Abstract
Mast cell degranulation affects many conditions, e.g., asthma and urticaria. We explored the potential role of the P2Y14 receptor (P2Y14R) and other P2Y subtypes in degranulation of human LAD2 mast cells. All eight P2YRs were expressed at variable levels in LAD2 cells (quantitative real-time RT-PCR). Gene expression levels of ADP receptors, P2Y1R, P2Y12R, and P2Y13R, were similar, and P2Y11R and P2Y4R were highly expressed at 5.8- and 3.8-fold of P2Y1R, respectively. Least expressed P2Y2R was 40-fold lower than P2Y1R, and P2Y6R and P2Y14R were ≤50 % of P2Y1R. None of the native P2YR agonists alone induced β-hexosaminidase (β-Hex) release, but some nucleotides significantly enhanced β-Hex release induced by C3a or antigen, with a rank efficacy order of ATP > UDPG ≥ ADP >> UDP, UTP. Although P2Y11R and P2Y4R are highly expressed, they did not seem to play a major role in degranulation as neither P2Y4R agonist UTP nor P2Y11R agonists ATPγS and NF546 had a substantial effect. P2Y1R-selective agonist MRS2365 enhanced degranulation, but ~1,000-fold weaker compared to its P2Y1R potency, and the effect of P2Y6R agonist 3-phenacyl-UDP was negligible. The enhancement by ADP and ATP appears mediated via multiple receptors. Both UDPG and a synthetic agonist of the P2Y14R, MRS2690, enhanced C3a-induced β-Hex release, which was inhibited by a P2Y14R antagonist, specific P2Y14R siRNA and pertussis toxin, suggesting a role of P2Y14R activation in promoting human mast cell degranulation.
Keywords: P2Y, Mast cells, Uracil nucleotide, Degranulation, GPCR, G protein-coupled receptors
Introduction
Mast cells are immune cells derived from hematopoietic precursors secreting prestored allergic mediators, such as histamine and tryptase. Allergic mediators released via degranulation are the major cause of allergy [1, 2]. The high affinity receptor for the Fc region of immunoglobulin E (IgE) (FcεRI) has been thought to be the major receptor mediating degranulation, but a growing number of other targets including ligand-gated ion channels (LGICs) and G-protein-coupled receptors (GPCRs) have recently been found to mediate degranulation [3–5]. The ability of activation of certain receptors to enhance antigen-mediated degranulation led to the suggestion that under some conditions; these receptors have the capacity to contribute to allergic responses in vivo [1].
The class of receptors for extracellular nucleotides comprises eight GPCRs (P2Y1,2,4,6,11,12,13,14) and seven LGICs (P2X1-7), many of which being expressed in blood cells [6]. The roles of P2 receptors in mast cell biology have recently been reviewed [7]. The expression and some functions of human P2X receptors have recently been characterized using both LAD2 mast cells and human lung mast cells [8]. Nucleotides, especially adenosine 5′-diphosphate (ADP) and adenosine 5′-triphosphate (ATP), have been studied previously in the context of mast cell degranulation [9, 10], but specific receptor subtypes mediating these functions have not been systematically explored. We recently demonstrated in RBL-2H3 basophillic cells, a degranulation model of rat mast cells, that P2Y13R and P2Y14R are involved in degranulation [11, 12]. However, the role of various P2YRs in human mast cells has been largely unexplored, although isolated studies indicate the possible involvement of certain P2YRs [10, 13]. Prominent species differences, which complicate this study, have been found both in P2YR function [14] and in mast cell function [15].
It is known that nucleotides may have different roles in different species. For example, ATP is an agonist for rat P2Y4R while an antagonist for human P2Y4R [14]. Furthermore, the P2Y gene expression profile is also different in various species. For example, the P2Y11R gene does not exist in rats and mice, although it is in human. We found previously that P2Y1R, P2Y13R, and P2Y14R are highly expressed in rat mast cells [11, 12]. It was found in the present study that P2Y11R and P2Y12R are also found highly expressed in human mast cells in addition to P2Y1R, P2Y13R, and P2Y14R. The roles of these receptors in degranulation of human LAD2 mast cells were subsequently explored by measuring release of β-Hex (β-hexosaminidase) induced by both antigen and C3a (protein formed by cleavage of complement component 3).
Materials and methods
Materials
ADP, ATP, uridine 5′-diphosphate (UDP), uridine 5′-triphosphate (UTP), uridine-5′-diphosphoglucose (UDP-glucose, UDPG), pertussis toxin (PTX), 8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl)oxy]phenyl]-1,3-dipropylxanthine (XAC), p-nitrophenyl-N-acetyl-β-d-glucosaminide, and NP-BSA (4-hydroxy-3-nitrophenylacetyl hapten amide conjugated to bovine serum albumin through lysine residues), Triton X-100 and anti-NP antibody were obtained from Sigma (St. Louis, MO, USA). MRS2211 (2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde disodium salt); MRS2365 ([[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl]diphosphoric acid mono ester trisodium salt), AR-C66096 (2-(propylthio)adenosine-5′-O-(β,γ-difluoromethylene)triphosphate tetrasodium salt), MRS2690 (2-thiouridine-5′-diphosphoglucose), NF340 (4,4′-(carbonylbis(imino-3,1-(4-methyl-phenylene)carbonylimino))bis(naphthalene-2,6-disulfonic acid) tetrasodium salt), NF546 (4,4′-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)carbonylimino))-bis(1,3-xylene-α,α′-diphosphonic acid tetrasodium salt), 3-phenacyl-UDP and MRS2500 ((1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt) were from Tocris (St. Louis, MO, USA). TaqMan Gene Expression Assays, High-Capacity cDNA Reverse Transcription Kit and were from Applied Biosystems (Foster City, CA, USA). C3a (catalog number 204881) was from EMD Biosciences (La Jolla, CA, USA). 4-(4-(Piperidin-4-yl)phenyl)-7-(4-(trifluoromethyl)phenyl)-2-naphthoic acid (mesylate salt form) (PPTN) was synthesized at NIDDK, National Institutes of Health as previously described [32, 33]. All other reagents were from standard sources and are of analytical grade.
Cell culture
The stem cell factor-dependent human mast cell line, LAD2, derived from bone marrow aspirates of a patient with sarcoma/leukemia, was provided by Drs. A. S. Kirshenbaum and D. D. Metcalfe of NIAID, National Institutes of Health. LAD2 cells were cultured using standard protocols [16]. Cells were cultured in serum-free media (StemPro-34; Life Technologies, Grand Island, NY, USA) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin, 50 μg/ml streptomycin (complete SFM), 100 ng/ml recombinant human (rh) SCF, with or without 100 ng/ml rhIL-6, and 30 ng/ml rhIL-3 (first week only) (Peprotech, Rocky Hill, NJ, USA).
Detection of gene expression level using real-time quantitative RT-PCR
Total cellular RNA was isolated from LAD2 cells using an RNA isolation kit (RNeasy, Qiagen, Valencia, CA, USA) and was reversed-transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The cDNA was then amplified with gene-specific primers for P2Y and GAPDH on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The TaqMan gene expression assays were from Applied Biosystems (Foster City, CA, USA). Quantitative analysis of data was performed by using the ΔΔCt method [17]. Values were normalized to GAPDH and were expressed as relative expression levels.
Measurement of the release of β-Hex in LAD2 cells
β-Hex release measured following crosslinking of FcεRI via IgE-antigen complexes
We measured the release of β-Hex, a granule-associated protein that parallels histamine release and is an indicator of degranulation of LAD2 cells, as previously described [11, 16]. In brief, LAD2 cells were split to 96-well plates (104 cells/well) and incubated overnight with 0.1 μg/ml anti-NP-specific IgE antibody. Cells were washed twice and then stimulated with the antigen NP-BSA for 30 min. P2YR agonists were added 20 min before NP-BSA. Release of β-Hex was measured in the medium and in cell lysates (in 0.1 % Triton X-100) by a colorimetric assay. Aliquots (20 μl) of samples were incubated with substrate (20 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide) at 37 °C in 0.1 M sodium citrate buffer (pH 4.5) for 1 h. The product, p-nitrophenol, was converted to the chromophore, 4-nitrophenoxide, by addition of 200 μl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer. Absorbance was read at 405 nm using a spectrophotometer. Results are reported as the percentage of intracellular β-Hex that was released into the medium.
C3a-induced β-Hex release
In addition to antigen, C3a, the agonist of Gi-coupled C3a receptors, was also shown to potently induce β-Hex release [3, 4]. LAD2 cells were washed twice with assay buffer and then split to 96-well plate (104 cells each well in 100 μl assay buffer). Various concentrations of C3a were incubated with cells for 30 min to stimulate β-Hex release in the presence or absence of various nucleotides, which were added 20 min in advance. After incubation for 30 min, cells were spun down. β-Hex release was measured in the medium and in cell lysates (in 0.1 % Triton X-100) by a colorimetric assay. Absorbance was read at 405 nm using a spectrophotometer (SpectraMax M5, Molecular Devices, CA, USA). To test the effect of P2Y14R siRNA (small interfering RNA) on C3a-induced β-Hex release, the siRNA was predesigned at Applied Biosystems. Transfection of siRNA was performed using Lipofectamine 2000 as previously described [12].
Data analysis
Data were analyzed with Prism 5 (GraphPad, San Diego, CA, USA). Statistical analyses were performed with ANOVA or t test where appropriate with P < 0.05 being considered significant.
Results
Effects of various nucleotides on antigen and C3a-mediated degranulation of LAD2 mast cells
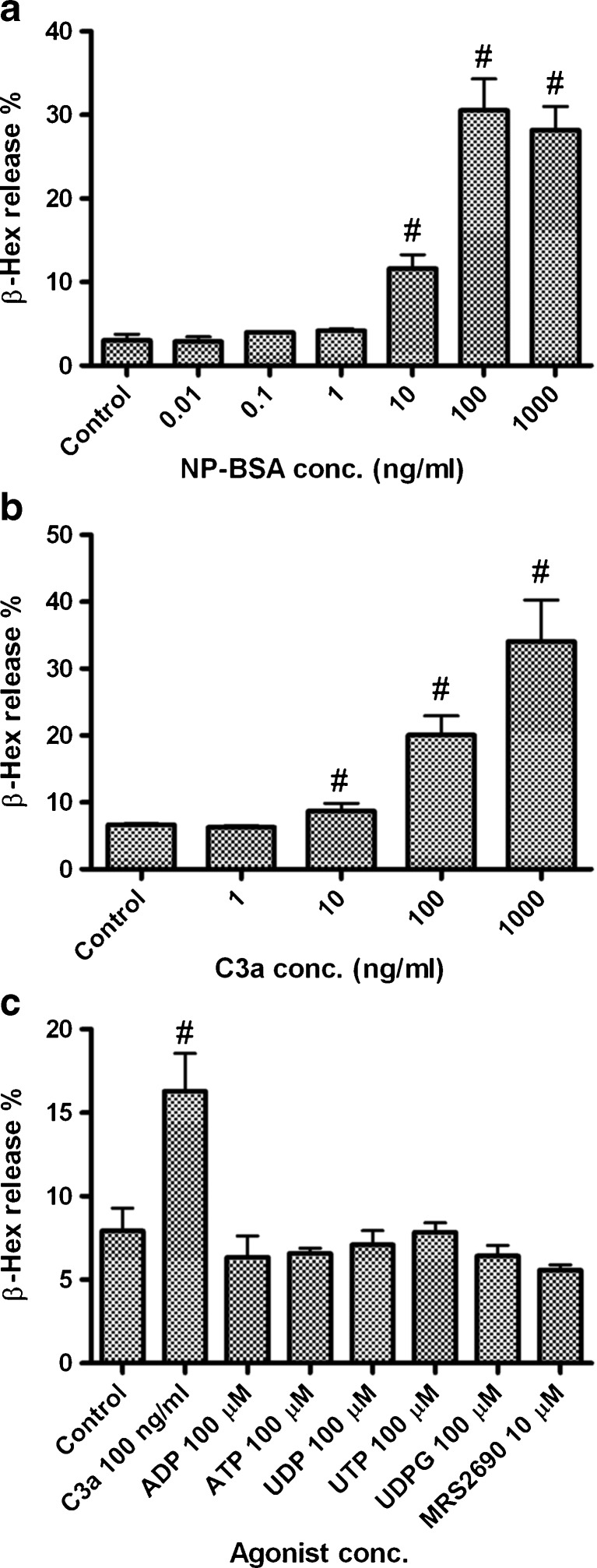
We first demonstrated antigen-induced release of β-Hex in LAD2 mast cells, which is mediated via FcεRI. Figure 1a shows that the antigen NP-BSA concentration-dependently induced the release of β-Hex in LAD2 cells primed overnight with anti-NP-specific IgE antibody (100 ng/ml). The maximal release was 30.5 ± 4.0 %. Figure 1b shows that C3a, at concentrations 10 ng/ml or higher, induced a significant amount of β-Hex release from LAD2 cells (P < 0.05, compared with control), with maximal release of 34.1 ± 6.2 % at 1 μg/ml. Figure 1c shows that, unlike C3a, none of the nucleotides alone induced a substantial release of β-Hex.
Fig. 1.
β-Hex release in LAD2 human mast cells induced by antigen NP-BSA (a), C3a (b), and nucleotides (c). For antigen NP-BSA-induced β-Hex release, LAD2 cells were split to 96-well plates (104 cells/well) and primed overnight with 0.1 μg/ml anti-NP-specific IgE antibody. Cells were washed twice and then stimulated with increasing concentrations of antigen NP-BSA for 30 min. β-Hex release was measured in the medium and in cell lysates (in 0.1 % Triton X-100) by a colorimetric assay. β-Hex release induced by C3a and nucleotides was measured in the same way without prior priming with antibody. Results are reported as the percentage of intracellular β-Hex that was released into the medium and are expressed as mean ± SE from at least of three separate determinations performed in triplicate. #P < 0.05, compared with control
We then examined the effects of various nucleotides, both native P2YR agonists and more selective synthetic derivatives, on antigen and C3a-mediated degranulation of LAD2 cells. We have shown previously that although ADP [11] or UDPG [12] alone did not induce β-Hex release in RBL-2H3 cells, they enhanced antigen-induced β-Hex release. It was not clear if the same phenomenon occurs in human LAD2 cells, as prominent differences have been found among various mast cell lines and in different species with respect to nucleotide effects. Thus, in the present study, we examined the occurrence and possible roles of all of the P2YRs in LAD2 cells.
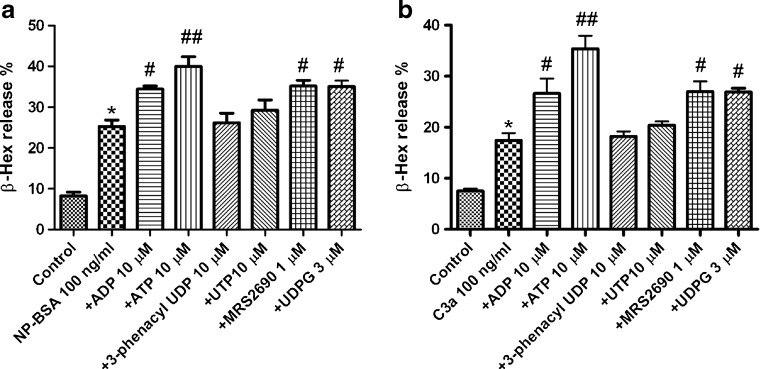
Figure 2a shows that ADP (10 μM), ATP (10 μM), UDPG (3 μM), and the newly synthesized P2Y14R agonist, MRS2690 (1 μM), but not the selective P2Y6R agonist 3-phenacyl-UDP (10 μM) or the agonist for P2Y2R and P2Y4R, UTP (10 μM), significantly enhanced antigen-induced β-Hex release. The increases induced by ADP, ATP, MRS2690, and UDPG over antigen alone were 35.6, 58.1, 39.1, and 35.1 %, respectively. Similar results were obtained on C3a-induced β-Hex release (Fig. 2b). ADP, ATP, and MRS2690 caused enhancement of 52.9, 103, and 54.9 %, respectively. UDPG (3 μM) also induced a significant enhancement (55.0 %). 3-Phenacyl-UDP and UTP did not cause a significant enhancement.
Fig. 2.
Effects of various nucleotides on β-Hex release induced by antigen (a) and C3a (b). Nucleotides were preincubated for 20 min with cells before the addition of NP-BSA (100 ng/ml) or C3a (100 ng/ml). The mixture was incubated for an additional 30 min. Results are reported as the percentage of intracellular β-Hex that was released into the medium and are expressed as mean ± SE from three to five separate determinations performed in triplicate. *P < 0.05 compared with control. #P < 0.05; ##P < 0.01, compared with NP-BSA group or C3a group. ADP is an agonist of P2Y1R, P2Y12R, and P2Y13R. ATP is an agonist for P2Y1R, P2Y2R, and P2Y11R (and P2X receptors as well). UTP is an agonist for P2Y2R and P2Y4R. 3-Phenacyl-UDP is a P2Y6R agonist, and UDPG and MRS2690 are P2Y14R agonists
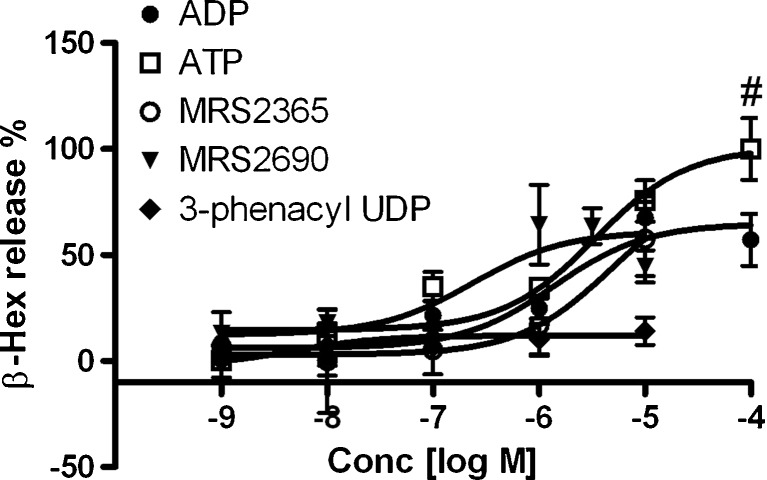
We next examined both the potency and efficacy of ADP, ATP, and MRS2690 in enhancing C3a-induced release of β-Hex. Figure 3 shows that ATP was more efficacious, although less potent, than ADP and MRS2690. The EC50 values of ADP, MRS2365, ATP, and MRS2690 are 1260 ± 380, 5960 ± 870, 3370 ± 700, and 229 ± 87 nM, respectively. The P2Y6R-selective agonist 3-phenacyl-UDP did not affect C3a-induced β-Hex release at a range of concentrations tested. The potency of MRS2365 in enhancing C3a-induced β-Hex release was much weaker than its affinity or potency at the human P2Y1R, which was greater by a factor of ~1,000 [11, 18]. Thus, the action of MRS2365 at this high concentration is not indicative of a P2Y1R-mediated effect. Therefore, these experiments suggested the involvement of the P2Y14R but not P2Y1R.
Fig. 3.
Concentration–response of nucleotides on C3a-induced β-Hex release from LAD2 cells. The EC50 values are listed in the text. Results are reported as the percentage of intracellular β-Hex that was released into the medium and are expressed as mean ± SE from at least of three separate determinations performed in triplicate. The slopes of these concentration–response curves are 0.4–0.8. #P < 0.05, compared with efficacy of ADP at 100 μM
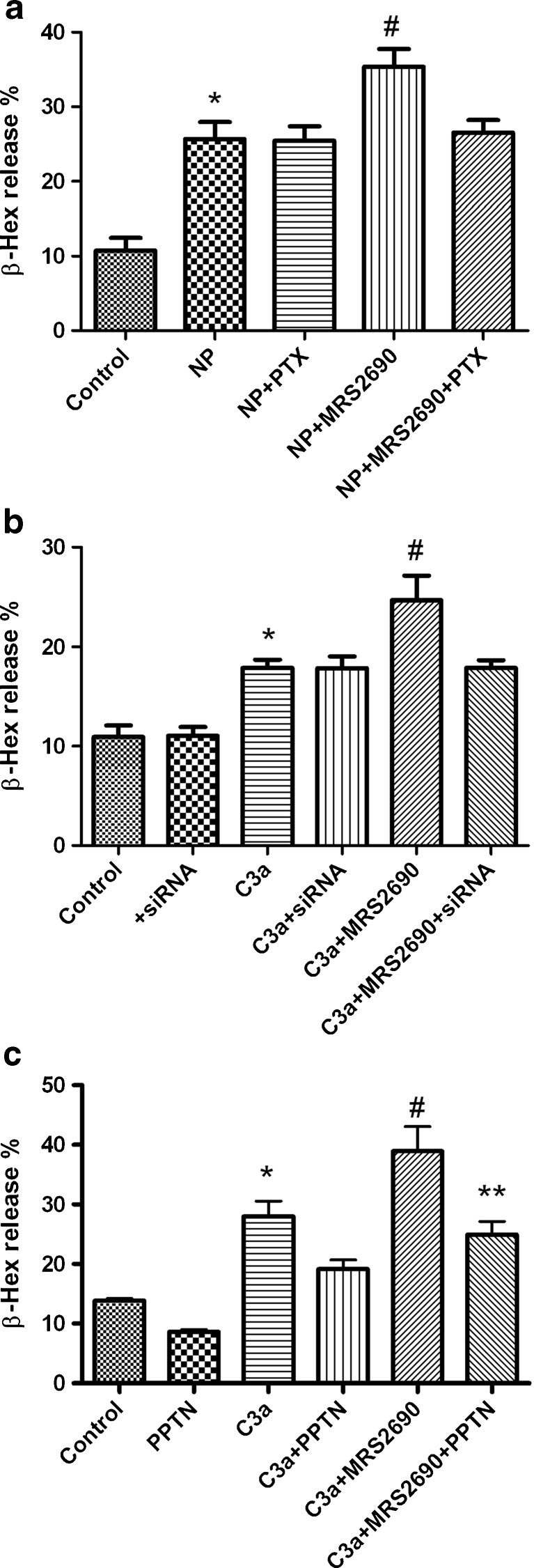
We further examined the possible receptor signaling mediating the effects of these nucleotides. Figure 4a shows that pertussis toxin (PTX) treatment (100 ng/ml) did not affect NP-induced β-Hex release but significantly blunted the enhancement of β-Hex release induced by MRS2690 (1 μM), consistent with a role of the Gi-coupled P2Y14R.
Fig. 4.
Effects of PTX, P2Y14R siRNA and a novel P2Y14R antagonist PPTN [32] on P2Y14R-mediated enhancement of β-Hex release. a LAD2 cells were split to 96-well plates (104 cells/well) and incubated overnight with 100 ng/ml anti-NP-specific IgE antibody and 100 ng/ml pertussis toxin (PTX). Cells were washed twice and then incubated with MRS2690 for 20 min followed by stimulation with the antigen NP-BSA (100 ng/ml) for 30 min. *P < 0.05, compared with control group; #P < 0.05, compare with NP, NP + PTX and NP + MRS2690 + PTX groups. b Effect of P2Y14R siRNA on C3a induced β-Hex release. The siRNA was predesigned at Applied Biosystems. Transfection of siRNA was performed using Lipofectamine 2000 as previously described [12]. *P < 0.05, compared with control group; #P < 0.05, compared with C3a or C3a + MRS2690 + siRNA group. siRNA, P2Y14R siRNA. c Effect of a novel P2Y14R antagonist PPTN on MRS2690-induced enhancement of C3a-induced β-Hex release. PPTN, 10 μM; C3a, 100 ng/ml; MRS2690, 1 μM. PPTN was added 20 min before the addition of C3a and MRS2690. *P < 0.05, compared with control. #P < 0.05, compared with C3a group. **P < 0.05, compared with C3a + MRS2690 group
Figure 4b shows that the P2Y14R siRNA significantly diminished MRS2690-caused enhancement of C3a-induced β-Hex release. Figure 4c shows that a novel, potent P2Y14R antagonist, PPTN [32], significantly inhibited MRS2690-induced enhancement of C3a-induced β-Hex release. PPTN did not significantly affect the effect of C3a (P > 0.05). The β-Hex release in the C3a + MRS2690 group was significantly different from that in C3a + MRS2690 + PPTN or C3a group (P < 0.05).
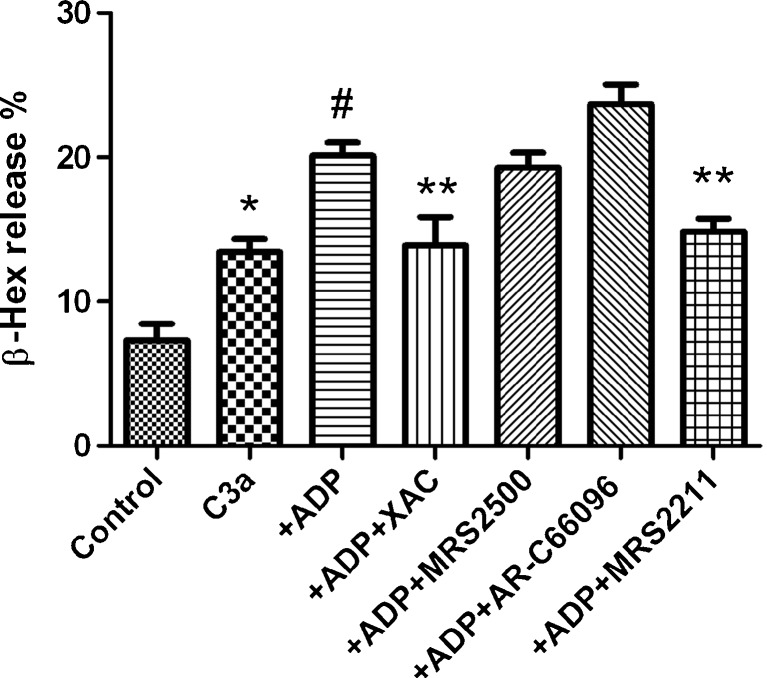
It has been suggested previously that the P2Y13R, but not the P2Y1R or P2Y12R, was involved in the ADP-induced release of β-Hex in RBL-2H3 cells [11]. In the present study, as shown in Fig. 3, the endogenous agonist ADP is more potent than the selective P2Y1R agonist MRS2365 in enhancing C3a-induced β-Hex release, further suggesting that the enhancing effect is probably not via the P2Y1R. However, it is not clear if the P2Y12R or P2Y13R or other receptors are responsible for the enhancement, as both are expressed in LAD2 cells. Figure 5 shows that the modest enhancement of β-Hex release induced by ADP was not affected by the P2Y1R agonist MRS2500 but was partially diminished by a selective P2Y13R antagonist MRS2211 (10 μM). However, a nucleotide antagonist of the P2Y12R, AR-C66096, tended to further enhance ADP-induced enhancement, although the enhancement was not statistically significant compared with the ADP group (P > 0.05). A nonselective adenosine receptor (AR) antagonist XAC completely blocked ADP-induced enhancement. Thus, it seems that the modest enhancement induced by ADP occurred possibly via both ARs and P2 receptors.
Fig. 5.
Effects of selected antagonists for ARs and P2Y receptors on ADP-induced enhancement of C3a-mediated β-Hex release in LAD2 cells. XAC is a non-selective adenosine receptor antagonist. MRS2500 and MRS2211 are antagonists for the P2Y1R and P2Y13R, respectively. AR-C66096 is a selective P2Y12R antagonist. The concentration of all antagonists used was 10 μM. For experiments with MRS2211, an additional wash step was included before the addition of C3a due to the color of MRS2211, which affects measurement of absorbance. Results are expressed as mean ± SE from three to five separate experiments performed in duplicate. *P < 0.05 compared with control group. #P < 0.05 compared with C3a group. **P < 0.05, compared with ADP group
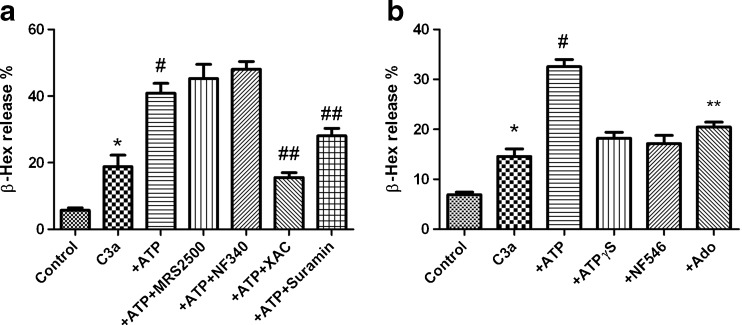
We next examined the possible receptors responsible for the ATP-induced enhancement of β-Hex release. Figure 6a shows that ATP-induced enhancement was minimally affected by potent P2Y1R antagonist MRS2500 or P2Y11R antagonist NF340, but was significantly inhibited by a potent, nonselective AR antagonist XAC, suggesting the involvement of ARs. The P2 antagonist suramin also partially diminished the effect of ATP.
Fig. 6.
a Effects of antagonists for adenosine receptors and P2Y receptors on ATP (10 μM)-induced enhancement of C3a-mediated β-Hex release in LAD2 cells. NF340 is a P2Y11R antagonist. Suramin is an antagonist for both P2X and P2Y receptors. The concentration of the antagonists used was 10 μM, except for suramin (100 μM). *P < 0.05, compared with control group; #P < 0.05, compared with C3a group. ##P < 0.05, compared with ATP group. b Comparison of effect of ATP (10 μM), ATPγS (10 μM), NF546 (10 μM) and adenosine (10 μM). Results were from four to six experiments performed in triplicate. *P < 0.05, compared with control group; #P < 0.01, compared with C3a group. **P < 0.05, compared with ATP or C3a group
Figure 6b shows that the effect of ATP (10 μM) could not be mimicked by the nonhydrolyzable P2Y11R agonist ATPγS (10 μM) or by adenosine (10 μM), i.e., the hydrolysis product of the action of multiple nucleotidases on ATP (Fig. 6b). A P2Y11R-selective agonist of atypical nonnucleotide structure NF546 (pEC50 = 6.27) also had no effect on degranulation at 10 μM. Together this suggests the possible involvement of both P2 receptors and ARs in combination in the effect of ATP.
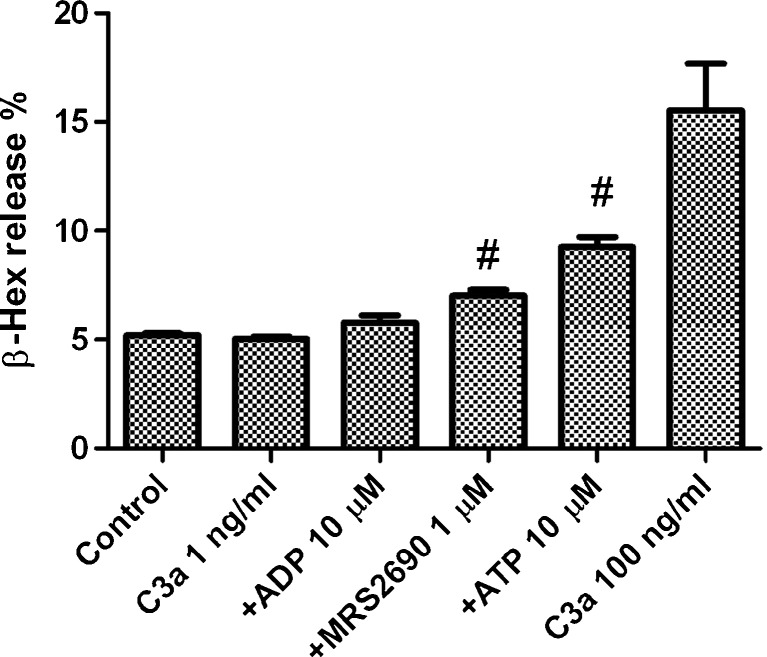
Figure 7 shows that C3a, at a concentration of 1 ng/ml, did not significantly induce the release of Hex. However, the combination of 1 ng/ml C3a with either MRS2690 or ATP produced a modest but significant β-Hex release, although these nucleotides did not produce any effect alone, suggesting cooperative effects between C3a and nucleotides. Combination of 1 ng/ml C3a and 10 μM ADP also produced an effect, but the difference was not significant (P > 0.05). This may indicate that under some specific conditions, these receptors may contribute to allergic responses in vivo. As a control, the effect of C3a at 100 ng/ml was included (Fig. 7).
Fig. 7.
Potential cooperative effects of C3a and nucleotides. Nucleotide agonists were added 20 min before the addition of C3a. The mixture was incubated at 37 °C for additional 30 min. Significantly different from the C3a group at 1 ng/ml, #P < 0.05. Results are expressed as mean ± SE and were from three to four separate experiments performed in duplicate
Comparison of the gene expression levels of P2Y receptors and the C3a receptor
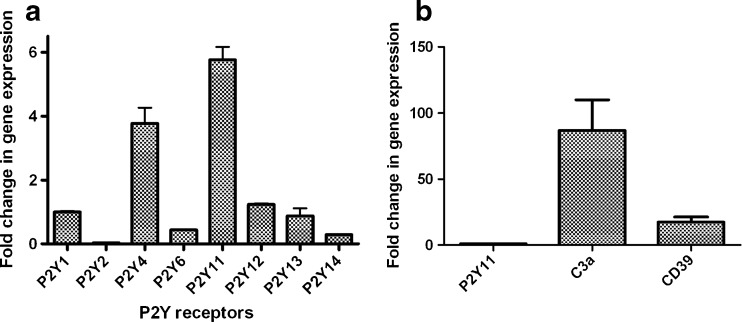
Figure 8a shows the gene expression level of various P2Y receptors. All P2YRs were found to express in LAD2 cells with variable levels. The P2Y11R and P2Y4R were among the highest, about 5.8- and 3.8-fold of P2Y1R. P2Y2R was the lowest in expression, which was about 40-fold lower than the P2Y1R receptor. The expression levels of P2Y6R and P2Y14R were roughly one third to half of the P2Y11R, and the expression levels of P2Y12R and P2Y13R were similar to the P2Y1R.
Fig. 8.
a Expression of P2Y receptor genes determined using qRT-PCR in LAD2 cells. GAPDH was used as an endogenous control in each separate experiment. The expression level of the P2Y receptors was calibrated based on the expression level of GAPDH. The expression level of the P2Y1R was arbitrarily expressed as 1. The gene expression levels of other P2YRs were expressed as their relative values to that of the P2Y1R. b Gene expression levels of C3a and CD39 in LAD2 cells in comparison to the most expressed P2Y receptor, P2Y11R. Quantitative analysis of data was performed by using the ΔΔCt method [17]. Results are expressed as mean ± SE from three separate experiments performed in triplicate
The expression level of the highly expressed P2Y11R was compared with that of the C3a receptor and the ecto-nucleotidase CD39, also known as ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1 and NTPDase1) (Fig. 8b). It was shown that the level of gene expression of the C3a receptor was about 80-fold higher than that of the P2Y11R, which may explain why C3a, but not ATP or UDPG, alone can induce β-Hex release. CD39, which indirectly contributes to the formation of extracellular adenosine from ATP, was found to be expressed at a 17-fold higher level than the P2Y11R. The expectation that there is robust enzymatic hydrolysis of ATP is consistent with the observation that the effect of ATP on degranulation, at least in part, occurs via ARs.
Discussion
In the present study, we used the LAD2 cells as a model to explore the expression and function of P2YRs in mast cell degranulation. LAD2 cells are the first reported human mast cell line that closely resembles primary cultures of CD34+-derived human mast cells; they respond to rhSCF and have functional FcεRI receptors [16].
Of all the P2YR subtypes studied, it seems only the role of the P2Y14R in degranulation was unambiguously defined, based on the potent facilitation by the synthetic P2Y14R agonist MRS2690 (EC50 229 nM, comparable to its known potency at the human P2Y14R [12]), the inhibition by PTX, the P2Y14R antagonist PPTN [32], and specific siRNA for the P2Y14R. Thus, human mast cells express an endogenous P2Y14R, which facilitates degranulation in a Gi-dependent manner and is therefore a potential therapeutic target for allergic conditions.
P2Y1R, P2Y2R, P2Y4R, P2Y6R, and P2Y11R did not seem to play a major role based on the pharmacological results in the current study. The role of the P2Y12R cannot be clearly defined due to the lack of a selective agonist, the relatively small window of enhancement by ADP and the ambiguous results with P2Y12R antagonist AR-C66096. Although it cannot be clearly defined, there is an indication that the P2Y13R might be involved, based on the effect of the P2Y13R antagonist MRS2211. However, it is clear that ARs are involved in both ADP- and ATP-mediated responses, which is not the focus of the current study. The results that ATP induced a large enhancement that could not be mimicked by ATPγS, NF546 or adenosine imply that more than one receptor is involved. The effect of suramin, a competitive P2X and P2Y antagonist [19–21], on the enhancement of degranulation by ATP suggests the possible involvement of certain P2X and/or P2Y subtypes. Several P2X (P2X1, P2X4, and P2X7) receptors were found expressed in LAD2 cells and human lung mast cells [8].
A growing number of GPCRs have been reported to mediate degranulation, either directly or via enhancement of antigen-induced degranulation. Activation of C3a and S1P (sphingosine-1-phosphate) receptors could induce degranulation directly [22–24]. However, adenosine and prostaglandin E2 (PGE2) alone could not induce degranulation, but they enhanced antigen-induced mast cell degranulation via the A3AR and EP3 prostanoid receptor, respectively [1, 5]. ADP and UDPG also enhanced antigen-mediated degranulaiton [11, 12]. It seems that most, if not all, GPCRs that mediate degranulation are coupled to Gi protein. In the present study, comparison of the expression level of the Gi-coupled P2Y12R, P2Y13R, and P2Y14R with the known Gi-coupled C3a receptor showed that the expression of P2YRs is much lower than that of the C3a receptor. Thus, we hypothesize that the lack of direct effect of P2YR agonists is likely related to the relatively low expression levels of these receptors in LAD2 cells.
The above hypothesis is supported by a recent study using the selective A3AR agonist 2-(1-hexynyl)-N6-methyladenosine, which potentiated antigen-induced degranulation in lung but not skin mast cells [25]. It was found in that study that the A3AR gene expression was three times higher in lung than in skin mast cells. Guhl et al. [26] found that the lack of degranulation induced by antigen in HMC-1 cells is mainly due to the low expression of FcεRI, not total histamine content. Indeed, transfection of FcεRI alpha subunit increased the antigen response [27].
The lack of effect of either C3a (at low concentration) or nucleotides alone, and the observation that their combination produced a significant effect, suggested a potential cooperative effect among C3a, P2Y, or ARs. Under some pathophysiological conditions, when nucleotide concentrations are elevated and/or P2YRs are upregulated, they may contribute to the allergic response. Thus, although activation of a particular adenosine or P2YR itself did not induce degranulation, it might contribute to the allergic response under certain specific circumstances in vivo.
The roles of ATP in mast cell degranulation have been previously reported. In human lung mast cells, ATP potentiated antigen-induced but not ionophore A23187-induced histamine release [10], which suggested a possible role of a P2Y receptors. In rat serosal mast cells, but not in cells from man and guinea pig, ATP was able to induce histamine release directly. The nonselective P2 antagonists Reactive blue 2 and suramin reduced the ATP effect [28]. ATP, but not ADP, has also been reported to induce histamine release from rat peritoneal mast cells via an energy-requiring mechanism [29]. The finding from the present study that ATP mediates degranulation via both P2 and ARs may imply that inhibitors of ecto-nucleotidases should also contribute to antiallergic effects.
Similar to the present finding, Feng et al. [13] also found the expression of three ADP-specific receptors, P2Y1R, P2Y12R, and P2Y13R (and P2Y2R and P2Y11R as well), in cord blood-derived human mast cells. Both ADP and ATP induced a small increase in antigen-induced Hex release. Neither blockade of P2Y1R nor P2Y12R diminished the effect, suggesting a possible role of the P2Y13R. However, unlike the present study, ATP only induced a very modest enhancement in that study [13]. It is suggested in the present study that responses induced by ADP and ATP might occur via P2YRs, P2XRs, or ARs, or a combination of these receptors.
It is noted in the present study that a non-selective AR agonist XAC blocked the effects of both ADP and ATP, suggesting the involvement of ARs in the degranulation. The involvement of adenosine in the degranulation of LAD2 cells has been observed, but the role of specific AR subtypes appears to be complex [30]. Consistent with this finding, an ectonucleotidase CD39, which cleaves ATP to AMP (a precursor of adenosine), was found to be highly expressed in LAD2 mast cells. Considering the ubiquitous presence of ATP, the use of inhibitors of CD39 and other ectonucleotidases would be useful in probing the relative involvement of P2 and ARs in the degranluation response. If the major effect of ADP and ATP is through ARs, inhibitors of CD39 could possibly be a means to control allergic responses. Alternately, targeting specific receptor(s), such as the P2Y14R, could be explored for treatment of allergic diseases. CD39 has been studied as a potential target for cancer, inflammation, and cardiovascular diseases [31], but its role in mast cell degranulation has not been well characterized, which could be an important subject of future study.
It is known that although LAD2 cells are considered mature mast cells compared with another human mast cell line HMC-1, they lack some proteases. For this reason, they can serve as surrogates of tissue mast cells only to a limited degree. It is of importance to confirm the results emerging from this study in future studies of primary human mast cells. Moreover, the relative involvement of P2YRs and ARs in mast cell degranulation demonstrated in the present study is only in an in vitro model; it remains to be investigated in an in vivo model.
In summary, the present study demonstrated that the P2Y14R is involved in degranulation in human mast cells, consistent with our previous study of rat mast cells. Selective P2Y14R antagonists have been reported [32] and therefore could be examined in models of asthma. Degranulation mediated via ADP or ATP likely involves multiple purine receptors, including ARs. The expression level of a given receptor may or may not indicate that it has a function in inducing or enhancing degranulation. For example, the P2Y11R was the most highly expressed, but its ligands had no effect in this model. It was found that an ectonucleotidase, CD39, is also highly expressed in the LAD2 human mast cells. It is assumed that under certain specific conditions, especially when the expression of purine receptors is elevated, the endogenous nucleotides may induce degranulation directly or cooperate with other allergic mediators, thereby contributing to allergic responses directly. Thus, both antagonists of certain purine receptors and inhibitors of ectonucleotidases may have antiallergic effects.
Acknowledgments
This work is supported by the NIDDK Intramural Research Program, National Institutes of Health. We thank Drs. Arnold Kirshenbaum and Dean Metcalfe (NIAID, NIH, Bethesda, MD, USA) for providing LAD2 cells. We thank Prof. Mortimer M. Civan (University of Pennsylvania) for helpful discussions.
Abbreviations
- ADP
Adenosine 5′-diphosphate
- AR-C66096
(2-(Propylthio)adenosine-5′-O-(β,γ-difluoromethylene)triphosphate tetrasodium salt)
- ATP
Adenosine 5′-triphosphate
- BSA
Bovine serum albumin
- (FcεRI)
Fc region of immunoglobulin E (IgE)
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G-protein-coupled receptor
- β-Hex
β-Hexosaminidase
- MRS2211
2-[(2-Chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde
- MRS2365
[[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl]diphosphoric acid mono ester trisodium salt
- MRS2500
(1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt
- MRS2690
2-Thiouridine-5′-diphosphoglucose
- NF340
4,4′-(Carbonylbis(imino-3,1-(4-methy l-phenylene)carbonylimino))bis(naphthalene-2,6-disulfonic acid) tetrasodium salt)
- NF546
4,4′-(Carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)carbonylimino))-bis(1,3-xylene-alpha,alpha′-diphosphonic acid tetrasodium salt)
- NP-BSA
4-Hydroxy-3-nitrophenylacetyl hapten amide conjugated to bovine serum albumin
- PTX
Pertussis toxin
- qRT-PCR
Quantitative real time polymerase chain reaction
- RBL
Rat basophilic leukemia
- UDP
Uridine 5′-diphosphate
- UTP
Uridine 5′-triphosphate
- UDPG
Uridine-5′-diphosphoglucose (UDP-glucose)
- XAC
8-[4-[[[[(2-Aminoethyl)amino]carbonyl]methyl)oxy]phenyl]-1,3-dipropylxanthine
- PPTN
4-(4-(Piperidin-4-yl)phenyl)-7-(4-(trifluoromethyl)phenyl)-2-naphthoic acid (mesylate salt form)
Contributor Information
Zhan-Guo Gao, FAX: +1-301-4808422, Email: zg21o@nih.gov.
Kenneth A. Jacobson, Email: kajacobs@helix.nih.gov
References
- 1.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okayama Y, Saito H, Ra C. Targeting human mast cells expressing g-protein-coupled receptors in allergic diseases. Allergol Int. 2008;57:197–203. doi: 10.2332/allergolint.R-08-163. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn HS, Jung MY, Beaven MA, Metcalfe DD, Gilfillan AM. Distinct PGE2-responder and non-responder phenotypes in human mast cell populations: "all or nothing" enhancement of antigen-dependent mediator release. Immunol Lett. 2011;141:45–54. doi: 10.1016/j.imlet.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 7.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wareham K, Vial C, Wykes RC, Bradding P, Seward EP. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br J Pharmacol. 2009;157:1215–1224. doi: 10.1111/j.1476-5381.2009.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudo N, Tanaka K, Koga Y, Okumura Y, Kubo C, Nomoto K. Extracellular ATP activates mast cells via a mechanism that is different from the activation induced by the cross-linking of Fc receptors. J Immunol. 1996;156:3970–3979. [PubMed] [Google Scholar]
- 10.Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, Mohanty J, Butterfield JH, Pelleg A. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–537. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- 11.Gao ZG, Ding Y, Jacobson KA. P2Y13 receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res. 2010;62:500–505. doi: 10.1016/j.phrs.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–879. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- 15.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 16.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/S0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leff P, Wood BE, O’Connor SE. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br J Pharmacol. 1990;101:645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashem SW, Subramanian H, Collington SJ, Magotti P, Lambris JD, Ali H. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2. Eur J Pharmacol. 2011;668:299–304. doi: 10.1016/j.ejphar.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivera A, Rivera J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv Exp Med Biol. 2011;716:123–142. doi: 10.1007/978-1-4419-9533-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez G, Zhao W, Schwartz LB. Disparity in FcεRI-induced degranulation of primary human lung and skin mast cells exposed to adenosine. J Clin Immunol. 2011;31:479–487. doi: 10.1007/s10875-011-9517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guhl S, Babina M, Neou A, Zuberbier T, Artuc M. Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells—drastically reduced levels of tryptase and chymase in mast cell lines. Exp Dermatol. 2010;19:845–847. doi: 10.1111/j.1600-0625.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- 27.Xia YC, Sun S, Kuek LE, Lopata AL, Hulett MD, Mackay GA. Human mast cell line-1 (HMC-1) cells transfected with FcεRIα are sensitive to IgE/antigen-mediated stimulation demonstrating selectivity towards cytokine production. Int Immunopharmacol. 2011;11:1002–1011. doi: 10.1016/j.intimp.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Jaffar ZH, Pearce FL. Histamine secretion from mast cells stimulated with ATP. Agents Actions. 1990;30:64–66. doi: 10.1007/BF01968999. [DOI] [PubMed] [Google Scholar]
- 29.Diamant B, Krüger PG. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5′-triphosphate. Acta Physiol Scand. 1967;71:291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 30.Leung CT, Li A, Gao ZG, Peterson-Yantorno K, Jacobson KA, Civan MM (2011) Purinergic regulation of degranulation by human LAD2 mast cells. Invest Ophthalmol Vis Sci. ARVO E-Abstract #2911
- 31.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011;7:21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier JY, Belley M, Deschênes D, Fournier JF, Gagné S, Gareau Y, et al. The identification of 4,7-disubstituted naphthoic acid derivatives as UDP-competitive antagonists of P2Y14. Bioorg Med Chem Lett. 2011;21:2836–2839. doi: 10.1016/j.bmcl.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 33.Belly M, Deschenes D, Fortin R, Fournier JF, Gagne S, Gareau Y, et al. (2009) Substituted 2-naphthoic acids as antagonists of GPR105 activity. WO 2009/070873A1