Abstract
The circuits that drive visually guided eye and arm movements transform generic visual inputs into effector-specific motor commands. As part of the effort to elucidate these circuits, the primate lateral intraparietal area (LIP) has been interpreted as a priority map for saccades (oculomotor-specific) or a salience map of space (not effector-specific). It has also been proposed as a locus for eye–hand coordination. We reversibly inactivated LIP while monkeys performed memory-guided saccades and reaches. Coordinated saccade and reach reaction times were similarly impaired, consistent with a nonspecific role. However, reaches made without an accompanying saccade remained intact, and the relative temporal coupling of saccades and reaches was unchanged. These results suggest that LIP contributes to saccade planning but not to reach planning. Coordinated reaches are delayed as a result of an eye–hand coordination mechanism, located outside of LIP, that actively delays reaches until shortly after the onset of an associated saccade. We conclude with a discussion of how to reconcile specificity for saccades with a possible role in directing attention.
Keywords: visuomotor, intraparietal sulcus, muscimol
Primates often react to the appearance of an object by looking at it, reaching for it, or both. A central goal of systems neuroscience is to elucidate the neural circuits responsible for visually guided saccades, reaches, and eye–hand coordination. A key part of this endeavor is to determine whether neural activity is linked to just one specific action or whether it reflects an earlier, more general stage of processing. Neurons in the lateral intraparietal area (LIP) fire robustly during saccade, reach, and peripheral attention tasks, consistent with their playing a role in a salience map of visual space (1–3). However, this activation is greater for saccades compared with reaches, consistent with a role in saccade planning (4–6). The weaker activation seen during reaching could reflect an additional role in yoking eye and arm movements together in time (7–10). There is evidence supporting such a role for LIP (11), but these issues remain unresolved.
Interventional approaches can provide direct evidence for functional relevance of a given area, which can augment correlational data provided by single unit recording studies (12, 13). Reversible inactivations of LIP have produced mixed results regarding the role of LIP (13–16). Recently, Liu et al. (17) found that inactivation of dorsal LIP (LIPd) impairs saccades but not an attention-demanding covert visual search task, whereas inactivation of ventral LIP (LIPv) impairs saccades and search. The saccade and search effects in LIPv were dissociable. Liu et al. suggested that LIPv plays a dual role in saccade planning and attention, whereas LIPd is saccade-specific (17). Because of this role in attention, we hypothesized that reaches would be impaired by lesions of LIPv but not LIPd. We further hypothesized that lesions might affect the temporal coordination of saccades and reaches. We found instead that lesions in LIPv and LIPd affect reaches that are accompanied by saccades, but do not affect reaches performed without accompanying saccades. Finally, temporal coordination was not affected by inactivation of either area. These results support the idea that LIP serves effector-specific (i.e., oculomotor) functions, and that temporal eye–hand coordination circuits must lie downstream of LIP.
Results
We unilaterally inactivated LIP to reveal its role in visuomotor processing and eye–hand coordination. Four monkeys performed interleaved saccade and reach trials (Fig. 1). In 16 experimental sessions, the reaches were accompanied by a coordinated saccade to the same target (“coordinated reach”; monkeys G, Q, and W). In 22 sessions, central ocular fixation was maintained during the reach (“dissociated reach”; monkeys G and S). Finally, in two sessions, coordinated and dissociated reaches were performed (monkey G). Dissociated saccades were performed in all 40 sessions.
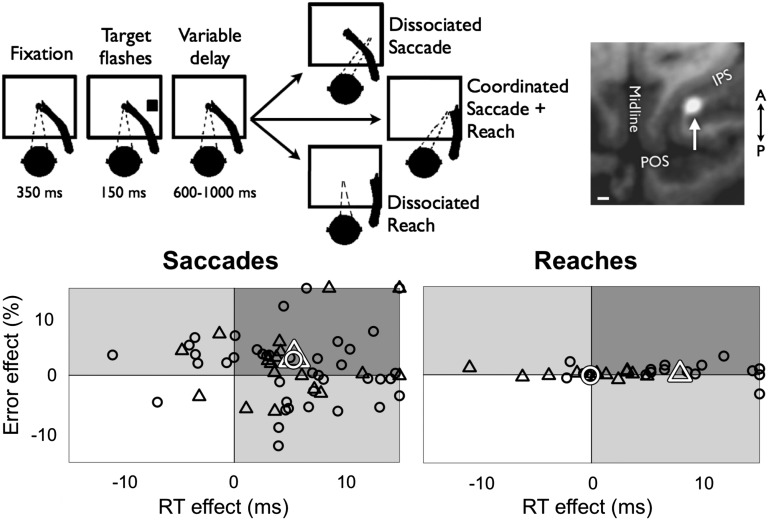
Fig. 1.
(Upper) Behavioral task. The target color instructed movement type, e.g., green for reach, red for saccade, and blue for coordinated reach (colors not shown). Within each block of trials, dissociated saccade trials were randomly interleaved with dissociated or coordinated reach trials (further details are provided in the text). (Inset) Example muscimol/manganese injection into LIP. MRI through horizontal plane shows the injection as a bright white halo on the lateral bank of the IPS. (Scale bar: 2 mm.) (Lower) Individual session effects of activation RT (abscissa) and error rate (ordinate) for saccades (Left) and reaches (Right). Circles indicate dissociated saccade or reach blocks, and triangles indicate coordinated saccade plus reach blocks. Black shapes show individual session means and white shapes show overall population means. LIP inactivation affected coordinated saccades, coordinated reaches, and dissociated saccades, but not dissociated reaches.
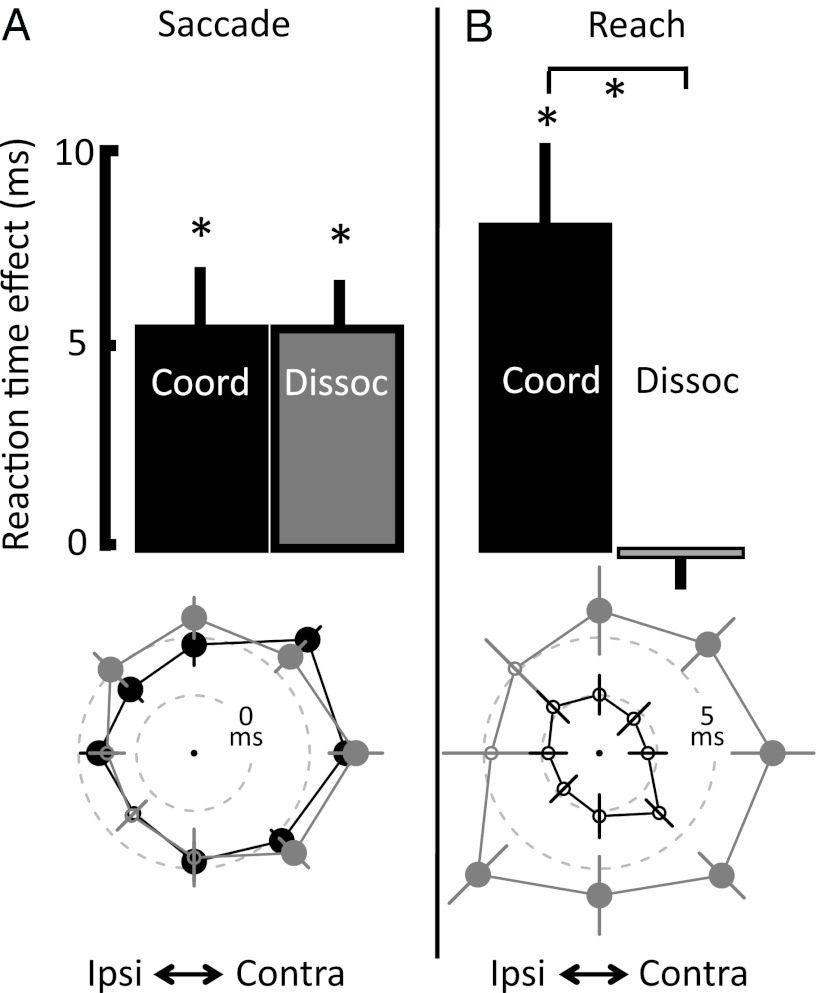
As expected, LIP inactivation slowed (i.e., delayed) saccade reaction time (RT) and increased saccade error rate (refs. 15–17, but see ref. 13). In individual sessions, RTs were slowed in 81% of cases and error rates were increased in 61%, with only two sessions in which both RT and error rate were decreased (Fig. 1, “saccades”). On average, dissociated saccades were delayed by 5.4 ms (P = 0.00003, two-tailed t test) and error rate was increased by 3.0% (P = 0.002, n = 40 sessions; Table 1 and Fig. 2A). The effect on coordinated saccades was nearly identical: a 5.4-ms slowing and a 2.7% increase in error rate (P < 0.01 for both effects; n = 18 sessions). Results were generally similar in LIPd and LIPv, with some differences in laterality (Table 2, SI Results, and Fig. S1).
Table 1.
Inactivation effects by movement type
| Saccade |
Reach |
|||
| Effect | Coordinated | Dissociated | Coordinated | Dissociated |
| RT, ms | 196.7, 5.4 (1.5)* | 202.7, 5.4 (1.2)* | 268.9, 7.9 (2.6)* | 227.6, 0.1 (0.9) |
| Error rate, % | 11.4, 2.7 (1.3)* | 14.1, 3.0 (1.0)* | 12.2, 0.1 (0.8) | 14.4, 0.1 (1.5) |
| Duration, ms | 61.8, 1.5 (0.5)* | 64.2, 0.8 (0.3)* | 159.8, 0.1 (2.4) | 131.3, 0.0 (1.6) |
| Accuracy, ° | 3.8, 0.1 (0.1)* | 3.9, 0.2 (0.0)* | 4.6, 0.2 (0.1)* | 5.0, 0.0 (0.3) |
| Endpoint scatter, ° | 1.9, 0.2 (0.1)* | 2.2, 0.3 (0.2) | 1.7, 0.1 (0.1) | 2.5, 0.0 (0.1) |
Each cell shows mean control values followed by the inactivation effects (SEM). Fig. S3 shows accuracy and scatter effects for each target.
*P < 0.05, two-tailed t test.
Fig. 2.
The effect of LIP inactivation on saccade and reach RT. (Upper) Mean effect of inactivation compared with controls for coordinated (black) and dissociated (gray) saccades (A) and reaches (B). Error bars represent SEM. (Lower) RT effects for each target direction. Dashed inner circle represents no effect, dashed outer circle represents a 5-ms slowing, and significance (P < 0.05, two-tailed t test) is indicated by large filled vs. small hollow symbols. Data from the contralateral visual field are plotted on the right side of each figure.
Table 2.
RT effect broken down by inactivations of LIPd or LIPv and by movement direction
| Saccade, ms (SEM) |
Reach, ms (SEM) |
|||
| Area | Coordinated | Dissociated | Coordinated | Dissociated |
| LIPd (n = 22) | ||||
| All | 6.1 (2.2)* | 6.7 (1.5)* | 10.8 (2.8)* | −0.3 (1.2) |
| Contralateral | 6.8 (1.7)* | 8.3 (1.2)* | 10.7 (2.2)* | −0.7 (1.3) |
| Ipsilateral | 4.7 (1.3)* | 5.1 (1.1)* | 11.4 (2.5)* | −1.6 (1.4) |
| LIPv (n = 20) | ||||
| All | 4.7 (2.3)* | 4.6 (1.6)* | 5.4 (4.4) | 0.3 (1.4) |
| Contralateral | 8.4 (1.7)* | 7.0 (1.5)* | 7.6 (3.2)* | 1.3 (1.5) |
| Ipsilateral | 1.2 (1.6) | −0.4 (1.3) | 0.0 (3.4) | 1.4 (1.2) |
Topmost and bottommost targets along midline were included only in “all.”
*P < 0.05, two-tailed t test.
Coordinated Reaches Are Delayed by LIP Inactivation.
On average, coordinated reach RT was delayed by 7.9 ms (P < 0.01; Fig. 2B). In individual sessions, coordinated reach RTs were significantly delayed in 11 of 18 sessions (P < 0.05, two-tailed t test) and were significantly faster in one session (triangles in Fig. 1, “reaches”). LIP inactivation did not affect reach error rate (increase of 0.1%; P > 0.5).
Based on the previous finding that LIPv inactivation affected attention-demanding visual search as well as saccades, whereas LIPd inactivation affected only saccades (17), we hypothesized that reach RT would be affected by LIPv inactivation but not LIPd inactivation. Instead, we found similar effects in the two areas (Table 2 and SI Results). The magnitudes of the coordinated reach RT effect (7.9 ± 2.6 ms) and the coordinated saccade RT effect (5.4 ± 1.5 ms) are statistically indistinguishable (P > 0.5, two-tailed paired t test). This holds even when errors are included in the comparison. In fact, when errors are taken into account, the effects on saccades and on reaches are nearly identical to one another (Fig. S2 and SI Results). One possible interpretation of this result is that it supports the hypothesis that LIP is not effector-specific, and instead plays a generic role in visually guided behavior. For example, it might form a general salience map for task-relevant spatial locations (1–3, 13, 14), which can subsequently be used to guide multiple types of movements.
An alternative possibility is that the observed slowing of coordinated reaches is only indirectly related to LIP inactivation. When human or nonhuman primates perform coordinated eye–arm movements, the eye and arm latencies are correlated with one another, but reach onset is often delayed 50 to 100 ms relative to saccade onset (18–20). There may exist a mechanism that coordinates the eye and hand by inhibiting the onset of the reach for some time after the onset of the saccade. In this case, an intervention that delays the execution of the saccade and operates at a point in the neural circuitry upstream of the eye–hand coordination mechanism might slow a concomitant reach. In other words, the slowing of coordinated reaches might reflect an indirect rather than direct effect of LIP inactivation.
Dissociated Reaches Are Not Affected by Inactivation.
To distinguish between direct vs. indirect effects of LIP inactivation (and between a general vs. effector-specific role for the area), we tested for effects when monkeys performed reaches without an accompanying saccade. The results were unambiguous. When reaches were performed without an accompanying saccade, there was no effect of LIP inactivation on reach RT (−0.1 ms; P = 0.95; circles in Reaches, Fig. 1; Fig. 2B). A direct comparison of coordinated and dissociated reach RTs reveals a highly significant difference (8.0 ms; P = 0.006). This result does not depend on grouping the data across animals. No effects of LIP inactivation on dissociated reach RTs were observed when data from the two animals were considered separately (monkey G, 0.5 ms, P = 0.96, n = 17 experimental sessions; monkey S, −0.7 ms, P = 0.75, n = 7; Table S1). In two sessions, monkey G performed dissociated and coordinated reach blocks, and, in each of those sessions, coordinated reaches were significantly delayed whereas dissociated reaches remained intact (Table S1, bottom row). The result does not depend on the hemifield in which the reach targets appeared. Specifically, there was no delay when dissociated reaches into either hemifield were considered separately (contralateral field, 0.2 ms, P = 0.81; ipsilateral field, −0.2 ms, P = 0.84; Table 2 and Fig. S1). There was no significant delay of dissociated reaches when injections into LIPd or LIPv were considered separately (−0.3 ± 1.2 ms and 0.3 ± 1.4 ms, respectively). Finally, there was no effect when dissociated reaches made with one or the other limb were considered separately (monkey G, contralateral limb, 0.5 ms, P = 0.62; ipsilateral limb, −0.4 ms, P = 0.82). The fact that reaches unaccompanied by saccades were not affected by LIP inactivation provides strong evidence against a direct contribution of LIP to reach RT, and instead supports the idea that reach initiation is linked to the timing of the saccade via an eye–hand coordination mechanism downstream of LIP.
Effects of Inactivation on Other Behavioral Measures.
Although RT was the most sensitive measure of the effect of LIP inactivation on saccades, error rate, duration, and accuracy were also significantly affected (Table 1). Similar effects were observed in coordinated and dissociated saccades. In contrast to saccades, coordinated reaches were impaired in RT and, to a lesser extent, in accuracy, whereas dissociated reaches were completely unaffected. Fig. S3 depicts mean reach endpoints and their 95% confidence ellipses for movements in each direction under control and inactivation conditions. Endpoints were often above the target, especially for lower targets, but the difference between control and experimental values was not more than 0.2° for saccades or reaches in coordinated or dissociated conditions. The impairment in reach accuracy, in particular, was significant only for coordinated reaches, and was not systematic across different directions (e.g., reaches were not consistently hypometric or hypermetric in any direction).
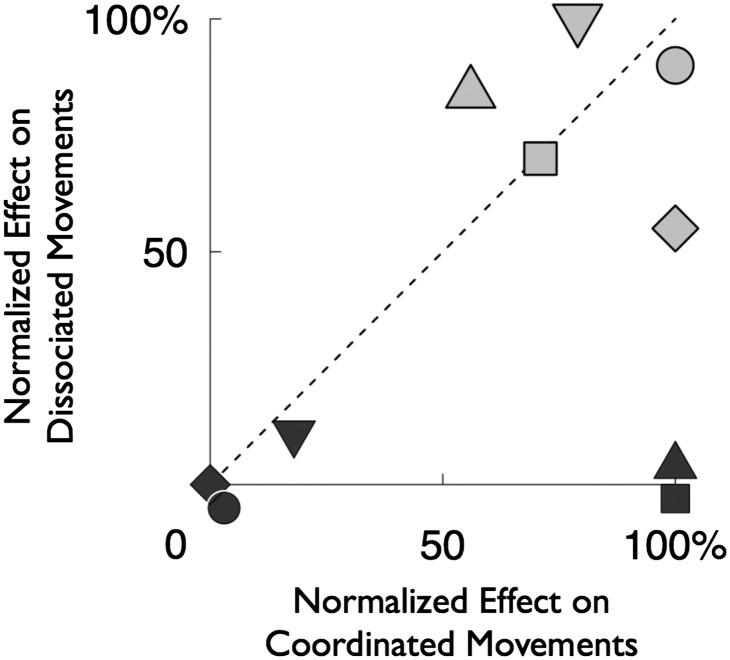
In Fig. 3, we compare inactivation effects in coordinated (abscissa) vs. dissociated (ordinate) movement conditions. Values are normalized to the maximum absolute effect found across the four movement types (Table 1 shows nonnormalized data). Inactivation effects that are independent of movement condition fall along the unity line whereas effects that are specific to coordinated or dissociated conditions fall along the x or y axis, respectively. For saccades, the data points for RT, error rate, accuracy, and endpoint scatter fall close to the unity line, indicating little or no effect of movement condition. In contrast, the data points for reaches fall on the x axis, indicating an effect specific to the coordinated condition (RT and accuracy), or are at the origin, indicating no effect in either condition (error rate, endpoint scatter, and duration). Thus, whereas effects on saccades were independent of condition, effects on reaches occurred only when the reach was accompanied by a saccade.
Fig. 3.
Comparison of the effect of LIP inactivation on coordinated (abscissa) vs. dissociated (ordinate) movements. Normalized effects on error rate (circle), RT (square), duration (diamond), accuracy (triangle pointing upward), and precision (triangle pointing downward) are shown for saccades (gray) and reaches (black).
Effects of Inactivation on Eye–Hand Coordination.
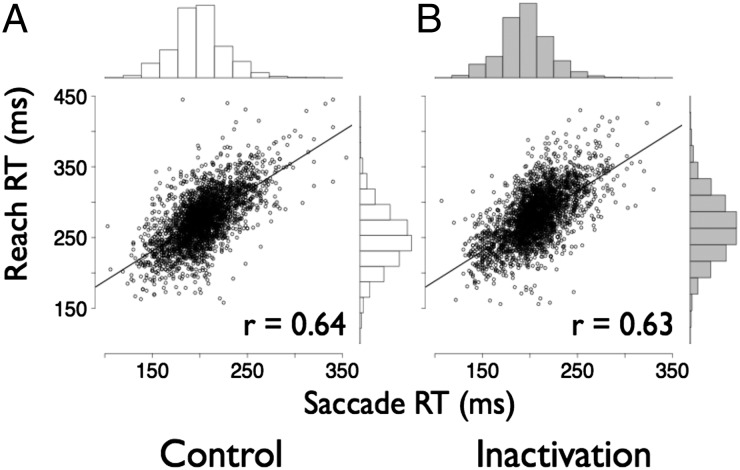
Previous studies in human and nonhuman primates have shown that coordinated reach and saccade latencies are tightly correlated on a trial-by-trial basis (7–10). Indeed, this was the case in our control data (Fig. 4A). We found strong correlations between coordinated saccade and reach RTs at the individual trial level (Pearson r = 0.637; P < 1 × 10−5; n = 3,441 trials) and at the individual session level (Pearson r = 0.869; P < 1 × 10−5; n = 18 sessions). If the circuits that control eye–arm coordination lie within LIP, LIP inactivation should reduce or even abolish the correlation. This was not the case (Fig. 4B). Instead, the strong correlation between saccade and reach RTs persisted after inactivation. In particular, the correlations seen in control and inactivation data were statistically indistinguishable from each other (across inactivation trials, Pearson r = 0.625; P < 1 × 10−6, not significantly different from control correlation of 0.637 with P = 0.68, Fisher Z-transformed r test). It is important to note that an effect on temporal coordination is entirely independent of the presence or absence of a mean effect on either saccade or reach RT.
Fig. 4.
Correlation of saccade and reach RT. Trial-by-trial RT for saccades and reaches are plotted for control (A) and inactivation (B) sessions. Histograms of the distributions of RT for saccades and reaches are shown above and to the right of the plots, respectively.
Another way to examine the trial-by-trial relationship of coordinated eye–arm movements is to examine the variability of the eye–arm offset, that is, the lag time between saccade and reach initiation. A looser coupling of the saccade and the reach would result in more variable eye–arm offsets. However, we found no change in offset (control SD, 28.3 ms; inactivation SD, 29.0 ms; P = 0.23, F test). The maintenance of trial-by-trial RT correlation after LIP inactivation does not support a role for LIP in coordinating the initiation of saccades and reaches. Furthermore, the fact that coordinated reaches are delayed by an amount similar to the delay of the accompanying saccade indicates that temporal eye–arm coordination is regulated downstream of area LIP. If coordination were imposed upstream of LIP, saccade delays produced by an LIP lesion would likely not affect coordinated reach RT.
Discussion
Single-unit recording studies have shown that LIP neurons are somewhat more active before saccades than reaches. This has led to a longstanding debate whether LIP contributes specifically to saccade planning or comprises a general purpose salience map (1–3, 13, 14). The results from the present study support a saccade-specific role for LIP. LIP lesions significantly impair saccades but have no effect on reaches unless the reach is accompanied by a saccade. Thus, although LIP is positioned early in the visuomotor hierarchy (21), the information processed in LIP is already motor-specific to saccades.
There are at least two explanations for why LIP lesions did not abolish saccades but instead merely delayed them by 5 to 10 ms. First, our LIP lesions were not complete. We used small injections to minimize inadvertent inactivation of adjacent areas. Based on the cortical extent of LIP (∼5 × ∼10 mm) and the diameters of the manganese halos (∼3–6 mm), we estimate that our injections inactivated no more than half of anatomically defined LIP, and, in some cases, much less. Second, slightly less rapid, parallel pathways may have compensated for the lost LIP function. For example, after an LIP lesion, saccades might still be driven by connections directly from the pulvinar, ventral intraparietal area, and area 7a to frontal eye field (FEF), or directly from the retina and early occipital areas to the superior colliculus (SC). It is likely that both mechanisms are at play in explaining the persistence of saccades.
The literature includes at least three previous studies of reversible lesions in LIP. Li et al. found saccade RT slowing (45 and 14 ms for contraversive and ipsiversive saccades, respectively) and hypometria (20%, e.g., 3° for a 15° movement) (15). In contrast, Wardak et al. found effects on visual search but no effects on saccade latency or accuracy, although it is unclear whether their study had enough power and precision to detect small effects (13, 14). A third study from our own laboratory (17) found latency effects of 5 to 10 ms, increases in errors of 1% to 5%, small effects on accuracy, and effects on visual search. In the present study, the effects on RT in particular were numerically small but highly reliable, with increases in RT 80% of the 60 inactivation data sets, and increases in either or both RT and error rate in all but two sessions. Why were the inactivation effects seen by Li et al. (15) larger than those seen in the present and other studies? One possibility is suggested by the fact that their animals had been used for previous parietal lobe recording studies, and that their control data appear to show substantial perturbations in saccade trajectory. It is possible that the recording studies caused some degree of permanent damage to LIP, area 7a, or neighboring areas, and that this damage then potentiated the effects of the subsequent reversible inactivations.
There are two possible explanations for why LIP lesions affected coordinated but not dissociated reaches. We argued earlier that a downstream mechanism might delay coordinated reaches by a fixed amount relative to saccades, so that any intervention that slows saccade onset will also slow reach onset. (A mechanism upstream of LIP would not compensate for the effect of the LIP lesion.) An alternative but less parsimonious possibility is that coordinated reaches are directly driven by LIP, but that dissociated reaches are supported by another pathway not involving LIP.
Inactivation of LIP Does Not Affect Temporal Eye–Arm Coordination.
Typically, the onset of the reach and the saccade are highly correlated, with the reach following the saccade by 50 to 100 ms (refs. 18–20, but see ref. 22). A neural mechanism might actively couple the two movements, or the correlation could arise passively as a result of a common input signal to the eye and arm movement pathways. LIP is a potential candidate for an active mechanism, as it contains saccade- and reach-related signals and there are signals within LIP related to eye–hand coordination (4, 11). However, our lesions did not reduce trial-by-trial temporal correlation (Fig. 4). The fact that a lesion that slows saccades also affects coordinated reaches (but not dissociated reaches) is good evidence for an active coupling mechanism that lies outside of LIP. We cannot rule out a second, redundant mechanism for temporal eye–hand coordination within LIP. Nor can we rule out LIP involvement in other aspects of eye–hand coordination that we have not tested. It is also possible that, by injecting a larger volume of muscimol and inactivating a greater percentage of LIP, one might observe an effect. However, our imaging method suggests that even slow infusions of volumes greater than 2 μL will often extend beyond the borders of LIP and thus compromise the experiment.
There may be behavioral advantages to coupling the timing of the reach and the saccade. Psychophysical studies show that foveating the target before or shortly after the reach begins increases reach accuracy and may benefit processes like the adjustment of grip aperture or the choice of how to grasp an object (23–25). Thus, we should not be surprised by a neural mechanism that conditions the start of the reach on the timing of the accompanying saccade.
Where might such a control mechanism be located? Representations of saccade and reach plans are required. LIP projects to FEF, which is in turn reciprocally connected with dorsal premotor cortex (PMd) (26, 27). Although the most prominent signals in FEF and PMd are oculomotor and somatomotor, respectively, modulation with regard to eye and hand movements has been described in each area (28–30). Either or both could be the locus of control. Alternatively, LIP also projects to the SC, which contains cells with both saccade and reach responses and provides another potential locus for coordinating circuitry (12, 31, 32).
Functional Divisions and Laterality Within LIP.
In the present study, inactivating LIPv produced deficits exclusively for saccades into the contralateral hemifield, whereas inactivating LIPd produced deficits in both hemifields with a bias for the contralateral hemifield (Fig. S1). Lesion studies (13–15), unit recording (33, 34), and imaging (35–37) support a contralateral hemifield organization for LIP. However, few of these studies differentiate between LIPd and LIPv, so it is possible that a bilateral representation in LIPd was obscured by the strong contralateral field bias in LIPv. A pair of imaging studies that did differentiate LIPv from LIPd did not test for laterality (38, 39). One recording study reported a bilateral spatial representation in LIP (40). In this study (40), most of the representative recording sites were located superficially, raising the possibility that LIPd may have been oversampled relative to LIPv.
LIPv may be more closely associated with visual sensory input than LIPd. Tract tracing studies report heavier input to LIPv than LIPd from extrastriate areas V2, V3, and V4, whereas LIPd receives heavier inputs from higher-order areas including area 7b, anteriomedial TE, and rostral temporal parietal occipital area, and prefrontal area 45 (27, 41). LIPv preferentially receives feedback projections from FEF whereas LIPd receives feedforward connections, consistent with a higher position in the cortical hierarchy for LIPd than LIPv (42). Although the overall role of LIP appears to be geared toward oculomotor planning, the differences observed in visual field representation in LIPd vs. LIPv could be related to a functional difference between the two areas. The stronger hemispheric lateralization in LIPv relative to LIPd suggests that LIPv may be more closely related to lateralized early visual sensory areas.
We speculate that there may be a general gradient within posterior parietal cortex, such that deeper sulcal areas are functionally and anatomically closer to the visual input, whereas superficial (i.e., gyral) areas are more advanced. For example, area 7a, on the gyral surface, lies late in the dorsal stream hierarchy (21). Additionally, on the medial side of the IPS, deeper cells respond primarily to visual input and/or somatosensation, whereas superficial cells and cells on the gyral surface are modulated before a reach but are much less likely to show visual responses (43, 44). Nearby posterior parietal area V6a recently has been cytoarchitectonically divided into dorsal and ventral subdivisions that demonstrate distinct patterns of connectivity (45, 46). Like LIP, the ventral subdivision is connected primarily with extrastriate visual areas, whereas the dorsal subdivision is more strongly connected with higher-order areas, including PMd and dorsolateral prefrontal cortex (47).
Role of LIP in Attention.
There is a long history of conflicting interpretations of the role of LIP. Unit recording studies show that LIP is more active when coding targets for upcoming saccades than for reaches (4–6). Other studies show that LIP activity is correlated with the spatial locus of attention during saccade tasks (2, 3) and that lesions of LIPv affect covert search among distractors even in a nonsaccadic task (14).
LIP is not the only region that signals saccadic intention yet may also play a role in attention. SC is a classic oculomotor area, yet unit recording and inactivation studies suggest a role in attention and reaching (12, 48, 49). Similarly, FEF is classically considered to play a role in generating saccades. However, FEF, like SC and LIP, has also been implicated in attentional control (50–54). Lesioning an area that is involved in visual attention should impair any spatially directed behavior (55). If we accept that the deficit in covert search among distractors after an LIPv lesion reflects a role for LIPv in directing attention, we would expect LIPv lesions to affect all visually guided movements. The fact that LIPv lesions do not impair (dissociated) reaches is somewhat surprising (Fig. S1). One explanation is that planning a reach to a single target does not require attention. An alternative is to reconsider the notion that attention is a unitary phenomenon with a single mechanism controlling all spatially directed tasks. A recent study has presented evidence for separate attentional systems for saccade and reach movements. Jonikaitis and Deubel (56) demonstrate that perceptual discrimination can be enhanced at two locations simultaneously, by asking subjects to plan an eye movement to one location and an arm movement to the other. Subjects do not split a single attentional resource in two: the degree of perceptual enhancement associated with each type of movement is the same, regardless of whether one or two types of movements are planned. If these results from humans apply to monkeys, LIPv might be involved in saccade planning and saccade-related attention, whereas other areas (e.g., parietal reach region, PMd) might mediate attentional effects that are associated with reaches.
Materials and Methods
Four adult male macaque monkeys were trained to make eye and arm movements to targets on a touch screen 17 cm away. Visual stimuli were back-projected onto a custom-designed IR touch screen. Touch position was detected every 2 ms by IR monitoring beams situated adjacent to the screen surface. Eye movements were monitored with a scleral search coil implant (CNC Engineering). Animals sat in complete darkness with their heads restrained in custom-made primate chairs (Crist Instruments). The fronts of the chairs were completely open so that the animals had free range of movement of the forelimbs. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Washington University Institutional Animal Care and Use Committee.
Behavioral Task.
All animals performed memory-guided, center-out saccades. Monkeys G, Q, and W performed combined reaches and saccades (coordinated reaches). Monkeys G and S performed reaches without saccades (dissociated reaches; Fig. 1). Reach and saccade trials were interleaved. A Plexiglas panel blocked the arm not in use. Trials started with the animal fixating and touching a central fixation cue (5.5° windows for the eye, 6° for the hand). After 350 ms of fixation, a peripheral target was flashed for 150 ms in one of eight equally spaced locations 20° (6.2 cm) from the fixation point. After a subsequent 1,000- to 1,600-ms delay, the fixation target was extinguished and the animal had 500 ms to initiate and complete a saccade and/or 750 ms to initiate and complete a reach to within 10° of the remembered target location. On coordinated trials, reaches were initiated an average of 72.8 ms after the onset of the saccade. On dissociated trials, the nonmoving effector was constrained to a 5.5° central fixation window. All windows were kept large in time and space so that lesion effects would not prevent the animals from performing the task. If the animal moved to within 10° of the target, a fluid reward was given. If the initial movement landed within 5.0° of the saccade target or 6.5° of the reach target, a second reward was given and the trial was ended. If not, the target reappeared 150 ms after the completion of the initial movement, and the animal had a maximum of 2 s to make a corrective movement to within 5.0° (saccade) or 6.5° (reach) of the (visible) target. Upon completion of a corrective movement, a second, smaller reward was given. Only the initial movement endpoint was used in data analysis; corrective movements to the visible target, along with the reward structure of the task, were used only to encourage the animals not to take advantage of the large windows but instead to move as accurately as possible. Animals performed ∼1,000 trials per session. Half these trials were dissociated saccade trials, and the other half were coordinated reach with saccade trials or dissociated reach trials.
Reversible Inactivation.
LIP was initially localized by using single-unit recording. LIP was defined as an area containing a high concentration of cells exhibiting visuospatial selectivity, with phasic visual activity and significant sustained activity during the delay period of memory-guided saccades. We use “LIP” to refer collectively to both dorsal and ventral divisions of LIP, even though functional criteria to distinguish LIPd from LIPv have not yet been established and anatomical boundaries remain difficult to assess because most studies do not report anatomical data. Inactivations were aimed well above or below the anatomical division at 53% of sulcal depth, as suggested by Lewis and Van Essen (27).
In each inactivation session, a 33-gauge cannula attached to a 25-µL Hamilton syringe was lowered to the desired depth. Ten minutes later, 0.5 to 2.0 µL of the inactivation solution was injected at a rate of 0.05 to 0.15 µL/min using a microinjection pump (Harvard Apparatus). The solution was composed of 8 mg/mL muscimol, an NMDA agonist, and 0.1 M of the MRI contrast agent manganese [19.8 mg/mL MnCl2(H2O)4 mixed in sterile water]. The cannula was left in place for 10 min after the end of each injection and then slowly retracted.
Inactivation and control sessions differed only in the presence of the muscimol/manganese injection. Each experimental session was paired with two controls, each on a separate day. Control sessions were the two sessions before each inactivation session. For control sessions, the experimenter would perform a sham injection in which the injection drive was mounted to the monkey’s head but not lowered down into the brain, and the microinjection pump was turned on. Control sessions were identical to inactivation sessions in number of trials, time of day, duration, and tasks performed. Control sessions never occurred on the day following an inactivation. No experimental sessions were excluded based upon behavior. Experimental sessions were excluded only on the basis of injection location.
Lesion Localization with MRI.
Following the behavioral session (2–4 h after injection), T1-weighted anatomical images (Fig. 1, Inset) were collected by using a magnetization-prepared rapid-acquisition gradient echo sequence conducted at 0.53 mm3 on a 3-T head-only system (Allegra; Siemens). A volume coil was used. Animals were fully anesthetized during the procedure. Injections were visible as a bright halo representing the manganese-induced T1 signal increase. Experiments in which there was no halo or a halo that overlapped the medial bank, the gyrus (area 7a), or the gray matter of the superior temporal sulcus were rejected (17).
Data Processing.
Reaches were defined as a change in hand position of at least 3°. Reach onset and offset were defined as the time at which the arm moved 1° from the starting or ending position, respectively. If an animal released the screen without first moving the criterion distance, reach onset was defined as the time of release. Saccades were defined as a change in eye position of at least 2°. Saccade onset and offset were defined as the time at which the velocity increased to 20°/s or decreased to 16°/s, respectively. Within each session, accuracy and precision (i.e., endpoint scatter) were computed for each target location. Accuracy was quantified as the Euclidian distance between the target and the mean endpoint. Endpoint scatter, which varies inversely with precision, was quantified as the average Euclidian distance between each individual movement endpoint and the mean endpoint. Errors included movements that occurred before or after the allotted movement period, failure to maintain fixation at the location of the peripheral target for at least 150 ms, movements that landed more than 10° away from the remembered peripheral target location, or failure to make a corrective movement to the peripheral target location after it flashed at the end of the trial. Trials in which an error occurred before the initial target appearance were excluded from the study.
Behavioral data from each inactivation session were compared with the data from the two previous control sessions. Unless otherwise noted, the significance of the effect of each inactivation vs. the two previous control sessions was computed by using a two-tailed Welch t test. The Welch t test computes independent variances for the control and injection data, and is therefore more conservative (i.e., fewer degrees of freedom) than a standard t test. The significance of inactivation effects across sessions was computed by using a two-tailed Student t test that compared the means from each session. A two-tailed χ2 test was used to determine the significance of a change in error rate.
Supplementary Material
Acknowledgments
We thank Jonathon Tucker, Thomas Malone, and the late Marcel Fremont for MRI technical assistance. This work was supported by National Eye Institute Grants EY012135 and EY002687, National Institute of Mental Health Grant MH088522, and National Science Foundation Integrative Graduate Education and Research Traineeship Grant 0548890.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220508110/-/DCSupplemental.
References
- 1.Colby CL, Duhamel JR. Spatial representations for action in parietal cortex. Brain Res Cogn Brain Res. 1996;5(1-2):105–115. doi: 10.1016/s0926-6410(96)00046-8. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391(6666):481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- 3.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299(5603):81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 4.Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386(6621):167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson AR, Calton JL, Snyder LH. Nonspatial saccade-specific activation in area LIP of monkey parietal cortex. J Neurophysiol. 2003;90(4):2460–2464. doi: 10.1152/jn.00788.2002. [DOI] [PubMed] [Google Scholar]
- 6.Quian Quiroga R, Snyder LH, Batista AP, Cui H, Andersen RA. Movement intention is better predicted than attention in the posterior parietal cortex. J Neurosci. 2006;26(13):3615–3620. doi: 10.1523/JNEUROSCI.3468-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prablanc C, Echallier JF, Komilis E, Jeannerod M. Optimal response of eye and hand motor systems in pointing at a visual target. I. Spatio-temporal characteristics of eye and hand movements and their relationships when varying the amount of visual information. Biol Cybern. 1979;35(2):113–124. doi: 10.1007/BF00337436. [DOI] [PubMed] [Google Scholar]
- 8.Fisk JD, Goodale MA. The organization of eye and limb movements during unrestricted reaching to targets in contralateral and ipsilateral visual space. Exp Brain Res. 1985;60(1):159–178. doi: 10.1007/BF00237028. [DOI] [PubMed] [Google Scholar]
- 9.Fischer B, Rogal L. Eye-hand-coordination in man: A reaction time study. Biol Cybern. 1986;55(4):253–261. doi: 10.1007/BF00355600. [DOI] [PubMed] [Google Scholar]
- 10.Dean HL, Martí D, Tsui E, Rinzel J, Pesaran B. Reaction time correlations during eye-hand coordination: Behavior and modeling. J Neurosci. 2011;31(7):2399–2412. doi: 10.1523/JNEUROSCI.4591-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean HL, Hagan MA, Pesaran B. Only coherent spiking in posterior parietal cortex coordinates looking and reaching. Neuron. 2012;73(4):829–841. doi: 10.1016/j.neuron.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JH, Rafal RD, McPeek RM. Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proc Natl Acad Sci USA. 2011;108(51):E1433–E1440. doi: 10.1073/pnas.1109656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22(22):9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42(3):501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 15.Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81(4):1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- 16.Balan PF, Gottlieb J. Functional significance of nonspatial information in monkey lateral intraparietal area. J Neurosci. 2009;29(25):8166–8176. doi: 10.1523/JNEUROSCI.0243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Yttri EA, Snyder LH. Intention and attention: Different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13(4):495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biguer B, Jeannerod M, Prablanc C. The coordination of eye, head, and arm movements during reaching at a single visual target. Exp Brain Res. 1982;46(2):301–304. doi: 10.1007/BF00237188. [DOI] [PubMed] [Google Scholar]
- 19.Helsen WF, Elliott D, Starkes JL, Ricker KL. Temporal and spatial coupling of point of gaze and hand movements in aiming. J Mot Behav. 1998;30(3):249–259. doi: 10.1080/00222899809601340. [DOI] [PubMed] [Google Scholar]
- 20.Snyder LH, Calton JL, Dickinson AR, Lawrence BM. Eye-hand coordination: Saccades are faster when accompanied by a coordinated arm movement. J Neurophysiol. 2002;87(5):2279–2286. doi: 10.1152/jn.00854.2001. [DOI] [PubMed] [Google Scholar]
- 21.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 22.Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vision Res. 2001;41(25-26):3559–3565. doi: 10.1016/s0042-6989(01)00102-x. [DOI] [PubMed] [Google Scholar]
- 23.Neggers SF, Bekkering H. Integration of visual and somatosensory target information in goal-directed eye and arm movements. Exp Brain Res. 1999;125(1):97–107. doi: 10.1007/s002210050663. [DOI] [PubMed] [Google Scholar]
- 24.Connolly JD, Goodale MA. The role of visual feedback of hand position in the control of manual prehension. Exp Brain Res. 1999;125(3):281–286. doi: 10.1007/s002210050684. [DOI] [PubMed] [Google Scholar]
- 25.Selen LP, Medendorp WP. Saccadic updating of object orientation for grasping movements. Vision Res. 2011;51(8):898–907. doi: 10.1016/j.visres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265(3):332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000b;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Thura D, Hadj-Bouziane F, Meunier M, Boussaoud D. Hand position modulates saccadic activity in the frontal eye field. Behav Brain Res. 2008;186(1):148–153. doi: 10.1016/j.bbr.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Fujii N, Mushiake H, Tanji J. Rostrocaudal distinction of the dorsal premotor area based on oculomotor involvement. J Neurophysiol. 2000;83(3):1764–1769. doi: 10.1152/jn.2000.83.3.1764. [DOI] [PubMed] [Google Scholar]
- 30.Pesaran B, Nelson MJ, Andersen RA. A relative position code for saccades in dorsal premotor cortex. J Neurosci. 2010;30(19):6527–6537. doi: 10.1523/JNEUROSCI.1625-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuphorn V, Hoffmann KP, Miller LE. Correlation of primate superior colliculus and reticular formation discharge with proximal limb muscle activity. J Neurophysiol. 1999;81(4):1978–1982. doi: 10.1152/jn.1999.81.4.1978. [DOI] [PubMed] [Google Scholar]
- 32.Lünenburger L, Kleiser R, Stuphorn V, Miller LE, Hoffmann KP. A possible role of the superior colliculus in eye-hand coordination. Prog Brain Res. 2001;134:109–125. doi: 10.1016/s0079-6123(01)34009-8. [DOI] [PubMed] [Google Scholar]
- 33.Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: A quantitative receptive field analysis. Exp Brain Res. 2001;140(2):127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- 34.Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66(3):1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- 35.Patel GH, et al. Topographic organization of macaque area LIP. Proc Natl Acad Sci USA. 2010;107(10):4728–4733. doi: 10.1073/pnas.0908092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagan I, Iyer A, Lindner A, Andersen RA. Space representation for eye movements is more contralateral in monkeys than in humans. Proc Natl Acad Sci USA. 2010;107(17):7933–7938. doi: 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arcaro MJ, Pinsk MA, Li X, Kastner S. Visuotopic organization of macaque posterior parietal cortex: A functional magnetic resonance imaging study. J Neurosci. 2011;31(6):2064–2078. doi: 10.1523/JNEUROSCI.3334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakola S, Gregoriou GG, Moschovakis AK, Savaki HE. Functional imaging of the intraparietal cortex during saccades to visual and memorized targets. Neuroimage. 2006;31(4):1637–1649. doi: 10.1016/j.neuroimage.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Savaki HE, Gregoriou GG, Bakola S, Raos V, Moschovakis AK. The place code of saccade metrics in the lateral bank of the intraparietal sulcus. J Neurosci. 2010;30(3):1118–1127. doi: 10.1523/JNEUROSCI.2268-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platt ML, Glimcher PW. Response fields of intraparietal neurons quantified with multiple saccadic targets. Exp Brain Res. 1998;121(1):65–75. doi: 10.1007/s002210050438. [DOI] [PubMed] [Google Scholar]
- 41.Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb Cortex. 2010;20(1):141–168. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- 42.Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23(1):161–179. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- 43.Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29(6):517–537. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- 44.Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol. 1997;78(5):2413–2426. doi: 10.1152/jn.1997.78.5.2413. [DOI] [PubMed] [Google Scholar]
- 45.Luppino G, Ben Hamed S, Gamberini M, Matelli M, Galletti C. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: A cytoarchitectonic study. Eur J Neurosci. 2005;21(11):3056–3076. doi: 10.1111/j.1460-9568.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- 46.Gamberini M, Galletti C, Bosco A, Breveglieri R, Fattori P. Is the medial posterior parietal area V6A a single functional area? J Neurosci. 2011;31(13):5145–5157. doi: 10.1523/JNEUROSCI.5489-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gamberini M, et al. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J Comp Neurol. 2009;513(6):622–642. doi: 10.1002/cne.21980. [DOI] [PubMed] [Google Scholar]
- 48.Robinson DL, Kertzman C. Covert orienting of attention in macaques. III. Contributions of the superior colliculus. J Neurophysiol. 1995;74(2):713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- 49.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13(2):261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98(3):1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bichot NP, Thompson KG, Chenchal Rao S, Schall JD. Reliability of macaque frontal eye field neurons signaling saccade targets during visual search. J Neurosci. 2001;21(2):713–725. doi: 10.1523/JNEUROSCI.21-02-00713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004;101(43):15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26(16):4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer RJ, Moore T. Selective attention from voluntary control of neurons in prefrontal cortex. Science. 2011;332(6037):1568–1571. doi: 10.1126/science.1199892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17(2):75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 56.Jonikaitis D, Deubel H. Independent allocation of attention to eye and hand targets in coordinated eye-hand movements. Psychol Sci. 2011;22(3):339–347. doi: 10.1177/0956797610397666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.