Abstract
Previous data suggest that nucleotides are important mitogens in the developing retina. Here, the effect of ATP on the death of cultured chick embryo retina cells was investigated. In cultures obtained from retinas of 7-day-old chick embryos (E7) that were cultivated for 2 days (E7C2), both ATP and BzATP induced a ∼30 % decrease in cell viability that was time- and dose-dependent and that could be blocked by 0.2 mM oxidized ATP or 0.3 μM KN-62. An increase in cleaved caspase-3 levels and in the number of TUNEL-positive cells was observed when cultures were incubated with 3 mM ATP and immunolabeling for cleaved-caspase 3 was observed over neurons but not over glial cells. ATP-dependent cell death was developmentally regulated, the maximal levels being detected by E7C2-3. Nucleotides were able to increase neuronal ethidium bromide and sulforhodamine B uptake in mixed and purified neuronal cultures, an effect that was blocked by the antagonists Brilliant Blue G and oxidized ATP. In contrast, nucleotide-induced cell death was observed only in mixed cultures, but not in purified cultures of neurons or glia. ATP-induced neuronal death was blocked by the glutamatergic antagonists MK801 and DNQX and activation of P2X7 receptors by ATP decreased the uptake of [3H]-d-aspartate by cultured glial cells with a concomitant accumulation of it in the extracellular medium. These results suggest that ATP induces apoptosis of chick embryo retinal neurons in culture through activation of P2X7 and glutamate ionotropic receptors. Involvement of a P2X7 receptor-mediated inhibition of the glial uptake of glutamate is suggested.
Keywords: P2X7 receptor, Apoptosis, Retinal neurons, Glutamate uptake, Glutamate receptors
Introduction
Programmed cell death, also called naturally occurring cell death, is a prominent phenomenon in the developing nervous system that, together with cell proliferation, determines the size of cell populations in the adult tissue. In the developing chick embryo retina, two consecutive periods of cell death were described, the first and the second waves of cell death peaking at E4-6 and E12, respectively [1–5]. Concomitant with cell death, cell proliferation and neurogenesis occur at the early stages of development in the vertebrate retina [6]. High proliferative activity occurs during the first 10 days of development in the chick embryo tissue, when all cell types cease mitosis in close and overlapping succession [7, 8].
Nucleotides are able to trigger cell death and/or cell proliferation in several biological systems and the balance between these processes during development depends on the expression and activity of different types of P2 receptors. In general, P2Y receptors, mainly P2Y1 and P2Y2/4 were associated with cell proliferation and growth [9]. In the retina, both ATP and UTP evoke calcium waves within early stages and induce the proliferation of retinal progenitors [10–15]. In the chick embryonic tissue, while UTP, through activation of P2Y2/4 receptors, induces the proliferation of early developing precursors of ganglion, amacrine, photoreceptor, and horizontal cells [12, 13], ATP, through activation of P2Y1 receptors, induces the proliferation of late developing glial/bipolar progenitors [14, 16]. This effect is primarily mediated by activation of PKC, MAP kinases, and the PI3K/AKT pathway and involves the regulation of cyclin D1 and p27 kip 1 levels [14, 15, 17, 18].
In contrast to P2Y receptors, the functional consequences of the activation of P2X receptors during development of the nervous system are still poorly explored, although mRNA for several P2X receptor subtypes were demonstrated in developing brain [9, 19, 20]. Both P2X3 and P2X7 receptors were shown to inhibit axonal growth and branching of developing motor axons [19], cultured hippocampal neurons [21], and neuroblastoma cells [22]. Moreover, activation of P2X7 receptors was shown to promote the survival of cerebellar granule cells [23, 24], but the death of cultured striatum-derived neural progenitors [25], a conflicting observation that now can be explained by the recent detection and characterization of P2X7 receptor splice variants with distinct functional properties [26, 27]. While P2X7A receptor induces cell permeabilization and death, the truncated P2X7B receptor that lacks the carboxy-terminal induces cell growth.
Besides P2Y receptors, the presence of several P2X receptor subtypes was characterized in the retina, including the P2X7 receptor [28]. Expression of this receptor subtype was demonstrated in the human [29] (Pannicke et al. 2000), rat [30–32], and monkey retina [33]. In the adult rat retina, prolonged activation of P2X7 induces the death of photoreceptors [32] and of purified ganglion cells in culture [34]. In the retina of newborn rats, activation of these receptors induces the death of cholinergic neurons that contributes to establish the density of these neurons in the adult tissue [35].
Although nucleotide-induced proliferation of retinal progenitors is well characterized during development of the retina, the effect of nucleotides on cell death and survival is still poorly characterized at the early stages of retinal development where both neurogenesis and natural cell death occur. In the present study, we investigated the effect of ATP on the survival of chick embryo retinal cells in culture. Our data show that activation of P2X7 receptors by ATP or BzATP induces apoptosis of developing retinal neurons in an early period of retinal development. Moreover, P2X7-dependent death of neurons is dependent on glutamate ionotropic receptors and occurs only when these cells are cultured in the presence of glial cells. A P2X7 receptor-dependent inhibition of [3H]-d-aspartate uptake was also observed in these mixed cultures.
Methods
Materials
Minimum essential medium (MEM) and fetal calf serum were from Life Technologies (São Paulo, SP, Brazil). ATP, BzATP, Tween 20, sodium dodecyl sulfate (SDS), periodate-oxidized ATP (oxiATP), dl-2-amino-5-phosphonopentanoic acid (AP5), MK 801, DNQX, ethidium bromide, poly-l-ornithine, Hepes, dimethyl sulfoxide, sulforhodamine B (SRB), penicillin, and streptomycin were purchased from Sigma-Aldrich (St Louis, MO, USA). Polyvinylidene difluoride (PVDF) membranes and enhanced chemiluminescence kit (ECL Plus) were from GE (São Paulo, SP, Brazil). Anti-β-tubulin III (TUJ-1) was from Millipore. Monoclonal rabbit anti-cleaved caspase-3 antibody and polyclonal anti-ERK antibody were from Cell Signaling Tech (MA, USA). Monoclonal anti-2M6 was kindly provided by Dr. B. Schlosshauer (Max-Planck-Institute, Tübingen, Germany). [3H]-Thymidine (5 Ci/mmol) and 2,3-[3H]-d-aspartate (11.3 Ci/mmol) were from Perkin-Elmer (São Paulo, SP, Brazil). All other reagents were of analytical grade. White-Leghorn chick embryos were from a local hatchery.
Primary mixed retinal cultures
The use of animals was in accordance with the “NIH guide for the Care and Use of Laboratory Animals” and approved by Commission for Animal Research of the Fluminense Federal University. Monolayer retinal cultures containing both neurons and glial cells (mixed cultures) were prepared according to a previously published procedure [14]. Briefly, retinas from E7 were dissected from other structures of the eye and immediately transferred to 1 mL of Ca2+ and Mg2+-free balanced salt solution (CMF). Trypsin (Worthington), at a final concentration of 0.1 %, was then added and the suspension incubated at 37 °C for 20 min. Trypsin solution was removed and retinas resuspended in MEM containing 2 % fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Tissues were mechanically dissociated by successive aspirations of the medium. For experiments measuring the incorporation of [3H]-thymidine and Western blot, 5 × 106 cells were seeded on plastic 35-mm culture dishes (1.04 × 104 cells/mm2). For 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) experiments measuring cell viability, 1.25 × 106 cells/well were seeded on plastic 24-well dishes. For immunocytochemistry, dye uptake and TUNEL assays, cells were seeded on culture dishes at a density of 3 × 106 cells/dish (3.1 × 103 cells/mm2). Drugs were added 48 h after the preparation of the cultures. Cells were incubated at 37 °C for the indicated periods of time, in humidified atmosphere of 5 % CO2 / 95 % air. The culture medium was changed every other day.
Glial cultures
Purified cultures of glia were obtained as described by Loiola and Ventura [36] and maintained for about 3 weeks. The initial density of cells was 5.0 × 106 cells / dish, and medium was changed regularly twice a week. Cultures were used after 20–22 days, when neurons were not detected in the cultures anymore.
Primary neuronal cultures
Glial-free purified cultures of retinal neurons were prepared from 7-day-old chick embryos as previously described [37]. In brief, retinas were dissected and incubated with 0.1 % trypsin in CMF (Ca2+- and Mg2+-free Hanks’ balanced salt solution) for 10 min at 37 °C. The cells were dissociated using a fire-polished Pasteur pipette, suspended in MEM, and then seeded in 35 mm plastic tissue culture dishes pre-coated with 25 μM poly-l-ornithine for 24 h. Cell density was approximately 0.8–1 × 106 cells/dish (832 cells/mm2). Cultures were incubated in MEM containing 2.5 % fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mm glutamine, for 2 days at 37 °C, in an atmosphere of 5 % CO2/95 % air. Medium of these cultures was not changed.
Macrophages cultures
Macrophages were obtained from the intra-peritoneal cavity of mice 4 days after thioglycollate injection and cultivated as previously described by Coutinho-Silva and Persechini [38]. Cultures with 2 × 105 cells/dish were used 4 days after their onset.
Cell viability assays
Cell viability was determined by the MTT reduction method first described by Mosmann [39]. Mixed, neuronal, or glial cultures were treated with 1.5 mg/mL of MTT and incubated for the specified periods of time, at 37 °C in Hanks’ salt solution (128 mM NaCl, 4 mM KCl, 1 mM Na2HPO4, 0.5 mM KH2PO4, 1 mM MgCl2, 3 mM CaCl2, 12 mM glucose, 20 mM HEPES, pH 7.4). One hour after the addition of MTT, cells were treated with ATP, BzATP, or H2O2. Inhibitors were always added 15–30 min before agonists, except for oxidized ATP that was added 2 h before ATP. After two washes with Hanks’ balanced salt solution, formazan product was dissolved with a mixture of HCl/isopropanol and estimated by the absorbance at 570 nm after subtracting absorbance at 650 nm.
To determine the viability of proliferating retinal progenitors, cultures at E7C2 were incubated for 90 min with 0.5 μCi [3H]-thymidine to label proliferating cells, washed four times with 2 mL of medium without serum and cultured for 4 h in MEM + 2 % FCS. After this incubation, cultures were treated with 3 mM ATP or 0.1 mM BzATP for an additional 3-h period. Cultures were then washed two times with 2 mL of BME buffered with 25 mM HEPES, pH 7.4 and cells dissolved with 0.4 N NaOH. Homogenates were precipitated with 10 % trichloroacetic acid (TCA) and the materials collected on Whatman GF/B glass fiber filters that were washed three times with 5 mL of 5 % TCA. Filters were dried and radioactivity determined by liquid scintillation spectroscopy. Experiments were performed in triplicate.
Immunofluorescence
Retinal cultures at E7C2 containing 3 × 106 cells/dish were treated with 3 mM ATP for 10 min. Cultures were then washed with phosphate buffered saline (PBS) and fixed for 15 min in 0.16 M phosphate buffer, pH 7.6 with 4 % paraformaldehyde, and 1 % picric acid. After three washes of 5 min with PBS, pH 7.6, nonspecific sites were blocked by incubating cells for 60 min in PBS/Triton X-100 containing 0.1 % NGS, and 5 % BSA. Cells were incubated overnight at 4 °C with cleaved caspase-3 (Asp175) primary antibody at a dilution of 1:100, washed, and incubated with Alexa secondary antibody (1:200) for 2 h at room temperature. Nuclei were counterstained with DAPI and cells examined and photographed under fluorescence illumination on a Nikon Eclipse TE 2000-U microscope.
For TUJ-1 and 2 M6 immunofluorescence, fixed retinal cultures were incubated for 1 h in PBS/triton-X100 blocking solution containing 10 % NGS, 1 % BSA, and 0.5 % Triton X-100 in 0.16 M PB, pH 7.6. Then cultures were incubated with mouse anti-β-tubulin III (TUJ1, 1:400) or mouse anti-2 M6 (1:200) in 0.16 M phosphate buffer, pH 7.6, containing 3 % NGS, 1 % BSA, and 0.5 % Triton X-100, overnight, at room temperature. After three washes with phosphate buffer, cultures were incubated with the appropriate Alexa secondary antibody, nuclei counterstained with DAPI, and labeled cells examined and photographed.
Dye uptake assays
Mixed (E7C2), neuronal (E7C2), glia (E7C18-21), and macrophage cultures were pre-incubated for 5 min at 37 °C in Hanks’ salt solution without Ca2+ or Mg2+ and then exposed to 5 μM ethidium bromide or 3 mM sulforhodamine B in the presence of 3 mM ATP or 0.1 mM BzATP for 10–15 min at 37 °C. Cultures were washed twice with Hanks’ solution and immediately observed and photographed on a Nikon TE 2000-U fluorescence microscope using a B-2E/C filter block for TRICT. Labeled cells were counted in ten micrographs of random fields from the cultures.
Western blotting assays
For detection of cleaved caspase-3 expression, mixed cell cultures were treated with 3 mM ATP for 5 and 10 min. Cells were washed and immediately transferred to sample buffer without bromophenol blue. Culture extracts were boiled and centrifuged for 10 min. Protein content was estimated in 2 μL samples by the Bradford protein assay [40], using a BSA solution containing 2 μL of sample buffer as standard. Extract samples (60 μg/lane) were size-fractionated on 9 % SDS polyacrylamide gels, transferred to PVDF membranes (GE Healthcare), and blocked with 5 % non-fat milk in Tris-buffered saline (pH 7.6) with 0.1 % Tween-20. Membranes were incubated with diluted primary antibody against cleaved caspase-3 (1:100) overnight, at 4 °C. Membranes were stripped and incubated with antibody against ERK 2 (1:2,000) for estimation of sample loading. Blots were developed using a secondary antiserum conjugated to horseradish peroxidase (Bio-Rad Labs Inc.) and enhanced chemiluminescence, according to the manufacturer’s protocol (ECL plus, GE Healthcare).
TUNEL assays
Mixed cultures were treated with 3 mM ATP for 3 h, fixed with 4 % paraformaldehyde + 1 % picric acid for 15 min and washed with PBS three times for 10 min. Apoptotic cells in the cultures were labeled with the APO-BrdU™ TUNEL Assay Kit (Molecular Probes) according to the provided protocol. Labeled cultures were photographed on a Nikon Eclipse microscope and apoptotic cells counted in ten photographs randomly obtained. Three independent experiments were performed.
Uptake and efflux of [3H]-d-aspartate
Retinal cultures were incubated with 3 mM ATP for 3 h at 37 °C and then incubated with 0.3 μCi/mL 2,3-[3H]-d-aspartate (11.3 Ci/mmol) for increasing periods of time in Hanks’ balanced salt solution without Ca2+ or Mg2+. For uptake experiments with purified glial cultures, 3 mM Ca2+ was added to the Hanks’ salt solution to avoid cell detachment. At the end of each incubation period, cultures were washed six times with 2 mL of cold Hanks’ solution for removal of non-incorporated radioactivity, and cells were disrupted with 0.5 mL of cold distilled water for at least 1 h. Samples of the intracellular radioactivity were determined by scintillation spectroscopy. Antagonists were added 10 min prior to ATP except oxidized ATP that was added 2 h before the nucleotide. To determine the efflux of pre-incorporated [3H]-d-aspartate, cultures at E7C2 were incubated with labeled d-aspartate for 1 h, at 37 °C, washed six times with 2 mL Hanks’ solution without Ca2+ or Mg2+, and then incubated in the absence or presence of 3 mM ATP for increasing periods of time. At the indicated incubation period, media of the cultures were collected and radioactivity estimated. Then cells were disrupted with 1 mL of cold water for at least 1 h and the remaining intracellular radioactivity determined. The efflux of [3H]-d-aspartate was expressed as the percent radioactivity present in the extracellular medium relative to the total radioactivity that was estimated as the sum of counts present in the extracellular medium plus the radioactivity remaining in cells.
Statistical analysis
Statistical comparisons were made by one-way analysis of variance followed by the Bonferroni post-test. When two groups were compared, the Student’s t test was used (graphs in Figs. 3, 6, 7, and 8a).
Fig. 3.
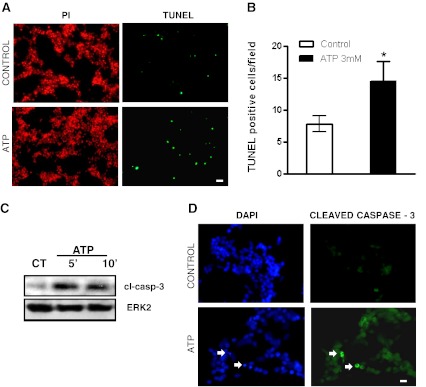
ATP induces cell apoptosis in chick embryo retinal cultures. a Representative photomicrographs showing mixed retinal cultures at E7C2, treated or not with 3 mM ATP for 3 h and labeled with TUNEL (green) or propidium iodide (red). b Quantification of the number of TUNEL-positive cells in control and ATP-treated cultures. Labeled cells in each culture were counted in ten micrographs randomly obtained. Data represent the mean ± SEM of three independent experiments performed in duplicate. c Detection by Western blotting of cleaved caspase-3 in protein extracts of retinal cell cultures at E7C2 stimulated with 3 mM ATP for 5 or 10 min. Experiments were replicated three times with similar results. CT = cultures incubated without ATP. d Representative photomicrographs showing cells immunolabeled for cleaved caspase-3 (arrows) in retinal cell cultures at E7C2 incubated with 3 mM ATP for 10 min. Cell nuclei staining was performed with DAPI. Bars represent 20 and 10 μm in a and d, respectively. *p < 0.05, versus control
Fig. 6.
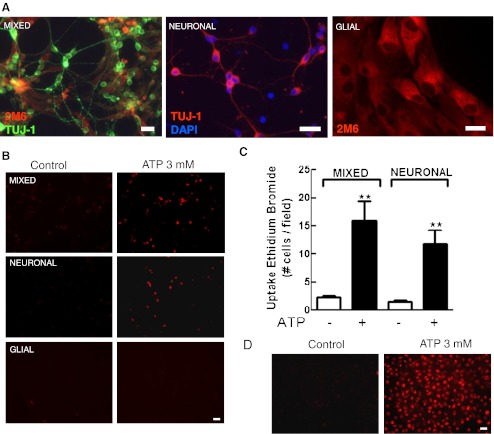
Uptake of ethidium bromide induced by ATP in three types of retinal monolayer cultures. Mixed and neuronal retinal cultures at E7C2 or glial cultures were treated with 3 mM ATP for 10–15 min in the presence of 5 μM ethidium bromide (EtBr) in Hanks’ balanced salt solution without Ca++/Mg++. After incubation, cells were washed and visualized under fluorescence illumination. a Representative photomicrographs of the three types of cultures labeled for β-tubulin III and the glial marker 2 M6. b Representative micrographs showing EtBr-labeled cells in the three types of cultures. c Quantification of EtBr-positive cells in mixed and neuronal retinal cultures. d Positive control showing the uptake of EtBr induced by 3 mM ATP in rat macrophage cultures. Labeled cells in each culture were counted in ten different micrographs randomly obtained. Data represent the mean ± SEM of five separate experiments performed in duplicate. ***p < 0.01 versus control. Bars = 20 μm
Fig. 7.
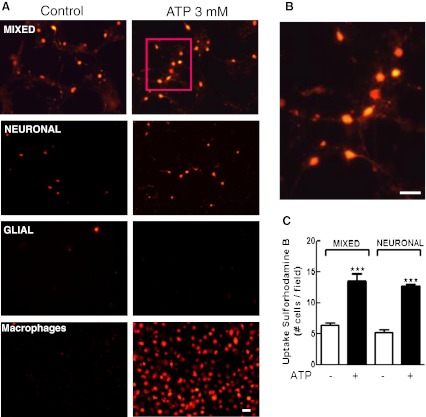
Uptake of sulforhodamine B (SRB) induced by ATP in the three types of retinal monolayer cultures. Mixed and neuronal retinal cultures at E7C2 or glial cultures were treated with 3 mM ATP for 10–15 min in the presence of 5 μM SRB in Hanks’ balanced salt solution without Ca++/Mg++. After incubation, cells were washed and visualized under fluorescence illumination. a Representative micrograph showing SRB labeled cells in mixed, purified neuronal, glial retinal cultures, and cultured macrophages. b High magnification of the cells shown in the red square in a. Note the presence of labeled neuronal processes. c Quantification of SRB-positive cells in mixed and neuronal retinal cultures. Labeled cells in each culture were counted in ten different micrographs randomly obtained. Data represent the mean ± SEM of five separate experiments performed in duplicate. ***p < 0.001, compared with control cultures. Bars = 20 μm
Fig. 8.
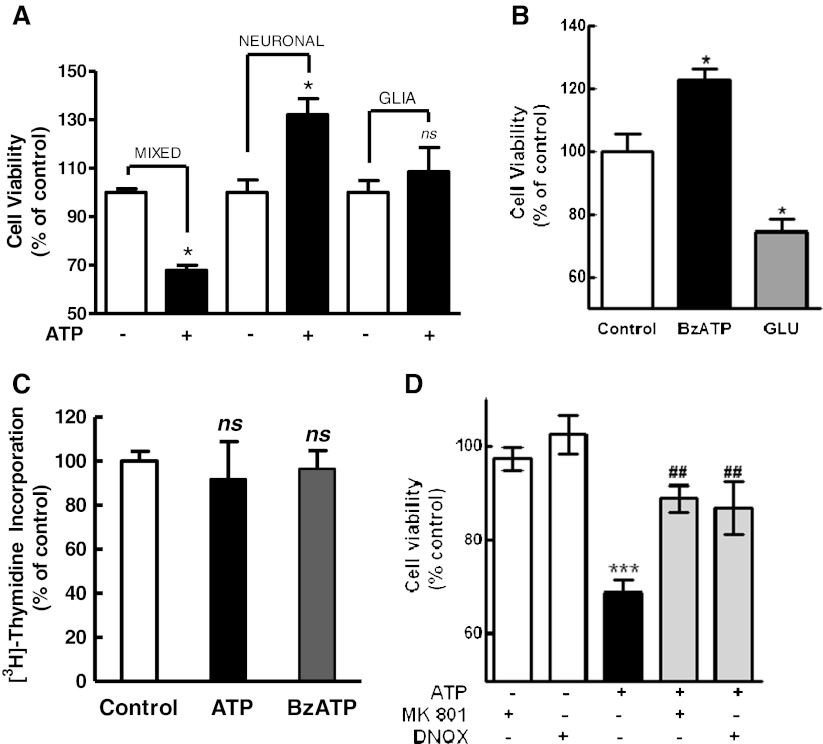
a Effect of ATP on the viability of retinal cells in the three types of retinal cultures. Mixed, neuronal, or glial purified retinal cultures were incubated with 3 mM ATP for 3 h. Cell viability was assessed by the MTT assay. b Effect of BzATP and glutamate on the viability of retinal neurons. Neuronal cultures at E7C2 were incubated with 0.1 mM BzATP or 1 mM glutamate for 3 h and cell viability assessed by MTT. c Effect of the NMDA and non-NMDA receptor antagonists MK801 and DNQX on the decrease in cell viability induced by ATP. Mixed cultures at E7C2 were incubated with 3 mM ATP for 3 h in the absence or presence of 50 μM MK801 or 50 μM DNQX. Antagonist was added 10 min prior to ATP. d Effect of ATP on the viability of retinal progenitors. Mixed cultures at E7C2 were incubated for 90 min with 0.5 μCi [3H]-thymidine to label proliferating progenitors, washed, and cultured for 4 h. Cells were then treated with 3 mM ATP or 0.1 mM BzATP for an additional 3-h period and processed for the detection of radioactivity as described in “Methods.” Data represent the mean ± SEM of three separate experiments performed in duplicate or triplicate (a) or three to four separate experiments performed in duplicate or triplicate (b, c, d). ***p < 0.001 and *p < 0.05, versus control cultures. ##p < 0.01, versus ATP-treated cultures. NS not statistically significant
Results
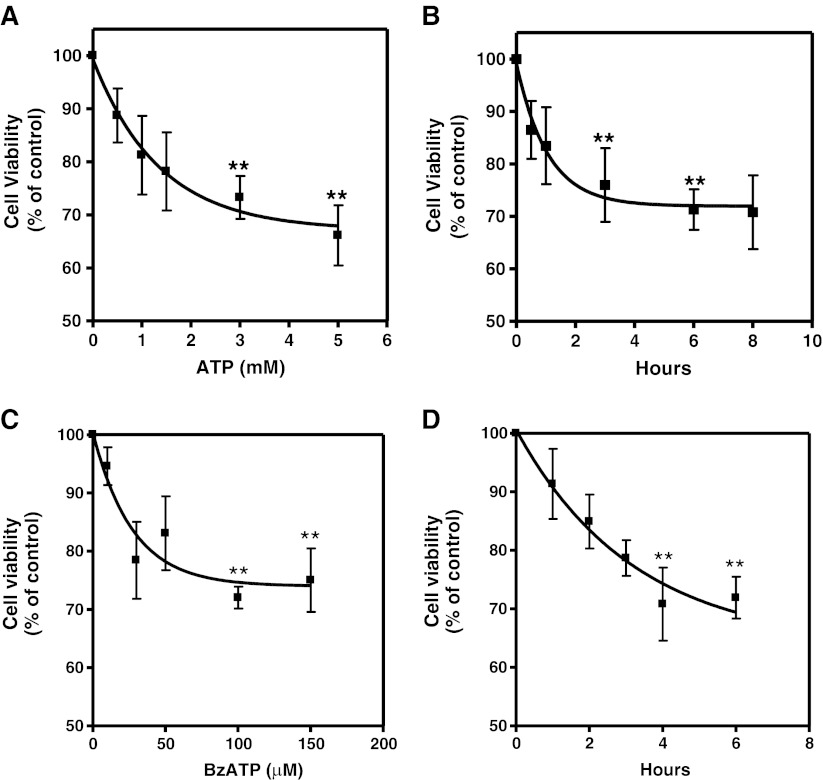
In order to verify if ATP could induce cell death in developing chick retinal cells, we investigated the cell viability in retinal cell cultures obtained from 7-day-old embryos and cultured for 2 days (E7C2). Cultures were incubated with increasing concentrations of ATP (Fig. 1a) or with 3 mM ATP for increasing periods of time (Fig. 1b). A progressive decline in cell viability was observed when higher concentrations of ATP, and longer periods of incubation were used. Although incubation of cells with increasing concentrations of H2O2 decreased cell viability by ∼70 % (not shown), a consistent decrease of ∼30 % was obtained when cultures were incubated for 3 h or longer periods with 3 mM or higher concentrations of ATP.
Fig. 1.
Effect of ATP and BzATP on cell survival as determined by MTT viability assays. Retinal cultures at E7C2 cultivated in complete medium were incubated with increasing concentrations of ATP or BzATP a, c for 3 h or with 3 mM ATP and 0.1 mM BzATP for increasing periods of time (b, d). After this period, MTT assays were performed as described in “Methods.” Data represent the mean ± SEM of at least three separate experiments performed in triplicate. **P < 0.01 compared with control
Since high concentrations of ATP are able to activate P2X7 receptors, the effect of BzATP, a potent agonist for these receptors, was evaluated in the retinal cell cultures (Fig. 1c). Similarly to ATP, cell viability decreased consistently as increasing concentrations of BzATP were used, an effect that was also time-dependent (Fig. 1d). Cell viability decreased to 72.8 ± 1.9 % of the control value when cultures were incubated with 0.1 mM BzATP for 3 h.
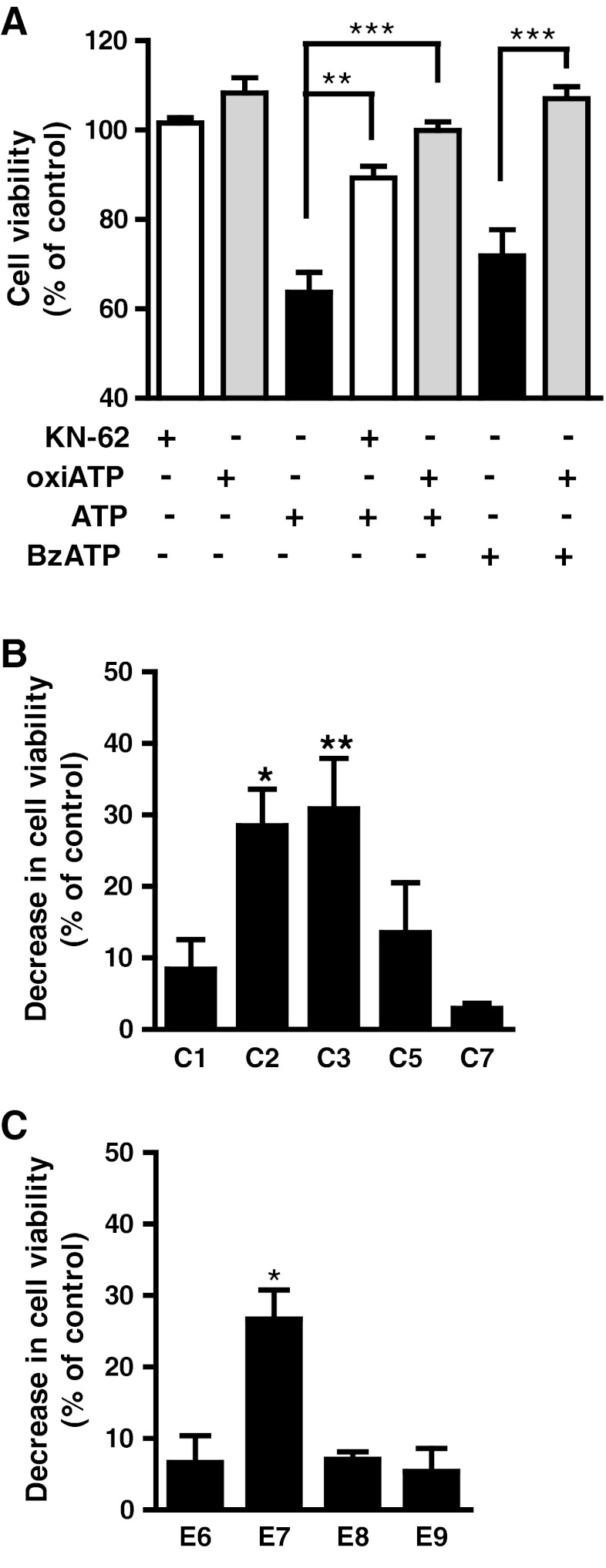
To confirm that ATP-induced decrease in cell viability was mediated by activation of P2X7 receptors, cell cultures at E7C2 were pre-incubated for 2 h with 0.2 mM oxidized ATP or with 0.3 μM KN-62 for 30 min prior to incubation of cells with 3 mM ATP or 0.1 mM BzATP, for 3 h. Figure 2a shows that antagonists were able to attenuate ATP-induced decrease in cell viability. While ATP decreased cell viability to 63.7 ± 4.5 % of control levels, cell viabilities of 89.3 ± 2.6 % and 99.9 ± 1.97 % of control levels were observed when cultures were incubated with ATP + KN62 and ATP + oxidized ATP, respectively. Oxidized ATP also attenuated the decrease in cell viability induced by 0.1 mM BzATP that increased from 71.8 ± 5.9 % to 107.0 ± 2.7 % of the control levels in cultures treated with the antagonist. No significant change in cell viability was observed in cultures treated with antagonists alone.
Fig. 2.
ATP and BzATP induced a decrease in the viability of retinal cells in culture. a Effect of P2X7 receptor antagonists. Retinal cell cultures at E7C2 were incubated with 3 mM ATP or 0.1 mM BzATP for 3 h in the absence or presence of 0.2 mM oxiATP or 0.3 μM KN-62 that were added 2 h or 30 min before the agonists, respectively. b ATP-induced decrease in cell viability as a function of the developmental stage of the cultures. Retinal cells obtained from 7-day-old embryos were cultured for several days and incubated with 3 mM ATP for 3 h at the indicated stage (C1 to C7). c ATP-induced decrease in retinal cell viability as a function of the developmental stage of the chick embryo. Retinas from chick embryos at different stages were dissociated and retinal cells cultured for 2 days. Cultures were incubated wht 3 mM ATP for 3 h and cell viability determined by MTT. The results in a and b represent the mean ± SEM of three or four separate experiments performed in duplicate or triplicate. Results in c represent the mean ± SEM of 2 (E6,E9) or 4 (E7,E8) separate experiments performed in duplicate or triplicate. In a, **p < 0.01 and ***p < 0.001, compared with ATP- or BzATP-treated cultures; in b and c, *p < 0.05 and **p < 0.01, compared with control, non-treated cultures. SEM values of control cultures were less than 10 %
ATP-induced decrease in cell viability as a function of the developmental stage of the cultures is shown in Fig. 2b. Cultures cultivated for the indicated periods of time were incubated with 3 mM ATP for 3 h and processed for the MTT assay as described in “Methods.” While ATP-induced loss of cell viability was 8.4 ± 4.1 % in the first day of cultivation (E7C1), it increased to 28.5 ± 5.1 % and 30.8 ± 7.0 % in cultures at E7C2 and E7C3, respectively. In the following periods of cultivation, ATP-induced decrease in cell viability declined and was 13.57 ± 6.9 % and 2.93 ± 0.75 % in cultures at E7C5 and E7C7, respectively.
A similar transient loss of retinal cell viability was observed when retinal cells were obtained from chick embryos at different stages of development and cultured for 2 days (Fig. 2c). While ATP-induced loss of cell viability was 6.6 ± 3.7 % of control levels in cultures from embryos at E6, it increased to 26.7 ± 4.0 % of control levels in cultures from embryos at E7. In retinal cultures obtained from older embryos, ATP-induced loss of cell viability decreased, attaining 5.4 ± 3.2 % of control levels in cultures from animals at E9.
The results above suggest that ATP, through activation of P2X7 receptors, induces the death of a population of chick developing retinal cells in culture. In order to verify if ATP-induced decrease in cell viability was due to the apoptosis of retinal cells, cultures at E7C2 were incubated with 3 mM ATP for 3 h and processed for TUNEL immunofluorescence assays (Fig. 3a, b). Propidium Iodide was used to label all nuclei of cells that were fixed with paraformaldehyde prior to terminal transferase reaction of the TUNEL assay. The number of TUNEL-positive cells was determined by counting labeled cells in ten randomly photographed fields/culture in experiments performed in duplicate. While the number of TUNEL-positive cells/ field was 7.8 ± 1.3 in control cultures, the number of labeled cells increased to 14.4 ± 1.8 when the cultures were treated with the nucleotide.
Classically, apoptosis triggers the activation of caspase proteolytic cascade that leads to the cleavage of executor caspases such as caspase-3. In order to investigate if ATP-induced death of cultured retinal cells was mediated by caspase processing, cultures at E7C2 were incubated with 3 mM ATP and analyzed for the expression of cleaved caspase-3 by Western blotting (Fig. 3c) and immunocytochemistry (Fig. 3d). An increase in the expression of this enzyme could already be detected in retinal cultures incubated for 5 or 10 min with 3 mM ATP as well as its labeling in cytoplasm of cells. Some labeled cells showing pyknotic nuclei could be noticed in ATP-treated cultures (arrows in Fig. 3d).
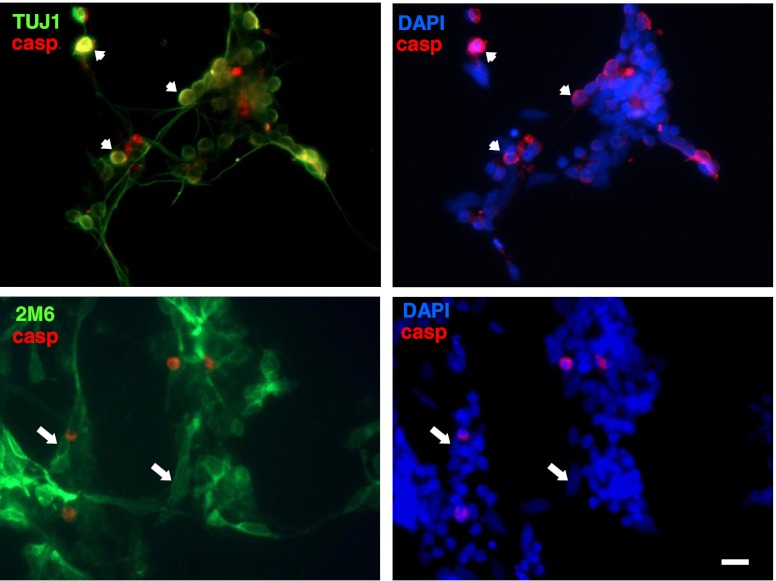
In order to characterize the retinal cell type that was affected by ATP in terms of apoptosis, retinal cultures at E7C2 were stimulated with 3 mM ATP for 10 min, fixed, and immunostained for cleaved caspase-3 (red) and anti-β-tubulin III or anti-2 M6 (green) to label neurons and glial cells, respectively (Fig. 4). Only neurons (short arrows) positive for β-tubulin III were labeled for cleaved caspase-3. No glial cells positive for 2 M6 antigen (long arrows) and cleaved-caspase-3 were noticed in the cultures.
Fig. 4.
Immunoreactivity for cleaved caspase-3 in ATP-treated retinal cultures. Retinal cultures at E7C2 were stimulated with 3 mM ATP for 10 min, fixed, and immunolabeled with anti-cleaved caspase-3 (red) and anti-β-tubulin III or anti-2 M6 (green) to label neurons and glial cells, respectively. Note that only neurons were labeled for cleaved-caspase 3 (short arrows at upper panels). No double-labeled glial cells were found (long arrows at lower panels). Experiments were replicated twice with similar results. Bar = 10 μm
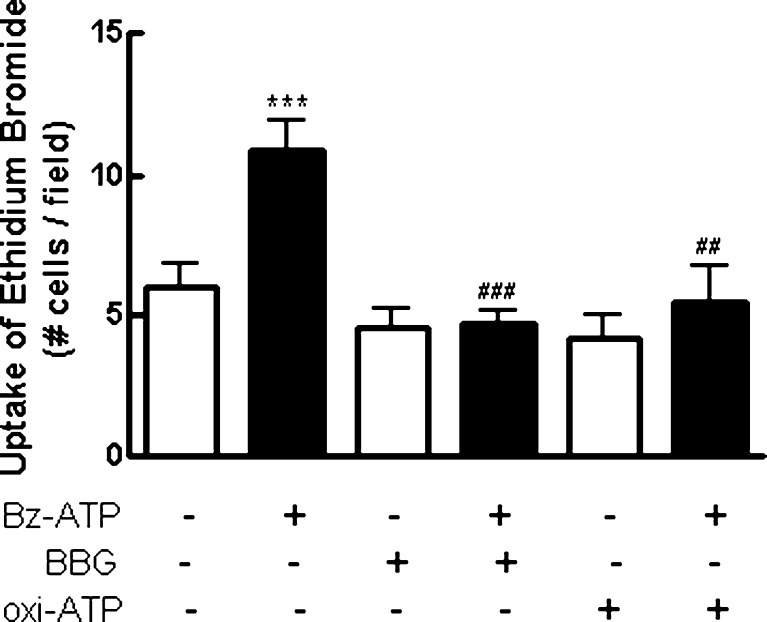
In several cell types, signaling through P2X7 receptors is concomitant with the opening of a non-selective pore that allows the influx of dyes with molecular weights lower than 900 Da such as ethidium bromide [41]. Mixed retinal cultures at E7C2 were incubated with 5 μM ethidium bromide for 5 min. Cultures were then incubated with 0.1 mM BzATP in Hanks’ solution without Ca2+ or Mg2+ for an additional 10–15 min and immediately inspected on a fluorescence microscope. As shown in Fig. 5, a significant increase of 75 % in the number of labeled cells was observed when cultures were incubated with the agonist (control, 6.0 ± 0.8 cells/field; BzATP, 10.5 ± 1.1 cells/field). Moreover, the pre-incubation of the cells with 5 μM Brilliant Blue G (BBG) for 15 min or 0.2 mM oxidized ATP for 2 h completely blocked BzATP-induced uptake of ethidium bromide, and the number of labeled cells decreased to 4.7 ± 0.8 cells/field and 5.5 ± 1.3 cells/field, respectively. No change in the number of labeled cells was observed when cultures were incubated only with antagonists or when uptake assays were performed in culture medium containing Ca2+ and Mg2+ (data not shown).
Fig. 5.
Uptake of ethidium bromide induced by BzATP in cultured retinal cells. Retinal cultures at E7C2 were stimulated with 0.1 mM BzATP for 10–15 min in the presence of 5 μM ethidium bromide in Hanks’ balanced salt solution without Ca++/Mg++. The P2X7 receptor antagonists oxiATP (0.2 mM) and BBG (5 μM) were added 2 h or 15 min before BzATP, respectively. After stimulation, cells were washed and visualized under fluorescence microscopy. In each culture, labeled cells were counted in ten micrographs randomly obtained. Data represent the mean ± SEM of five separate experiments performed in duplicate. ***p < 0.001, compared with control cultures. ###p < 0.001 and ##p < 0.01, compared with BzATP-treated cultures
Dye uptake assays described above were performed in mixed cultures of retinal cells containing both neurons and glia. In order to verify if neurons and glial cells take up ethidium bromide after activation of P2X7 receptors, uptake assays were performed in purified cultures containing only neurons or glial cells (Fig. 6). While no uptake of ethidium bromide was detected in purified glial cells at E8C21, when purified neuronal cultures at E7C2 were stimulated with 3 mM ATP for 10–15 min, a similar increase in the number of ethidium bromide labeled cells was observed, as compared with mixed cultures (in cells/field: mixed cultures, control = 2.3 ± 0.3; ATP = 16.0 ± 3.3; purified neuronal cultures, control = 1.5 ± 0.3; ATP = 11.7 ± 2.6). As described previously by others [38, 41, 42], positive control cultures of peritoneal macrophages also took up ethidium bromide when stimulated with 3 mM ATP (Fig. 6d).
Ethidium bromide is a cationic DNA dye that does not accumulate in the cytoplasm of permeabilized cells. Figure 7 shows that both mixed and purified neuronal cultures also accumulated SRB, a cationic dye that accumulates in cytoplasm of cells, when stimulated with 3 mM ATP for 10–15 min. A consistent increase in the number of SRB-stained cells was observed when both cultures were treated with the nucleotide (in cells/field: mixed cultures, control = 6.4 ± 0.4; ATP = 13.5 ± 1.2; purified neuronal cultures, control = 5.3 ± 0.5; ATP = 12.7 ± 0.3). Moreover, SRB labeling in mixed cultures occurred in neurons as their labeled processes could be easily distinguished in higher magnification micrographs (Fig. 7b). No dye uptake was observed in purified glial cultures at E8C21, but positive control cultures of macrophages also showed labeling for SRB when stimulated with ATP.
Although purified neuronal cultures accumulated ethidium bromide or sulforhodamine B when incubated with ATP, no decrease in cell viability induced by 3 mM ATP was observed in this type of culture (Fig. 8a). While incubation of mixed cultures at E7C2 with ATP decreased cell viability to 70.9 ± 6.7 % of the levels of control cultures, cell viability in purified neuronal cultures treated with ATP was 132.0 ± 6.7 % of the control levels. An increase in cell viability of 122.6 ± 3.9 % of the control levels was also observed when purified neuronal cultures were treated with 0.1 mM BzATP (Fig. 8b). Conversely, as reported elsewhere [43], incubation of these purified neuronal cultures with 1 mM glutamate decreased cell viability to 74.3 ± 4.1 % of the control levels. No significant ATP-induced decrease in cell viability was noticed in purified glial cultures (108.5 ± 9.9 %).
Besides differentiating neuroblasts and glial cells, mixed cultures at E7C2 have glial/bipolar progenitors that are still proliferating in the cultures ([16]). Since ATP did not decrease cell viability in purified neuronal cultures, the effect of ATP on the viability of retinal progenitors was assessed by labeling retinal progenitors of mixed cultures at E7C2 with [3H]-thymidine prior to 3 mM ATP or 0.1 mM BzATP treatment. As shown in Fig. 8c, no significant decrease in pre-incorporated [3H]-thymidine was observed in the mixed cultures treated with both agonists, discarding the possibility that retinal progenitors died upon nucleotide treatment.
It is well known that glutamate is excitotoxic to developing chick retinal neurons in culture [43]. In order to verify if glutamate was mediating the P2X7-induced cell death, the effect of the N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists MK 801 and DNQX, respectively, on ATP-induced decrease in retinal cell survival was investigated (Fig. 8d). While incubation of mixed retinal cultures at E7C2 with 3 mM ATP decreased cell viability to 68.7 ± 2.7 % of the control levels, cell viability decreased only to 88.8 ± 2.8 % and 86.9 ± 5.6 % of the control levels in cultures incubated with ATP plus 50 μM MK 801 and ATP plus 50 μM DNQX, respectively. No significant effect of MK 801or DNQX was observed when cultures were incubated with the antagonists alone (97.3 ± 2.5 % and 102.5 ± 4.1 % of the control levels, respectively).
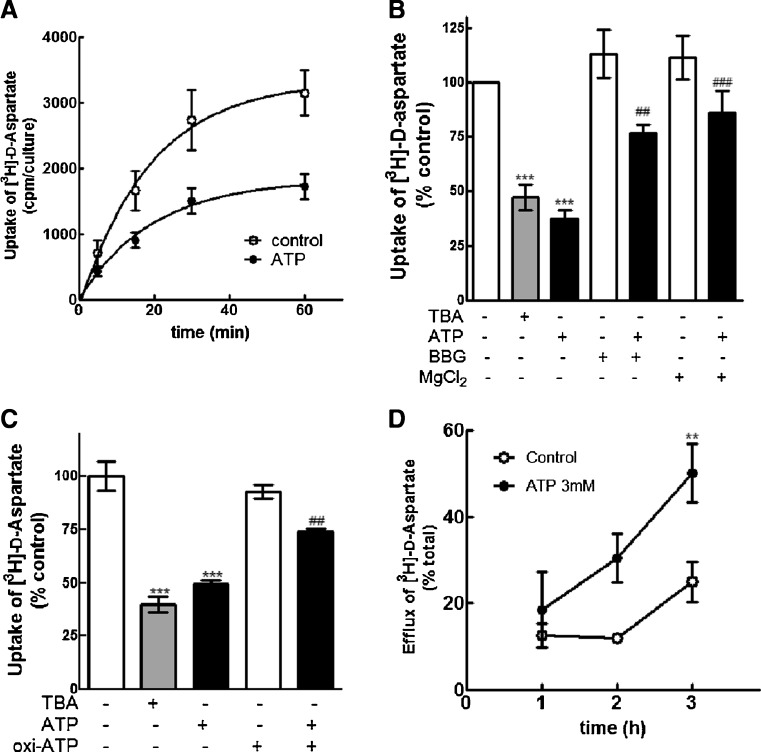
The effect of ATP on the uptake of [3H]-d-aspartate in mixed retinal cultures is shown in Fig. 9a. Cultures at E7C2 were incubated in the absence or presence of 3 mM ATP and then with 0.3 μCi/mL [3H]-d-aspartate for increasing periods of time. Cultures were then washed and the intracellular radioactivity determined as described in “Methods.” A time-dependent increase in the uptake of [3H]-d-aspartate was observed in control as well as in ATP-treated cultures. However, while the level of intracellular radioactivity attained 3,150 ± 348 cpm/culture in non-treated cultures by 60 min, a 45 % lower level of 1,721 ± 188 cpm/culture was observed in the ATP-treated cultures at the same time point (*p < 0.05).
Fig. 9.
Effect of ATP on the uptake of [3H]-d-aspartate in mixed and purified glial retinal cultures. a Effect of ATP on the uptake of [3H]-d-aspartate in mixed cultures. Cultures at E7C2 were pre-incubated for 3 h in the absence or presence of 3 mM ATP and incubated for the indicated periods with 0.3 μCi/mL of [3H]-d-aspartate. At the end of each period, cells were lysed and the intracellular radioactivity determined as described in “Methods.” c Effect of TBA, BBG, and MgCl2 on the uptake of [3H]-d-aspartate. Retinal cultures were incubated for 3 h in the presence of 100 μM TBA, 3 mM ATP in the presence or not of 10 μM BBG or 5 mM MgCl2. Cultures were then incubated for 30 min with 0.3 μCi/mL [3H]-d-aspartate, cells were lysed and intracellular radioactivity determined. c Purified glial cultures at E8C20 were incubated for 3 h in the presence or absence of 100 μM TBA or 3 mM ATP and processed for [3H]-d-aspartate uptake as described for mixed cultures. Oxidized ATP was added 2 h before ATP. d Effect of ATP on the efflux of pre-incorporated [3H]-d-aspartate. Retinal cultures were loaded with 0.3 μCi/mL [3H]-d-aspartate, washed, and incubated for the indicated periods of time in the absence or presence of 3 mM ATP. Radioactivity present in the extracellular medium was estimated as described in “Methods.” Data represent the mean ± SEM of three or four separate experiments performed in triplicate (a, b, c). In d, data represent the mean ± SEM of two or three separate experiments performed in triplicate. ***p < 0.001 and **p < 0.01, compared with control cultures. ##p < 0.01 and ###p < 0.001, compared with ATP-treated cultures
The ATP-induced decrease in [3H]-d-aspartate uptake was significantly attenuated when retinal cultures were incubated for 3 h in the presence 3 mM ATP together with 10 μM of the P2X7 antagonist BBG or 5 mM MgCl2 and then with 0.3 μCi/mL [3H]-d-aspartate for 30 min (Fig. 9b). While ATP decreased the uptake of [3H]-d-aspartate to 36.8 ± 5.2 % of the control levels, the uptake of the labeled compound was 76.7 ± 3.8 % and 85.9 ± 10.4 % of the control levels when cells were incubated with ATP + BBG and ATP + MgCl2, respectively. Moreover, a significant decrease in the uptake of [3H]-d-aspartate was observed when retinal cultures were incubated with 100 μM β-threo-benzyl-aspartate (TBA), a glutamate transporter inhibitor that preferentially blocks EAATs 1, 2, and 3 (Fig. 9b). Intracellular radioactivity decreased to 47.0 ± 5.9 % of the control levels in this condition.
The decrease in the uptake of [3H]-d-aspartate induced by 3 mM ATP that was obtained in the mixed retinal cultures was also observed in purified glial cultures at E8C20, an effect that was significantly attenuated by incubating the glial cultures in the presence of 0.2 mM oxidized ATP (Fig. 9c). While ATP decreased the uptake of [3H]-d-aspartate to 49.5 ± 3.2 % of the control levels, the intracellular content of 3H]-d-aspartate was 74.1 ± 2.3 % of the control levels when cultures were incubated with ATP plus oxidized ATP. The effect of ATP was mimicked by 100 μM TBA and intracellular radioactivity decreased to 39.8 ± 6.4 % of the control levels in this condition.
The ATP-induced decrease in the uptake of [3H]-d-aspartate in the mixed retinal cultures was concomitant with an accumulation of the labeled amino-acid in the extracellular medium (Fig. 9d). Retinal cultures at E7C2 were incubated with 0.3 μCi/mL [3H]-d-aspartate, washed, and then incubated with 3 mM ATP (Fig. 9d). At the indicated periods of time, samples of the extracellular medium were collected and the percent accumulation of extracellular radioactivity estimated as described in “Methods.” While the extracellular levels of radioactivity increased to 24.9 ± 4.6 % of the total radioactivity in the control cultures after 3 h of incubation, levels of extracellular [3H]-d-aspartate increased to 50.1 ± 6.7 % in the cultures incubated with 3 mM ATP for the same period of time.
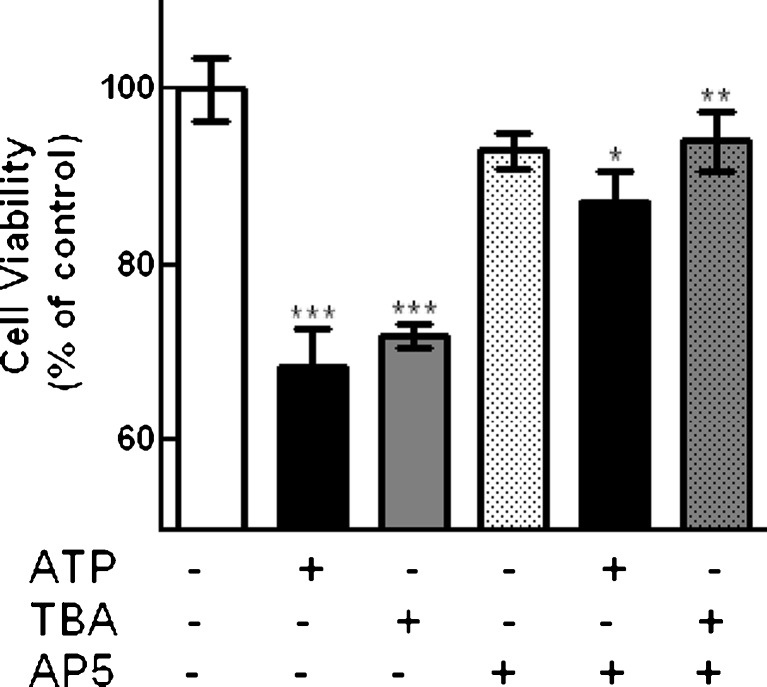
In order to verify if ATP-induced decrease in cell viability in mixed retinal cultures was associated with the inhibition of glutamate/d-aspartate transport into retinal cells, the effect of the glutamate transporter inhibitor TBA on cell viability was determined. Mixed retinal cultures at E7C2 were incubated with 3 mM ATP or 100 μM TBA (Fig. 10). Similarly to ATP that decreased viability of cultured cells to 68.3 ± 4.3 % of the control levels, TBA significantly decreased cell viability to 72.0 ± 1.4 % of the control levels. Moreover, the effects of both ATP and TBA were significantly attenuated by incubating cells in the presence of 200 μM AP5, a NMDA receptor antagonist. Cell viability levels were 87.2 ± 3.6 % and 94.1 ± 3.4 % of the control levels when incubations were performed with ATP or TBA in the presence of AP5, respectively. No significant effect of AP5 was observed when cultures were incubated with the antagonist alone (93.1 ± 2.0 % of the control levels).
Fig. 10.
Effect of TBA on cell viability in retinal cultures. Mixed cultures at E7C2 were incubated for 3 h with 3 mM ATP or 100 μM TBA, in the absence or presence of 200 μM AP5. Data represent the mean ± SEM of three independent experiments performed in triplicate or quadruplicate. ***p < 0.001, versus control; *p < 0.05, versus ATP-treated cultures; **p < 0.01, versus TBA-treated cultures
Discussion
In the present study, we show that ATP, in the millimolar range of concentration, or BzATP induced a decrease in the viability of retinal cells in culture that was dose- and time-dependent and that could be blocked by oxidized ATP or KN-62, two antagonists for the nucleotide P2X7 receptor. Moreover, ATP induced an increase in the number of TUNEL-positive cells as well as an increase in the expression of cleaved caspase-3 in the cultures that was observed only in neurons but not in glial cells. Since no increase in extracellular LDH release was observed when cultures were treated with 3 mM ATP for 3 h (data not shown), these data strongly suggest that extracellular ATP can induce apoptosis of developing chick retinal neurons in culture. They also suggest that ATP-mediated neuronal apoptosis occurs through the activation of P2X7 receptors. This idea is supported by other studies showing that activation of these receptors induces the death of specific neuronal populations in the adult or developing mammalian retina [32, 34, 35].
In several cell types, sustained activation of P2X7 receptors results in the formation of membrane non-selective pores permeable to molecules up to 900 Da [38, 41, 42, 44] that can lead to cell death [41, 45–47]. Our data show that ATP or BzATP induces the uptake of the cationic dyes ethidium bromide or SRB in cultured chick retinal cells. While the effect of BzATP was completely blocked by BBG and oxidized ATP, suggesting that it was mediated by activation of P2X7 receptors, the uptake of SRB induced by ATP could be easily visualized in retinal neurons in cultures containing both neurons and glial cells (mixed cultures). These results suggest that retinal neurons express P2X7 receptors that upon stimulation with ATP induce the uptake of cationic dyes. Accordingly, ATP was able to induce an increase in the number of ethidium bromide or SRB labeled cells in purified cultures of neurons.
In contrast to mixed and purified neuronal cultures, no ATP-induced uptake of fluorescent dyes was observed in purified glial cultures, suggesting that cultured chick embryo Müller glia did not express P2X7 receptors. This possibility, however, can be ruled out since ATP significantly reduced the uptake of [3H]-d-aspartate in purified glial cultures, an effect that was blocked by oxiATP, suggesting that the lack of dye uptake in chick embryo Müller cells might have explanations other than absence of P2X7 receptor expression. Similar evidence for expression of P2X7 receptors but no ATP-dependent uptake of fluorescent dyes was obtained previously by Pannicke et al. [29] in human Müller cells.
Zhang et al. [34] reported that activation of P2X7 receptors induces a time-dependent death of ganglion cells in rat retinal cultures. Moreover, Sugiyama et al. [48] have shown that P2X7 receptor antagonists are able to inhibit the death of rat retinal neurons in purified cultures. Here, we showed that a decrease in cell viability induced by ATP or BzATP was obtained only in mixed cultures containing glial cells. When purified neuronal cultures were used, ATP and BzATP induced a consistent increase in cell viability, suggesting that activation of P2X7 receptors in retinal neurons in the absence of glial cells might be neuroprotective. These observations are in good agreement with recent findings showing that activation of P2X7 receptors is neuroprotective against PI3K/Akt inhibition in purified cultures of rat cerebellar granule cells [23, 24] and the discrepancies between our findings and those mentioned before could be due to differences in the methodological conditions used. Zhang et al. observed significant levels of ganglion cell death when freshly dissociated retinal cultures were incubated with BzATP. In the present work, we used mixed and purified neuronal cultures 2 days after they were established, a condition devoid of ganglion cells due to their axotomy. Furthermore, Sugiyama et al. observed that ATP and BzATP induced neuronal death when purified rat retinal neuronal cultures were incubated with agonists for 24 h, a much longer incubation time than the period used in the present study.
The lack of nucleotide-induced decrease in cell survival in the neuronal cultures used here could have other explanations. One possibility would be that nucleotides affected retinal glial cells or glial progenitors that are present in mixed [16] but not in purified neuronal cultures [37]. This possibility, however, can be ruled out since no decrease in the culture levels of radioactivity was observed when retinal progenitors were labeled with [3H]-thymidine and then stimulated with ATP or BzATP, suggesting that labeled progenitors were still present at the end of incubation with agonists. Moreover, expression of cleaved caspase-3 was not detected in 2 M6+ glial cells in mixed cultures, and no decrease in cell viability was observed when purified glial cell cultures were stimulated with ATP, reinforcing the idea that either proliferating or post-mitotic glial cells are not affected by ATP in terms of cell death.
Activation of P2X7 receptors in rat and human retinal Müller cells induces the release of neurotransmitter such as GABA and glutamate by a reduction in Na+ gradient and a concomitant inhibition of the neurotransmitter uptake [29, 49]. Another possibility to explain the lack of ATP- or BzATP-mediated cell death in purified neuronal cultures would be that nucleotide-induced decrease in retinal neuronal viability was indirect, mediated by factors released from glial cells. In this scenario, nucleotide-induced neuronal death would occur only in mixed, glial containing cultures, but not in purified neuronal cultures, although neurons in this type of culture take up fluorescent dyes when stimulated with nucleotides for 10–15 min, an observation that suggests that retinal neurons might express P2X7 receptors. Glutamate is a particular interesting candidate to mediate ATP-induced decrease in retinal neuronal survival since, as in other areas of the CNS, it is excitotoxic in the chick embryo retina, decreasing the viability of retinal neurons in culture through activation of NMDA or non-NMDA ionotropic receptors [43]. Accordingly, the present results showed that the NMDA and non-NMDA receptor antagonists MK801 and DNQX, at a concentration of 50 μM, induced a significant attenuation of ATP-induced decrease in the viability of cultured retinal cells, suggesting that P2X7 receptor-mediated neuronal death involved activation of ionotropic glutamate receptors. In good agreement with this hypothesis is our observation that direct stimulation of purified neuronal cultures with glutamate induced a significant decrease in neuronal viability.
Incubation of mixed retinal cultures with 3 mM ATP induced a substantial decrease the amount of [3H]-d-aspartate taken up by cultured retinal cells, an effect that was blocked by the P2X7 receptor antagonist BBG and by increasing the concentration of Mg2+ ions in the Hanks’ solution. A corresponding increase in the content of this labeled amino-acid in the extracellular medium was also observed in ATP-treated cultures. These results suggest that activation of P2X7 by ATP can inhibit the uptake of glutamate in our cultures and induce the accumulation of this amino-acid in the extracellular medium. Previous evidence showed that Bz-ATP inhibits the eletrogenic uptake of glutamate in human Müller cells [29]. Thus, it is reasonable to suggest that ATP-induced neuronal death during development of the chick retina could involve the efflux of glutamate mediated by P2X7-induced inhibition of glutamate/d-aspartate transporter expressed in glial Müller cells. In good agreement with this hypothesis is our finding that activation of P2X7 receptors induced a significant decrease in [3H]-d-aspartate uptake in purified glial cultures, an effect that was mimicked by TBA, a preferential inhibitor of EAAT1-3 that does not inhibit glutamate-induced release of [3H]-d-aspartate from chick retinal neurons in culture [50]. Moreover, incubation of mixed retinal cultures with the glutamate transporter inhibitor induced a significant decrease in the viability of cultured cells, an effect that was attenuated by the NMDA receptor antagonist AP5, suggesting that inhibition of glutamate/d-aspartate uptake into retinal cells can lead to activation of NMDA receptors and induce cell death in the cultures. Since GLAST is a major sodium-dependent glutamate transporter expressed by Müller cells in several species [51, 52], this protein is a good candidate to be regulated by P2X7 receptors in the chick retina. This point deserves to be further explored.
Glutamate-induced increase in intracellular calcium rises between embryonic days 7 and 9 and is well correlated both temporally and spatially with the decline in mitotic activity and increase in cell death in the inner portion of the developing chick embryo retina [53]. In this tissue, at least two waves of natural cell death were described so far [1, 4]. While the first wave occurs very early, between embryonic stages E4 and E6, the second rise in cell death occurs between stages E8 and E12, mainly at the inner nuclear and ganglion cell layers of the retina [4]. Our present results showed that ATP-induced decrease in neuronal viability occurs in the same discrete period of retinal development that ranges from E8 to E10, no matter if retinal cells develop “in vivo” or “in vitro” during this period. This differential effect of ATP could have several explanations including changes in the expression of P2X7 receptors, NMDA receptor subunits, or glutamate transporters during retinal development. One interesting hypothesis would be that the transient nucleotide-induced cell death observed in the present study occurred only at stages or conditions where the uptake of glutamate that can be affected by activation of P2X7 receptors was the predominant mechanism of clearance of extracellular glutamate in the developing retina. It is known that malfunction of glutamate transport into glial cells leads to increased levels of extracellular glutamate that can be toxic to retinal neurons through over-stimulation of glutamate ionotropic receptors [52]. This hypothesis, as well as the other possibilities mentioned, is worthy to be investigated in the near future.
Acknowledgments
We would like to thank Maria Leite Eduardo and Sarah A. Rodrigues for technical assistance. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Programa de Núcleos de Excelência (PRONEX-CNPq), POM/IOC (Fiocruz), Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), Pró-reitoria de Pesquisa, Pós-graduação e Inovação (Proppi-UFF) and Instituto Nacional para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica, Conselho Nacional de Desenvolvimento Científico e Tecnológico/MCT (INCT-INPeTAm/CNPq/MCT), Brazil. Roxana M. Anccasi is the recipient of a fellowship from CAPES/CNPq-Brazil.
References
- 1.Frade JM, Bovolenta P, Martínez-Morales JR, Arribas A, Barbas JA, Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124:3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- 2.Mayordomo R, Valenciano AI, de la Rosa EJ, Hallböök F. Generation of retinal ganglion cells is modulated by caspase-dependent programmed cell death. Eur J Neurosci. 2003;7:1744–1750. doi: 10.1046/j.1460-9568.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 3.Díaz B, Pimentel B, de Pablo F, de la Rosa EJ. Apoptotic cell death of proliferating neuroepithelial cells in the embryonic retina is prevented by insulin. Eur J Neurosci. 1999;11:1624–1632. doi: 10.1046/j.1460-9568.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- 4.Chavarria T, Valenciano AI, Mayodormo R, Egea J, Comella JX, Haällboök F, de Pablo F, de la Rosa EJ. Differential, age-dependent ERK and PI3K activation by insulin acting as a survival factor in early retinal development. Dev Neurobiol. 2007;67:1777–1788. doi: 10.1002/dneu.20554. [DOI] [PubMed] [Google Scholar]
- 5.Valenciano AI, Boya P, De La Rosa EJ. Early neural cell death: numbers and cues from the developing neuroretina. Intl J Dev Biol. 2009;53(8–10):1515–1528. doi: 10.1387/ijdb.072446av. [DOI] [PubMed] [Google Scholar]
- 6.Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:32–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 7.Khan AJ. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Devl Biol. 1974;38:30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- 8.Prada C, Puga J, Pérez-Méndez L, López R, Ramírez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann H. Purinergic signaling in neural development. Sem Cell Devl Biology. 2011;22:194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol. 1996;493:855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugioka M, Zhou WL, Hoffmann HD, Yamashita M. Ca2+ mobilization and capacitive Ca2+ entry regulate DNA synthesis in cultured chick retinal neuroepithelial cells. Intl J Dev Neurosci. 1999;17(3):163–172. doi: 10.1016/S0736-5748(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 12.Pearson RA, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Sanches G, Alencar LS, Ventura ALM. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Intl J Dev Neurosci. 2002;20(1):21–27. doi: 10.1016/S0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 15.Sholl-Franco A, Fragel-Madeira L, Macama ACCM, Linden R, Ventura ALM. ATP controls cell cycle and induces proliferation in the mouse developing retina. Intl J Dev Neurosci. 2010;28:63–73. doi: 10.1016/j.ijdevneu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.França GR, Freitas RC, Ventura ALM. ATP-induced proliferation of developing retinal cells: regulation by factors released from postmitotic cells in culture. Intl J Dev Neurosci. 2007;25(5):283–291. doi: 10.1016/j.ijdevneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Nunes PH, Calaza KC, Albuquerque LM, Fragel-Madeira L, Sholl-Franco A, Ventura ALM. Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. Intl J Dev Neurosci. 2007;25(8):499–508. doi: 10.1016/j.ijdevneu.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Ornelas IM, Ventura ALM. Involvement of the PI3K/AKT pathway in ATP-induced proliferation of developing retinal cells in culture. Intl J Dev Neurosci. 2010;28:503–511. doi: 10.1016/j.ijdevneu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neurosci. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann H. Nucleotide signaling in nervous system development. Eur J Physiol. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Hernandez M, del Puerto PA, Diaz-Hernandez JI, Diez-Zaera M, Lucas JJ, Garrido JJ, Miras-Portugal MT. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci. 2008;121:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Villafuertes R, del Puerto PA, Diaz-Hernandez M, Bustillo D, Diaz-Hernandez JI, Huerta PG, Artalejo AR, Garrido JJ, Miras-Portugal MT. Ca2+/calmodulin-dependent kinase II signalling cascade mediates P2X7 receptor-dependent inhibition of neuritogenesis in neuroblastoma cells. FEBS J. 2009;276:5307–5325. doi: 10.1111/j.1742-4658.2009.07228.x. [DOI] [PubMed] [Google Scholar]
- 23.Ortega F, Perez-Sen R, Delicado EG, Miras-Portugal MT. P2X7 nucleotide receptor is coupled to gsk-3 inhibition and neuroprotection in cerebellar granule neurons. Neurotox Res. 2009;15:193–204. doi: 10.1007/s12640-009-9020-6. [DOI] [PubMed] [Google Scholar]
- 24.Ortega F, Perez-Sen R, Morente V, Delicado EG, Miras-Portugal MT. NMDA and BDNF receptors converge on GSK3 phosphorylation and cooperate to promote survival in cerebellar granule neurons. Cell Mol Life Sci. 2010;67:1723–1733. doi: 10.1007/s00018-010-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109:846–857. doi: 10.1111/j.1471-4159.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheewatrakoopong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 27.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, Di Virgilio F. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 28.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Pannicke T, Fischer W, Biedermann B, Schadlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Muller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brändle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res. 1998;62(1):106–109. doi: 10.1016/S0169-328X(98)00254-X. [DOI] [PubMed] [Google Scholar]
- 31.Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E. ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci. 2004;24(39):8577–8583. doi: 10.1523/JNEUROSCI.2812-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puthussery T, Fletcher E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J Comp Neurol. 2009;513:430–440. doi: 10.1002/cne.21964. [DOI] [PubMed] [Google Scholar]
- 33.Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol. 2003;459(3):267–277. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- 35.Resta V, Novelli E, Di Virgilio F, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- 36.Loiola EC, Ventura ALM. Release of ATP from avian Müller glia cells in culture. Neurochem Intl. 2011;58:414–422. doi: 10.1016/j.neuint.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984;99:1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann T. Rapid colorimetric assay for celullar growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of the microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. Macrophages possess probenecid-inhibitable organic anion transporters that remove fluorescent dyes from the cytoplasmic matrix. J Cell Biol. 1987;105:2695–2702. doi: 10.1083/jcb.105.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira IL, Duarte CB, Carvalho AP. Ca2+ influx through glutamate receptor-associated channels in retina cells correlates with neuronal cell death. Eur J Pharmacol. 1996;302:153–162. doi: 10.1016/0014-2999(96)00044-1. [DOI] [PubMed] [Google Scholar]
- 44.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 45.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/S0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 46.Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Inv Ophthalmol Vis Sci. 2010;51:3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- 49.Neal MJ, Cunningham JR, Dent Z. Modulation of extracellular GABA levels in the retina by activation of glial P2X-purinoceptors. Br J Pharmacol. 1998;124:317–322. doi: 10.1038/sj.bjp.0701841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stutz B, Yamasaki EN, de Mello MC, de Mello FG. Exchange of extracellular l-glutamate by intracellular d-aspartate: the main mechanism of d-aspartate release in the avian retina. Neurochem Intl. 2011;58:767–775. doi: 10.1016/j.neuint.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Reis RAM, Ventura ALM, Schitine CS, de Mello MC, de Mello FG. Müller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res. 2008;33:1466–1474. doi: 10.1007/s11064-008-9604-1. [DOI] [PubMed] [Google Scholar]
- 52.Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, Wiedermann P, Albrecht J, Reichenbach A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Intl. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Sugioka M, Fukuda Y, Yamashita M. Development of glutamate-induced intracellular Ca2+ rise in the embryonic chick retina. J Neurobiol. 1998;34:113–125. doi: 10.1002/(SICI)1097-4695(19980205)34:2<113::AID-NEU2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]