Significance
The first retinal gene therapy in human blindness from RPE65 mutations has focused on safety and efficacy, as defined by improved vision. The disease component not studied, however, has been the fate of photoreceptors in this progressive retinal degeneration. We show that gene therapy improves vision for at least 3 y, but photoreceptor degeneration progresses unabated in humans. In the canine model, the same result occurs when treatment is at the disease stage equivalent to humans. The study shows the need for combinatorial therapy to improve vision in the short term but also slow retinal degeneration in the long term.
Keywords: neurodegeneration, outer nuclear layer, retinal structure
Abstract
Leber congenital amaurosis (LCA) associated with retinal pigment epithelium-specific protein 65 kDa (RPE65) mutations is a severe hereditary blindness resulting from both dysfunction and degeneration of photoreceptors. Clinical trials with gene augmentation therapy have shown partial reversal of the dysfunction, but the effects on the degeneration are not known. We evaluated the consequences of gene therapy on retinal degeneration in patients with RPE65-LCA and its canine model. In untreated RPE65-LCA patients, there was dysfunction and degeneration of photoreceptors, even at the earliest ages. Examined serially over years, the outer photoreceptor nuclear layer showed progressive thinning. Treated RPE65-LCA showed substantial visual improvement in the short term and no detectable decline from this new level over the long term. However, retinal degeneration continued to progress unabated. In RPE65-mutant dogs, the first one-quarter of their lifespan showed only dysfunction, and there was normal outer photoreceptor nuclear layer thickness retina-wide. Dogs treated during the earlier dysfunction-only stage showed improved visual function and dramatic protection of treated photoreceptors from degeneration when measured 5–11 y later. Dogs treated later during the combined dysfunction and degeneration stage also showed visual function improvement, but photoreceptor loss continued unabated, the same as in human RPE65-LCA. The results suggest that, in RPE65 disease treatment, protection from visual function deterioration cannot be assumed to imply protection from degeneration. The effects of gene augmentation therapy are complex and suggest a need for a combinatorial strategy in RPE65-LCA to not only improve function in the short term but also slow retinal degeneration in the long term.
Hereditary blindness can be caused by genetic defects in retinal pigment epithelium (RPE) cells, where a continuous supply of 11-cis-retinal chromophore is produced by the visual cycle, or in photoreceptors, where light photons captured by chromophore molecules signal vision (1). One of the key components of the visual cycle is the retinoid isomerase encoded by the RPE-specific protein 65 kDa (RPE65) gene (2). Mutations in RPE65 cause Leber congenital amaurosis (LCA), a severe childhood blindness (3). RPE65-associated LCA (RPE65-LCA) is a complex disease in which vision loss results from two pathological mechanisms—dysfunction and degeneration of photoreceptors (3–6). RPE65-LCA has been proclaimed to be the first successfully treated inherited retinopathy using gene augmentation therapy (7–10), and the treatment has resulted in substantial improvement in vision that endures at least 3 y (11).
Whereas we showed that gene therapy corrects the biochemical blockade leading to visual dysfunction and converts RPE65-LCA from a two-mechanism disease into a simple retinal degeneration (9), it has not been determined if therapy also treats the retinal degeneration component. It is hypothesized that correction of the underlying cellular dysfunction in a human recessive retinal degeneration will promote greater neuronal survival by slowing the natural rate of photoreceptor loss. Gene therapy experiments that have evaluated photoreceptor degeneration have been performed mainly in mouse and dog models treated at young ages (12–18) and have implied that at least a subset of photoreceptors could be rescued from degeneration. However, accumulating evidence has suggested that human RPE65-LCA, which can show degeneration prenatally or within the first years of life (19, 20), is better modeled at the more advanced disease stages in older animals (3, 21).
We evaluated the visual function and retinal degeneration in treated and untreated eyes of patients with RPE65-LCA and in the dog model of this disease. Gene therapy resulted in remarkable and lasting improvements in visual function, but serial measurements of outer photoreceptor nuclear layer (ONL) thickness showed continuing progression of photoreceptor loss. Our human and canine results are consistent with the hypothesis that the majority of photoreceptors in the degenerative phase of RPE65 disease is destined for progressive loss. Treatment of RPE65-LCA should evolve into a two-pronged intervention that not only leads to visual restoration in the short term but also, improved photoreceptor survival in the long term.
Results
Human RPE65-LCA: Natural History of Photoreceptor Degeneration.
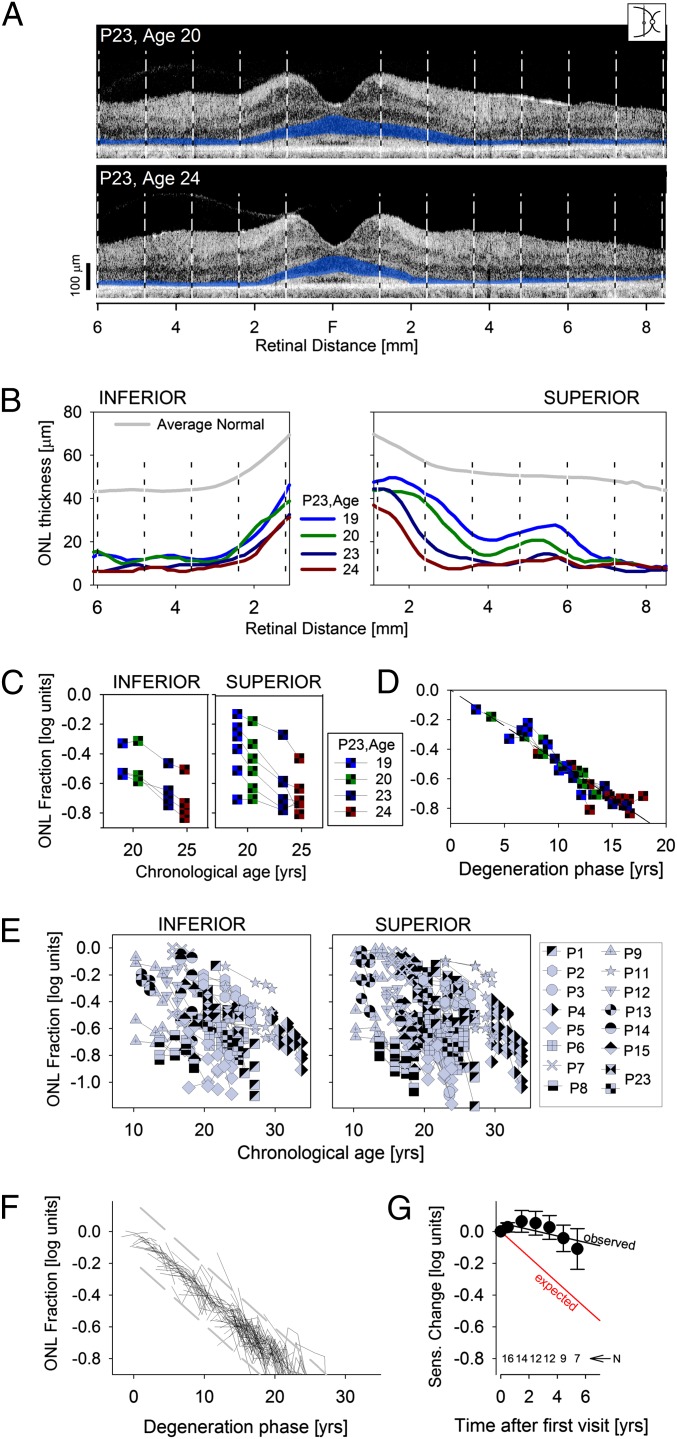
Our cohort of RPE65-LCA patients (Tables S1 and S2), including those patients as young as 3 y of age, had abnormal ONL thickness at a majority of sampled retinal locations (Fig. S1), consistent with previous reports of loss of photoreceptors detectable at the earliest ages evaluated to date (6, 19, 20, 22–24). Untreated RPE65-LCA eyes were examined longitudinally to measure the rate of progression of this early-onset retinal degeneration. Patient 23 (P23) illustrates the spatiotemporal progression of retinal degeneration (Fig. 1 A–D). Optical coherence tomography (OCT) scans across a 15-mm extent along the vertical meridian crossing the fovea (Fig. 1A) show the intraretinal backscattering differences that delineate retinal sublaminae corresponding to different cell layers (20). Quantitatively, the ONL thickness shows changes at most retinal locations (Fig. 1 A and B). Thinning of the ONL fraction (ONL thickness of the patient divided by mean normal thickness at the same retinal location) at most extrafoveal retinal locations showed log-linear progression (Fig. 1C), which is consistent with a model wherein the photoreceptor degeneration follows an exponential decay after a retinal locus-specific onset of photoreceptor degeneration (25, 26). To a first approximation, kinetics of ONL loss followed similar exponential decay rates irrespective of retinal location. This invariant exponential decay was estimated from the median value of the population of slopes calculated from each of the consecutive pairs of measurements. The onset of progressive degeneration phase was estimated at each retinal location by extrapolating along the median slope to 0 log ONL fraction, and all of the data was replotted to illustrate how a single underlying natural history can predict disease progression across multiple retinal locations at different disease stages (Fig. 1D).
Fig. 1.
Natural history of disease in untreated human RPE65-LCA. (A) Cross-sectional OCT retinal scans in P23 performed along the vertical meridian (Inset) at ages 20 and 24 y. Vertical dashed lines delimit the 10 extrafoveal regions quantified. ONL is highlighted in blue for visibility. F, fovea. (B) ONL thickness of P23 as a function of eccentricity at ages 19, 20, 23, and 24 y shows progressive thinning at most retinal locations. (C) Logarithm of the ONL fraction against age supports an underlying exponential decay function describing progression. (D) Data from C replotted after horizontal shifts accounting for differences in the onset of degeneration at each retinal region. (E) ONL thickness in untreated RPE65-LCA eyes shows an extremely wide spectrum of results with a weak tendency of thinning as a function of chronological age. (F) Data from E replotted after horizontal shifts accounting for differences in age of onset. Results show a predictable natural history underlying the progressive degeneration in most patients. Dashed lines delimit the range of deviation (±2 SD) of the data from the model. (G) Average visual sensitivities measured at the same retinal locations in the same patients are stable over time and do not conform to the expected slope (red) estimated from the ONL degeneration. Error bars are SE. Numbers of eyes contributing to each data point are shown.
Fifteen patients (ages 7–29 y at first visit) (Table S1) were examined serially (over periods ranging from 1.2 to 6.7 y, average = 4.6 y) to understand better the inter- and intraretinal variation of the natural history of retinal degeneration in RPE65-LCA (Fig. 1E). ONL thickness fraction from all retinal locations and all patients showed a wide variation when plotted against chronological age (Fig. 1E). There was a weak negative correlation with age both at inferior (slope = −0.015 log/y; r2 = 0.12) and superior (slope = −0.016 log/y; r2 = 0.11) retinal loci (Fig. 1E). To form a predictive natural history of disease applicable to RPE65-LCA, we calculated the median of the exponential rates across all patients, all retinal locations, and all consecutive pairs of measures (n = 426). On adjustment for the individual and locus-specific onset of the progressive degeneration phase, the underlying natural history (Fig. 1F) was highly consistent (r2 = 0.91) with a steeper slope (−0.04 log10/y) compared with the same data plotted against chronological age.
Natural History of Visual Sensitivity in Human RPE65-LCA.
Patients with RPE65-LCA show a substantial loss of light sensitivity unexplained by the amount of underlying photoreceptors (3, 6, 10, 22, 27). It is important to emphasize, however, that the patients do not lose all visual function. Often, there is measurable remnant vision that is mediated by rods, cones, or both (11, 22, 27). The source of this remnant vision or its natural history is not understood in RPE65-LCA. We next asked the question: what would be the expected change in sensitivity during the time span of measured ONL thinning? For simple retinal degenerations, a change in sensitivity should be proportional to the square of the change in ONL thickness (6). For the median exponential degeneration rate of −0.04 log/y, the corresponding change in sensitivity would be −0.08 log/y (Fig. 1G, expected). Progression of vision loss is expected to be similar to simple retinal degenerations, although the absolute sensitivity is dissociated from ONL thickness (6). To evaluate this hypothesis, we measured the change in light sensitivity along the vertical meridian at the same retinal locations and in the same patients as those patients included for the study of ONL change over time (Table S1). Unexpectedly, there was little detectable change measured in sensitivity. A regression line fit to the visual function data over a time horizon extending to 6 y showed a slope of −0.02 log/y (Fig. 1G, observed; −0.007 log/y if the regression line was forced to go through the origin), which was more shallow than the slope expected from the underlying photoreceptor degeneration.
Gene Therapy Does Not Slow the Progressive Retinal Degeneration in RPE65-LCA.
To determine the consequences of subretinal gene therapy on retinal degeneration, three analyses were performed: (i) intraocular comparisons were made of ONL fractions between treated and untreated retinal regions of the same eye; (ii) interocular comparisons were made of ONL fractions between study eyes and contralateral control eyes; and (iii) the progression of the ONL fraction in study eyes was compared with the expected natural history of RPE65-LCA. All analyses were performed along the vertical meridian. Treated regions in study eyes received the subretinal therapeutic vector and showed a significant improvement in visual function during the 90-d period after surgery. Control regions in study eyes did not receive the subretinal therapeutic vector and did not show change in visual function parameters during the same time period.
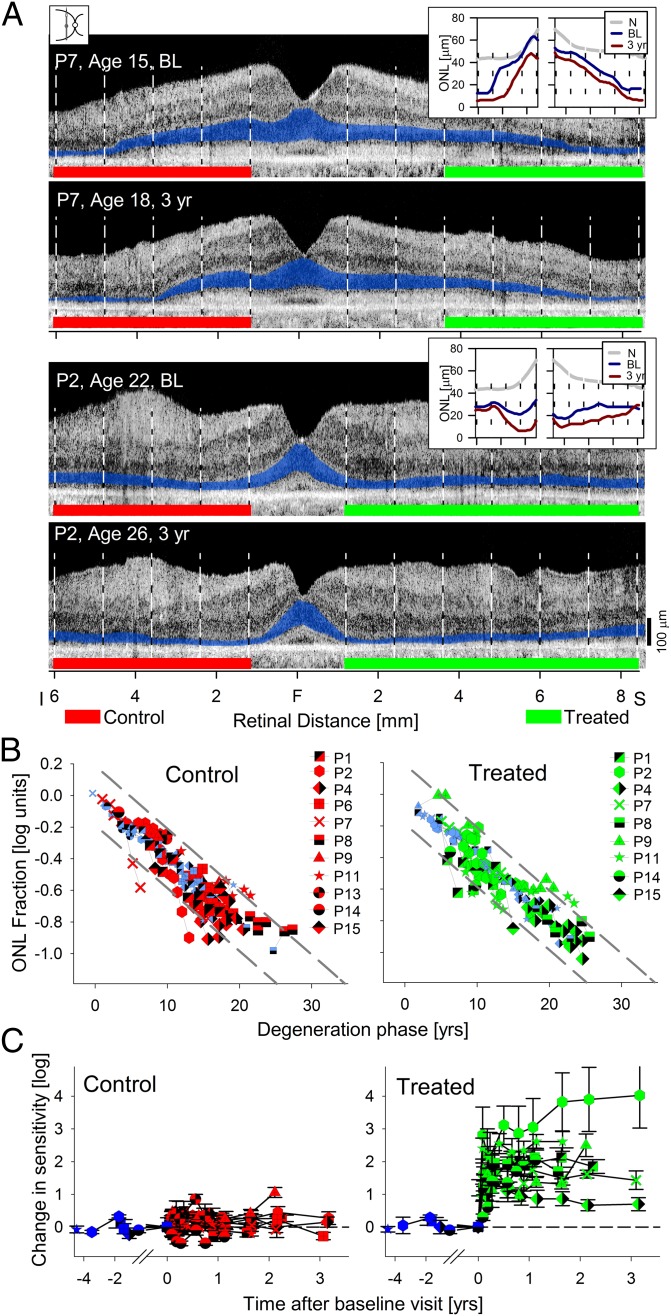
The first analysis is illustrated by the intraocular comparison data from the study eyes of P7 and P2; control [Fig. 2A, red bars (inferior retina)] and treated [Fig. 2A, green bars (superior retina)] regions are shown. For the control region in P7, the mean ONL fraction was 0.73 at baseline and reduced to 0.45 at 3 y later. For the treated region, the ONL fractions were 0.52 and 0.32 at baseline and 3 y later, respectively. Results in the study eye of P2 showed a similar reduction of ONL fraction at 3 y compared with baseline for both types of regions (from 0.57 to 0.38 at the control region and from 0.49 to 0.35 at the treated region). When ONL fractions from study eyes were compared, there was no significant difference (P = 0.91, Mann–Whitney rank sum) at baseline between regions (treated quartiles = 0.22, 0.40, 0.57; untreated quartiles = 0.24, 0.29, 0.57). At the last available visit after the treatment (Table S2), retinal regions showed thinner ONL compared with baseline, but there remained no significant difference (P = 0.66) between treated and untreated regions (treated quartiles = 0.17, 0.25, 0.36; untreated quartiles = 0.16, 0.26, 0.42).
Fig. 2.
Photoreceptor degeneration in human RPE65-LCA eyes that have had gene therapy. (A) Cross-sectional OCT scans in two patients, P7 and P2, at baseline (BL) and 3 y after treatment. Green lines demarcate the region superior to the fovea that received a subretinal injection and that visual function measurements showed significant improvements in sensitivity. Red lines demarcate the untreated control retinal regions. ONL is highlighted in blue for visibility. (Insets) Thickness of the ONL at BL and posttreatment time points compared with mean normal (N) shows degeneration occurring at most loci. (B) ONL measures in control (Left) and treated (Right) retinal regions plotted on the natural history of RPE65-LCA disease defined in Fig.1. The progression in control and treated regions is, for the most part, indistinguishable from the natural history. (C) Visual sensitivities measured at control (Left) and treated (Right) retinal locations in the same patients. Control locations show stable sensitivities before and after surgery. Treated locations show a dramatic improvement after the surgery that is retained over time. Pretreatment measures are shown with purple symbols in B and C.
In the second analysis, ONL fractions from treated study eyes and untreated control eyes (Tables S1 and S2) were compared. There was no significant difference (P = 0.70, Mann–Whitney rank sum) at baseline between eyes (study eye quartiles = 0.22, 0.40, 0.57; control eye quartiles = 0.22, 0.33, 0.51) when considering matched retinal regions (n = 36 regions in nine patients) that were later found to have been successfully treated in study eyes. At the last available visit, retinal regions showed thinner ONL compared with baseline, but there remained no significant difference (P = 0.99) between the eyes (study eye quartiles = 0.17, 0.25, 0.36; control eye quartiles = 0.16, 0.26, 0.44). Similarly, for untreated regions in study eyes and their matched regions in control eyes (n = 37 regions in nine patients), there were no significant differences in ONL fraction at baseline (P = 0.79) or at the last visit (P = 0.52).
In the third analysis, data from study eyes were compared with the expected model of the natural history of degeneration (Fig. 2B). For these analyses, the start of the degeneration phase was estimated from the ONL thickness fraction measured at baseline, and all posttreatment time points were then plotted with respect to the baseline value. The great majority of 45 control regions (11 eyes) (Fig. 2B, Left) showed progressive thinning of the ONL that was not substantially different from the natural history observed in untreated eyes. There were rare locations that showed no change or greater than expected deviation. For the 36 treated regions, the results were similar (nine eyes) (Fig. 2B, Right); the great majority progressed along the expected natural history, with rare regions showing no change or greater than expected deviation. When the ONL fraction deviations between the measured data and the natural history model were compared, there were no statistically significant differences between treated regions in study eyes, control regions in study eyes, and all loci in untreated eyes (P = 0.81, Kruskal–Wallis ANOVA on ranks). In summary, all three analysis methods supported the conclusion that gene therapy has not modified the natural history of progressive retinal degeneration in the RPE65-LCA patients.
Early Visual Improvements Persist Up to 3 y After Human Gene Therapy.
Visual sensitivity at the control regions of treated eyes was not significantly changed compared with baseline (Fig. 2C). The regression line showed a nearly zero slope (0.02 log/y) instead of a slope of −0.08 log/y expected based on the retinal degeneration rate estimated at the same retinal locations. In a subset of patients, visual function data for up to 4.5 y (average = 2.1 y, n = 6) before treatment were available (Fig. 2C, purple symbols) and consistent with the stability observed posttreatment. These results mirrored our observations in untreated control eyes (Fig. 1G). At the treated retinal locations, there were substantial improvements in visual sensitivity averaging 1.6 (range = 0.8–2.4) log units better than baseline within a short (30–90 d) period (Fig. 2C). Long-term evaluation of the treated locations showed mostly stability or additional improvement of the sensitivities (Fig. 2C, regression slope 0.04 log/y), results that were in contrast to the continuing degeneration measured at the same locations of the same eyes (Fig. 2B).
Natural History of Photoreceptor Degeneration in RPE65-Mutant Dogs.
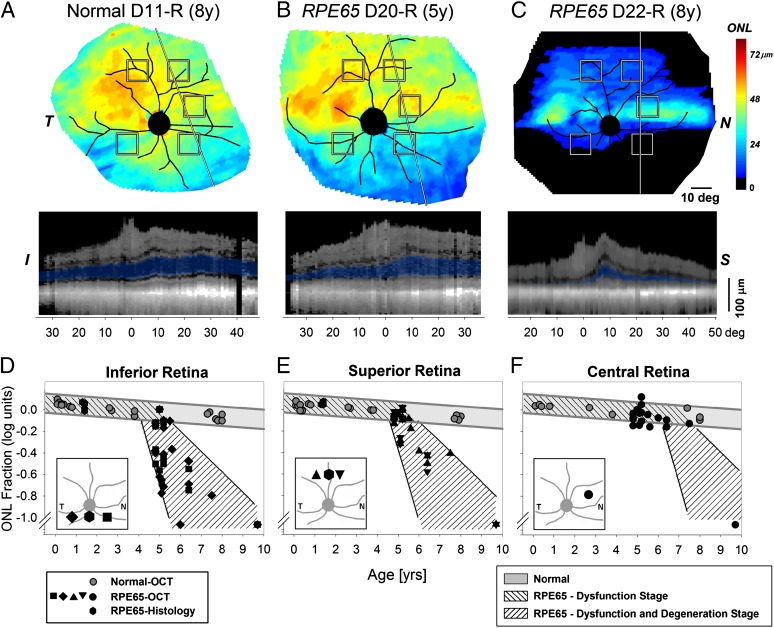
Next, we asked whether the posttreatment results of advancing retinal degeneration despite enduring visual improvement were unique to human RPE65-LCA or also occurred in an animal model of the disease. RPE65-mutant dogs are the only large animal model for RPE65-LCA and are known to show congenital visual dysfunction followed later by retinal degeneration (5, 17, 28–32). First, we defined the natural history of the canine disease. The thickness of the ONL layer recorded with noninvasive cross-sectional OCT retinal imaging was used to estimate the spatial pattern of the onset and progression of the retinal degeneration. Histological data in a limited number of dogs were also used in the analyses. Normal dogs showed thicker ONL in the superior (tapetal; nonpigmented RPE) retina compared with inferior (nontapetal; pigmented RPE) retina (Fig. 3A). RPE65-mutant dogs at 5 y of age could show nearly normal ONL thickness topography (Fig. 3B). By 8 y of age, there was substantial thinning of the ONL, with detectable photoreceptors remaining across the central (visual streak) retina extending into the superior retina (Fig. 3C).
Fig. 3.
Natural history of retinal degeneration in Rpe65-mutant dogs shows regional effects. (A–C) Photoreceptor (ONL) thickness topography in a normal dog compared with two Rpe65-mutant dogs at different ages. ONL thickness topography is mapped to a pseudocolor scale. I, inferior; N, nasal; S, superior retina; T, temporal. Reconstituted OCT scans along a superior–inferior meridian (line) are shown in Lower with the ONL layer highlighted in blue. Retinal distances are specified relative to the point closest to the optic nerve. Note that the OCTs shown are reconstituted from ultra-wide angle maps covering more than 80° × 80° (∼24 × 24 mm) sampled at 1°. (D–F) ONL thickness quantified as a function of age at five retinal locations (shown as squares in A–C): two inferior loci (D), two superior loci (E), and a nasal central visual streak locus (F). Gray symbols represent normal animals; black symbols represent RPE65-mutant dogs. Gray lines approximate the expected range of normal results. Hashed regions within gray lines show the dysfunction-only stage, and hashed regions within black lines show the dysfunction and degeneration stage of RPE65 disease. Insets are 60° × 60° and show the five retinal locations sampled with OCT and two locations sampled with histology. Overlapping symbols have been moved horizontally to aid in their visibility.
Five locations in inferior, superior, and central retina were chosen (squares in Fig. 3 A–C) to quantify the natural history of ONL changes in RPE65-mutant dogs compared with normal dogs (Table S3). At all five locations, normal eyes showed a small amount of ONL thinning (−0.014 log/y) over the first 8 y of dog life (Fig. 3 D–F, gray circles and lines). In RPE65-mutant eyes, at the two inferior retinal loci, ONL fraction measurements were consistent with the onset of the photoreceptor degeneration occurring near 4.9 y (Fig. 3D). At the two superior retinal loci, there was a similar natural history, but disease onset was delayed to 5.3 y (Fig. 3E). At the central retinal locus (nasal to the optic nerve on or near the visual streak), most of the eyes showed normal or near-normal ONL thickness. Limited data from a 10-y-old dog were consistent with the onset of photoreceptor degeneration being delayed to as much as 7 or more y (Fig. 3F). In summary, RPE65-mutant dogs displayed only the dysfunction component of the RPE65 disease for an extended period ranging to at least one-quarter of their lifespan. Onset of retinal degeneration in RPE65-mutant dogs occurs relatively late at ages equivalent to ∼30 human y (33).
Gene Therapy in RPE65-Mutant Dogs Rescues Progressive Retinal Degeneration only When Delivered Before the Onset of Retinal Degeneration.
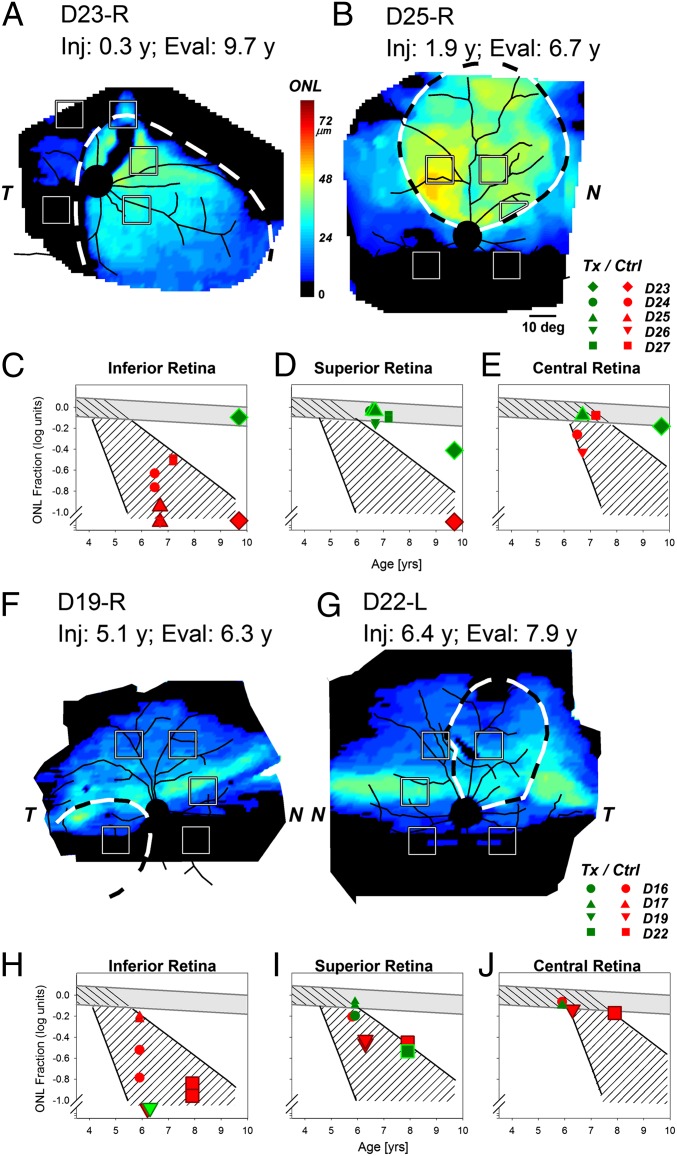
Results from two dogs illustrate the long-term success in preventing the photoreceptor loss when RPE65 gene therapy is delivered before the onset of retinal degeneration (Fig. 4 A and B). Dog 23 (D23) received a subretinal injection in the central and inferior nasal retina of the right eye at the age of 0.3 y. Electroretinogram (ERG) recordings for the first 3 y after treatment were markedly improved for rod and cone amplitudes compared with untreated eyes (34, 35). ERGs at age 9.7 y remained sizable, suggesting a durability of functional gains by rods and cones for nearly a decade (Fig. S2). By OCT, ONL thickness was undetectable outside the injected region, but there was substantial ONL remaining within the treated region (Fig. 4A). Quantitative results were consistent with nearly normal ONL thickness within the treated region in infero-nasal and central retina (Fig. 4 C and E, green diamonds), relatively retained ONL in the supero-nasal retina (Fig. 4D, green diamond), and severe retinal degeneration consistent with the natural history of disease in untreated supero-temporal and infero-temporal retinal locations (Fig. 4 C and D, red diamonds). D25 received, at age 1.9 y, a subretinal injection that extended from near the optic nerve into superior retina. ERG recording nearly 5 y after the treatment at 6.7 y showed significant functional improvement (Fig. S2). ONL thickness topography at age 6.7 y indicated that there was a retained area corresponding to the treatment region (Fig. 4B). Quantitative results in the inferior untreated retina were consistent with the natural history (Fig. 4C, red up triangles). In the treated superior retina, however, ONL thickness in D25 was within the normal range and substantially greater than expected from the natural history of RPE65 disease (Fig. 4D, green up triangles). The treated one-half of the central retinal region of D25 had near-normal ONL thickness, which was expected from the natural history. These results underscore the importance of understanding spatiotemporal distribution of the disease to interpret therapeutic effects (Fig. 4E).
Fig. 4.
Gene therapy applied before and after the onset of retinal degeneration in Rpe65-mutant dogs. (A and B) Photoreceptor (ONL) thickness topography in two dogs (D23 and D25) treated before the onset of the degeneration and evaluated ∼5–9 y later. There is much better retention of ONL thickness within the treatment region (dashed lines) compared with outside the treatment region. (C–E) ONL thickness quantified as a function of age at five retinal locations in five dogs treated before the initiation of degeneration. Red symbols correspond to retinal locations outside the treatment region, and green symbols correspond to locations within the treatment region. (F and G) ONL thickness topography in two dogs (D19 and D22) treated after the onset of degeneration. There is no evidence for thicker ONL within the treatment regions compared with outside the treatment regions. (H–J) ONL thickness quantified as a function of age at five retinal locations in treated eyes. Both untreated control (red symbols) and treated (green symbols) regions are not substantially different compared with the natural history of disease. Larger symbols in C–E correspond to data shown in A and B, and larger symbols in H–J correspond to data shown in F and G.
ONL thickness mapping and quantitative measurements were performed in three additional eyes (D24, D26, and D27) (Table S3) injected subretinally in the superior retina at ages 1.8–2.4 y and evaluated at ages 6.6–7.2 y (Fig. 4 C–E). All three eyes showed significant ERG improvements nearly 5 y after the treatment at ages of ∼7 y (Fig. S2). ONL fraction in control regions outside of the injection region was not different from the natural history of RPE65 disease in dogs (Fig. 4 C and E, smaller red symbols). In treated superior retinal regions, ONL fraction was normal or near normal, showing substantial rescue compared with the natural history of RPE65 disease (Fig. 4D, smaller green symbols).
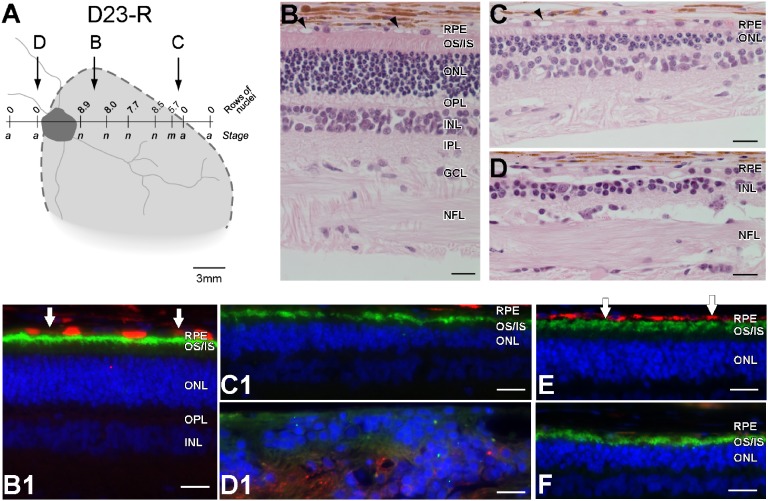
The eyes of the five early-treated dogs were examined by histology at the end of the study (Table S3). Histology results were well-correlated with those results obtained with noninvasive OCT imaging. The findings for D23, D25, and D26 are illustrated (Fig. 5 and Fig. S3). In general, photoreceptor morphology was normal in the center of the treatment area, with an intact ONL and preservation of inner retinal layers (Fig. 5B and Fig. S3 B and E). Towards the margins of the treatment areas, the ONL gradually thinned, and photoreceptors became shorter, broader, and more disoriented (Fig. 5C and Fig. S3C). Outside the treatment boundary, there was an abrupt loss of photoreceptors, with prominent retinal gliosis and loss of layer organization (Fig. 5D). RPE65 immunolabeling showed distinct cytoplasmic labeling within the treatment areas (Fig. 5B1), but none outside (Fig. 5D1). It appeared that RPE65 expression was most intense in the center of the treatment area and less intense and uniform towards the edges. Of interest, regardless of the expression of the RPE65 transgene in the treated regions, there were no apparent changes in the size and number of the vacuolated RPE inclusions that are characteristic of the disease (35).
Fig. 5.
Histologic evaluation of the RPE65-mutant dog D23 treated at 0.3 y before the onset of retinal degeneration shows remarkable rescue of photoreceptors from degeneration when assessed over a decade later. (A) Schematic representation of the en face image showing the treatment area (dashed lines), on which is superimposed the ONL rows (mean of three values in area sampled) and disease staging (a, advanced atrophy with gliosis and loss of retinal layer organization; m, moderate photoreceptor loss with 1/3 to 1/2 of ONL remaining; n, normal) assessed at 11.2 y. (B–D) Representative photomicrographs taken from areas identified in A. In the treatment area, there is normal retinal preservation (B), although the RPE shows vacuolated inclusions typical of the disease (arrowheads). At the edge of the treatment border (C), the photoreceptor layer becomes markedly attenuated and is absent (D) outside of the treatment region. (B1, C1, and D1) Double immunolabeling with RPE65 (red) and rod opsin (green) taken from regions corresponding to B–D. Labeling in RPE is present inside the treatment region (B), with some cells intensely labeled and others (vertical arrows) showing weaker immunolabeling. In the transition zone (C1), RPE65 labeling is absent, and opsin labeling reflects rod OS shortening and disorientation; outside the treatment area (D1), the photoreceptor layer is absent, and the inner nuclear layer (INL) is disorganized. (E and F) Control sections from WT dog (E) and RPE65-mutant dog in early stages of degeneration (F) show, respectively, the presence (red) or absence of specific RPE65 immunolabeling in the RPE. GCL, ganglion cell layer; NFL, nerve fiber layer; OPL, outer plexiform layer; OS/IS, outer and inner segment layer; INL, inner nuclear layer; IPL, inner plexiform layer. (Calibration: 20 µm.)
When gene therapy was performed in eyes of mutant dogs older than 4.8 y (Table S3), an age when at least part of the retina shows photoreceptor loss in most of the animals, visual function improved within 1 mo (Fig. S2), but retinal structural results obtained 1 y later did not show a positive modification of the natural history of photoreceptor degeneration (Fig. 4 F–J). Illustrating these results are D19 injected in the infero-temporal retina (Fig. 4F) and D22 injected in the supero-nasal retina (Fig. 4G) at ages 5.1 and 6.4 y, respectively. When imaged more than 1 y later, there was no obvious retention of ONL thickness corresponding to the treated regions. Quantitative results in dogs treated after 4.8 y of age (D16, D17, D19, and D22) showed ONL fraction consistent with the expected natural history of disease both in treated (Fig. 4 H–J, green symbols) and untreated (Fig. 4 H–J, red symbols) locations. These results are consistent with the hypothesis that gene therapy after the onset of retinal degeneration did not substantially modify the natural history of disease in the mutant dog.
Discussion
Gene augmentation therapy for human RPE65-LCA, as performed in four independent clinical trials, has resulted in substantially improved visual function within days to weeks after treatment (7–10), and the functional gain has been durable for as long as 3 y (11, 36) (Fig. 2C). These positive results bode well for the ability of gene therapy to partially ameliorate the severe and lifelong visual impairment experienced in RPE65-LCA. It is important to remember, however, that this vision loss is caused by a combination of a biochemical chromophore deficiency and a progressive degeneration of cone and rod photoreceptor cells (3). Restoration of RPE65 expression through gene therapy is thought to treat the chromophore deficiency, but it has also been assumed that treating the primary biochemical deficiency would slow or arrest the (presumed) secondary degeneration. Here, we test this assumption by determining if there is progression of retinal degeneration in patients with RPE65-LCA treated with gene therapy. We find that gene therapy causes substantial and durable vision improvement but does not slow the natural history of photoreceptor degeneration. Our findings have similarities to treatments applied to the CNS that lessen symptoms but do not slow the neurodegeneration (37, 38). The practical implication of our results is that human treatments of RPE65-LCA should address the need to slow or arrest the retinal degeneration that is present and ongoing in these patients, no matter how young they are when considered for enrollment in clinical trials to treat the dysfunction component of their complex disease.
To determine success of any treatment intended to slow the rate of retinal degeneration, an estimate should first be made of the rate of natural progression without treatment. For RPE65-LCA, the momentum toward treatment in the clinic was so great after proof-of-concept success in animals that there was no waiting for natural history data to emerge. Treatment for a genetic retinal disease, for the first time in history, took priority, and early reports of visual improvement seemed to validate that it was unnecessary to establish a rate of retinal degeneration. Now, as plans are announced to extend these initial trials to treating very young patients, both eyes, and greater retinal areas, we felt the need to revisit first principles. We made serial measurements of ONL thickness in untreated eyes of RPE65-LCA patients and estimated the rate of photoreceptor loss to be, on average, −0.04 log10/y (−9.6%/y) under a delayed exponential model of disease (25, 39–41). This progression rate is comparable with the rate estimated in a different retinal degeneration caused by MYO7A mutations using similar methods and longitudinal data (26) and within the range of progression rates estimated from visual function measures in other retinal degenerative diseases (25, 40, 41).
How do our estimates of the rate of progression of retinal degeneration in human patients compare with animal models of RPE65-LCA? Rpe65−/− mice show an onset of degeneration near 3–4 mo (4, 42) and a degeneration rate averaging −0.22 log10/y (21). Assuming an allometric relationship between rates of neurodegeneration and maximum lifespan (33), the Rpe65−/− mouse disease is much slower than human disease (6, 19–24, 43–45), with an onset that is equivalent to 9–12 human y and a rate that corresponds to −0.006 log10/human y. RPE65-mutant dogs were also known to have slow photoreceptor degeneration (5, 29, 30, 32), but the details of spatiotemporal progression were unknown. Our results showed that the onset of degeneration ranges from 5 to 8 y depending on retinal location. By allometric scaling, this range corresponds to 31–48 human y. The progression rate in RPE65-mutant dogs averaged −0.33 log10/y, corresponding to −0.05 log10/human y. Thus, both murine and canine models show an extended period (up to 25% of their lifespan) with a retina-wide dysfunction-only phase that does not seem to have an equivalent in human RPE65-LCA at any age observed to date (6, 19–24, 43–45). Human RPE65-LCA is best modeled in older animals clearly displaying the dysfunction as well as degeneration phases of RPE65 disease. During this combined phase, the rate of progression in the canine model seems to be more similar to the human disease compared with the rate observed in the murine model.
Preclinical experiments of gene augmentation therapy have been mostly performed in young RPE65-mutant animals at a disease stage preceding the onset of photoreceptor degeneration, and most investigations have focused on the restoration of visual function and safety of the intervention (12, 13, 34, 35, 46–52). Here, we evaluated the retinal degeneration in five younger dogs treated during the dysfunction-only stage of disease by longitudinally following them for 5–11 y after treatment. The results showed unequivocal survival of the photoreceptors within the treated region that would have otherwise degenerated. Noninvasive OCT studies localized the region of preservation, and histological studies showed intact ONL and preservation of inner retinal layers. Our studies confirm and extend previous studies (14, 17, 35), and they support the hypothesis that, in RPE65-mutant dogs, early treatment of the primary RPE defect prevents the later onset of photoreceptor degeneration. Similar experiments in mutant mice treated early with gene therapy have shown survival of cone (12, 13, 15, 16) and rod photoreceptors (49). Investigation of the mechanism(s) causing photoreceptor cell loss (18, 53–56) has been mostly performed in untreated eyes to date. Extension of such studies to eyes undergoing gene therapy (18) would be important to clarify molecular underpinnings of the observed modification of natural history. Furthermore, the newly discovered vitamin A isomerization pathway originating in Muller cells and subserving cones (57) may be found to contribute to the differences in (cone or rod) photoreceptor survival.
Our cohorts of RPE65-LCA patients who received gene therapy (9, 11, 36) showed remarkable and lasting improvements in visual function, but our serial measurements of ONL thickness now show that photoreceptor loss has continued along a trajectory no different from the expected natural history. In parallel experiments that we performed in older RPE65-mutant dogs, gene therapy applied after the onset of retinal degeneration did not seem to increase photoreceptor survival. To explain both findings, we postulate that there exists a critical limit in terms of accumulating molecular changes that are detrimental to photoreceptor survival under this condition of severe RPE65 dysfunction. There is evidence for molecular changes occurring in rod and cone photoreceptors in the predegenerative phase of RPE65 disease (17, 18, 32, 54, 58), and a specific translocation of one of the proapoptotic Bax isoforms from the cytosol to mitochondria has been observed to correlate with the initiation of photoreceptor cell loss (54, 55). To date, there has been a paucity of murine studies of gene therapy in older animals after the onset of retina-wide rod degeneration (6, 59). Expansion of such studies is required to better understand the molecular changes that are associated with inevitable loss of photoreceptors or successful protection from degeneration following gene augmentation therapy.
RPE65-mutant animals are not alone in requiring gene augmentation therapies to be applied in a predegenerative phase. Several murine models of primary photoreceptor diseases—such as mice with Aipl1, Rpgrip1, Pde6b, Bbs4, and Cngb1 defects—show improved photoreceptor survival when therapies were applied before or at the early stages of retinal degeneration (60–64). To date, there has been only one demonstration of gene augmentation therapy applied after the onset of retinal degeneration that showed improved survival of photoreceptors (65). It remains to be determined whether this difference in outcome of canine retinitis pigmentosa GTPase regulator (RPGR) disease vs. other photoreceptor or RPE diseases in mouse models is because of species differences in the pathophysiology of disease or other treatment variables.
Untreated eyes of RPE65-LCA patients showed abnormal but stable visual function at the same retinal locations that were showing progressive loss of photoreceptors over the same observation period. Similarly, in treated eyes, the improved visual function in the short term did not diminish in the longer term with the progression of degeneration. These pairs of seemingly paradoxical findings may be explained by a number of hypotheses alone or combined. Two of these hypotheses involve the source of the remnant function driven by a chromophore, the identity, source, and concentration of which are currently unknown. A first hypothesis proposes that in RPE65-LCA, unliganded opsin molecules are in excess to chromophore molecules. Then the available chromophore would effectively limit detection of light, and visual function would be lost only when degeneration reduces the number of opsin molecules to be equal or less than the chromophore molecules available. A second hypothesis is that the chromophore production increases with progression of RPE65 disease and associated accumulation of retinyl esters within the RPE. This hypothesis has been raised to explain an age-related increase in sensitivity observed in Rpe65−/− mice (21). An increase in chromophore production could partially compensate for the loss of photoreceptors by increasing the proportion of regenerated opsin molecules in remaining cells. A third hypothesis would involve cones as the basis of stable visual function and the majority of rods showing the progressive degeneration. All three of these hypotheses could possibly explain the results in untreated eyes with ∼5 log units loss of light sensitivity but are unlikely to explain the observations in the treated eyes, where (rod-mediated) sensitivities can approach within 1 log unit of normal. A fourth hypothesis, more consistent with our data, is that RPE65-LCA photoreceptors show a spectrum of preapoptotic cellular stress states. At one end of the spectrum are functionally silent cells that are next in line to be lost to degeneration, and at the other end are healthier and functionally potent cells that will survive the longest. In this case, degeneration would progress with a constant rate of exponential decay after the disease onset (25, 26, 39–41). Initially, there would be little loss of function, but later, vision would be affected more and more at the late-disease stages with RPE atrophy, when there is loss of a critical number of last-remaining functionally potent (rod and cone) photoreceptors. Preliminary evidence for such a progression is observed as falling off a “cliff” of visual function near the peripheral boundaries of the visual field (27).
Gene augmentation therapy in RPE65-mutant patients, mice, and dogs has shown relative safety and remarkable improvement in visual function (11, 16, 35). Future human studies are being proposed to continue and expand the same therapeutic strategy. However, there is mounting evidence for a need to refine the initial approaches. One need for refinement is to understand sufficiently the visual cycle of human foveal cones to make the treatment efficacious for visual acuity (11). Another need, revealed in the present study, would be to seek a more complete therapeutic outcome that involves both visual improvement and structural rescue in extrafoveal retinal regions. The latter direction can be determined experimentally in older Rpe65-mutant mice and dogs that show both dysfunction and degeneration phases of the disease. In other words, there is a need to advance the therapy by taking a step back to proof-of-concept studies in animals at ages that better model the human patients. Among the possibilities to consider for refinement is delivery of agents to prevent the loss of retinal cells, such as neuroprotective, prosurvival, or antiapoptotic factors or antioxidants (66–69), sequentially or simultaneously with a more advanced version of gene augmentation therapy to increase chromophore to the remaining rod, extrafoveal cone, and foveal cone photoreceptors.
Materials and Methods
Human Subjects.
Patients (n = 23) with RPE65-LCA were included (Tables S1 and S2). Informed consent was obtained. Procedures followed the Declaration of Helsinki guidelines and were approved by the institutional review board. Fifteen of the patients were enrolled in the clinical trial (NCT00481546). Methodological details are provided in SI Materials and Methods.
Dogs.
RPE65-mutant (n = 16) and normal (n = 11) dogs were included (Table S3). All procedures involving animals (34, 35, 48, 65, 70) were performed in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and had Institutional Animal Care and Use Committee approval. Methodological details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
The clinical trial was supported by National Eye Institute Grant U10 EY 017280. Experimental work with the canine disease was funded by National Eye Institute Grants EY 06855, 017549, 019304, and 022012, and the Foundation Fighting Blindness.
Footnotes
Conflict of interest statement: W.W.H. and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The remaining authors declare no conflict of interest. University of Pennsylvania, University of Florida, and Cornell University hold a patent on the described gene therapy technology (United States Patent 20070077228, “Method for Treating or Retarding the Development of Blindness”).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218933110/-/DCSupplemental.
References
- 1.Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287(3):1612–1619. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29(5):398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre GD, et al. Congenital stationary night blindness in the dog: Common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- 6.Jacobson SG, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci USA. 2005;102(17):6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 8.Maguire AM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cideciyan AV, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banin E, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: Human gene therapy initiated in Israel. Hum Gene Ther. 2010;21(12):1749–1757. doi: 10.1089/hum.2010.047. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson SG, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice. Invest Ophthalmol Vis Sci. 2006;47(3):1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 13.Bemelmans AP, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3(10):e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narfström K, et al. Morphological aspects related to long-term functional improvement of the retina in the 4 years following rAAV-mediated gene transfer in the RPE65 null mutation dog. Adv Exp Med Biol. 2008;613:139–146. doi: 10.1007/978-0-387-74904-4_15. [DOI] [PubMed] [Google Scholar]
- 15.Kostic C, et al. Gene therapy regenerates protein expression in cone photoreceptors in Rpe65(R91W/R91W) mice. PLoS One. 2011;6(2):e16588. doi: 10.1371/journal.pone.0016588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, et al. Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse: A model for midcourse RPE65 leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011;52(1):7–15. doi: 10.1167/iovs.10-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mowat FM, et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther. 2012 doi: 10.1038/gt.2012.63. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Q, et al. Differential proteomics and functional research following gene therapy in a mouse model of Leber congenital amaurosis. PLoS One. 2012;7(8):e44855. doi: 10.1371/journal.pone.0044855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porto FB, et al. Prenatal human ocular degeneration occurs in Leber’s congenital amaurosis (LCA2) J Gene Med. 2002;4(4):390–396. doi: 10.1002/jgm.278. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson SG, et al. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49(10):4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso RC, et al. Retinal disease in Rpe65-deficient mice: Comparison to human leber congenital amaurosis due to RPE65 mutations. Invest Ophthalmol Vis Sci. 2010;51(10):5304–5313. doi: 10.1167/iovs.10-5559. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson SG, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz B, et al. A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest Ophthalmol Vis Sci. 2008;49(12):5235–5242. doi: 10.1167/iovs.07-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda T, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18(12):2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cideciyan AV, et al. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18(5):931–941. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson SG, et al. Retinal disease course in Usher syndrome 1B due to MYO7A mutations. Invest Ophthalmol Vis Sci. 2011;52(11):7924–7936. doi: 10.1167/iovs.11-8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson SG, et al. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50(5):2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narfström K, Wrigstad A, Nilsson SE. The Briard dog: A new animal model of congenital stationary night blindness. Br J Ophthalmol. 1989;73(9):750–756. doi: 10.1136/bjo.73.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wrigstad A, Nilsson SE, Narfström K. Ultrastructural changes of the retina and the retinal pigment epithelium in Briard dogs with hereditary congenital night blindness and partial day blindness. Exp Eye Res. 1992;55(6):805–818. doi: 10.1016/0014-4835(92)90007-f. [DOI] [PubMed] [Google Scholar]
- 30.Wrigstad A, Narfström K, Nilsson SE. Slowly progressive changes of the retina and retinal pigment epithelium in Briard dogs with hereditary retinal dystrophy. A morphological study. Doc Ophthalmol. 1994;87(4):337–354. doi: 10.1007/BF01203343. [DOI] [PubMed] [Google Scholar]
- 31.Veske A, Nilsson SE, Narfström K, Gal A. Retinal dystrophy of Swedish briard/briard-beagle dogs is due to a 4-bp deletion in RPE65. Genomics. 1999;57(1):57–61. doi: 10.1006/geno.1999.5754. [DOI] [PubMed] [Google Scholar]
- 32.Hernández M, Pearce-Kelling SE, Rodriguez FD, Aguirre GD, Vecino E. Altered expression of retinal molecular markers in the canine RPE65 model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51(12):6793–6802. doi: 10.1167/iovs.10-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright AF, et al. Lifespan and mitochondrial control of neurodegeneration. Nat Genet. 2004;36(11):1153–1158. doi: 10.1038/ng1448. [DOI] [PubMed] [Google Scholar]
- 34.Acland GM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 35.Acland GM, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cideciyan AV, et al. Human RPE65 gene therapy for Leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20(9):999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes C, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson D, et al. Memantine and brain atrophy in Alzheimer’s disease: A 1-year randomized controlled trial. J Alzheimers Dis. 2012;29(2):459–469. doi: 10.3233/JAD-2011-111616. [DOI] [PubMed] [Google Scholar]
- 39.Clarke G, et al. A one-hit model of cell death in inherited neuronal degenerations. Nature. 2000;406(6792):195–199. doi: 10.1038/35018098. [DOI] [PubMed] [Google Scholar]
- 40.Herrera W, et al. Retinal disease in Usher syndrome III caused by mutations in the clarin-1 gene. Invest Ophthalmol Vis Sci. 2008;49(6):2651–2660. doi: 10.1167/iovs.07-1505. [DOI] [PubMed] [Google Scholar]
- 41.Stone EM, et al. Autosomal recessive retinitis pigmentosa caused by mutations in the MAK gene. Invest Ophthalmol Vis Sci. 2011;52(13):9665–9673. doi: 10.1167/iovs.11-8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai CM, et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65-/- knockout mouse eye results in limited rescue. Genet Vaccines Ther. 2004;2(1):3. doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Hooser JP, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA. 2000;97(15):8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenz B, et al. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111(8):1585–1594. doi: 10.1016/j.ophtha.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Pasadhika S, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51(5):2608–2614. doi: 10.1167/iovs.09-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narfström K, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44(4):1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 47.Dejneka NS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9(2):182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson SG, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13(6):1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Pang JJ, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3):565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Aguirre GK, et al. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4(6):e230. doi: 10.1371/journal.pmed.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Meur G, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 52.Annear MJ, et al. Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther. 2011;18(1):53–61. doi: 10.1038/gt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodruff ML, et al. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35(2):158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 54.Cottet S, Schorderet DF. Triggering of Bcl-2-related pathway is associated with apoptosis of photoreceptors in Rpe65-/- mouse model of Leber’s congenital amaurosis. Apoptosis. 2008;13(3):329–342. doi: 10.1007/s10495-008-0180-2. [DOI] [PubMed] [Google Scholar]
- 55.Hamann S, Schorderet DF, Cottet S. Bax-induced apoptosis in Leber’s congenital amaurosis: A dual role in rod and cone degeneration. PLoS One. 2009;4(8):e6616. doi: 10.1371/journal.pone.0006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Métrailler S, Schorderet DF, Cottet S. Early apoptosis of rod photoreceptors in Rpe65(-/-) mice is associated with the upregulated expression of lysosomal-mediated autophagic genes. Exp Eye Res. 2012;96(1):70–81. doi: 10.1016/j.exer.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Kaylor JJ, et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat Chem Biol. 2013;9(1):30–36. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cottet S, et al. Biological characterization of gene response in Rpe65-/- mouse model of Leber’s congenital amaurosis during progression of the disease. FASEB J. 2006;20(12):2036–2049. doi: 10.1096/fj.06-6211com. [DOI] [PubMed] [Google Scholar]
- 59.Nusinowitz S, et al. Cortical visual function in the rd12 mouse model of Leber Congenital Amarousis (LCA) after gene replacement therapy to restore retinal function. Vision Res. 2006;46(22):3926–3934. doi: 10.1016/j.visres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Tan MH, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: Effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009;18(12):2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pawlyk BS, et al. Replacement gene therapy with a human RPGRIP1 sequence slows photoreceptor degeneration in a murine model of Leber congenital amaurosis. Hum Gene Ther. 2010;21(8):993–1004. doi: 10.1089/hum.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang JJ, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19(2):234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simons DL, Boye SL, Hauswirth WW, Wu SM. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci USA. 2011;108(15):6276–6281. doi: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch S, et al. Gene therapy restores vision and delays degeneration in the CNGB1/mouse model of retinitis pigmentosa. Hum Mol Genet. 2012;21(20):4486–4496. doi: 10.1093/hmg/dds290. [DOI] [PubMed] [Google Scholar]
- 65.Beltran WA, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA. 2012;109(6):2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA. 2010;107(13):5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalkara D, et al. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Mol Ther. 2011;19(9):1602–1608. doi: 10.1038/mt.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohnaka M, et al. Long-term expression of glial cell line-derived neurotrophic factor slows, but does not stop retinal degeneration in a model of retinitis pigmentosa. J Neurochem. 2012;122(5):1047–1053. doi: 10.1111/j.1471-4159.2012.07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trifunović D, et al. Neuroprotective strategies for the treatment of inherited photoreceptor degeneration. Curr Mol Med. 2012;12(5):598–612. doi: 10.2174/156652412800620048. [DOI] [PubMed] [Google Scholar]
- 70.Komáromy AM, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.