Abstract
Age-related hearing loss and noise-induced hearing loss are major causes of human morbidity. Here we used genetics and functional studies to show that a shared cause of these disorders may be loss of function of the ATP-gated P2X2 receptor (ligand-gated ion channel, purinergic receptor 2) that is expressed in sensory and supporting cells of the cochlea. Genomic analysis of dominantly inherited, progressive sensorineural hearing loss DFNA41 in a six-generation kindred revealed a rare heterozygous allele, P2RX2 c.178G > T (p.V60L), at chr12:133,196,029, which cosegregated with fully penetrant hearing loss in the index family, and also appeared in a second family with the same phenotype. The mutation was absent from more than 7,000 controls. P2RX2 p.V60L abolishes two hallmark features of P2X2 receptors: ATP-evoked inward current response and ATP-stimulated macropore permeability, measured as loss of ATP-activated FM1-43 fluorescence labeling. Coexpression of mutant and WT P2X2 receptor subunits significantly reduced ATP-activated membrane permeability. P2RX2-null mice developed severe progressive hearing loss, and their early exposure to continuous moderate noise led to high-frequency hearing loss as young adults. Similarly, among family members heterozygous for P2RX2 p.V60L, noise exposure exacerbated high-frequency hearing loss in young adulthood. Our results suggest that P2X2 function is required for life-long normal hearing and for protection from exposure to noise.
Keywords: channel, deafness, genomics, presbycusis
Hearing loss is a socially and economically important cause of human morbidity, affecting 538 million people worldwide, including 36 million people in the United States (1, 2). Hearing loss is often related to inherited genetic lesions, with 60 of the critical genes identified and more than 100 others mapped (http://hereditaryhearingloss.org). The identification of genes for inherited forms of hearing loss that progress with age is particularly important for understanding age-related hearing loss, or presbycusis, which affects more than 40% of people over age 65 y (3). Age-related hearing loss is influenced both by genetics and by environmental factors, including exposure to noise in the workplace, in the environment, or recreationally (4–7). Noise-induced hearing loss is also influenced by genetic factors (8, 9), although causal genes have not heretofore been identified. An important focus of current hearing research is on the biological mechanisms underlying age-related and noise-induced hearing loss.
Here we report the identification of a mutation in the P2RX2 gene as the cause of dominantly inherited, progressive hearing loss in two families. P2RX2 encodes the P2X2 receptor, which assembles as a trimer to form a channel gated by extracellular ATP. P2X2 receptors mediate a variety of cellular responses, including excitatory postsynaptic responses in sensory neurons (10, 11). In the inner ear, P2X2 receptors are thought to regulate sound transduction and auditory neurotransmission (12–14), outer hair cell electromotility (15, 16), inner ear gap junctions (17), and K+ recycling (17, 18). We explored the effect of the inherited mutation in P2RX2 on function of the P2X2 receptors and the consequences of loss of function of these ATP-gated channels on progressive and noise-induced hearing loss.
Results
Discovery of P2RX2 as Responsible for Progressive Hearing Loss DFNA41.
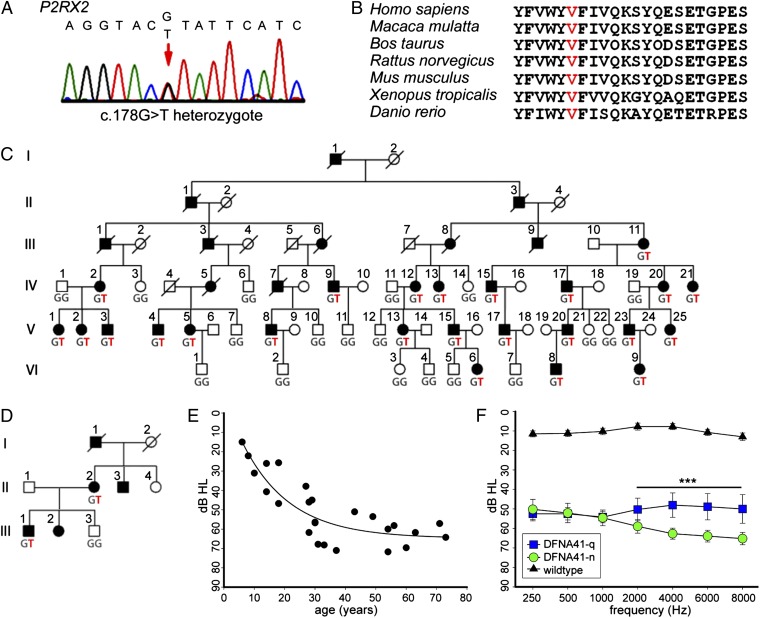
DFNA41 is an autosomal dominant, progressive, sensorineural hearing loss defined in a six-generation kindred living in Sichuan, China (19). Audiologic evaluation of family members revealed bilateral and symmetrical sensorineural hearing loss, with age at onset ranging from 12 y to 20 y, generally accompanied by high-frequency tinnitus. Hearing loss was progressive and ultimately involved all frequencies. The gene responsible for hearing loss in the family was localized by linkage analysis to the region between D12S1609 and 12qter (19), but Sanger sequencing of eight genes in this interval did not identify the responsible gene (20).
The DFNA41 linkage interval corresponds to the 4.80-MB genomic region chr12:129,051,849–133,851,895 (hg19) and includes at least 34 genes. To identify the gene responsible for hearing loss in the family, we captured and sequenced all 427 coding exons, noncoding exons, and documented regulatory regions in the linked interval from genomic DNA of three affected family members. Using a pipeline for variant discovery from massively parallel sequences (21), we identified all variants in the targeted region. In the 4.80-MB region, the three affected individuals shared 216 variants, of which 206 were common polymorphisms also present in multiple other family members and unrelated persons. The 10 rare variants included 5 intronic variants, 3 intergenic variants, 1 synonymous coding sequence change, and 1 nonsynonymous change. Only the nonsynonymous change altered a conserved nucleotide site: chr12:133,196,029 G > T (hg19), corresponding to P2RX2 c.178G > T (p.V60L NM_174873). This variant was confirmed by Sanger sequencing (Fig. 1A). The residue is conserved as valine in all sequenced vertebrates, from zebrafish to mammals (Fig. 1B). The mutation has been predicted to affect protein function (22).
Fig. 1.
Identification of P2RX2 as the gene responsible for DFNA41. (A) P2RX2 sequence from a family member with hearing loss. (B) Evolutionary conservation of the region of the P2X2 receptor protein including the variant site. (C) DFNA41 family with genotypes at P2RX2 c.178G > T (p.V60L). Filled symbols represent persons with progressive sensorineural hearing loss. Genomic capture and sequencing were carried out for individuals IV:12, IV:20, and V:04. (D) A second family with the DFNA41 phenotype, also carrying P2RX2 c.178G > T (p.V60L). (E) Progression of DFNA41 hearing loss with age. Among mutation carriers, average hearing thresholds (PTA250–8,000 Hz) declined until the fourth decade then remained approximately stable. (F) Noise exposure and high-frequency hearing loss. Mutation carriers who reported a history of occupational noise exposure as young adults (n = 12; green symbols) had significantly poorer hearing in the 2,000–8,000 Hz range compared with mutation carriers who reported no history of noise exposure (n = 9; blue symbols). ***P = 0.001.
In the family defining DFNA41, P2RX2 c.178G > T (p.V60L) cosegregated perfectly with hearing loss, with an LOD score of 13.3, corresponding to odds of 1013:1 in favor of linkage (Fig. 1C). Penetrance of the phenotype among heterozygous mutation carriers was 100%. Sequencing of the entire P2RX2 coding sequence in 65 other families with autosomal dominant nonsyndromic sensorineural hearing loss yielded one other family, also Chinese, carrying P2RX2 c.178G > T (Fig. 1D). This family lives in a different region of China than the DFNA41 family. The two families were not aware of any relationship between them. All affected individuals in the second family had bilateral moderate to severe progressive sensorineural hearing loss with onset in the second decade, similar to affected members of the original DFNA41 family. A computed tomography scan of one affected family member revealed normal temporal bones. Middle ear status was normal, and clinical examinations revealed no other abnormalities. The mutation was absent from 7,000 controls, including 4,300 persons of European ancestry, 2,200 persons of African-American ancestry (evs.gs.washington.edu/EVS/), and 500 persons of Chinese ancestry. Thus, the P2RX2 c.178G > T allele has an estimated frequency of <0.001 in the Chinese population and of <0.0001 in a mixed-ancestry population.
Among the DFNA41 family members heterozygous for P2RX2 p.V60L, hearing loss progressed with age. Hearing loss was first detected in the second decade of life and was severe (60–70 dB) by age 20 y, with little further progression thereafter (Fig. 1E). Some family members exhibited elevated thresholds at one or more frequencies as early as age 6–10 y, suggesting that this loss may begin at an even earlier age. Members of the DFNA41 family had significantly varied exposure to noise as young adults. The family lives in a rural area of Sichuan province with traditional agriculture and little noise exposure, but many young adults work in industrial areas, where they were exposed to continuous noise in construction trades. All participating family members were interviewed for their history of noise exposure after age 12 y. Mutation carriers reporting daily exposure in construction trades (and age of exposure between age 12 and 25 y) were subjects IV:15 (16–21 y), IV:17 (15–18 y), V:4 (14–21 y), V:8 (15–19 y), V:15 (14–21 y), V:17 (15–20 y), V:20 (14–16 y), and V:23 (16–17 y). Subjects III:9, IV:9, and V:1 reported similar exposure but did not provide age of exposure. Subject IV:21 reported frequent exposure to explosions in a fireworks factory beginning at age 20 y. Mutation carriers III:11, IV:2, IV:12, IV:13, IV:20, V:2, V:5, V:13, and V:25 reported no exposure to noise. Among DFNA41 family members heterozygous for P2RX2 p.V60L, those exposed to noise as young adults had significantly poorer hearing at high frequencies compared with those not exposed (P = 0.001) (Fig. 1F).
P2RX2 p.V60 is known to be critical to the function of the P2X2 receptor by forming a disulfide bond with P2RX2 p.I339 (NP_174873), creating the gate that opens the channel on stimulation by ATP. Experimental substitution of cysteine at residues 60 and 339 results in a channel that is unresponsive to ATP (23). We explored the consequences of the naturally occurring substitution of leucine at P2RX2 p.V60.
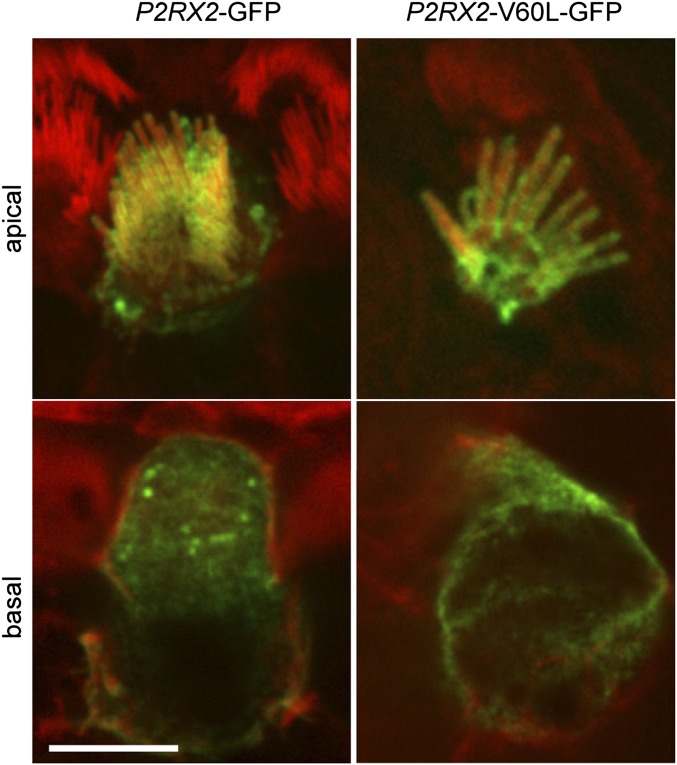
P2RX2 Localizes to Plasma Membranes of Hair Cells.
Explant cultures of neonatal rat organ of Corti and of vestibular tissues were transfected with GFP-tagged WT P2RX2 or with GFP-tagged P2RX2 p.V60L. Within 9 h after transfection, WT and mutant P2X2 receptors localized identically to the apical membranes of hair cells of the organ of Corti, including the stereociliary bundles (Fig. 2). GFP fluorescence was demonstrated in cell bodies, indicating that P2X2 receptors are synthesized and compartmentalized in the endoplasmic reticulum and Golgi complex. This distribution is consistent with the localization of P2RX2 to the endolymphatic surface of the sensory hair cells and other epithelial cells of the cochlear partition.
Fig. 2.
Localization of P2X2 receptors in hair cells. Both WT and mutant P2X2 receptors preferentially target the plasma membranes of auditory hair cells. Fluorescence confocal optical sections of the organ of Corti of rat inner ear tissue culture are shown, with GFP-tagged P2RX2 WT and mutant constructs stained in green and actin counterstained in red. Images are of the hair cell apical surface and 7–8 µm deep through the basolateral pole of the cell body. (Scale bar: 5 µm.)
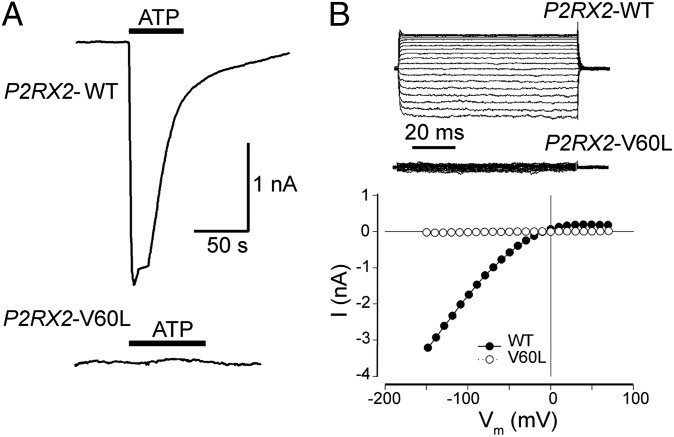
P2RX2 p.V60L Abolishes Response of P2X2 to ATP.
Patch clamp recording showed that stimulation with ATP (36 μM–1 mM) evoked a large inward current in HEK293 cells transfected with WT P2RX2, whereas cells transfected with P2RX2 p.V60L demonstrated no response to 1 mM ATP (n = 15; Fig. 3A). At a holding potential of −80 mV, the average 1 mM ATP-evoked current in cells expressing WT P2RX2 was 2.17 ± 0.46 nA (n = 16). Inward rectification of the ATP-gated current was evident in cells transfected with WT P2RX2, but not in cells transfected with P2RX2 p.V60L (Fig. 3B).
Fig. 3.
P2RX2 p.V60L-transfected HEK293 cells lack ATP-gated inward currents. (A) An ATP-evoked inward current is visible in an HEK293 cell transfected with the WT P2RX2 plasmid. The cell was clamped at −80 mV under the whole-cell configuration. The horizontal line represents ATP perfusion. In contrast, no current was detectable in a cell transfected with P2RX2 p.V60L. (B) An example of the inward rectifying ATP-gated current across the applied voltage range (−150 to +70 mV) in an HEK293 cell transfected with WT P2RX2. In contrast, a cell transfected with the P2RX2 p.V60L plasmid lacked this ATP-activated membrane conductance. The current–voltage (I-V) relationship was determined as the average current over the last 20 ms at the various voltage steps during the steady-state phase of the ATP-gated inward current.
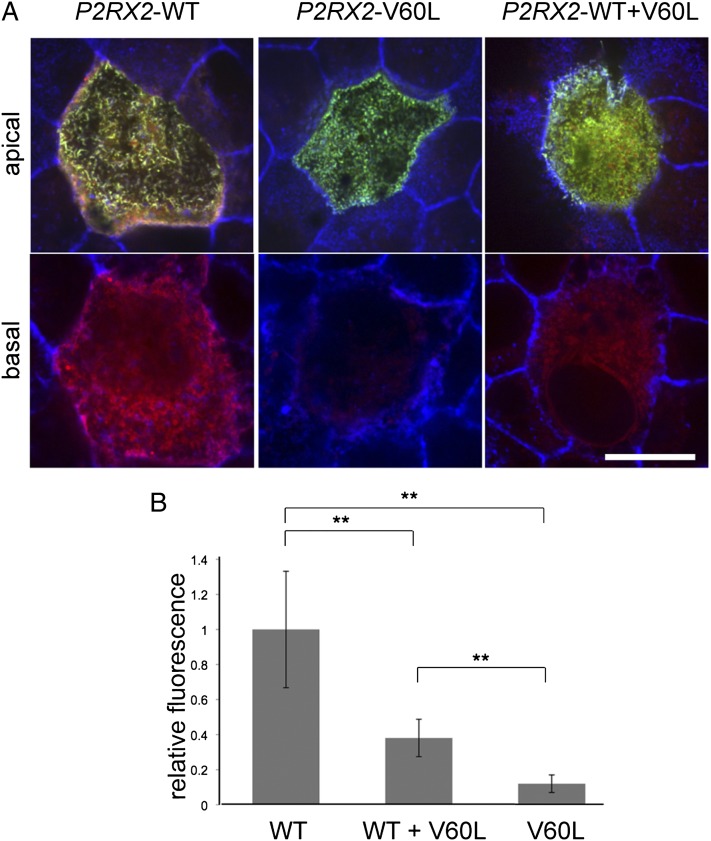
P2RX2 p.V60L Abolishes ATP-Stimulated Permeability of P2X2 Receptors.
When stimulated by extracellular ATP, P2X2-type ATP-gated ion channels form large pores that allow passage of cationic dyes (24–26). To determine whether ion channel permeability was altered by P2RX2 p.V60L, we transfected MDCK-II cells with GFP-tagged WT P2RX2, GFP-tagged P2RX2 p.V60L, or both forms simultaneously, then tested for permeability to the cationic dye FM1-43 in the presence or absence of ATP (Fig. 4). When cells expressing homomeric WT P2X2 receptors were stimulated with 300 µM ATP for 1 min in the presence of FM1-43, their internal membranes, including microvilli, were stained by FM1-43 (Fig. 4A, Left). When cells expressing homomeric mutant P2X2 receptors were similarly stimulated with ATP, no FM1-43 fluorescence signal was detected (Fig. 4A, Center). DFNA41 family members heterozygous for P2RX2 p.V60L express both WT and mutant P2X2 subunits. In MDCK-II cells expressing heteromeric channels composed of both WT and mutant P2X2 subunits, FM1-43 uptake in the presence of ATP was reduced by ∼60% compared with cells expressing only WT P2X2 channels (Fig. 4 A, Right and B).
Fig. 4.
P2RX2 p.V60L abolishes ATP-induced permeability of P2X2 receptors. Confocal optical sections showing staining of FM1-43 dye (red) on the apical surface and 4–5 µm deep through the cell body of polarized MDCK-II cells expressing GFP-tagged P2X2 constructs (green) in the presence of ATP. (A) (Left) Cells expressing WT P2X2 were permeable to FM1-43, with staining present in both apical and basal cell regions. (Center) Cells expressing only mutant P2RX2 p.V60L showed no FM1-43 staining. (Right) Cells expressing both WT and P2RX2 p.V60L showed significantly reduced FM1-43 staining compared with WT. (Scale bar: 5 µm.) (B) FM1-43 uptake was assayed by fluorescence intensity and normalized to that of cells expressing only WT P2RX2. One hundred cells were measured for each condition. FM1-43 permeability in cells expressing both WT and P2RX2 p.V60L simultaneously was reduced by ∼60% compared with cells expressing only WT P2RX2. **P < 0.001.
Age-Related Hearing Loss in Mice Lacking the P2X2 Receptor.
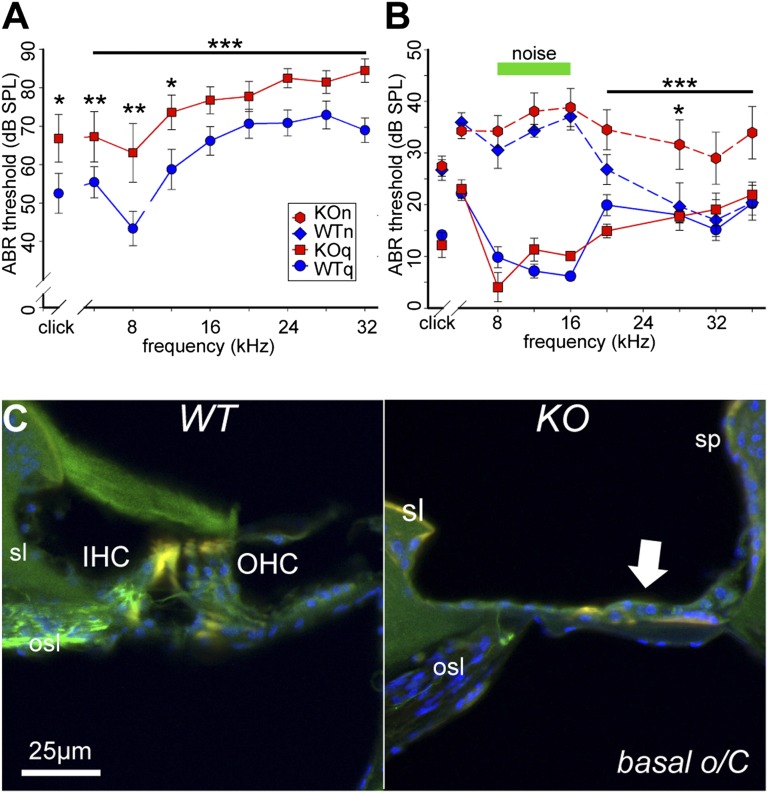
The P2RX2-null mouse was developed to evaluate the role of the P2X2 receptor in mediating sensory effects of ATP (27). In the absence of exposure to noise, auditory thresholds of WT and P2RX2-null mice are the same until at least age 19–23 wk (Figs. S1–S3). We assessed the potential contribution of P2X2 receptor signaling to protection from age-related hearing loss (presybycusis) by analyzing 17-mo-old mice raised in a quiet environment (Fig. 5A). Age-related hearing loss was more severe in P2RX2-null mice compared with WT mice across the frequency range tested (4- to 32-kHz tone pips, P < 0.001; clicks, P = 0.03), with the greatest difference at 8 kHz (P = 0.003). This broad hearing loss is consistent with sensorineural deafness, including the impact on the inner hair cell–type I spiral ganglion nexus. These P2RX2-null mice had significantly higher cubic DPOAE thresholds than the WT mice (P = 0.008) above 8 kHz (Fig. S2), reflecting differential loss of outer hair cell function in the mid-basal region of the cochlea over the WT controls. At higher frequencies (28–32 kHz), thresholds for both WT and mutant mice were ∼70 dB, our upper limit for reliable determination of cubic DPOAE thresholds. These results suggest that loss of P2X2 receptors aggravates the progressive hearing loss of the DFNA41 human subjects.
Fig. 5.
Age-related and noise-induced hearing loss in P2RX2-null and WT mice. (A) After 17 mo residence in the quiet chamber, P2RX2-null mice (n = 12; KOq) had poorer hearing than WT mice (n = 8; WTq) across all frequencies. *P < 0.05; **P < 0.01; ***P < 0.001. (B) As 11- to 15-wk-old young adults, WT and P2RX2-null mice born and raised in the quiet chamber did not differ in hearing acuity (P = 1.0). With continuous exposure to 75-dB SPL moderate noise at 8–16 kHz (green bar), both WT mice (WTn) and P2RX2-null mice (KOn) exhibited substantial and similar hearing loss, evident with a click stimulus as well (P = 0.001, noise-exposed vs. unexposed animals). However, only P2RX2-null mice exhibited additional significant hearing loss at frequencies above the noise band. *P < 0.05; ***P < 0.001. (C) Confocal fluorescence images of cryosections of the cochlear base from 17-mo-old WT mice (WTq) and P2RX2-null mice (KOq) raised in a quiet environment. Spiral ganglion neurons and their central and peripheral processes were immunolabeled for neurofilament 200K (green). Cell nuclei were labeled with DAPI (blue). o/C, organ of Corti; sp, spiral prominence; sl, spiral limbus; sg, spiral ganglion; IHC, inner hair cells; OHC, outer hair cells. In the P2X2-null mice, loss of hair cells and supporting cells led to flattening of the sensory epithelium (arrow).
Histological analysis of cochlear tissue from 17-mo-old mice raised in quiet indicated substantial deterioration of the organ of Corti in the P2RX2-null mice, particularly in the high-frequency–encoding basal region. Loss of hair cells and supporting cells was evident as a flattening of epithelial lining on the basilar membrane (Fig. 5 and Fig. S4). Associated with loss of sensory epithelium was a profound loss of spiral ganglion neurons within Rosenthal’s canal (Fig. S2). Hair cells were retained in the more apical cochlear regions, whereas spiral ganglion density appeared diffuse in P2RX2-null mice compared with WT mice.
Noise-Induced Hearing Loss in Mice Lacking the P2X2 Receptor.
As 11- to 15-wk-old young adults, both P2X2-null and WT mice raised in a quiet environment with minimal exposure to sound had normal hearing for mice of this age (28, 29). Exposure to moderate background noise led to significant hearing loss in both P2RX2-null and WT mice. Animals of both genotypes raised in a moderately noisy environment had an average 30-dB higher ABR threshold at 8–16 kHz compared with animals raised in a quiet environment (Fig. 5B).
In contrast, P2RX2-null and WT mice differed significantly in terms of the effect of noise on hearing at higher frequencies (Fig. 5B). At 20–36 kHz, P2RX2-null mice raised in a noisy environment had an average 13-dB higher ABR threshold than WT mice raised in a noisy environment and mice of either genotype raised in a quiet environment (P < 0.001). These results suggest that loss of P2X2 renders mice more susceptible to high-frequency hearing loss induced by moderate noise levels, as observed in the DFNA41 human subjects (Fig. 1F).
Discussion
P2RX2 p.V60L cosegregates with dominantly inherited progressive hearing loss in two families and profoundly affects the ATP-evoked inward current response of P2X2 receptors and the permeability of ATP-gated ion channels assembled as trimers from P2X2 subunits. Our data suggest that mutant and WT P2X2 receptor subunits assemble together, hindering conductance in the heteromeric channel. Both WT and mutant P2X2 receptors localize to the plasma membranes of hair cells and supporting cells, excluding mislocalization of the heteromeric channel as the cause of hearing loss in DFNA41 subjects. Rather, we suggest that improper docking or defective gating within or between subunits is the more likely mechanism for impaired function of the heteromeric channels.
Extracellular ATP is a purine signaling molecule that influences the developing and mature cochlea via both ionotropic P2X receptors and metabotropic P2Y receptors (13, 30–32). P2X2 receptors are expressed in epithelial cells lining the cochlear partition, including the sensory hair cells and supporting cells of the organ of Corti and the afferent spiral ganglion neurons (12, 33). In the organ of Corti, sustained noise exposure causes up-regulation of P2RX2 transcripts and P2X2 protein (34, 35), most likely mediated by release of ATP into the cochlear partition (36). During periods of sustained noise exposure, ATP released into the endolymphatic compartment (the scala media) activates P2X2 receptors, producing a cation shunt across the cochlear partition and reducing the driving force for both inner and outer hair cell-mediated sound transduction (12, 32, 37). Old mice (18 mo) have blunted regulation of cochlear partition resistance mediated by P2X2 receptor-type conductance (35). This might have contributed to the development of hearing loss in the older WT mice in the present study, but genetic deletion of the P2X2 receptor resulted in significantly more pronounced hearing loss with age.
Exacerbation of hearing loss after noise exposure of both P2RX2 p.V60L heterozygous human subjects and P2RX2-null mice provides a link between P2X2 receptor signaling in the cochlea and protection from noise and progressive hearing loss. The phenotype of the families segregating P2RX2 p.V60L is progressive sensorineural hearing loss, generally with onset in the second decade. Among P2RX2 heterozygotes with significant noise exposure, initial hearing loss involves both low and high frequencies, whereas among P2RX2 heterozygotes with no history of noise exposure, high frequencies (>2,000 Hz) are affected only some years later. The P2RX2-null mouse models the effect of the human mutation. Similar to their human counterparts, P2RX2-null mice continuously exposed to moderate noise developed hearing loss both at the frequency of the noise exposure and at higher frequencies. It is reasonable to conclude that signaling via the cochlear P2X2 receptor/ATP-gated ion channel pathway confers protection from noise-induced hearing loss.
Taken together, our findings suggest that in persons with loss of function of P2X2 receptors, levels of noise exposure that are nominally safe for other individuals may cause significant noise-induced hearing loss and accelerated presbycusis. In many countries, 85 dBA is the legislated safe working level for noise exposure in an 8-h workday (e.g., www.safeworkaustralia.gov.au). This nominally safe daily noise exposure is based on a dose regimen in which the safe daily broadband exposure doubles as sound intensity halves with every 3 dB. Thus, the 75-dB bandpass noise (one octave) to which mice were exposed in this study may reflect common human environmental exposures (29). Our results thus suggest that P2X2 receptors contribute significantly to the ability of the cochlea to sustain function within the normal physiological range of background noise. In the absence of this signaling pathway, the cochlea is far more susceptible to noise-related and age-related hearing loss.
Genetic dissection of additional molecular mechanisms of hearing impairment can provide valuable insights into the molecular triggers for sensorineural degeneration. Therapeutic strategies targeting the mutant P2X2 receptor also might provide a platform for treating the progression of hearing loss in DFNA41 individuals. More generally, a focus on loss of function of P2X2 receptors could represent a promising direction for possible intervention in noise-induced hearing loss in the wider population.
Materials and Methods
Human Subjects.
This study was approved by the Institutional Review Boards of the University of Miami (protocol 2001-0415), University of Washington (protocol 33468), and PLA General Hospital, Beijing. Informed consent was obtained from each adult subject and from a parent of each subject under age 18 y. Clinical histories, interviews, physical examinations, and audiometric evaluations were carried out as described previously (19). In particular, each subject was asked to provide a detailed occupational and residence history before age 25 and information on exposure to noise during each year of this period, the job context of the noise exposure, and whether exposure was chronic or occasional.
Gene Discovery.
A total of 3,636 cRNA 120-mer overlapping probes were designed to capture the 4.80-MB DFNA41 linkage interval. Sequencing was performed on a HiSeq2000 genome analyzer (Illumina) to a median read depth of 350×, with 99.2% of the targeted regions covered by ≥10 reads. Variants were classified as missense, nonsense, frameshift, or splice-site alleles, validated by Sanger sequencing, and tested for cosegregation with hearing loss in the family. All experimental procedures are described in more detail in SI Materials and Methods.
Rat Inner Ear Tissue Cultures and P2RX2 Transfection.
Organ of Corti and vestibular sensory organs were dissected from postnatal day 2 (P2) rats in accordance with National Institutes of Health guidelines (protocol 1215-08) and maintained for 2 d in culture, then transfected with GFP-P2RX2 WT or P2RX2 p.V60L using a Helios Gene Gun system (BioRad), as described previously (14).
Patch-Clamp Analysis of ATP-Evoked Current.
HEK293 cells were transfected with WT or mutant P2RX2 plasmid. Whole-cell recording was performed using an Axopatch 200B patch clamp amplifier (Molecular Devices) (15, 16, 18), and data were analyzed with jClamp.
Measurement of FM1-43 Permeability Through P2X2 Receptor Channels.
MDCK-II cells were cultured as described previously (38) and transfected with GFP-P2RX2 WT or P2RX2 p.V60L. Transfected cells were incubated with media including FM1-43 FX (Invitrogen), then fixed, permeabilized, and counterstained with Alexa Fluor 647 phalloidin. GFP and FM1-43 were excited by a 488-nm laser and detected by separate emission filters.
Animals, Hearing Assessment, and Immunolabeling.
All experiments were approved by the Animal Care and Ethics Committee of the University of New South Wales. P2RX2-null and background strain C57BL/6J WT mice were born and raised either in a quiet environmental chamber or in an environmental chamber with a continuous noise level of 75 dB SPL, 8–16 kHz. Assessments of hearing function were performed using established protocols (39). Mid-modiolar cochlear cyrosections from the quiet-chamber 17-mo old P2RX2-null and WT mice were examined by immunofluorescence confocal microscopy as described previously (40).
Supplementary Material
Acknowledgments
We thank the families for their participation in the study. We thank Menwei Cai, Pu Dai, Dongyi Han, Kaisun Li, Chunyu Liang, Zian Xiao, and Shiming Yang for contributions to the fieldwork in China; Anne Thornton, Ming Lee, Xiaomai Ouyang, and Suleyman Gulsuner for contributions to genomics and bioinformatics; Kwang Pak, Eduardo Chavez, Jeremy Pinyon, Rachel Morton-Jones, Sherif Tadros, and Yogeesan Sivakumaran for contributions to the mouse experiments; Debra Cockayne for supporting the establishment of the mouse model; Edward Crawford for designing and engineering the environmental chambers; and Karen B. Avraham, Walter Nance, Yanbin Zhang, and Jianxin Bao for helpful discussions. This work was supported by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders [Grants R01 DC012546 (to X.Z.L.), R01 DC005575 (to X.Z.L.), R01 DC005989 (to H.-B.Z.), R01 HL105631 (to Y.Z.), R01 DC009645 (to M.T.), R01 DC000139 (to A.F.R.), and R01 DC005641 (to M.-C.K. and T.W.), and the intramural program (B.K.)], the Veterans’ Administration (A.F.R.), the Australia National Health and Medical Research Council [Grant 630618 (to G.D.H. and A.F.R.)], the New Zealand Marsden Fund (G.D.H.) and Health Research Council and Deafness Research Foundation (P.R.T., G.D.H., and S.M.V.), and the People’s Republic of China National Natural Science Foundation [Grant 30528025 (to X.Z.L.)].
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222285110/-/DCSupplemental.
References
- 1.Stevens G, et al. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health. 2011 doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HJ, Dobie RA, Ko CW, Themann CL, Murphy WJ. Americans hear as well or better today compared with 40 years ago: Hearing threshold levels in the unscreened adult population of the United States, 1959-1962 and 1999-2004. Ear Hear. 2010;31(6):725–734. doi: 10.1097/AUD.0b013e3181e9770e. [DOI] [PubMed] [Google Scholar]
- 3.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 4.Masterson EA, et al. Prevalence of hearing loss in the United States by industry. Am J Ind Med. 2012 doi: 10.1002/ajim.22082. doi: 101002/ajim.22082. [DOI] [PubMed] [Google Scholar]
- 5.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: Evidence of a misspent youth. J Neurosci. 2006;26(7):2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao F, Manchaiah VK, French D, Price SM. Music exposure and hearing disorders: An overview. Int J Audiol. 2010;49(1):54–64. doi: 10.3109/14992020903202520. [DOI] [PubMed] [Google Scholar]
- 7.Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211(2):188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 8.Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091(1):89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Konings A, Van Laer L, Van Camp G. Genetic studies on noise-induced hearing loss: A review. Ear Hear. 2009;30(2):151–159. doi: 10.1097/AUD.0b013e3181987080. [DOI] [PubMed] [Google Scholar]
- 10.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 11.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 12.Housley GD, et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: Implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19(19):8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32(3):128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Salles FT, et al. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol. 2009;11(4):443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102(51):18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu N, Zhao HB. ATP activates P2x receptors and requires extracellular Ca(++) participation to modify outer hair cell nonlinear capacitance. Pflugers Arch. 2008;457(2):453–461. doi: 10.1007/s00424-008-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Zhao HB. ATP activates P2X receptors to mediate gap junctional coupling in the cochlea. Biochem Biophys Res Commun. 2012;426(4):528–532. doi: 10.1016/j.bbrc.2012.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Zhao HB. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010;6(2):221–229. doi: 10.1007/s11302-010-9184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanton SH, et al. A novel locus for autosomal dominant non-syndromic deafness (DFNA41) maps to chromosome 12q24-qter. J Med Genet. 2002;39(8):567–570. doi: 10.1136/jmg.39.8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan D, et al. Refinement of the DFNA41 locus and candidate genes analysis. J Hum Genet. 2005;50(10):516–522. doi: 10.1007/s10038-005-0286-0. [DOI] [PubMed] [Google Scholar]
- 21.Walsh T, et al. Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. Am J Hum Genet. 2010;87(1):90–94. doi: 10.1016/j.ajhg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang LH, et al. Subunit arrangement in P2X receptors. J Neurosci. 2003;23(26):8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2(4):322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 25.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2(4):315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 26.Meyers JR, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23(10):4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockayne DA, et al. P2X2 knockout mice and P2X2/P2X3 double- knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130(1-2):94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. Noise in a laboratory animal facility from the human and mouse perspectives. J Am Assoc Lab Anim Sci. 2010;49(5):592–597. [PMC free article] [PubMed] [Google Scholar]
- 30.Kujawa SG, Erostegui C, Fallon M, Crist J, Bobbin RP. Effects of adenosine 5′-triphosphate and related agonists on cochlear function. Hear Res. 1994;76(1-2):87–100. doi: 10.1016/0378-5955(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 31.Housley GD, et al. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7(1):55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- 32.Thorne PR, et al. Potential role of purinergic signalling in cochlear pathology. Audiol Neurootol. 2002;7(3):180–184. doi: 10.1159/000058307. [DOI] [PubMed] [Google Scholar]
- 33.Järlebark LE, Housley GD, Thorne PR. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. J Comp Neurol. 2000;421(3):289–301. doi: 10.1002/(sici)1096-9861(20000605)421:3<289::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang JC, et al. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport. 2003;14(6):817–823. doi: 10.1097/00001756-200305060-00008. [DOI] [PubMed] [Google Scholar]
- 35.Telang RS, et al. Reduced P2x(2) receptor-mediated regulation of endocochlear potential in the ageing mouse cochlea. Purinergic Signal. 2010;6(2):263–272. doi: 10.1007/s11302-010-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahne M, Gale JE. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent Ca waves. Purinergic Signal. 2010;6(2):189–200. doi: 10.1007/s11302-010-9193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorne PR, Muñoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea pig. J Assoc Res Otolaryngol. 2004;5(1):58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci USA. 2011;108(28):11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cederholm JM, et al. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear Res. 2012;292(1-2):71–79. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong AC, Velamoor S, Skelton MR, Thorne PR, Vlajkovic SM. Expression and distribution of creatine transporter and creatine kinase (brain isoform) in developing and mature rat cochlear tissues. Histochem Cell Biol. 2012;137(5):599–613. doi: 10.1007/s00418-012-0922-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.