Abstract
The Caenorhabditis elegans vulval precursor cells (VPCs) offer a paradigm for investigating how multipotency of progenitor cells is maintained during periods of quiescence. The VPCs are born in the first larval stage. When hermaphrodites are grown under favorable conditions, the EGF-mediated “inductive” signal and the LIN-12/Notch-mediated “lateral” signal confer a precise spatial pattern of distinct vulval cell fates in the third larval stage, a day after hatching. Under adverse conditions, hermaphrodites undergo a prolonged quiescent period as dauer larvae, which can endure for several months with progenitor cells such as VPCs in developmental arrest. If favorable conditions ensue, larvae recover and resume development as postdauer third stage larvae, with the same VPC spatial-patterning events as in continuously developing third stage larvae. Here, we identify several consequences of dauer life history for VPC specification. In wild-type dauers, VPCs undergo a phenomenon reminiscent of natural direct reprogramming to maintain or reestablish multipotency; they acquire an active block to signal transduction by EGF receptor and LIN-12/Notch and have a different mechanism for regulating transcription of the lateral signal. Furthermore, DAF-16/FoxO, a target of insulin/insulin-like growth factor signaling, is required to promote VPC fate plasticity during dauer and for normal vulval patterning after passage through dauer, suggesting that DAF-16/FoxO coordinates environment and life history with plasticity of cell fate.
The life history of Caenorhabditis elegans depends on environmental conditions (1, 2). Under favorable conditions, C. elegans larvae develop rapidly and continuously through four larval stages (L1 through L4) to adulthood. However, adverse environmental conditions in the L1 stage cause developing larvae to undergo what we refer to here as “dauer life history.” Dauer life history encompasses an alternative L2 stage, called the “L2d,” which is prolonged relative to continuous L2 stage. L2d larvae molt into the stress-resistant dauer larva stage if conditions do not improve. Dauer larvae are developmentally arrested and can withstand even many months of adverse conditions. If conditions become favorable, they undergo a recovery period and resume development through postdauer (PD)L3 and PDL4 stages. Postdauer development is indistinguishable from the development of continuously grown larvae in terms of cell lineage and overall morphology (3–5). Thus, during dauer quiescence, there must be a mechanism that enables progenitor cells such as the vulval precursor cells (VPCs) to remain multipotent for long periods.
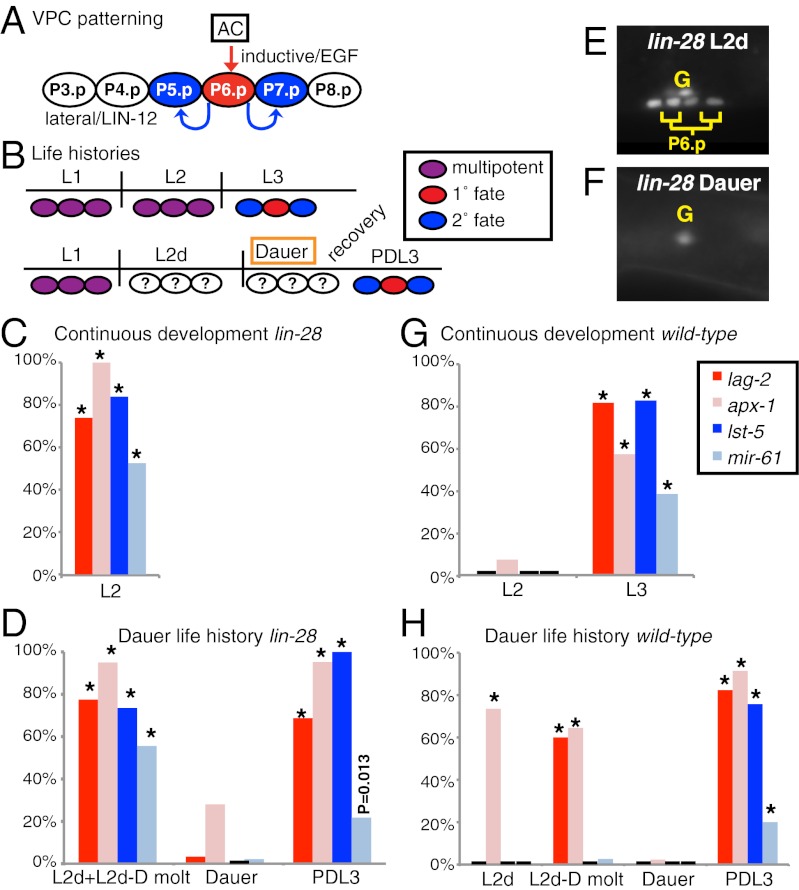
Six VPCs, named P3.p through P8.p, have the potential to generate vulval cells (Fig. 1A). During continuous development, the VPCs remain quiescent and multipotent throughout the L2 stage until vulval induction is initiated in the L3 stage by an EGF-like signal produced by the gonadal anchor cell (6). Activation of EGF receptor (EGFR) by the inductive signal causes P6.p to adopt the “1° fate,” and to produce a lateral signal that activates LIN-12/Notch in the neighboring cells, P5.p and P7.p, which adopt the “2° fate.” These key aspects of vulval development must also be managed during dauer life history (Fig. 1B).
Fig. 1.
Cell-fate plasticity in dauer larvae. (A) VPC specification in wild-type hermaphrodites. An EGF-like signal (red) from the anchor cell (AC) activates Ras signaling in P6.p, causing it to adopt 1° fate and produce ligands, including LAG-2 and APX-1, which activate LIN-12/Notch in P5.p and P7.p (6). (B) Comparison of cell fate in P5.p, P6.p, and P7.p (ovals) during continuous development (Upper) and dauer life history (Lower). Vertical lines represent molts. Red, 1° fate; blue, 2° fate; purple, multipotential; white, unknown before this work. (C–H) Expression of VPC specification markers in different conditions. Reddish bars indicate 1° fate markers, bluish bars indicate 2° fate markers, and thickened black lines indicate no expression. Expression of VPC identity markers in dauer life history is shown in Fig. S1. (C) *P < 0.01, compared with wild-type L2 (G) (n = 23–42; Fisher's exact test). (D) *P < 0.01, compared with Dauer (n = 16–46; Fisher's exact test). (E and F) Representative images of lag-2p::yfp expression before (E) and during (F) dauer in lin-28(0) larvae. P6.p has generated four descendants in both larvae shown. lag-2p::yfp is also expressed in the gonad (labeled “G”) in continuous and dauer life histories. (G) *P < 0.01, compared with wild-type L2 (n = 22–39; Fisher's exact test). (H) *P < 0.01, compared with Dauer (n = 20–43; Fisher's exact test).
During continuous development, loss of the heterochronic gene lin-28 causes a “precocious” phenotype in which developmental events that normally occur in the L3 stage, including vulval induction, instead occur prematurely in the L2 stage (4, 7, 8). In an elegant study using lineage analysis, Euling and Ambros (4) found that some VPCs are prematurely induced in the L2d stage, generating daughters or even granddaughters before entering dauer. Remarkably, when such dauers recover and resume development in the postdauer L3 stage, the lineages do not resume from the point where they left off; instead, three of these descendants generate the vulva after a new inductive signaling event, suggesting that the cells of the vulval lineages may be reprogrammed back to multipotency during dauer diapause. Here, using cell fate markers and other tools not available at the time of the study of Euling and Ambros, we have investigated the consequences of dauer life history on VPCs of wild-type dauers, finding that they also undergo a process that maintains or reestablishes multipotency. In addition, we identify differences in transcriptional regulatory circuitry, blocks to transduction of spatial patterning signals, and a requirement for daf-16/FoxO in maintaining quiescence and multipotentiality during dauer.
Results
Dauer State Reverses Expression of VPC-Specification Markers.
In the L3 stage of continuous development, the EGF-like inductive signal activates a canonical EGFR-Ras-ERK cascade and specifies the nearest VPC, P6.p, to adopt the 1° fate and to produce ligands that activate LIN-12/Notch in the two flanking “2°” VPCs (normally P5.p and P7.p) (Fig. 1A) (see ref. 6 for review). Reporters expressed specifically in 1° or 2° VPCs and their descendants in the L3 stage in response to these patterning signals are “specification markers”: for 1° fate, lag-2p::yfp and apx-1p::yfp, expressed in response to EGFR activation (9, 10), and for 2° fate, lst-5p::yfp and mir-61/250p::yfp, direct transcriptional targets of LIN-12 (11, 12) (Figs. 1 A and G). Reporters expressed uniformly in all multipotent VPCs during the L2 stage, and before induction in the L3 stage, such as lin-31p::cfp and LIN-12::GFP, are “VPC identity markers” (Fig. S1) (13–15). Using these markers, we can assess the state of each VPC and its descendants in every phase of dauer life history.
In lin-28(0) null mutant hermaphrodites experiencing dauer life history, specification markers are precociously expressed in the L2d stage and are also expressed in the postdauer L3 stage but are not expressed in dauer larvae (Fig. 1 C–F). Expression of specification markers in the somatic gonad is not affected in dauer (Figs. 1 E and F), indicating that expression of these transgenes is not generally repressed in dauer. In addition, expression of VPC identity markers is evident throughout dauer life history, indicating that the VPCs have not lost their identity and that transgenes are not generally repressed in the VPCs of dauer larvae (Fig. S1). Our results support the inference of Euling and Ambros (4) that the specified fates of VPC descendants in lin-28(0) mutants is reversed by passage through dauer.
These markers enabled us to examine the consequences of dauer life history on vulval fate specification in an otherwise wild-type background, where the VPCs do not divide before entry into dauer. We observed that both 1° fate markers were expressed as L2d larvae molt into dauer (Fig. 1H and Fig. S2), implying that EGFR-mediated inductive signaling is initiated at the L2d-Dauer molt during dauer life history, rather than in the L3 stage as in continuous development. The vulva is not fully induced, because 2° fate markers were not expressed in the neighboring VPCs (Fig. 1H). The expression of 1° fate markers was extinguished in the VPCs of dauer larvae whereas the VPC identity markers were maintained (Fig. S1). Thus, in wild-type dauers, as in heterochronic mutants, specification is reversed by passage through dauer.
Differences in Transcriptional Regulation of the Lateral Signal Gene lag-2 During Continuous Development and Dauer Life History.
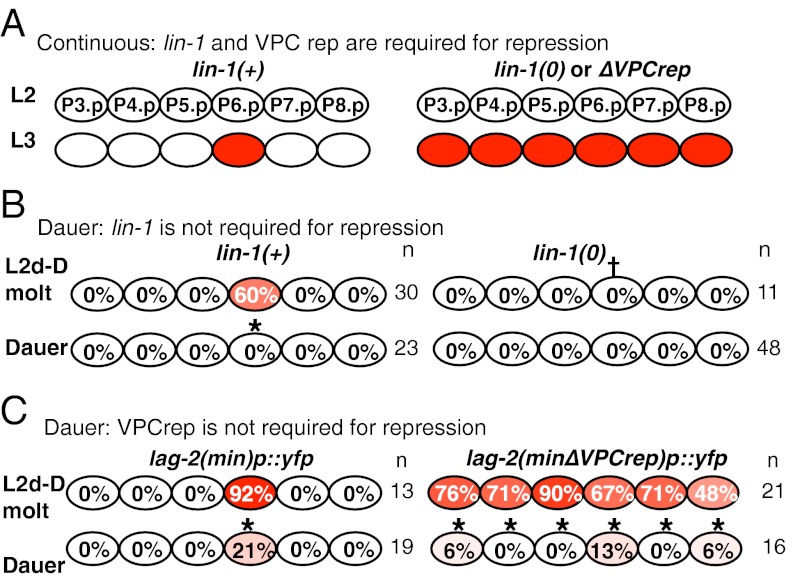
The regulated transcription of three lateral signal genes in P6.p in response to the inductive signal is a critical event in spatial patterning (9, 10). The genetic circuitry governing transcription of the lateral signal gene lag-2 in P6.p has been described in continuous development. In the L3 stage, the C. elegans Elk1 ortholog LIN-1 represses lag-2 in all VPCs before vulval induction, counteracting a positive factor present in all VPCs (10). In P6.p, the inductive signal leads to ERK-mediated phosphorylation of LIN-1 (16), relieving repression and permitting transcription of lag-2 (10). Thus, in continuous development, if transcriptional repression is abrogated through loss of lin-1 or deletion of a LIN-1–binding consensus site called “VPCrep,” lag-2 is transcribed in all six VPCs in the L3 stage (10) (Fig. 2A).
Fig. 2.
Differences in regulation of lag-2 in continuous development and dauer life history. (A) lag-2 regulation during continuous development (10). (Left) LIN-1 represses lag-2 expression in VPCs in which Ras signaling is low via a cis-acting sequence called VPCrep. (Right) Deletion of VPCrep or loss of lin-1/Elk1 activity permits transcription of lag-2 in all VPCs (10). (B and C) Effect of dauer life history on repression of lag-2 in VPCs. Percentage of larvae in which each VPC expresses the indicated reporter out of the total larvae examined (n). *P < 0.01 compared with L2d-Dauer molt (Fisher’s exact test); †P < 0.01 compared with P6.p at L2d-Dauer molt in lin-1(+) (Fisher's exact test).
A simple hypothesis to account for the loss of lag-2 transcription in dauer is that loss of EGFR-Ras-ERK activity in P6.p enables continued LIN-1 repression of lag-2 transcription. However, we found that lag-2::yfp was not derepressed in dauer larvae in lin-1(0) mutants (Fig. 2B) or after deletion of VPCrep (Fig. 2C), even though lin-1 activity is again required to repress ectopic lag-2 expression in postdauer L3 hermaphrodites: 10/10 postdauer L3 lin-1(0) larvae displayed ectopic lag-2 expression. These observations suggest that repression of lag-2 in dauer is independent of LIN-1, the repressor that governs spatial patterning.
We observed another difference in the genetic circuitry for lag-2 regulation. In continuous development, there is no evidence for a positive role for lin-1 in promoting lag-2 transcription (10). In contrast, during dauer life history, lin-1 has a positive role as hermaphrodites molt into the dauer stage: the expression of lag-2 at the L2d-Dauer molt seen in a wild-type background was not observed in lin-1(0) mutant (Fig. 2B). Furthermore, this positive role may be indirect, because deletion of VPCrep resulted in expression of lag-2 in all VPCs as animals molt into dauer (Fig. 2C), suggesting that a factor whose activity requires LIN-1 may be responsible for positive regulation at that time.
Different Consequences of Constitutive EGFR- and Notch-Transducing Activity in VPCs During Continuous Development and Dauer Life History.
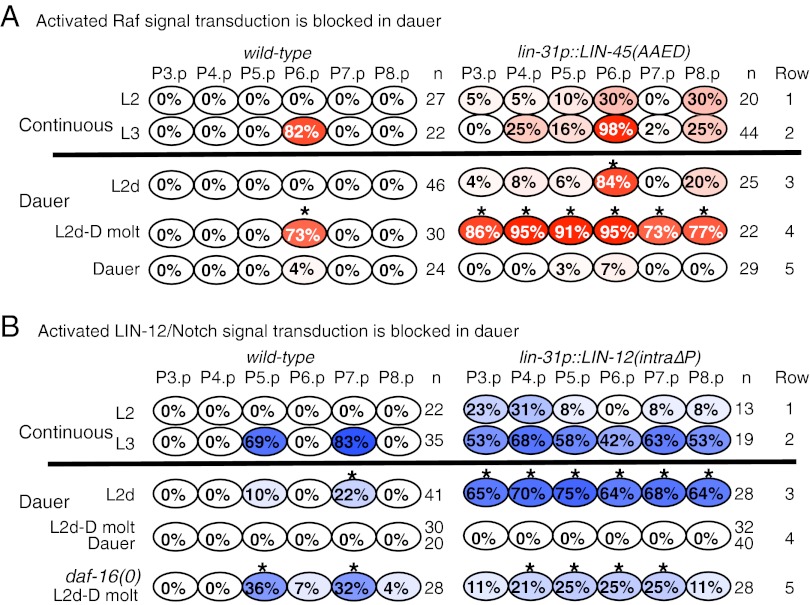
The loss of 1° fate marker expression in the dauer stage suggests that inductive signal transduction ceases or is blocked in wild-type hermaphrodites, and the loss of 2° fate marker expression in lin-28(0) dauers suggests that there may be a mechanism for abrogating the effects of the lateral signal as well. To gain insight into the interaction between dauer diapause and transduction of VPC patterning signals, we expressed constitutively active components of the spatial patterning pathways in VPCs and examined the effects on marker gene expression in hermaphrodites that experienced continuous development or dauer life history. Our analysis suggests that signal transduction by both EGFR and LIN-12/Notch is actively blocked in dauer larvae.
We found that VPC-specific expression of LIN-45(AAED), a stable, constitutively active form of LIN-45/Raf (17) caused precocious expression of lag-2p::yfp during the L2d stage (Fig. 3A, row 3), with strong expression evident in all six VPCs through the L2d-Dauer molt (Fig. 3A, row 4), indicating that signal transduction is active before the dauer stage. In dauer, LIN-45(AAED) protein is stable (Fig. S3 A and B), but expression of lag-2p::yfp declines, albeit gradually, until it is largely extinguished from all VPCs (Fig. 3A, row 5).
Fig. 3.
Constitutive activity of LIN-12/Notch or LIN-45/Raf appears to be blocked in dauer larvae. (A and B) Percentage of larvae in which each VPC expresses a 1° fate reporter (lag-2p::yfp, red) or a 2° fate reporter (lst-5p::yfp, blue) out of the total larvae examined (n). daf-7(e1372) is present in larvae scored during dauer life history. Similar results were seen in dauer larvae isolated from starved and crowded plates in daf-7(+) strains. All strains were grown at 25 °C. (A) lin-31p::LIN-45[AAED], transgene expressing activated LIN-45/RAF in all VPCs. *P < 0.01 compared with Dauer (row 5) (Fisher’s exact test). (B) lin-31p::LIN-12(intra∆P), transgene expressing activated LIN-12 in all VPCs. *P < 0.01 compared with L2d-D molt (row 4) (Fisher’s exact test).
We also found a rapid block to constitutive LIN-12 activity. VPC-specific expression of LIN-12(intraΔP), a stable, constitutively active form of LIN-12 (18), caused precocious expression of the 2° fate marker and direct lin-12 target, lst-5p::yfp in L2d (Fig. 3B, row 3, compare Left and Right). This result suggests that L2d hermaphrodites lack the normal inhibition against lin-12 activity observed in continuous L2 (18), (Fig. 3B, compare rows 1 and 3). However, expression of lst-5::yfp was completely extinguished as early as the L2d-Dauer molt and remained off in the VPCs of dauer larvae (Fig. 3B, compare rows 3 and 4, Right), consistent with a strong block to signal transduction. We note that a fluorescent-tagged form of LIN-12(intra∆P) is stable and nuclear in dauer larvae, indicating that the block to target gene activation operates downstream of the release and nuclear translocation of the intracellular domain of LIN-12/Notch (Fig. S3 C and D).
Cell-Fate Plasticity and Multipotency in Mutants That Regulate Entry into Dauer.
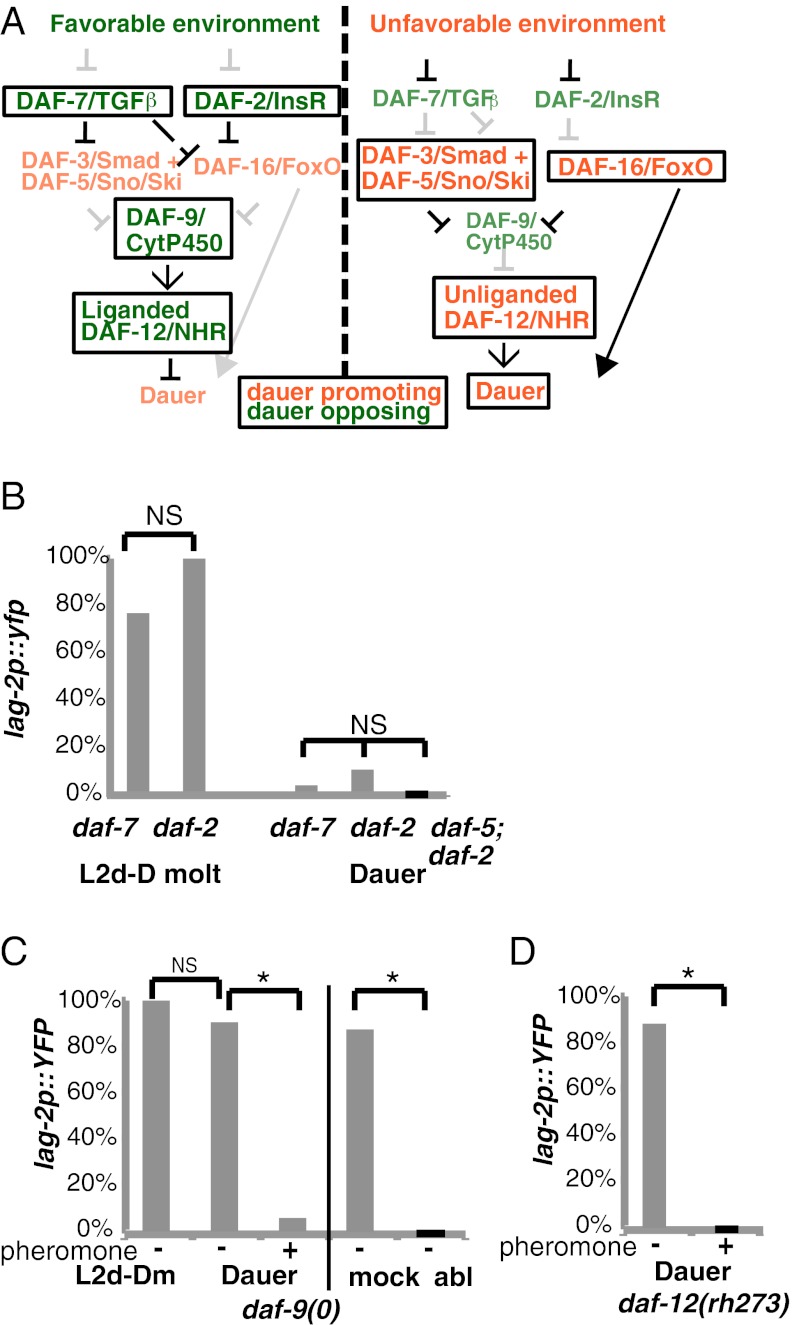
The decision to enter dauer depends on environmental conditions. In a population, each individual secretes dauer pheromone; in unfavorable conditions, a high pheromone-to-food ratio promotes dauer formation (see ref. 1 for review). Three major signaling systems have input into this decision: TGFβ, insulin/insulin-like signaling (IIS), and nuclear hormone receptor (NHR). These three pathways have independent inputs and outputs but also display crosstalk at various points (Fig. 4A). Mutants that lack dauer-opposing components of these pathways form dauers constitutively even in favorable conditions, whereas mutants that lack dauer-promoting components do not form dauers regardless of environmental conditions.
Fig. 4.
Effect of dauer formation mutants on down-regulation of lag-2 transcription. (A) Schematic representation of the major signaling pathways that regulate the dauer formation decision (1). Boxes indicate active components under the indicated conditions. (B) Percentage of larvae expressing lag-2p::yfp in P6.p at 25 °C (n = 13–37). (C) Percentage of larvae expressing lag-2p::yfp in P6.p at 25 °C (“+” indicates exogenous pheromone was added to create an unfavorable environment condition as in A, Right). (Left) n = 13–34. (Right) Laser ablation (abl) of the gonad (n = 8) compared with mock-ablated (mock) larvae (n = 7). (D) Percentage of larvae expressing lag-2p::yfp in P6.p at 25 °C (“+” indicates exogenous pheromone was present; n = 23–44). *P < 0.01 (Fisher’s exact test). NS, not significant.
We asked whether the major dauer-regulating pathways also impart plasticity of VPC fate during the dauer stage by examining whether down-regulation of lag-2p::yfp occurs normally in dauer larvae formed constitutively by mutants deficient in daf-7/TGFβ, daf-2/insulin receptor (InsR), or daf-9/CytP450. daf-9 is required for production of dafachronic acid, the ligand for DAF-12/NHR. The activity of daf-12 governs the decision to enter dauer: liganded DAF-12 promotes continuous development, whereas unliganded DAF-12 is required for dauer formation (19, 20).
In favorable conditions, daf-7(e1372), daf-2(e1370), and daf-9(dh6) [daf-9(0)] mutants all become dauer larvae at 25 °C. In all three mutants, we observed lag-2p::yfp expression at the L2d-Dauer molt, as in wild-type (Figs. 4 B and C). In two mutants, daf-7(e1372) and daf-2(e1370), lag-2p::yfp expression was subsequently lost in dauer, as in wild-type dauers (Fig. 4B). In contrast, most daf-9(0) dauer larvae continued to express lag-2p::yfp in P6.p (Fig. 4C), and in many (20/34) daf-9(0) dauer larvae, P6.p also divided. Persistence of lag-2 expression in daf-9(0) is likely attributable to the presence of unliganded DAF-12 because lag-2p::yfp is also observed in daf-12(rh273) dauer larvae (Fig. 4D); daf-12(rh273) encodes a mutant protein that binds ligand poorly, causing constitutive dauer formation (20, 21). Finally, laser ablation of the gonad, the normal source for the inductive signal, abrogated lag-2p::yfp expression in daf-9(0) dauers (Fig. 4C). These observations indicate that inappropriate vulval induction occurs during the quiescent dauer state in daf-9(0) dauers in favorable conditions.
We then looked at the mutant dauer larvae in the presence of exogenous pheromone, an unfavorable condition under which wild-type animals form dauers. If daf-9 and daf-12 are key components of the mechanism that promotes lag-2 down-regulation in wild-type dauer larvae, they should be required in adverse conditions as well. Instead, we found that in the presence of exogenous dauer pheromone, lag-2::yfp was down-regulated normally in daf-9(0) and daf-12(rh273) dauer larvae (Fig. 4 C and D). These results demonstrate that daf-9 and daf-12 activity are not required per se to promote lag-2 down-regulation and indicate that another factor, the activity of which differs depending on environmental conditions, must be responsible.
daf-16/FoxO Activity Is Required to Block EGFR and Notch Signal Transduction and for Normal Vulval Development in Dauer Life History.
The DAF-7/TGFβ and DAF-2/InsR pathways respond to the environment: in favorable environments signal transduction is active, and in unfavorable environments, transduction is inactive (see ref. 1 for review). The immediate downstream transcription factors of these pathways are active only in unfavorable environments and are also genetically upstream of daf-9 and daf-12. Thus, either of these factors could have an output that is independent of the DAF-12/NHR pathway for dauer entry.
Low TGFβ activity results in high activity of the DAF-3/Smad and DAF-5/Sno transcription complex, and low IIS activity results in high activity of the DAF-16/FoxO transcription factor. Null mutations for either daf-5 or daf-16 are dauer-defective, but constitutive dauer entry can be achieved through compromising the parallel pathway, i.e., daf-5(0); daf-2(e1370) and daf-16(0); daf-7(e1372) double mutants form dauer larvae (Fig. 4A) (22, 23), which can be readily identified based on SDS resistance (Materials and Methods). We found that loss of daf-5 did not prevent normal down-regulation of lag-2p::yfp (Fig. 4B), but loss of daf-16 had a significant effect: 60% of daf-16(0); daf-7(e1372) dauers failed to down-regulate lag-2 transcription, regardless of environmental conditions (i.e., in the presence or absence of dauer pheromone; Fig. 5A). Therefore, daf-16 appeared to be a strong candidate for mediating the link between dauer quiescence and cell-fate plasticity.
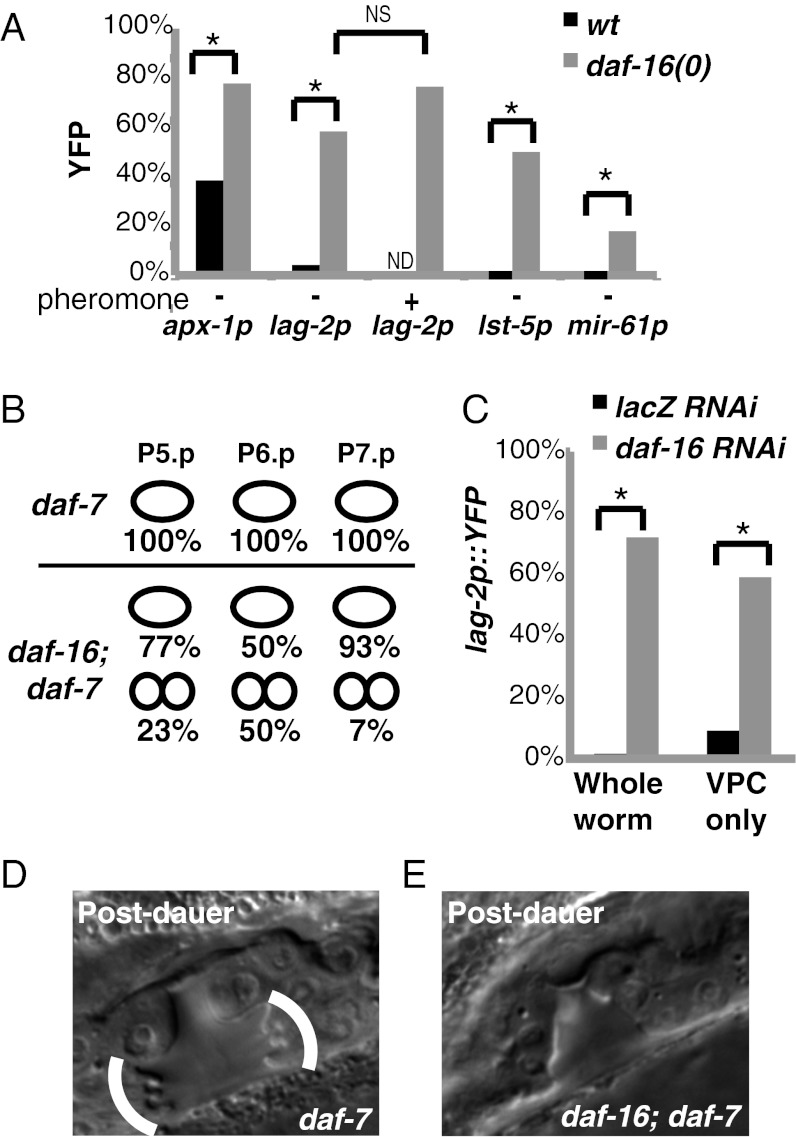
Fig. 5.
daf-16 promotes cell-fate plasticity in dauer larvae. (A) Percentage of dauer larvae expressing lag-2p::yfp in P6.p at 25 °C. daf-7(e1372) is present in all strains (“+” indicates exogenous pheromone was present; n = 17–71). (B) Percentage of the indicated VPCs that have divided one or more rounds in dauer larvae (n = 30). These divisions occur during dauer because they are never observed at the L2d-Dauer molt (0/29). (C) “Whole worm”: daf-7(e1372) lag-2p::yfp larvae were subject to RNAi for either lacZ (control) or daf-16 (n = 43–63). RNAi works efficiently in all nonneuronal tissues because these larvae are wild-type for rde-1 (25, 46, 47). “VPC only”: daf-7 lag-2p::yfp; rde-1(0); lin-31p::RDE-1 larvae were subject to RNAi for either lacZ (control) or daf-16 (n = 22). These larvae are unable to carry out RNAi in any tissue except the VPCs where lin-31p drives expression of rde-1 (indicated by “VPC only”) (15, 45, 48). (D) A total of 33/33 daf-7 postdauer L4 larvae displayed normal vulval morphology including the characteristic “Christmas-tree” structure (bracketed in image). In addition, normal vulval morphology was observed in 13/13 daf-16 larvae grown continuously. By contrast, 13/15 postdauer daf-16(0); daf-7 larvae displayed overtly abnormal morphology, such as the lack of Christmas tree. *P < 0.01 (Fisher’s exact test). NS, not significant.
If DAF-16/FoxO opposes specification and promotes multipotency of VPCs in dauer, we would expect that both inductive and lateral signaling would occur when daf-16 activity is removed. Indeed, in daf-16(0) dauer larvae, P6.p expressed both 1° specification markers (Fig. 5A) and often divided (Fig. 5B), and P5.p and P7.p expressed both 2° specification markers (Fig. 5A) and sometimes divided (Fig. 5B). Removing daf-16 also abrogated the otherwise robust and rapid inhibition of LIN-12(intraΔP) activity that occurs as early as the L2d-Dauer molt (Fig. 3B, compare rows 4 and 5). Finally, in daf-16(0) dauer larvae, VPCs not only divided, they did so asynchronously; for example, P6.p granddaughters were found adjacent to undivided P5.p and P7.p in daf-16(0) dauer larvae (Fig. S4A). We note that premature VPC division per se does not cause vulval abnormalities, because reducing the activity of the cell-cycle regulator cdc-14 caused premature cell division in the L2 stage without causing precocious expression of fate markers or overt effects on vulval morphology (24) (Fig. S4B). These observations suggest that daf-16 is required for blocking transduction of the spatial patterning signals to maintain multipotency and to maintain quiescence during the dauer state.
The effects of daf-16 are specific for the dauer life history, as continuously developing daf-16(0) larvae displayed normal expression of 1° and 2° fate markers and appeared to have synchronous VPC divisions. Postdauer daf-16(0) hermaphrodites displayed overt abnormalities in vulval development at high penetrance (Fig. 5 D and E), as well as egg-laying defects, indicating that daf-16 function in dauer life history has lasting implications for vulval organogenesis.
Finally, we assessed whether daf-16 is likely to act in VPCs or in neurons, where daf-16 activity is sufficient to regulate the dauer formation decision (25). To assess neurons, we examined daf-16(RNAi); daf-7 dauer larvae and found that, as in daf-16(0); daf-7 dauers, lag-2::yfp expression persisted in P6.p (Fig. 5C). These results suggest that the relevant cellular focus is likely not neurons, because neurons have been shown to be refractory to daf-16(RNAi) (25). To assess a potential focus of action in the VPCs, we created a strain in which the RNAi defect of rde-1(0) was rescued specifically in VPCs by using lin-31p to express RDE-1(+) (Fig. 5C and Materials and Methods). In this background, daf-16(RNAi) dauer larvae displayed significant lag-2 expression, suggesting that there is a VPC-autonomous component to daf-16 function in VPCs for preventing inductive signal transduction.
Discussion
Although VPC fate patterning is precise whether hermaphrodites develop continuously or experience dauer life history, we have identified numerous differences in the regulation of the activity of the EGFR and LIN-12/Notch signaling systems that specify vulval fates. Differences begin to be apparent before dauer formation: L2d hermaphrodites express lateral signal genes, which are not expressed until the L3 stage in continuous development, and display an apparent weakening of the mechanism that blocks lin-12 activity in the L2 stage of continuous development. Most striking, we find that dauer diapause reverses the effects of EGFR and LIN-12/Notch signal transduction and activates a mechanism that is powerful enough to counter potent constitutively active forms of core components of these pathways.
In addition, we have found that daf-16 activity is required for an effective block to EGFR and LIN-12/Notch signal transduction, as well as for VPC quiescence in dauer. DAF-16 is a downstream effector of insulin signaling, one of the systems that controls entry into the dauer state. Because DAF-16 is primarily active in dauer life history (26–29), our findings suggest that plasticity of vulval cell fate is coordinated with the environmental inputs into the decision to become a dauer larva, ensuring the maintenance of multipotency during a potentially long period of dauer quiescence.
The role of daf-16 for proper vulval development in dauer life history is important, because postdauer daf-16 hermaphrodites have highly penetrant and overt vulval defects, whereas continuously developing daf-16 hermaphrodites do not have any vulval abnormalities. Although the daf-16 postdauer vulval defect may reflect more than just the effects in dauer, the cell-autonomous requirement for daf-16 in silencing lag-2 expression in dauer is consistent with the dauer state itself being the point at which environmental input is coordinated with maintenance of multipotency.
DAF-16 is the sole C. elegans FoxO ortholog. In vertebrates, FoxO has been implicated in maintaining somatic and cancer stem cells, which share two key properties with the dauer VPCs: they are multipotent, and they exist in a quiescent state. Both DAF-16 and FoxO have been well characterized as factors that promote quiescence (for reviews, see refs. 30–32). In contrast, the contribution of FoxO in promoting multipotency is only beginning to emerge (33–36). For example, recently, FoxO was shown to promote pluripotency of human embryonic stem cells, at least in part, by activating transcription of the pluripotency factors OCT4 and SOX2 (37). Here, we have shown that DAF-16 promotes multipotency as well as quiescence of the VPCs. The elucidation of the mechanism by which daf-16 allows VPCs to retain or reacquire multipotency as they enter dauer quiescence may prove to be relevant to human stem cell biology.
Materials and Methods
Strains and Transgenes.
A complete list of all strains and transgenes can be found in Tables S1 and S2. We note that daf-16(mgDf50), lin-28(n719), daf-5(m512), lin-1(n304), and daf-9(dh6) are null alleles; daf-2(e1370) and daf-7(e1372) are hypomorphs; and daf-12(rh273) encodes a protein that binds ligand poorly (20, 21).
Microscopy.
Larvae were examined using a Zeiss Axio Imager D1 with an AxioCam MRm camera and an X-Cite 120Q light source (EXFO Photonic Solutions). Cell-fate markers were visualized using a 63× objective and a 500-ms exposure time, except lin-31p::cfp (Fig. S1), which was visualized using a 40× objective and a 100-ms exposure time. Expression of a marker in the relevant cell(s) using the above criteria was categorized as “on.”
Dauer life history.
For strains lacking a dauer-opposing mutation, predauer larvae were generated using crude dauer pheromone (38). Dauer larvae were isolated from crowded and starved plates and either examined directly or allowed to recover to generate postdauer larvae. Dauer larvae created by addition of dauer pheromone to the medium looked similar to dauers isolated from crowded and starved plates, although for some markers several days were required for any predauer expression to be down-regulated (Fig. S2).
daf-7(e1372) was also used to promote the dauer life history, because these larvae enter dauer constitutively at 25 °C. Marker expression was the same whether crude pheromone or daf-7(e1372) caused larvae to enter L2d and dauer (e.g., compare Fig. 1H and Figs. 4B and 5A). Dauers formed by either addition of dauer pheromone or daf-7(e1372) were scored at particular times: 1° fate markers were scored in two-day dauers, after the L2d-Dauer molt expression is down-regulated in wild-type (Fig. S2), whereas 2° fate markers were scored in one-day dauers.
daf-16 dauers.
daf-7(e1372) was used to drive daf-16(0) larvae into dauer (22, 23). Although daf-16(0) dauer larvae form, they do not remain in dauer indefinitely even in unfavorable conditions, unlike wild-type or daf-7(e1372) dauers (27, 39). The most stringent test of the dauer state is resistance to 1% SDS (40); thus, all daf-16(0) dauers scored here were selected by SDS resistance.
In addition to SDS resistance, daf-16(0) dauer larvae have the characteristic dauer lateral alae, although they do not have a fully constricted pharynx (22, 27). This pharyngeal morphogenesis defect, however, does not appear to be linked to VPC defects. When dauers are formed by daf-16(RNAi) rather than daf-16(0), the daf-16(RNAi); daf-7(e1372) dauer larvae have a constricted pharynx (Fig. S5) and display the same VPC phenotype as is generated by daf-16(0) (Fig. 5 A and C). In addition, lin-28(0) dauer larvae also display incomplete dauer morphology (41) and yet down-regulate lag-2 normally (Fig. 1D). Together, these observations suggest that persistent lag-2 expression and inappropriate VPC division is unrelated to the dauer morphogenesis defect in daf-16(0) dauer larvae.
Laser ablations.
daf-9(0) early-L1–staged larvae were mounted on agarose slides in a drop of 10 mM sodium azide. The four cells of the gonad primordium (Z1 to Z4) were ablated using a laser microbeam. Mock-ablated larvae were handled in the same manner, but no ablations were performed. Larvae were recovered, grown at 25 °C, and scored at 2 d following dauer formation. The success of the ablation was confirmed by lack of gonad formation.
RNAi.
RNAi by feeding was carried out as described (42). The daf-16 RNAi clone is from the Ahringer library (43), and the lacZ clone is pXK10 (44).
Strain GS6810, of genotype daf-7(e1372) arIs131; rde-1(ne300); arEx1720[lin-31p::RDE-1(+)] (Table S2), was used for VPC-specific RNAi (15, 45). The VPC specificity of RNAi in this strain is supported by the following observations: gfp(RNAi) specifically eliminated YFP in P6.p but not in the anchor cell of the gonad; tsp-15(RNAi), which targets a gene active in the major hypodermal syncytium, did not result in a Blister phenotype; unc-22(RNAi), which targets a muscle gene, did not cause a Twitcher phenotype; and pos-1(RNAi), which targets a germ-line–expressed gene, did not cause maternal-effect embryonic lethality.
Supplementary Material
Acknowledgments
We thank Victor Ambros for inspiration and encouragement. We also thank Claire de la Cova, Oliver Hobert, Eric Moss, Ann Rougvie, Maria Sallee, and Daniel Shaye for valuable discussions and comments on this manuscript and Richard Ruiz and Xinlan Zhou for technical assistance. Some of the strains used in this study were provided by the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (Grant P40 OD010440). This work was supported, in part, by NIH Grant R01CA095389 (to I.G.). I.G. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222377110/-/DCSupplemental.
References
- 1.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22(16):2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu PJ. 2007. Dauer. WormBook, ed The C. elegans research community. Available at www.wormbook.org. Accessed January 9, 2013.
- 3.Liu Z, Ambros V. Alternative temporal control systems for hypodermal cell differentiation in Caenorhabditis elegans. Nature. 1991;350(6314):162–165. doi: 10.1038/350162a0. [DOI] [PubMed] [Google Scholar]
- 4.Euling S, Ambros V. Reversal of cell fate determination in Caenorhabditis elegans vulval development. Development. 1996;122(8):2507–2515. doi: 10.1242/dev.122.8.2507. [DOI] [PubMed] [Google Scholar]
- 5.Braendle C, Félix M-A. Plasticity and errors of a robust developmental system in different environments. Dev Cell. 2008;15(5):714–724. doi: 10.1016/j.devcel.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg PW. Vulval development. WormBook. ed The C. elegans research community. Available at www.wormbook.org. Accessed January 9, 2013. [DOI] [PMC free article] [PubMed]
- 7.Euling S, Ambros V. Heterochronic genes control cell cycle progress and developmental competence of C. elegans vulva precursor cells. Cell. 1996;84(5):667–676. doi: 10.1016/s0092-8674(00)81045-4. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226(4673):409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 9.Chen N, Greenwald I. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev Cell. 2004;6(2):183–192. doi: 10.1016/s1534-5807(04)00021-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Greenwald I. Spatial regulation of lag-2 transcription during vulval precursor cell fate patterning in Caenorhabditis elegans. Genetics. 2011;188(4):847–858. doi: 10.1534/genetics.111.128389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo AS, Greenwald I. LIN-12/Notch activation leads to microRNA-mediated down-regulation of Vav in C. elegans. Science. 2005;310(5752):1330–1333. doi: 10.1126/science.1119481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi MS. 2009 Genes that act in specification of the vulval secondary fate in Caenorhabditis elegans. [Google Scholar]
- 13.Levitan D, Greenwald I. LIN-12 protein expression and localization during vulval development in C. elegans. Development. 1998;125(16):3101–3109. doi: 10.1242/dev.125.16.3101. [DOI] [PubMed] [Google Scholar]
- 14.Tan PB, Lackner MR, Kim SK. MAP kinase signaling specificity mediated by the LIN-1 Ets/LIN-31 WH transcription factor complex during C. elegans vulval induction. Cell. 1998;93(4):569–580. doi: 10.1016/s0092-8674(00)81186-1. [DOI] [PubMed] [Google Scholar]
- 15.Myers TR, Greenwald I. lin-35 Rb acts in the major hypodermis to oppose ras-mediated vulval induction in C. elegans. Dev Cell. 2005;8(1):117–123. doi: 10.1016/j.devcel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs D, Beitel GJ, Clark SG, Horvitz HR, Kornfeld K. Gain-of-function mutations in the Caenorhabditis elegans lin-1 ETS gene identify a C-terminal regulatory domain phosphorylated by ERK MAP kinase. Genetics. 1998;149(4):1809–1822. doi: 10.1093/genetics/149.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Cova C, Greenwald I. SEL-10/Fbw7-dependent negative feedback regulation of LIN-45/Braf signaling in C. elegans via a conserved phosphodegron. Genes Dev. 2012;26(22):2524–2535. doi: 10.1101/gad.203703.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Greenwald I. LIN-14 inhibition of LIN-12 contributes to precision and timing of C. elegans vulval fate patterning. Curr Biol. 2010;20(20):1875–1879. doi: 10.1016/j.cub.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14(12):1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 20.Antebi A, Culotti JG, Hedgecock EM. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development. 1998;125(7):1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- 21.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124(6):1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130(1):105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139(4):1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito RM, Perreault A, Peach B, Satterlee JS, van den Heuvel S. The CDC-14 phosphatase controls developmental cell-cycle arrest in C. elegans. Nat Cell Biol. 2004;6(8):777–783. doi: 10.1038/ncb1154. [DOI] [PubMed] [Google Scholar]
- 25.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115(4):489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 26.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 27.Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 28.Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11(24):1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 30.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: Insights from the hematopoietic system. Cell Stem Cell. 2007;1(2):140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20(2):126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17(15):4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm A-M, et al. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proc Natl Acad Sci USA. 2012;109(48):19697–19702. doi: 10.1073/pnas.1209714109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik J-H, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1(1):101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Renault VM, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13(9):1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138(2):303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: Genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics. 1994;137(1):107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46(2):326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZC, Ambros V. Heterochronic genes control the stage-specific initiation and expression of the dauer larva developmental program in Caenorhabditis elegans. Genes Dev. 1989;3(12B):2039–2049. doi: 10.1101/gad.3.12b.2039. [DOI] [PubMed] [Google Scholar]
- 42.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395(6705):854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 43.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 44.Karp X, Greenwald I. Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans. Genes Dev. 2003;17(24):3100–3111. doi: 10.1101/gad.1160803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jose AM, Garcia GA, Hunter CP. Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Nat Struct Mol Biol. 2011;18(11):1184–1188. doi: 10.1038/nsmb.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabara H, et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99(2):123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 47.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263(1-2):103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 48.Qadota H, et al. Establishment of a tissue-specific RNAi system in C. elegans. Gene. 2007;400(1-2):166–173. doi: 10.1016/j.gene.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.