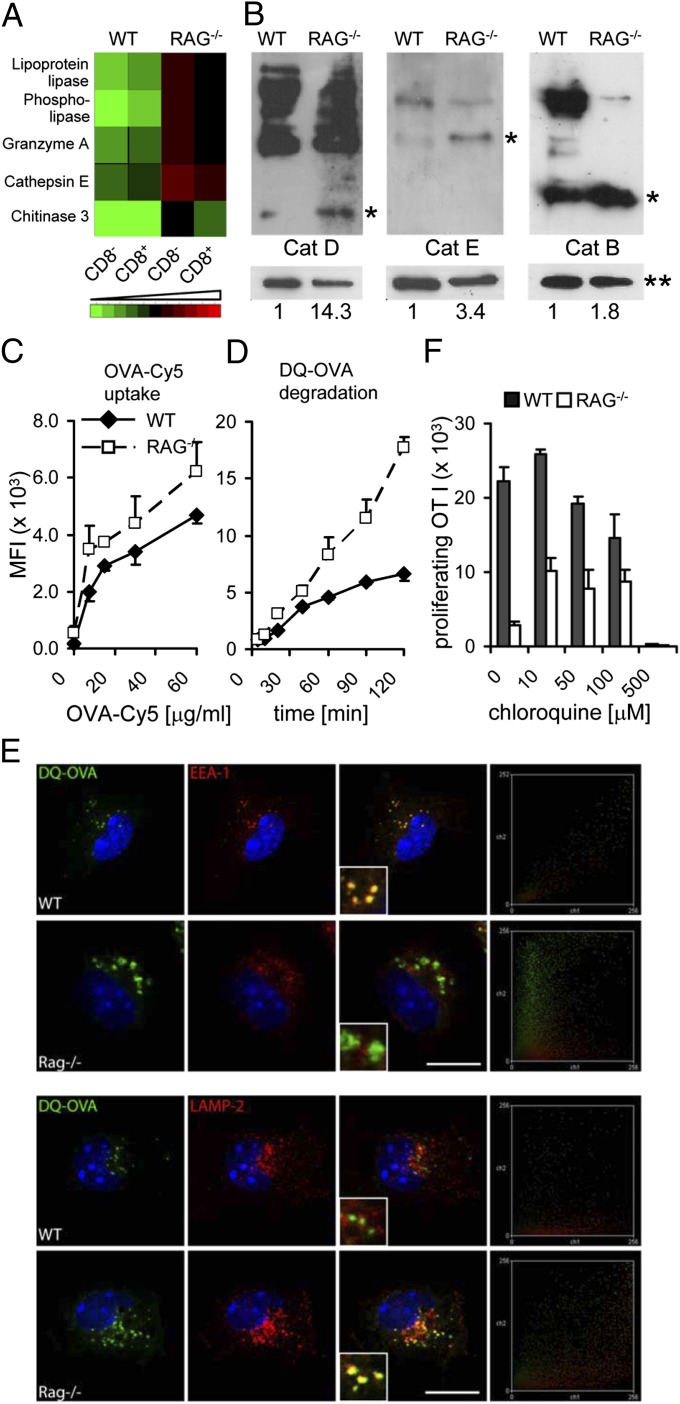
We set out to identify the mechanism underlying the deficiency in cross-presentation by cDCs from RAG−/− mice. First, we performed gene expression analysis by using sorted splenic CD8α− and CD8α+ cDCs from RAG−/− and WT mice. This experiment revealed that among others several CLRs were expressed at elevated levels in cDCs from lymphopenic mice as were genes encoding endolysosomal enzymes including Cathepsins (Fig. 2A). Because Cathepsins require proteolytic processing for activation, the analysis of mRNA expression and immunofluorescent microscopy (Fig. S1) are only of limited value. Western blot analysis revealed that in splenic cDCs from RAG-deficient mice Cathepsins D, E, and B are present at highly elevated levels in catalytically active form compared with cDCs from WT mice (Fig. 2B). Thus, more efficient antigen uptake and more aggressive degradation of soluble antigen could be a possible consequence. Indeed, analysis of antigen uptake using fluorophore-coupled OVA (OVA-Cy5) demonstrated increased endocytosis of OVA by cDCs from RAG−/− mice (Fig. 2C). Similarly, as predicted, antigen processing was increased in RAG−/− cDCs (Fig. 2D). Antigen processing was assessed by measuring fluorescence of DQ-OVA, the emission of which depends on degradation. Together these experiments suggest that in the absence of T and B cells, cDCs acquire antigen more efficiently, but their increased proteolytic activity ultimately results in reduced presence of MHC I–peptide complexes. To substantiate this hypothesis, we visualized OVA trafficking in cDCs by using fluorescence microscopy. Colocalization of DQ-OVA degradation products with EEA1-positive early endosomes was observed only in WT cDCs (Fig. 2E, Upper). In contrast, cDCs from RAG-deficient mice exhibited DQ-OVA degradation products mainly in LAMP2-positive late endosomal/lysosomal compartments (Fig. 2E, Lower). These results prompted us to test the hypothesis that inhibition of lysosomal acidification should restore cross-presentation ability in cDCs from RAG-deficient mice. To this end, we treated sorted DCs with graded concentrations of chloroquine before loading with soluble OVA and tested their ability to activate OT I T cells. Consistent with our hypothesis, inhibition of lysosomal acidification in cDCs from RAG-deficient mice improved their capacity to cross-present soluble OVA (Fig. 2F).
Fig. 2.
Absence of T and B cells during DC development leads to aberrant trafficking and enhanced degradation of antigen. (A) Expression analysis of endolysosomal enzymes in splenic cDCs. (B) Western blot analysis of Cathepsins D, E, and B in DCs sorted from WT and RAG−/− mice. Numbers below plots indicate relative quantification of band intensity of Cathepsins D and B [25-kDa bands (*)] and of Cathepsin E [40-kDa band (*)]. **, loading control. Data are representative of two independent experiments. (C) WT and RAG−/− splenic cDCs were incubated for 1 h with the indicated concentrations of OVA-Cy5. (D) WT and RAG−/− splenic cDCs were loaded with 62.5 μg/mL DQ-OVA for 45 min, incubated at 37 °C for the indicated time points, and analyzed by flow cytometry. Data are representative of three independent experiments with a minimum three mice per group. (E) WT and RAG−/− cDCs were loaded with DQ-OVA, incubated at 37 °C for 2 h, fixed, and stained for EEA1 (Upper) or LAMP2 (Lower). Pictures are representative of multiple cells (sorted from 12 animals per group) analyzed in two independent experiments. Right shows colocalization scatter plots. DAPI was used as nuclear staining. (Scale bars: 10 μm.) (F) Splenic cDCs from WT and RAG−/− mice were pretreated with the indicated concentrations of chloroquine, loaded with OVA (1 mg/mL), and incubated with CFSE-labeled OT I cells for 2.5 d. The proliferative response of T cells was enumerated by flow cytometry. Numbers of proliferating cells are shown (mean + SEM). Data are representative of two independent experiments.
We conclude that in splenic cDCs from lymphopenic mice, antigen is mistargeted to late endosomal/lysosomal compartments, resulting in enhanced degradation of antigen and, consequently, in inefficient cross-presentation.
Soluble Ig Is Sufficient To Restore Efficient Cross-Presentation by cDCs from Lymphopenic Mice.
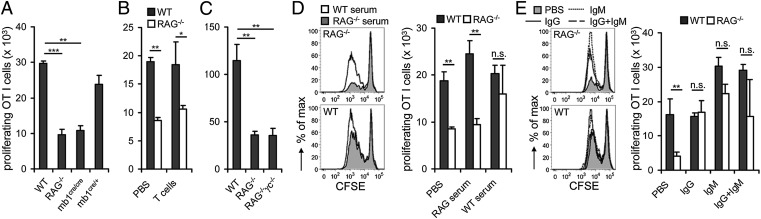
We next wanted to delineate the critical factors required for proper maturation of splenic cDCs that are lacking in RAG-deficient mice. First, we determined the individual contribution of B and T cells. OVA-sensitized cDCs from B-cell–deficient mb1cre/cre mice were hampered to support proliferation of OT I T cells to a similar extent as cDCs from RAG−/− mice, in contrast to cDCs from WT and B-cell–sufficient mb1cre/+ mice (Fig. 3A). Similar to cDCs from RAG−/− mice, cDCs from mb1cre/cre mice did not display any defect in presenting exogenously administered cognate peptide (Fig. S2A). T cells, however, when transferred into RAG-deficient mice were not able to fully reconstitute the cross-presentation capacity of splenic cDCs (Fig. 3B). These data indicate that B cells alone are able to modulate the efficiency of cross-presentation of soluble antigen by splenic cDCs.
Fig. 3.
Soluble Ig is sufficient to restore efficient cross-presentation by cDCs from lymphopenic mice. (A) Splenic cDCs from WT, RAG−/−, mb1cre/cre, and mb1cre/+ mice were loaded with OVA (500 μg/mL) and incubated with CFSE-labeled OT I cells for 2 d. (B) WT and RAG−/− mice were i.v. injected with splenic WT T cells (CD3+CD4+ and CD3+CD8+). Three weeks after T-cell transfer, splenic DCs were sorted from recipient mice, loaded with OVA (500 μg/mL), and incubated with CFSE-labeled OT I cells for 2 d. (C) Splenic cDCs isolated from WT, RAG−/−, or RAG2−/−γc−/− were loaded with OVA (500 μg/mL) and incubated with CFSE-labeled OT I cells for 2 d. (D) WT and RAG−/− mice were injected three times within 21 d i.v. with serum collected from WT and RAG−/− mice. (E) WT and RAG−/− mice were injected twice within 21 d i.v. with murine IgG (7.5 μg per mouse) and/or murine IgM (7.5 μg per mouse). The proliferative response of T cells was enumerated by flow cytometry. Numbers of proliferating cells are shown (mean + SEM). Data are representative of two or three independent experiments with minimum three mice per group. Statistical significance was determined by using paired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.005. n.s., not significant.
RAG−/− mice still contain natural killer (NK) cells, which could negatively influence cDCs. However, cDCs derived from RAG2−/−γc−/− mice, which lack NK cells in addition to T and B cells, had not acquired cross-presentation capacity (Fig. 3C). Thus, NK cells do not negatively influence cross-presentation by cDCs in RAG-deficient mice.
To evaluate candidate factors of B cells able to modulate cross-presentation, we first reconstituted RAG−/− mice or WT mice with serum from RAG-deficient or normal mice. Cross-presentation ability of RAG−/− cDCs was completely restored by transfer of WT serum in contrast to transfer of RAG−/− serum (Fig. 3D). This observation indicates that soluble factors rather than cell–cell interactions between B cells and cDCs modulate cross-presentation. Importantly, transfer of serum from RAG-deficient mice into WT mice did not reduce cross-presentation by WT cDCs, excluding the presence of inhibitory factors in serum of RAG-deficient mice.
Ig, a major component of serum, could act on such cDCs. Thus, WT and RAG−/− mice were reconstituted with purified monoclonal IgG, IgM, or both. Either type of Ig restored the cross-presentation capacity of cDCs from RAG-deficient mice to WT levels (Fig. 3E). Of note, purified IgM even enhanced cross-presentation by splenic cDCs isolated from WT mice. Addition of serum or Ig did not alter the presentation of exogenously added peptide (Fig. S2 B and C). Taken together, secreted Ig from B cells directly promotes the capacity of splenic cDCs to cross-present soluble antigen both by restoring cross-presentation in cDCs from Ig-deficient hosts and by enhancing cross-presentation in cDCs from Ig-competent hosts.
Secreted Ig Promotes Cross-Presentation by cDCs via Interaction with Lectin Receptors in Vivo.
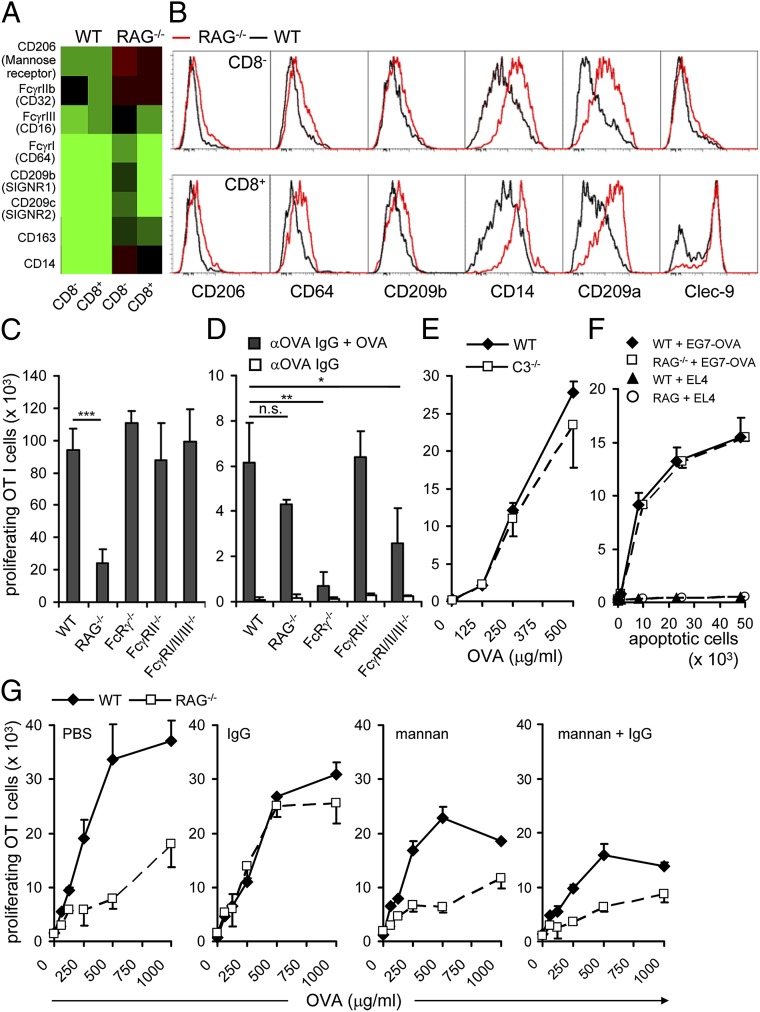
Gene expression analysis revealed several differentially regulated candidate genes whose products might serve as receptors for Ig. The genes encoding FcγRI, FcγRIIb, FcγRIII, and CLRs, including mannose receptor (MR, CD206), were up-regulated in cDCs from RAG−/− mice (Fig. 4A). Surface staining for Fcγ receptors (CD64), CLRs (CD206, CD209a, CD209b), and CD14 essentially confirmed our mRNA expression data (Fig. 4B). We also included the CLR Clec9a in our analysis, because it had been shown to bind dead-cell associated antigens and regulate cross-presentation (14, 15). Whereas in WT mice a large fraction of CD8+ DCs was virtually Clec9a-negative, this population of Clec9a-negative CD8+ DCs was almost absent from RAG-deficient mice (Fig. 4B).
Fig. 4.
Ig promotes cross-presentation by cDCs via interaction with lectin receptors. (A) Expression analysis of candidate surface receptors in splenic cDCs. (B) Splenic cDCs of WT and RAG−/− mice were analyzed for expression of the indicated C-type lectin receptors. Data are representative of four mice per group in two independent experiments. (C) Splenic cDCs from WT, FcRγ−/−, FcγRII−/−, or FcγRI/II/III−/− mice were loaded with OVA (500 μg/mL) and incubated with CFSE-labeled OT I cells for 2 d. (D) Sorted splenic cDCs from RAG−/−, WT, Fcγc−/−, RcRγIIB−/−, and FcRγI/II/III−/− were loaded with αOVA IgG + OVA. Cross-presentation abilities were tested by coincubation with OT I cells. (E) Splenic cDCs from WT or C3−/− mice were analyzed as in C. (F) Sorted splenic cDCs from RAG−/− and WT mice were loaded for 1h with 5 × 104 UV-irradiated EG7-OVA or EL4 cells. Further cells were coincubated with CFSE-labeled OT I cells. (G) WT and RAG−/− recipient mice were first injected i.v. with mannan (200 μg per mouse) and, subsequently, with IgG (7.5 μg per mouse) every 3 d for 21 d. Data are representative of two independent experiments with minimum three mice per group. The proliferative response of T cells was enumerated by flow cytometry. Numbers of proliferating cells are shown (mean +SEM). Statistical significance was determined by using paired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.005; n.s., not significant.
Next, we addressed a possible contribution of activating or inhibitory FcγR to Ig-dependent regulation of cross-presentation. cDCs isolated from FcRγ−/−, FcγRII−/−, and FcγRI/II/III−/− mice were able to cross-present OVA as efficiently as WT cDCs, suggesting that signals provided via FcγR are not required for functional maturation of splenic cDCs (Fig. 4C). These results are in line with our finding that not only IgG, but also IgM, can restore cross-presentation. Consistent with previous reports (12, 13), cDCs isolated from FcRγ−/− and FcγRI/II/III−/− mice were impaired in cross-presenting OVA-IC (Fig. 4D). Alternatively, ICs associated with components of the complement system could engage complement receptors present on splenic cDCs (16, 17). The component C3 is essential for the activation of the complement system and is required for all three pathways—the classical, the alternative, and the lectin pathway. Therefore, we analyzed cross-presentation of cDCs from complement component 3 (C3) deficient mice. However, splenic cDCs from such mice were as efficient as WT cDCs in cross-presentation of OVA, thus, excluding ICs and engagement of complement receptors as modulators of cross-presentation (Fig. 4E). Of note, presentation of other than soluble Ag, like cell-associated OVA, was not affected in cDCs from RAG−/− mice (Fig. 4F). Taken together, these data indicate that Ig affects DC function in at least two distinct levels: IC–FcR interaction and, more fundamentally, FcR-independent in a noncomplexed form. The dichotomy of Ig-dependent and Ig-independent cross-presentation of soluble antigen and apoptotic cells, respectively, is consistent with the assumption that humoral immunity has a greater role in clearance of soluble antigen than in removal of infected cells.
CLRs, like DC-SIGN or SIGN-R1, constitute a large group of surface receptors, more than 20 of which can be expressed on myeloid cells (18). Such receptors have been shown to engage Ig via their carbohydrates and potentially interfere with immune reactions (19). Hence, they could be involved in the functional maturation of splenic DCs. Mannan derived from Saccharomyces cerevisiae is widely considered to be a specific inhibitor of CLRs (20). Thus, we administered mannan to RAG−/− or WT mice before administration of IgG and tested the influence on the recovery of cross-presentation capacity by cDCs. Importantly, administration of mannan alone did not induce maturation of DCs as assessed by surface staining for MHC I, CD40, and the costimulatory ligands CD80 and CD86 (Fig. S3). Interestingly, in the presence of mannan, IgG could not restore cross-presentation in cDCs from RAG−/− mice (Fig. 4G). Mannan treatment reduced cross-presentation to some extent in cDCs from WT mice, because uptake of soluble OVA depends on MR (6). To closer investigate the receptor candidates, we tested bone marrow derived DCs (BMDCs) from MR-deficient mice. However, in the absence of MR, binding of Ig was not impaired compared with WT BMDCs (Fig. S4). This finding indicates that MR alone is not responsible for functional Ig binding to DCs. It also suggests that the contribution of a particular CLR might be masked by redundancy.
These experiments show that nonspecific Ig, independent of IC formation, engage mannan-inhibitable receptors, possibly of the CLR family, on splenic cDCs to induce functional maturation by dampening excessive degradation of soluble antigen and promoting efficient cross-presentation.
Discussion
Investigation of signals that control the function of DCs is critical to understand their role in regulation of the immune response and homeostasis. Mutual interactions between DCs with T and B cells during induction of adaptive immune response have been described (2, 10, 21). Nevertheless, still little is known how the separate lymphocyte populations modulate the development and function of tissue resident DCs in steady state. Cross-presentation of exogenous self-antigens is necessary to delete autoreactive CD8+ T cells but also in triggering CD8+ T-cell responses against pathogens that do not directly infect DCs (22, 23). Therefore, DCs, the main APCs that possess the ability to cross-present, play a crucial role in maintaining the subtle balance between tolerance and autoimmunity, and orchestrate protective T-cell responses. Factors that influence this balance might be very important targets for therapeutic interventions.
In our studies, we used the lymphopenic mouse model where a deficiency in the RAG gene leads to a developmental blockade and results in the complete absence of mature T and B lymphocytes. We could show that tissue resident DCs, which developed in such lymphopenic hosts exhibit severely impaired capacity to cross-present soluble OVA and prime CD8+ T cells. Interestingly, splenic cDCs isolated from B-cell–deficient mice, mb1cre/cre (24) showed similar dysfunction like cDCs isolated from RAG-deficient mice. Consistent with the importance of B cells injection of WT serum or soluble Ig into RAG knockout mice could recover deficiency in cross-presentation of cDCs isolated from such lymphopenic hosts. Therefore, we concluded that mature B cells and B-cell–derived Ig are necessary to maintain proper function of splenic cDCs in steady state.
Our experiments showed that transfer of naïve T cells into RAG-deficient mice only mildly enhanced the cross-presenting abilities of splenic cDCs. This finding does not correlate with a previous report by Shreedhar et al. (25), in which the authors showed that adoptive transfer of immune T cells can restore the disturbed antigen presentation capacity of DCs from lymphopenic mice. However, the two systems are difficult to compare. Immune T cells were essential in the in vivo assays, and hapten was used as antigen for skin sensitization. Thus, most likely skin derived Langerhans cells from lymphopenic mice were targeted. Such DCs are known to follow a different developmental pathway compared with normal cDCs (26). In our case, the DCs from lymphopenic hosts showed properties of their monocytic precursor. For instance, they still expressed CD14 and exhibited a high capacity for lysosomal degradation. The signal elicited by circulating Ig was presumably required for a full differentiation after acquisition of residency in the spleen.
Differential expression of CLRs and endolysosomal enzymes in splenic cDCs that had developed in a lymphopenic environment suggested that such cDCs might exhibit altered antigen uptake and target soluble antigens into degradation pathways different from WT DCs. Indeed, we could show that cDCs isolated from RAG-deficient mice have a slightly higher ability to acquire soluble antigen in comparison with WT cDCs. However, cDCs from lymphopenic mice degraded antigen much more vigorously. In addition, microscopic analysis showed that degradation of OVA takes place in different cellular compartments in the cDCs from RAG-deficient mice compared with WT cDCs. Splenic cDCs isolated from RAG−/− mice targeted OVA into lysosomes, whereas in WT cDCs, antigens remained in early endosomes. It had been shown that early endosomes provide a milder environment for antigen degradation that enables DCs to form MHC–peptide complexes (27). In contrast, Mφ and other phagocytes target Ag into lysosomes where degradation is more efficient. More rapid degradation of the antigen prevents formation of MHC–peptide complexes and blocks efficient T-cell stimulation (28).
FcγR had been shown to regulate the maturation of DCs. Especially FcγRII was proposed to prevent spontaneous maturation of DCs, thereby promoting steady-state tolerance (13, 29). Nevertheless, experiments using splenic cDCs isolated from different FcγR-deficient mice did not support the hypothesis that i.v. administration of Ig provides a FcγR-dependent signal to restore the impaired function of cDCs from RAG-deficient mice. An FcR-independent mechanism of Ig function in this context was also suggested by our observation that IgM acted essentially identical to IgG. Recently, it was shown that expression of the Fc receptor for IgM, FcµR, is restricted to B cells and not expressed on T cells or DCs (30). Taken together, this report and our data strongly suggest that Ig acts independently of Fc receptors, FcγR or FcµR.
Because our experiments essentially excluded Fc receptors and complement as effectors of Ig and also pointed toward mechanisms different from the uptake of ICs and apoptotic cells, we explored whether CLRs might mediate Ig-induced amelioration of cross-presentation. This idea was further prompted by our observation that CLRs were up-regulated on DCs from RAG-deficient mice. Moreover, Anthony et al. demonstrated that anti-inflammatory activity of i.v. Ig (IVIG) is mediated by CLRs, like DC-SIGN and SIGN-R1 (31, 32). To test the hypothesis of a CLR-dependent effect, we performed a bulk blockade of CLRs by injection of mannan before administration of Ig. We observed that Ig were able to rescue efficient cross-presentation only when CLRs were not blocked. Mannan is widely considered to be a specific inhibitor for CLRs (20, 33, 34). However, it cannot ultimately be excluded that an intervention using mannan might result in nonspecific effects beyond inhibition of CLRs. Thus, it has been proposed by others that mannan induced functional maturation of bone-marrow derived DCs in vitro and of lymph node DCs in vivo (35). However, these effects were evoked at much higher doses of mannan than those used in our experiments. Of note, the reported effects of mannan on maturation of DCs were dramatically lower in comparison with effects mediated by LPS and, accordingly, we did not observe any phenotypic maturation of DCs after administration of mannan at the concentration used throughout our study.
More than 20 CLRs have been reported to be expressed on myeloid cells, which exhibit partially overlapping binding capacities for N-glycans and also engage similar downstream signaling pathways (18). Thus, the CLR system is likely to be highly redundant also with respect to Ig binding. We detected elevated expression of the MR, SIGN-R1, SIGN-R2, and Clec9a on DCs from RAG-deficient mice, raising the possibility that one or more of these or a different receptor might mediate Ig-induced amelioration of cross-presentation. Analysis of Ig binding to DCs from MR-deficient mice did not reveal any difference in binding compared with WT DCs. However, uptake of soluble OVA depends on MR, thereby precluding functional analysis of MR deficiency in this experimental system (6).
In summary, our studies excluding FcR-dependent and complement-dependent mechanisms and mechanisms mediating the uptake of ICs or apoptotic cells, in addition to our findings that the Ig-induced capacity to cross-present can be inhibited by the bona fide CLR-specific inhibitor mannan, leads us to suggest that interaction of soluble Ig with CLRs is required to endow splenic DCs with an antigen-processing machinery optimized for cross-presentation.
Why is such a simple signal required for functional maturation of splenic DCs? We attribute this requirement to the plasticity of the monocyte/macrophage/DC lineage. The highly diversified functions of cells from this lineage requiring a multitude of differentiation and activation stages have to be matched by a multitude of differentiation and maturation signals. Thus, migratory and homing molecules might be giving directives and, finally, the Ig molecules via CLRs might give the final functional cue for such DCs.
Materials and Methods
Mice.
Mouse lines used in this study were C57BL/6 (WT), OT I, RAG1−/−, RAG2−/−, RAG2−/−γc−/−, mb1-cre, FcRγ−/−, FcγRII−/−, FcγRI/II/III−/−, and C3−/− and are described in detail in SI Materials and Methods. All animal experiments were conducted under approval of the local authority Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES) 33.9–42502-04–10/0148.
Flow Cytometry and Cell Sorting.
cDCs were sorted as cells that were CD11chiCD8α−/+CD11b−/+. All B cells were CD19+. Abs used in this work and detailed procedures are described in SI Materials and Methods. Purity of APCs was always >97% as judged by reanalysis.
Analysis of Antigen Presentation.
For the experiments using soluble OVA or peptides, cDCs were plated in 96-well plates (Nunc) at 1 × 104 cells per well with the indicated amount of soluble EndoGrade OVA (Profos) or OVA257–264 (Ana Spec) for 1 h. Proliferation of T cells was analyzed by flow cytometry after 1.5 or 2.5 d of culture. The number of divided cells (CFSElo CD8+) was determined as described (36) (SI Materials and Methods).
Microarrays.
Data sets have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus: accession no. GSE17989. See also SI Materials and Methods.
Additional Methods.
A detailed description of assays for antigen uptake and processing, cell isolation and culture, Western blot, fluorescence microscopy, and in vivo administration of cells and reagents is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Regina Lesch and Susanne zur Lage for excellent technical assistance; J. Sjef Verbeek, Andreas Klos, and Christian Kurts for providing mouse strains; Gordon D. Brown, Luisa Martinez-Pomares, and Philip R. Taylor for providing cell lines; and Harald von Boehmer and Jonathan Yewdell for critical reading of the manuscript. This work was supported in part by the Kultusministerium of Niedersachsen—Georg Lichtenberg PhD program (N.Z.); Marie Curie Action Miditrain Grant MEST-CT-2004-504990 (to M.L.); the Helmholtz Gemeinschaft via Helmholtz International Research School for Infection Biology; the Hannover Biomedical Research School; the Deutsche Krebshilfe; the German Research Council (Deutsche Forschungsgemeinschaft) (S.W.); the German Research Foundation (Deutsche Forschungsgemeinschaft) Grants KR2320/2-1 (Emmy-Noether Program), SFB738-A7, and EXC62 (“Rebirth“) (to A.K.); and a Helmholtz Young Investigator Grant (to M.G.G. and G.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Microarray data in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE17989).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210654110/-/DCSupplemental.
References
- 1.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 2.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9(5):558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 4.Segura E, Villadangos JA. A modular and combinatorial view of the antigen cross-presentation pathway in dendritic cells. Traffic. 2011;12(12):1677–1685. doi: 10.1111/j.1600-0854.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316(5824):612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176(11):6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 7.Saveanu L, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325(5937):213–217. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 9.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 10.Wykes M, MacPherson G. Dendritic cell-B-cell interaction: Dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100(1):1–3. doi: 10.1046/j.1365-2567.2000.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayry J, et al. Modulation of dendritic cell maturation and function by B lymphocytes. J Immunol. 2005;175(1):15–20. doi: 10.4049/jimmunol.175.1.15. [DOI] [PubMed] [Google Scholar]
- 12.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196(6):817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnault A, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189(2):371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(7240):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JG, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36(4):646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Ben Nasr A, et al. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): Uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukoc Biol. 2006;80(4):774–786. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 17.Morelli AE, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: Dependence on complement receptors and effect on cytokine production. Blood. 2003;101(2):611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 18.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: Shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulin V, et al. B lymphocytes regulate dendritic cell (DC) function in vivo: Increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurts C, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186(12):2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186(2):239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobeika E, et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103(37):13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shreedhar V, et al. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11(5):625–636. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DH, et al. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204(11):2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol. 2009;9(12):871–882. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- 28.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 29.Nimmerjahn F, Ravetch JV. Fcgamma receptors: Old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Ouchida R, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. 2012;109(40):E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGreal EP, et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16(5):422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 34.Turville SG, Vermeire K, Balzarini J, Schols D. Sugar-binding proteins potently inhibit dendritic cell human immunodeficiency virus type 1 (HIV-1) infection and dendritic-cell-directed HIV-1 transfer. J Virol. 2005;79(21):13519–13527. doi: 10.1128/JVI.79.21.13519-13527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng KC, et al. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118(3):372–383. doi: 10.1111/j.1365-2567.2006.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson NS, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102(6):2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.