Abstract
The master checkpoint kinase ATR (ATM and Rad3-related) and its partner ATRIP (ATR-interacting protein) exist as a complex and function together in the DNA damage response. Unexpectedly, we found that the stability of the ATR–ATRIP complex is regulated by an unknown kinase independently of DNA damage. In search for this regulator of ATR–ATRIP, we found that a single member of the NIMA (never in mitosis A)-related kinase family, Nek1, is critical for initiating the ATR response. Upon DNA damage, cells lacking Nek1 failed to efficiently phosphorylate multiple ATR substrates and support ATR autophosphorylation at threnine 1989, one of the earliest events during the ATR response. The ability of Nek1 to promote ATR activation relies on the kinase activity of Nek1 and its interaction with ATR–ATRIP. Importantly, even in undamaged cells, Nek1 is required for maintaining the levels of ATRIP, the association between ATR and ATRIP, and the basal kinase activity of ATR. Thus, as an ATR-associated kinase, Nek1, enhances the stability and activity of ATR–ATRIP before DNA damage, priming ATR–ATRIP for a robust DNA damage response.
The ability of cells to sense and signal DNA damage is crucial for genomic stability. In human cells, the ataxia telangiectasia-mutated (ATM) and the ATM- and Rad3-related (ATR) checkpoint kinases are central players in DNA damage signaling (1). In contrast to ATM, which primarily responds to double-stranded DNA breaks (DSBs), ATR is elicited by a broad spectrum of DNA damage and DNA replication stress (2, 3). ATR functions in a complex with its regulatory partner ATRIP (4). RPA-coated single-stranded DNA (RPA–ssDNA), a common intermediate of DNA replication and repair, plays a key role in recruiting and activating the ATR–ATRIP kinase complex (5). Once activated, ATR phosphorylates and activates its downstream effector kinase Chk1 (checkpoint kinase 1) with the help of a group of mediator proteins. Together, activated ATR and Chk1 phosphorylate a number of proteins involved in DNA replication, DNA repair, and cell-cycle transitions, thereby coordinating these cellular processes to suppress genomic instability.
Although the ATR-Chk1 kinase cascade is clearly the backbone of the ATR signaling pathway, several other protein kinases, such as ATM (6, 7), CDKs (cyclin-dependent kinases) (8–10), PLK1 (polo-like kinase) (11-14), AKT (15, 16), and casein kinases (17, 18), have been implicated in tuning the strength and dynamics of ATR signaling in different contexts. The effects of these kinases on ATR signaling suggest that the ATR pathway is intertwined with other signaling pathways and cellular programs. In addition to the aforementioned kinases, the NIMA (never in mitosis A)-related kinases have recently emerged as a new class of checkpoint regulator (19).
NIMA was originally discovered in Aspergillus nidulans as a protein kinase essential for mitosis (20). In human cells, 11 NIMA-related kinases have been identified, which were dubbed Nek1 to Nek11. The human Nek kinases have apparently adapted to a variety of functions (21). For example, Nek2 is critical for centrosome duplication, whereas Nek6, 7, and 9 are important regulators of the mitotic spindle and cytokinesis (19). Interestingly, several members of the Nek family have been linked to the ATR-mediated DNA damage response. Nek1, through unknown mechanisms, promotes Chk1 activation (22, 23) and repair of several types of DNA damage (24). Nek11, on the other hand, is a substrate of Chk1, and it promotes the G2/M checkpoint arrest by phosphorylating Cdc25A (cell division cycle 25A) and targeting Cdc25A for degradation (25). Nek6 may also be a substrate of Chk1 and contribute to the G2/M checkpoint arrest (26). Although these studies have suggested a functional link between some of the Nek kinases and the ATR checkpoint, how the Nek kinases as a family regulate the signaling events along the ATR pathway is still largely unknown.
In this study, we unexpectedly found that the stability of ATR–ATRIP complex is regulated by an unknown kinase in the absence of DNA damage. In search for this regulator of ATR–ATRIP, we tested all Nek family members using a panel of siRNAs. We found that Nek1 is the only Nek kinase that functions upstream of Chk1. We showed that Nek1 not only associates with the ATR–ATRIP kinase complex physically, but also regulates multiple phosphorylation events along the ATR pathway. In particular, cells lacking Nek1 failed to undergo efficient ATR autophosphorylation at Thr-1989 (threnine 1989) after DNA damage, suggesting that Nek1 is required for the initial step of ATR response (27). Both the association of Nek1 with ATR–ATRIP and the kinase activity of Nek1 are required for efficient ATR signaling. Importantly, even in the absence of DNA damage, Nek1 is required for maintaining normal levels of ATRIP, the ATR–ATRIP interaction, and ATR basal kinase activity, suggesting that ATR–ATRIP needs to be primed by Nek1 to be fully activated in response to DNA damage.
Results
Stabilization of the ATR–ATRIP Complex by Phosphorylation.
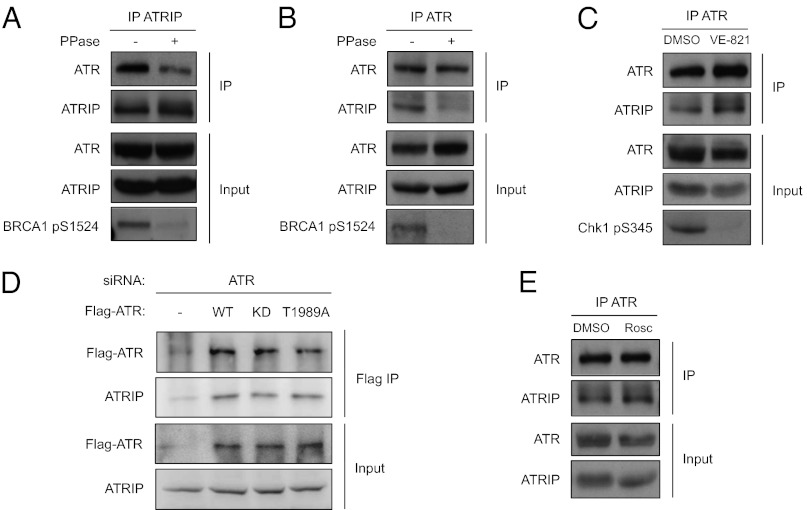
We and others recently showed that ATR is phosphorylated at Thr-1989 in response to DNA damage (27, 28). To investigate whether the phosphorylation status of ATR influences the stability of ATR–ATRIP complex, we tested the effects of phosphatase on the interaction between ATR and ATRIP in cell extracts. Unexpectedly, even in extracts derived from undamaged cells, the interaction between ATR and ATRIP was significantly reduced by phosphatase treatment (Fig. 1 A and B). These results suggest that the ATR–ATRIP complex is stabilized in a phosphorylation-dependent manner, even when ATR is not activated by DNA damage.
Fig. 1.
Stabilization of the ATR–ATRIP complex by phosphorylation. (A and B) Extracts derived from undamaged HCT116 cells were treated with Calf Intestinal Phosphatase (CIP) or mock treated. The effectiveness of CIP treatment was confirmed by the dephosphorylation of Brca1 Ser-1524. Endogenous ATRIP (A) or ATR (B) was immunoprecipitated with antibodies, and the coprecipitated proteins were analyzed by Western blot using the indicated antibodies. (C) Cells were treated with 10 μM VE-821 or mock treated with DMSO for 6 h. The effectiveness of VE-821 was confirmed by the dephosphorylation of Chk1 Ser-345. Endogenous ATR was immunoprecipitated and the coprecipitated proteins were analyzed as in B. (D) Cells were transfected with ATR siRNA and plasmids expressing siRNA-resistant, Flag-tagged ATRWT, ATRKD, or ATRT1989A. The Flag-tagged ATR proteins were immunoprecipitated with anti-Flag antibody, and the coprecipitated ATRIP was analyzed by Western blot. (E) Cells were treated with 50 μM roscovitine for 6 h or mock treated. The increase of ATRIP mobility in the input confirms the dephosphorylation of ATRIP. The interaction between ATR and ATRIP was analyzed as in B.
To directly test whether the kinase activity of ATR is required for stabilizing the ATR–ATRIP complex, we treated cells with the ATR inhibitor VE-821 and tested its effects on the ATR–ATRIP complex (29). VE-821 abolished the baseline phosphorylation of Chk1 in undamaged cells, but did not reduce the interaction between ATR and ATRIP (Fig. 1C). Furthermore, when transiently expressed in cells where endogenous ATR was depleted by siRNA, both the kinase-deficient ATR mutant (KD) and the ATR mutant lacking the autophosphorylation site (T1989A) interacted with ATRIP as efficiently as wild-type ATR (WT) (Fig. 1D). ATRIP is known to be phosphorylated by CDK2 in the absence of DNA damage. As shown previously (9), prolonged treatment of cells with the CDK inhibitor roscovitine led to ATRIP dephosphorylation (Fig. 1E). However, roscovitine treatment did not reduce the interaction between ATR and ATRIP (Fig. 1E). Together, these results suggest that the kinase activity of neither ATR nor CDK2 is responsible for the stabilization of ATR–ATRIP complex in undamaged cells.
In search for the kinase that stabilizes ATR–ATRIP, we considered a number of kinases that have been implicated in the ATR response (see introduction). Among these kinases are several members of the Nek kinase family (22, 25, 26). The implication of multiple Nek kinases in the ATR response and the requirement of an unknown kinase for the stability of ATR–ATRIP complex prompted us to investigate how the Nek kinase family regulates ATR activation. As described below, our studies on the Nek kinases eventually led us to identify a kinase that stabilizes the ATR–ATRIP complex.
Nek1 Is Critical for Initiating the ATR Response.
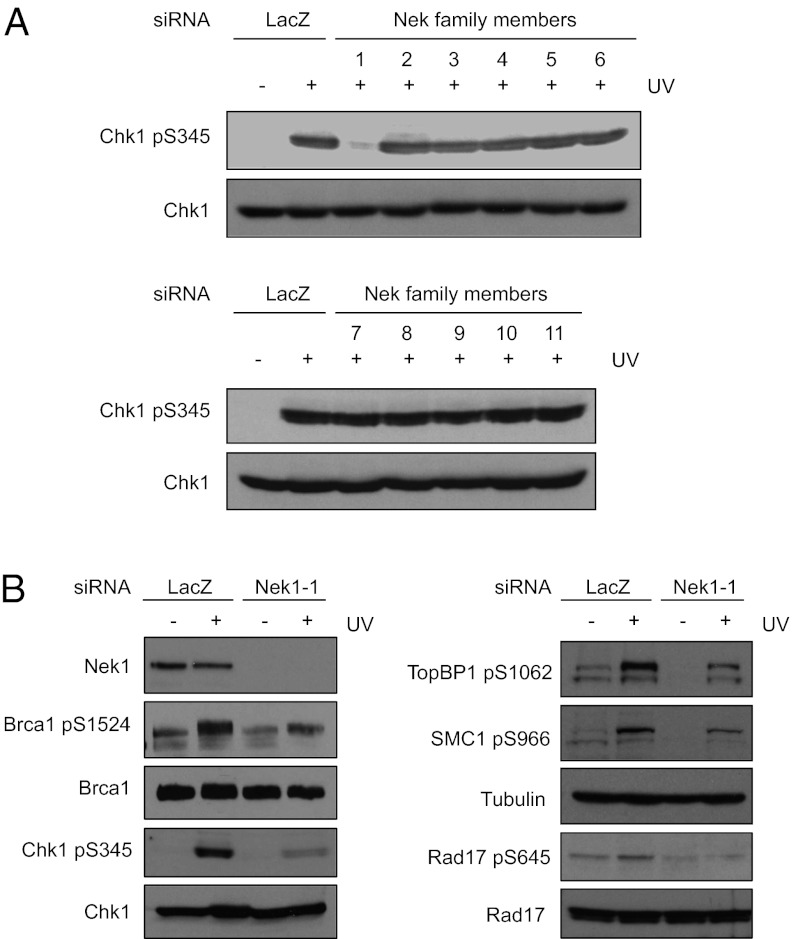
To obtain a comprehensive view of how the Nek kinase family contributes to the early events of ATR signaling, we asked which Nek kinases are required for efficient Chk1 phosphorylation in response to DNA damage and replication stress. Using a panel of siRNAs (SMART pools of ON-TARGET siRNAs from Dharmacon) targeting all members of the human Nek kinase family, we knocked down each of the 11 Nek kinases in HCT116 cells. Knockdown of Nek1, but not the other ten human Nek kinases, significantly reduced Chk1 phosphorylation in response to UV light (UV) (Fig. 2A). Hydroxyurea (HU)-induced Chk1 phosphorylation was also significantly reduced by Nek1 knockdown, but was unaffected or modestly affected by knockdown of the other Nek kinases (Fig. S1). These results suggest that Nek1 is the only Nek kinase that functions upstream of Chk1.
Fig. 2.
Nek1 regulates multiple phosphorylation events along the ATR pathway. (A) HCT116 cells were transfected with LacZ siRNA (control siRNA) or siRNA pools targeting each of the 11 human Nek kinases (Nek1-11). Two days after transfection, cells were irradiated with UV (15 J/m2), and Chk1 phosphorylation was analyzed 2 h after UV treatment using phospho-specific antibody. (B) Cells were transfected with LacZ or Nek1-1 siRNA, and treated with UV as in A. UV-induced phosphorylation of Chk1, Rad17, TopBP1, Brca1, and SMC1 was analyzed using the indicated phospho-specific antibodies.
To confirm the results of Fig. 2A, we used two independent Nek1 siRNAs to knock down endogenous Nek1 in HCT116 cells (Fig. S2A). Both Nek1 siRNAs reduced UV-induced Chk1 phosphorylation compared with control siRNA. Importantly, the cell cycle profile of Nek1 knockdown cells was not significantly different from control cells (Fig. S2B), ruling out cell cycle arrest as the cause of compromised Chk1 phosphorylation. Furthermore, expression of siRNA-resistant, Flag-tagged Nek1 in Nek1 knockdown cells significantly suppressed the reduction in Chk1 phosphorylation (Fig. S2C). These results are consistent with the results of a previous study using mouse cells (22), and they suggest that Nek1 is required for efficient Chk1 activation in human cells.
We next used Nek1 siRNA to investigate whether Nek1 regulates the phosphorylation of other ATR substrates. Rad17, TopBP1, and Brca1 are known to function upstream of Chk1 activation in the ATR signaling pathway (30–32). In response to UV, Rad17, TopBP1, and Brca1 were all phosphorylated at reduced levels in Nek1 knockdown cells (Fig. 2B). In addition, SMC1, a substrate of ATR that is required for the S-phase checkpoint (33), was also phosphorylated less efficiently in Nek1 knockdown cells (Fig. 2B). These results reveal that Nek1 is required for the phosphorylation of multiple signaling proteins along the ATR pathway, suggesting a role of Nek1 in the initiation of ATR response.
Nek1 Promotes ATR Signaling as a Kinase.
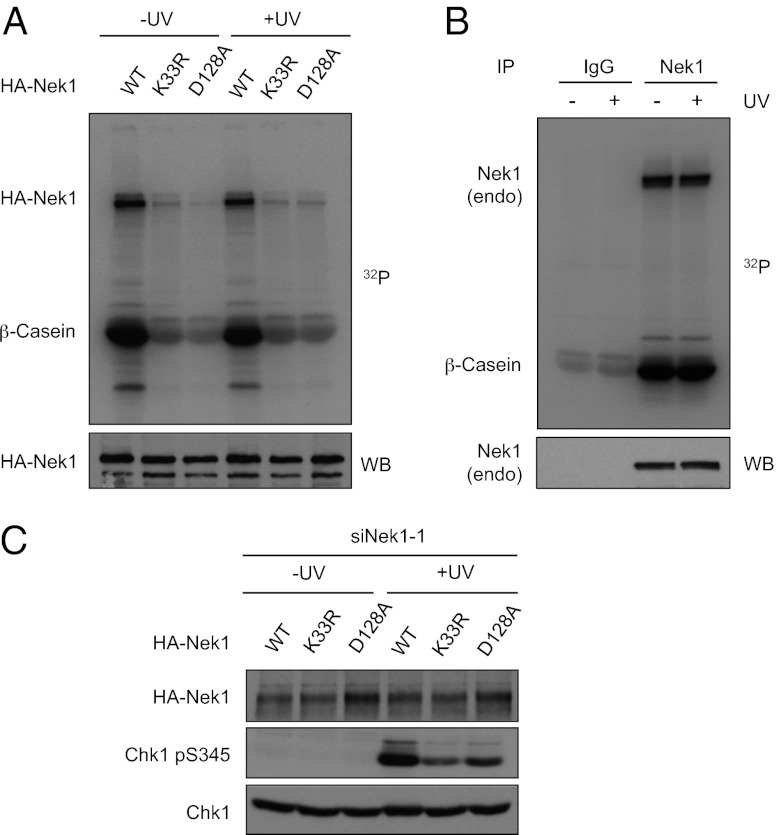
To further understand the role of Nek1 in ATR regulation, we asked whether the kinase activity of Nek1 is important for ATR signaling. To address this question, we mutated two key residues in the kinase domain of Nek1 (Nek1K33R and Nek1D128A). When tested in the in vitro kinase assays, HA-tagged wild-type Nek1 (HA–Nek1WT) underwent robust autophosphorylation and, as reported (34), phosphorylated β-casein efficiently (Fig. 3A). In marked contrast, neither HA–Nek1K33R nor HA–Nek1D128A was active for these phosphorylation events, showing that these Nek1 mutants are indeed kinase defective.
Fig. 3.
Nek1 kinase activity is required for ATR signaling. (A) HEK 293T cells were transfected with plasmids expressing HA–Nek1WT, HA–Nek1K33R, or HA–Nek1D128A. Transfected cells were irradiated with UV (15 J/m2) or mock treated, and HA–Nek1 was immunoprecipitated with HA antibody. In vitro Nek1 kinase assays were performed using β-casein as substrate in the presence of γ-32P ATP. (B) HEK 293T cells were irradiated with UV or mock treated as in A. Endogenous Nek1 was immunoprecipitated with Nek1 antibody, and its kinase activity was analyzed as in A. (C) HCT116 cells were first transfected with LacZ or Nek1-1 siRNA, and then transfected again with plasmids expressing HA–Nek1WT, HA–Nek1K33R, or HA–Nek1D128A as indicated. Transfected cells were irradiated with UV or mock treated, and Chk1 phosphorylation was analyzed using phospho-specific antibody.
Because Nek1 is required for efficient Chk1 activation in response to DNA damage, we asked whether Nek1 itself is stimulated by UV. In contrast to a previous report (23), we found that HA–Nek1WT was active before and after UV damage and that its activity was not significantly altered by UV (Fig. 3A). To exclude the possibility that the activity of HA–Nek1WT did not accurately reflect the activity of endogenous Nek1, we immunoprecipitated endogenous Nek1 from UV-treated or untreated cells and analyzed its activity (Fig. 3B). Consistent with our experiments using HA–Nek1WT, the kinase activity of endogenous Nek1 was not significantly altered by UV. Thus, although Nek1 is required for the efficient ATR response, its kinase activity is not regulated by DNA damage.
To directly address whether the kinase activity of Nek1 is important for ATR signaling, we expressed siRNA-resistant HA–Nek1WT, Nek1K33R, and Nek1D128A in cells where endogenous Nek1 was knocked down by siRNA (Fig. 3C). UV-induced Chk1 phosphorylation occurred much more efficiently in cells expressing Nek1WT than in cells expressing Nek1K33R or Nek1D128A. These results suggest that, although Nek1 is not stimulated by DNA damage, its kinase activity is important for ATR signaling.
Nek1 Promotes ATR Signaling Through Its Interaction with ATR–ATRIP.
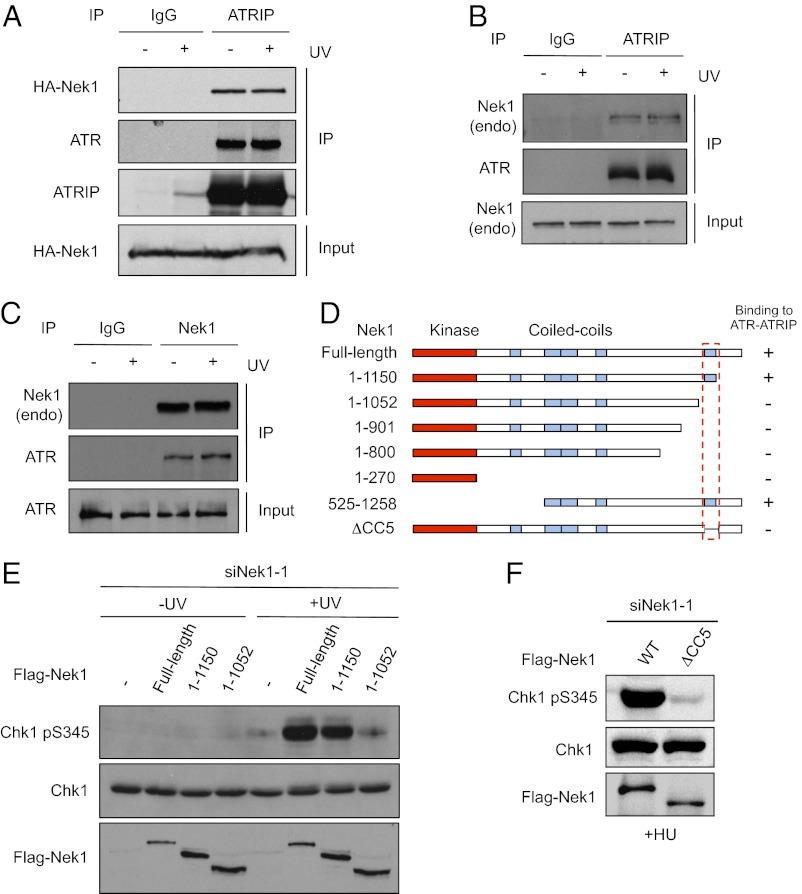
The requirement of Nek1 kinase activity for ATR signaling prompted us to test whether Nek1 interacts with ATR and ATRIP. Immunoprecipitation of endogenous ATRIP coprecipitated both ATR and HA–Nek1WT (Fig. 4A). In contrast, immunoprecipitation of TopBP1 did not capture HA–Nek1WT (see Fig. S4B). The interaction between ATRIP and HA–Nek1 was observed in UV-treated and untreated cells, suggesting that Nek1 associates with ATR–ATRIP before and after DNA damage (Fig. 4A). Similar to HA–Nek1WT, endogenous Nek1 also associated with both ATR and ATRIP before and after UV treatment (Fig. 4 B and C and Fig. S3 A and B). In contrast to Nek1, Nek11, another Nek kinase implicated in ATR signaling, did not interact with ATR (Fig. S3A). Furthermore, unlike ATR, ATM was unable to bind Nek1 (Fig. S3B). Collectively, these results suggest that Nek1 associates with the ATR–ATRIP complex irrespective of DNA damage.
Fig. 4.
Nek1 promotes ATR signaling by interacting with ATR–ATRIP. (A) HEK 293T cells expressing HA–Nek1WT were irradiated with UV (15 J/m2) or mock treated, and ATRIP was immunoprecipitated 2 h after UV treatment. ATRIP and coprecipitated ATR and HA–Nek1 were analyzed with the respective antibodies. (B and C) Untransfected HEK 293T cells were irradiated with UV or mock treated as in A. Endogenous ATRIP (B) and Nek1 (C) were immunoprecipitated, and the coprecipitated proteins were analyzed with the indicated antibodies. (D) Schematic of the Nek1 fragments and mutants that were tested for binding to ATR or ATRIP. The ability of these Nek1 derivatives to bind ATR or ATRIP was summarized. (E) HCT116 cells were first transfected with Nek1-1 siRNA, and then transfected again with plasmids expressing Flag–Nek1WT, Flag–Nek1-1150, or Flag–Nek1-1052. UV-induced Chk1 phosphorylation was analyzed as above. (F) HCT116 cells were transfected with siNek1-1 and then with plasmids expressing Flag–Nek1WT or Flag–Nek1ΔCC5. Cells were treated with 1 mM HU, and Chk1 phosphorylation was analyzed in 2 h.
To understand whether the interaction between Nek1 and ATR–ATRIP is important for ATR signaling, we sought to map the ATR–ATRIP-interacting domain of Nek1. Nek1 contains a kinase domain at the N terminus, a cluster of four coiled-coil domains in the central region, and a single coiled-coil domain near the C terminus (Fig. 4D). A series of truncation mutants of Nek1 were tagged with HA or Flag epitope and tested for binding to ATR or ATRIP (Fig. 4D and Fig. S4 A and B). A Nek1 C-terminal truncation mutant (Nek11-1052) lacking the coiled-coil domain near the C terminus was unable to interact with ATR (Fig. 4D and Fig. S4A). In contrast, another Nek1 C-terminal truncation mutant (Nek11-1150) that retains the coiled-coil domain interacted with ATR as efficiently as the full-length Nek1. When expressed in Nek1 knockdown cells, the Nek11-1150 mutant that retained the ability to interact with ATR–ATRIP rescued Chk1 phosphorylation as Nek1WT (Fig. 4E). In contrast, the Nek11-1052 mutant that failed to interact with ATR–ATRIP was unable to rescue Chk1 phosphorylation (Fig. 4E). These results suggest that the interaction between Nek1 and ATR–ATRIP is required for ATR signaling. To confirm this conclusion, we generated an internal deletion mutant of Nek1 lacking only the C-terminal coiled-coil domain (amino acids 1053–1150) (Fig. 4D). The resulting Nek1ΔCC5 mutant failed to interact with ATR and was unable to rescue Chk1 phosphorylation in Nek1 knockdown cells (Fig. 4F and Fig. S4C).
Nek1 Enhances ATR–ATRIP Stability and ATR Basal Activity.
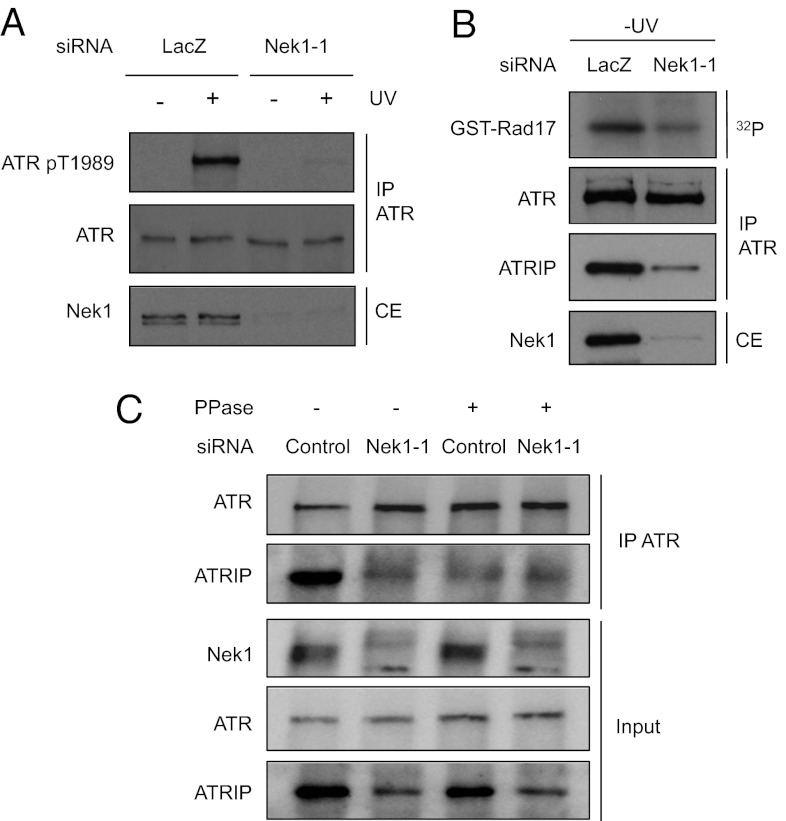
Although dispensable for the stabilization of ATR–ATRIP complex (Fig. 1D), ATR autophosphorylation at Thr-1989 is one of the earliest markers for the ATR response. The autophosphorylation of ATR at Thr-1989 relies on the recruitment of ATR–ATRIP to RPA–ssDNA and the kinase activity of ATR (27). Thus, if Nek1 is important for the stability of ATR–ATRIP complex, it is likely required for efficient ATR autophosphorylation. Indeed, UV-induced ATR phosphorylation at Thr-1989 was significantly reduced in Nek1 knockdown cells (Fig. 5A). To assess whether Nek1 phosphorylates ATR at Thr-1989 directly, we tested whether HA–Nek1WT and HA–Nek1K33R (kinase defective) were able to phosphorylate a GST-fused peptide encompassing Thr-1989 and its surrounding amino acids (GST–T1989) (Fig. S5). A mutant form of the peptide with Thr-1989 changed to Ala (GST–T1989A) was used as a negative control. Similar to the kinase-defective Nek1K33R, Nek1WT did not phosphorylate GST–T1989 and GST–T1989A above the background levels (Fig. S5, lanes 1–2 and 4–5). In contrast, Nek1WT, but not Nek1K33R, efficiently phosphorylated β-casein even when β-casein was present at a much lower concentration than GST-T1989 (Fig. S5, lanes 3 and 6). These results show that Nek1 does not phosphorylate Thr-1989 directly, suggesting that Nek1 functions as a prerequisite for ATR autophosphorylation in cells.
Fig. 5.
Nek1 promotes the stability of ATR–ATRIP and ATR basal activity. (A) HCT116 cells were transfected with LacZ or Nek1-1 siRNA. Transfected cells were irradiated with UV, and the levels of ATR and phospho-ATR (T1989) were analyzed with the respective antibodies 2 h after UV treatment. (B) HCT116 cells were transfected with LacZ or Nek1-1 siRNA. Endogenous ATR was immunoprecipitated, and its kinase activity was analyzed in vitro using GST-Rad17 as substrate. The levels of Nek1 in cell extracts (CE), and the levels of ATR and ATRIP in ATR immunoprecipitates, were analyzed with respective antibodies. (C) HCT116 cells were transfected with LacZ or Nek1-1 siRNA, and cell extracts were treated with CIP or mock treated. The interaction between ATR and ATRIP was analyzed as in B.
Next, we directly analyzed the effects of Nek1 knockdown on the basal activity of ATR and the stability of ATR–ATRIP complex. To monitor ATR kinase activity, we immunoprecipitated endogenous ATR and tested it using a substrate that contains an ATR phosphorylation site of Rad17 (GST–Rad17). To validate the specificity of the ATR kinase assay, we first performed this assay using ATRflox/- cells infected with adenovirus expressing Cre (Ad–Cre) or GFP (Ad–GFP) (Fig. S6A). The kinase activity captured by ATR antibody was abolished when ATR was depleted in Ad–Cre-infected ATRflox/- cells. Furthermore, the kinase activity was substantially reduced by the ATR-specific inhibitor VE-821 (29), but not by the ATM inhibitor KU55933 (Fig. S6B). Using this assay, we found that the kinase activity of ATR was lower in Nek1 knockdown cells than in control cells in the absence of DNA damage (Fig. 5B). This reduction in ATR basal activity was not due to reduced ATR levels (Fig. 5B). However, the amount of ATRIP associated with ATR was significantly reduced in Nek1 knockdown cells (Fig. 5B). In Nek1 knockdown cells, the levels of ATRIP were reduced, and the residual ATRIP did not interact with ATR efficiently (Fig. 5C). Given that ATRIP is known to be stabilized by ATR in cells (4), these results suggest that the stability of ATR–ATRIP complex is compromised in the absence of Nek1.
If Nek1 is responsible for the phosphorylation that stabilizes ATR–ATRIP, this phosphorylation should be lost in Nek1 knockdown cells and phosphatase treatment should not further weaken the interaction between ATR and ATRIP in extracts. Consistent with this possibility, the amounts of ATRIP coprecipitated by ATR in extracts of Nek1 knockdown cells were not further reduced by phosphatase treatment of the extracts (Fig. 5C, lanes 2 and 4), supporting the notion that the ATR–ATRIP complex is stabilized by Nek1-mediated phosphorylation in undamaged cells.
Discussion
The ATR checkpoint signaling pathway is known to be crucial for maintenance of genomic stability, yet how the ATR pathway is elicited by DNA damage and genomic instability is still not fully understood (2, 3). In this study, we show that Nek1 plays an important role in ATR signaling. Both the interaction between Nek1 and ATR–ATRIP and the kinase activity of Nek1 are required for efficient Chk1 activation, suggesting that Nek1 functions as an ATR–ATRIP-associated kinase. Interestingly, Nek1 interacts with ATR–ATRIP before and after DNA damage, and its kinase activity is not obviously stimulated by DNA damage. These findings suggest that Nek1 may exert its effects on ATR–ATRIP before DNA damage, providing a prerequisite for efficient ATR activation after DNA damage. Although the bulk of Nek1 is already active in the absence of DNA damage and able to interact with ATR–ATRIP, we cannot exclude the possibility that a minor fraction of Nek1 is stimulated by DNA damage and interact with ATR–ATRIP at sites of DNA damage. Nek1 could potentially regulate ATR–ATRIP both before and after DNA damage.
The full activation of ATR–ATRIP by DNA damage is driven by a sequence of molecular events. The recruitment of ATR–ATRIP and its regulators to sites of DNA damage creates a “microenvironment” that allows these proteins to interact with each other efficiently and to form an active signaling complex. During this process, ATR autophosphorylates in trans at Thr-1989 using its basal kinase activity, enabling TopBP1 to engage ATR, to stimulate the kinase activity of ATR, and to act as a scaffold in the signaling complex (27). Using ATR autophosphorylation at Thr-1989 as a functional marker for ATR activation, we revealed a role of Nek1 in the initiation of ATR signaling, challenging the conclusion of a recent study that relied on a marker irrelevant to ATR activation (23, 27, 28). Consistent with its role in promoting ATR autophosphorylation, Nek1 is required for maintaining the basal kinase activity of ATR in undamaged cells.
How does Nek1 regulate the kinase activity of ATR–ATRIP? Our results show that the steady-state levels of ATRIP were reduced in Nek1 knockdown cells. Furthermore, the interaction between ATR and ATRIP is compromised by phosphatase treatment and by Nek1 knockdown. Importantly, the effects of phosphatase and Nek1 knockdown are not additive, suggesting that the ATR–ATRIP complex is stabilized by Nek1-mediated phosphorylation. A further understanding of the role of Nek1 will require identification of the Nek1 substrate(s) involved in ATR–ATRIP regulation. Potential substrates of Nek1 include ATR, ATRIP, Nek1 itself, and other proteins that associate with them. Phosphorylation of Nek1 substrate(s) likely elevates the basal kinase activity of ATR–ATRIP by stabilizing the kinase complex. Furthermore, stabilization of the ATR–ATRIP complex may enhance its ability to recognize RPA–ssDNA, promoting its full activation in response to DNA damage.
As a regulator of the stability of ATR–ATRIP complex, Nek1 may influence the magnitude and duration of ATR response in different biological contexts. For example, Nek1 could fine tune the ATR pathway in different cell types, different differentiation stages, different cell cycle phases, and different cellular compartments. Moreover, the function of Nek1 could be negatively regulated by ATR through a feedback loop in the late phase of ATR response. The inhibition of Nek1, or the dephosphorylation of relevant Nek1 substrates, could help turn off the ATR–ATRIP at sites of DNA damage and promote termination of the ATR response.
Although Nek1 is an important regulator of ATR signaling, it should be noted that the effects of Nek1 depletion are not identical to the effects of ATR depletion. Deletion of ATR, but not NEK1, results in embryonic lethality in mouse (35, 36), indicating that Nek1 loss compromises but does not eliminate ATR function. Nek1−/− mutant mice displayed several phenotypes similar to those of the hypomorphic ATR-Seckel mutant mice, such as dwarfism, sterility, and anemia (37). Similar to some Seckel patients, Nek+/− mutant mice developed lymphoma (38, 39). Interestingly, loss of Nek1 has been linked to defective ciliogenesis and progressive kidney failure in both mouse and human (40–43). Several other genes involved in the DNA damage response were recently linked to similar cellular and tissue defects (44–46), raising the possibility that the function of Nek1 in DNA damage signaling is critical in specific tissues.
Materials and Methods
Cell Culture and Cell Lines.
HEK293T cells were cultured in DMEM (Invitrogen) supplemented with 10% (vol/vol) FBS. HCT116 cells were cultured in McCoy’s medium (Invitrogen) supplemented with 10% (vol/vol) FBS.
siRNAs.
Transfections of siRNA were performed using Oligofectamine (Invitrogen) following the manufacturer’s instructions. The siRNAs targeting the Nek family kinases were SMART Pools of ON-TARGET Plus siRNA from Dharmacon. The other siRNAs were made by Invitrogen. Nek1-1 siRNA: GGUCUGUUUGAUGCAAACAACCCAA. Nek1-2 siRNA: ACAUCAGCAUCUUUAUGCCAAGAUU. LacZ siRNA: GUGGUUGUAACAGCG-CAUCUU.
Plasmids.
To generate Nek1 expression plasmids, Nek1 coding sequence was PCR amplified from the I.M.A.G.E. clone 40082305, and cloned into the pEGFP-N1 vector. To express HA-tagged Nek1, the coding sequence of an HA epitope followed by a stop codon was inserted to the 3′ end of Nek1 coding sequence. To render HA–Nek1 resistant to Nek1-1 siRNA, wobble mutations were introduced to the siRNA target sequence by site-directed mutagenesis, resulting in the plasmid HA–Nek1WT. HA–Nek1K33R and HA–Nek1D128A were derived from HA–Nek1WT by site-directed mutagenesis. To express siRNA-resistant Nek1 with an N-terminal Flag tag, Nek1 coding sequence was PCR amplified from HA–Nek1WT and cloned into the pFlag–CMV2 vector. Flag–Nek11-1150, Flag–Nek11-1052, Flag–Nek11-901, Flag–Nek11-800, and Flag–Nek11-270 were derived from Flag–Nek1WT by creating stop codons at the respective termination sites. To generate the Flag–Nek1ΔCC5 mutant lacking the C-terminal (the fifth) coiled-coil domain, amino acids 1053–1150 were deleted from Flag–Nek1WT. To express GST–Nek11057-1258 in Escherichia coli and purify the protein, the coding sequence of Nek1 amino acids 1057–1258 was PCR amplified from HA–Nek1WT and cloned into the pGEX–2TKcs vector.
Antibodies.
Nek1 antibody was generated by Bethyl against purified GST–Nek11057-1258, and was affinity purified. ATRIP and phospho-ATR Thr-1989 antibodies were as described (4, 27). ATR, TopBP1, and phospho-Rad17 antibodies were from Bethyl. Phospho-Chk1 antibody was from Cell Signaling. Rad17, Chk1, and HA antibodies were from Santa Cruz Biotechnology. Flag M2 antibody was from Sigma.
Immunoprecipitation.
HEK293T or HCT116 cells were lysed in NETN buffer (20 mM Tris⋅HCl, pH 8.0/100 mM NaCl/1 mM EDTA/0.5% Nonidet P-40) containing 1 mM DTT, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and the protease inhibitor mixture (Sigma). Cell extracts were spun at 20,817 × g (Eppendorf, Germany, 5417c) for 10 min, and the resulting supernatants were precleared with Protein G/A Sepharose beads. Antibodies to ATR, ATRIP, or Nek1 were added to the extracts with Protein G/A Sepharose beads, and were incubated overnight at 4 °C. Subsequently, the Sepharose beads were sedimented and washed four times with NETN buffer. The immunoprecipitates were then subjected to SDS/PAGE and Western blot analysis.
In Vitro Kinase Assays.
Nek1 kinase assays were performed as described (22). In ATR kinase assays, HEK293T or HCT116 cells were suspended in PBS buffer and lysed by sonication. Endogenous ATR protein was immunoprecipitated with ATR antibody from precleared cell extracts. ATR immunoprecipitates were sequentially washed with PBS buffer containing 350 mM, 250 mM, and 137 mM NaCl. Subsequently, the immunoprecipitates were washed three times with kinase buffer (10 mM Hepes, pH 7.5/50 mM Glycerolphosphate/50 mM NaCl/10 mM MgCl2/10 mM MnCl2). Washed ATR immunoprecipitates were incubated with 1 μg of GST-Rad17, 5 μCi of [32P]ATP, and 1 μM ATP in 25 μL of kinase buffer at 30 °C for 30 min.
To examine the effects of ATR and ATM inhibitors on ATR kinase assays, HCT116 cells were treated with 10 μM VE-821, 10 μM KU55933, or DMSO for 1 h. Cells were then irradiated with UV (15 J/m2), lysed 2 h after UV treatment, and subjected to ATR immunoprecipitation. ATR immunoprecipitates were washed as described above, and treated with the corresponding inhibitors again (20 μM VE-821 or 20 μM Ku55933) on ice for 1 h. The inhibitor-treated or mock-treated ATR immunoprecipitates were incubated with substrate and ATP as above.
Supplementary Material
Acknowledgments
We thank members of the Zou laboratory for helpful discussions. This work was supported by the National Institutes of Health Grant GM076388 and a grant funded by the Federal Share of Proton Income (to L.Z.). L.Z. is a Jim and Ann Orr Massachusetts General Hospital Research Scholar.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217781110/-/DCSupplemental.
References
- 1.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn RL, Zou L. ATR: A master conductor of cellular responses to DNA replication stress. Trends Biochem Sci. 2011;36(3):133–140. doi: 10.1016/j.tibs.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: Partners in checkpoint signaling. Science. 2001;294(5547):1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 5.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 6.Jazayeri A, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8(1):37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 7.Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM. Mol Biol Cell. 2009;20(9):2351–2360. doi: 10.1091/mbc.E08-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu N, et al. Cdk-mediated phosphorylation of Chk1 is required for efficient activation and full checkpoint proficiency in response to DNA damage. Oncogene. 2012;31(9):1086–1094. doi: 10.1038/onc.2011.310. [DOI] [PubMed] [Google Scholar]
- 9.Myers JS, Zhao R, Xu X, Ham AJ, Cortez D. Cyclin-dependent kinase 2 dependent phosphorylation of ATRIP regulates the G2-M checkpoint response to DNA damage. Cancer Res. 2007;67(14):6685–6690. doi: 10.1158/0008-5472.CAN-07-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson SE, et al. Cdk1 uncouples CtIP-dependent resection and Rad51 filament formation during M-phase double-strand break repair. J Cell Biol. 2011;194(5):705–720. doi: 10.1083/jcb.201103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamely I, et al. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol. 2006;16(19):1950–1955. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Peschiaroli A, et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell. 2006;23(3):319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Mailand N, Bekker-Jensen S, Bartek J, Lukas J. Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol Cell. 2006;23(3):307–318. doi: 10.1016/j.molcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell. 2004;117(5):575–588. doi: 10.1016/s0092-8674(04)00417-9. [DOI] [PubMed] [Google Scholar]
- 15.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7(2):193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Xu N, et al. Akt/PKB suppresses DNA damage processing and checkpoint activation in late G2. J Cell Biol. 2010;190(3):297–305. doi: 10.1083/jcb.201003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeishi Y, et al. Casein kinase 2-dependent phosphorylation of human Rad9 mediates the interaction between human Rad9-Hus1-Rad1 complex and TopBP1. Genes Cells. 2010;15(7):761–771. doi: 10.1111/j.1365-2443.2010.01418.x. [DOI] [PubMed] [Google Scholar]
- 18.Meng Z, Capalbo L, Glover DM, Dunphy WG. Role for casein kinase 1 in the phosphorylation of Claspin on critical residues necessary for the activation of Chk1. Mol Biol Cell. 2011;22(16):2834–2847. doi: 10.1091/mbc.E11-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div. 2011;6:18. doi: 10.1186/1747-1028-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osmani SA, Pu RT, Morris NR. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- 21.Fry AM, O’Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125(Pt 19):4423–4433. doi: 10.1242/jcs.111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Chen PL, Chen CF, Jiang X, Riley DJ. Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle. 2008;7(20):3194–3201. doi: 10.4161/cc.7.20.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Chen CF, Riley DJ, Chen PL. Nek1 kinase functions in DNA damage response and checkpoint control through a pathway independent of ATM and ATR. Cell Cycle. 2011;10(4):655–663. doi: 10.4161/cc.10.4.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelegrini AL, et al. Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest. Mutagenesis. 2010;25(5):447–454. doi: 10.1093/mutage/geq026. [DOI] [PubMed] [Google Scholar]
- 25.Melixetian M, Klein DK, Sørensen CS, Helin K. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat Cell Biol. 2009;11(10):1247–1253. doi: 10.1038/ncb1969. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, et al. Nek6 is involved in G2/M phase cell cycle arrest through DNA damage-induced phosphorylation. Cell Cycle. 2008;7(17):2705–2709. doi: 10.4161/cc.7.17.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, et al. ATR autophosphorylation as a molecular switch for checkpoint activation. Mol Cell. 2011;43(2):192–202. doi: 10.1016/j.molcel.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam EA, et al. Thr-1989 phosphorylation is a marker of active ataxia telangiectasia-mutated and Rad3-related (ATR) kinase. J Biol Chem. 2011;286(33):28707–28714. doi: 10.1074/jbc.M111.248914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reaper PM, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011;7(7):428–430. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 30.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30(3):285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- 31.Zou L, Cortez D, Elledge SJ. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 2002;16(2):198–208. doi: 10.1101/gad.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, et al. Claspin operates downstream of TopBP1 to direct ATR signaling towards Chk1 activation. Mol Cell Biol. 2006;26(16):6056–6064. doi: 10.1128/MCB.00492-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Qin J. MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci USA. 2003;100(26):15387–15392. doi: 10.1073/pnas.2536810100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letwin K, et al. A mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells. EMBO J. 1992;11(10):3521–3531. doi: 10.1002/j.1460-2075.1992.tb05435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA. 2000;97(1):217–221. doi: 10.1073/pnas.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14(4):397–402. [PMC free article] [PubMed] [Google Scholar]
- 37.Murga M, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41(8):891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler MG, Hall BD, Maclean RN, Lozzio CB. Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am J Med Genet. 1987;27(3):645–649. doi: 10.1002/ajmg.1320270318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, et al. Mutation of NIMA-related kinase 1 (NEK1) leads to chromosome instability. Mol Cancer. 2011;10(1):5. doi: 10.1186/1476-4598-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahjoub MR, Trapp ML, Quarmby LM. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol. 2005;16(12):3485–3489. doi: 10.1681/ASN.2005080824. [DOI] [PubMed] [Google Scholar]
- 41.Shalom O, Shalva N, Altschuler Y, Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett. 2008;582(10):1465–1470. doi: 10.1016/j.febslet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 42.White MC, Quarmby LM. The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol. 2008;9:29. doi: 10.1186/1471-2121-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiel C, et al. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet. 2011;88(1):106–114. doi: 10.1016/j.ajhg.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaki M, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150(3):533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lans H, Hoeijmakers JH. Genome stability, progressive kidney failure and aging. Nat Genet. 2012;44(8):836–838. doi: 10.1038/ng.2363. [DOI] [PubMed] [Google Scholar]
- 46.Zhou W, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet. 2012;44(8):910–915. doi: 10.1038/ng.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.