Abstract
Inosine is the first metabolite of adenosine. It exerts an antinociceptive effect by activating the adenosine A1 and A2A receptors. We have previously demonstrated that inosine exhibits antinociceptive properties in acute and chronic mice models of nociception. The aim of this study was to investigate the involvement of pertussis toxin-sensitive G-protein-coupled receptors, as well as K+ and Ca2+ channels, in the antinociception promoted by inosine in the formalin test. Mice were pretreated with pertussis toxin (2.5 μg/site, i.t., an inactivator of Gi/0 protein); after 7 days, they received inosine (10 mg/kg, i.p.) or morphine (2.5 mg/kg, s.c., used as positive control) immediately before the formalin test. Another group of animals received tetraethylammonium (TEA) or 4-aminopyridine (4-AP) (1 μg/site, i.t., a non-specific voltage-gated K+ channel blockers), apamin (50 ng/site, i.t., a small conductance Ca2+-activated K+ channel blocker), charybdotoxin (250 pg/site, i.t., a large-conductance Ca2+-activated K+ channel blocker), glibenclamide (100 μg/site, i.t., an ATP-sensitive K+ channel blocker) or CaCl2 (200 nmol/site, i.t.). Afterwards, the mice received inosine (10 mg/kg, i.p.), diclofenac (10 mg/kg, i.p., a positive control), or morphine (2.5 mg/kg, s.c., a positive control) immediately before the formalin test. The antinociceptive effect of inosine was reversed by the pre-administration of pertussis toxin (2.5 μg/site, i.t.), TEA, 4-aminopyridine, charybdotoxin, glibenclamide, and CaCl2, but not apamin. Further, all K+ channel blockers and CaCl2 reversed the antinociception induced by diclofenac and morphine, respectively. Taken together, these data suggest that the antinociceptive effect of inosine is mediated, in part, by pertussis toxin-sensitive G-protein coupled receptors and the subsequent activation of voltage gated K+ channel, large conductance Ca2+-activated and ATP-sensitive K+ channels or inactivation of voltage-gated Ca2+ channels. Finally, small conductance Ca2+-activated K+ channels are not involved in the antinociceptive effect of inosine.
Keywords: Inosine, Pain, Potassium channels, Calcium channels, Pertussis toxin, Formalin test
Introduction
Inosine, a metabolite of the purinergic system, is an endogenous nucleoside produced through the breakdown of adenosine in intracellular and extracellular spaces [1–3]. Purine nucleosides, such as adenosine and its primary metabolite, inosine, are low-molecular-weight molecules that participate in a wide variety of intracellular biochemical processes. Nucleosides also have important roles as extracellular signaling molecules [4]. Inosine acts directly on the coronary artery, causing relaxation [5] and reducing the total peripheral resistance [6, 7]. Besides cardiovascular effects, inosine also exhibits immunomodulatory and neuroprotective effects and stimulates the production of the anti-inflammatory IL-10 [8]. Inosine potently inhibits the release of inflammatory cytokines and chemokines [9], reduces the migration of human monocytes and neutrophils in vitro and decreases TNF-α production [10].
We have previously demonstrated that inosine exhibits antinociceptive properties in acute and chronic rodent models of nociception. Furthermore, we have shown that the antinociceptive action of inosine involves the activation of the A1 and A2A adenosine receptors, but not the A2B receptor [11].
Notably, the activation of adenosine receptors involves various intracellular transduction mechanisms, including pathways activated by Gi/0 and Gs/q. A1 receptor is coupled to Gi/0 protein family. Most of the biological effects induced by A1 receptor activation, including antinociceptive effect, are due to inhibition of cAMP second messenger, consequence of the inhibition of adenylate cyclase activity by the α inhibitory G-protein subunit [12–14]. Furthermore, A1 receptor agonists can directly activate K+ channels, promoting the hyperpolarization of the cell membrane and reduction of neuronal excitability. A2A receptor is coupled to Gs protein family. A2A receptor activation induces cAMP increased production due to increased activity of the enzyme adenylate cyclase promoted by the α excitatory G-protein subunit. With the enhancement in cAMP intracelular levels, PKA becomes able to activate several pathways through PKC, calcium channels, cAMP responsive element-binding (CREB), MAPK, PLC activation, which are all involved in the activation of pain response. Furthermore, A2A receptor agonists can directly open K+ channels, promoting the hyperpolarization of the cell membrane and reduction of neuronal excitability, which are all involved in the inhibition of pain response [12, 13, 15, 16]. Thus, the role of A1R in pain control is well described; however, there are conflicting results regarding the role of the A2AR in pain and nociception.
According to the above, several studies have shown that agonists that activates Gi/o-protein-coupled receptors, such as adenosine receptors, open specific K+ channels and inhibit voltage-gated Ca2+ channels in neurons. This inhibition reduces Ca2+ influx and thereby decreases the Ca2+ intracellular concentration to promote antinociception [17–19]. For this reason, development of new antinociceptive drugs has begun targeting K+ and Ca2+ channels themselves. Because inosine exerts its effects through the activation of adenosine receptors, the purpose of the present study was to investigate the role of pertussis toxin-sensitive G-protein coupled receptors as well as the participation of different types of K+ channels and voltage-gated Ca2+ channels in the antinociception caused by inosine. Furthermore, this study used morphine and diclofenac as positive controls because many literature data have indicated that its antinociceptive effects involve, at least in part, the mechanisms here investigated.
Material and methods
Animals
Experiments were conducted using male Swiss mice (25–35 g) maintained at 22 ± 2 °C with free access to water and food and housed under a 12:12 h light:dark cycle. Animals were acclimatized to the laboratory for at least 1 h before testing and were used only once throughout the experiments. All experiments were approved by the Ethics Committee for Animal Research of the Universidade Federal de Santa Catarina (Protocol No. PP00484), and all efforts were made to minimize animal suffering and to reduce the number of animals used in the experiments.
Drugs
Reagents were purchased from suppliers as indicated: formalin (Merck, Darmstadt, Germany), inosine and pertussis toxin (Sigma Chemical Co., St. Louis, USA), diclofenac (Medley S/A, São Paulo, Brazil), morphine hydrochloride (Merck, Darmstadt, Germany), tetraethylammonium (TEA), 4-aminopyridine, charybdotoxin, apamin, and glibenclamide (Tocris Cookson Inc., Ellisville, USA). Diclofenac and glibenclamide were dissolved in saline with 5 % Tween 80, whereas all the other drugs were dissolved in isotonic (0.9 % NaCl) saline solution immediately before use.
Drug administration
Pertussis toxin, K+ channel blockers and CaCl2 were administered by intrathecal (i.t.) injection in a volume of 5 μl per mouse. To perform an i.t. drug injection, a 30½-gauge stainless needle attached to a 50-μL Hamilton microsyringe was inserted between the L5 and L6 vertebrae of conscious mice, and 5 μL of drug solution was injected into the subarachnoid space (modified from Hylden and Wilcox [20]). Inosine (10 mg/kg) and diclofenac (10 mg/kg, used as a positive control) were administered by i.p. injection in a volume of 10 ml/kg body weight. Morphine (2.5 mg/kg, s.c., used as a positive control) was injected by s.c. injection in a volume of 10 ml/kg body weight. All procedures, doses, and administration routes of the various drugs were chosen on the basis of previous studies [22–25, 36] or in preliminary experiments carried out in our laboratory (data not shown).
Formalin test
The procedure used was essentially the same as that described previously [21] with minor modifications. Animals received 20 μl of a 2.5 % formalin solution (0.92 % formaldehyde) made up in saline, injected intraplantarly (i.pl.) into the ventral surface of the right hindpaw. Animals were observed from 0–5 min (neurogenic phase) and from 15–30 min (inflammatory phase). Animals received inosine (10 mg/kg, i.p.), diclofenac (10 mg/kg, i.p.) or morphine (2.5 mg/kg, s.c.) 30 min beforehand. Control animals received vehicle (10 ml/kg, i.p.). After the i.pl. formalin injection, animals were immediately placed in a glass cylinder 20 cm in diameter. The time each spent licking the injected paw was recorded with a chronometer and considered indicative of nociception. In the current study, we noticed the behavior only in the inflammatory phase of the formalin test due to the occurrence of antinociception caused by inosine solely in this phase [11].
Role of pertussis toxin-sensitive G-protein coupled receptors
To determine the involvement of Gi/0 protein-coupled receptors in the antinociceptive action of inosine, mice were pretreated with pertussis toxin (2.5 μg/site, i.t.), an inactivator of Gi/o protein. A control group was pretreated with saline (5 μl/site, i.t.). The experiment was carried out as described by Meotti et al. [22]. Seven days after the pretreatment, mice received vehicle (10 ml/kg), inosine (10 mg/kg, i.p.), or morphine (2.5 mg/kg, s.c.) as a positive control. After 30 min, the animals received an injection of formalin 2.5 % (20 μl/site, i.pl.).
Role of K+ and Ca2+ channels on the antinociceptive effect of inosine
We also investigated the possible role played by different types of potassium and voltage-gated Ca2+ channels in the antinociceptive action of inosine during the second phase of the formalin test. Animals were pretreated by intrathecal (i.t.) route with TEA (1 μg/site, a non-selective blocker of voltage-sensitive K+ channels), 4-aminopyridine (1 μg/site, a non-selective blocker of voltage-sensitive K+ channels), apamin (50 ng/site, a blocker of small (or low)-conductance Ca2+-sensitive K+ channels), charybdotoxin (250 pg/site, a blocker of large (or fast)-conductance Ca2+-sensitive K+ channels), glibenclamide (100 μg/site, a blocker of ATP-sensitive K+ channels), and CaCl2 (200 nmol/site). After 15 min, they received inosine (10 mg/kg, i.p.), diclofenac (10 mg/kg, i.p.), or morphine (2.5 mg/kg, s.c.). The nociceptive responses to formalin were recorded 30 min after the administration of inosine, diclofenac, or morphine (used as positive control).
Statistics
The results are presented as the mean (S.E.M.) obtained using GraphPad software (GraphPad software, San Diego, CA). Data were analyzed by one-way ANOVA followed by the Newman–Keuls test. P values less than 0.05 were considered significant.
Results
Involvement of pertussis toxin-sensitive G-protein coupled receptors in the inosine antinociceptive effect
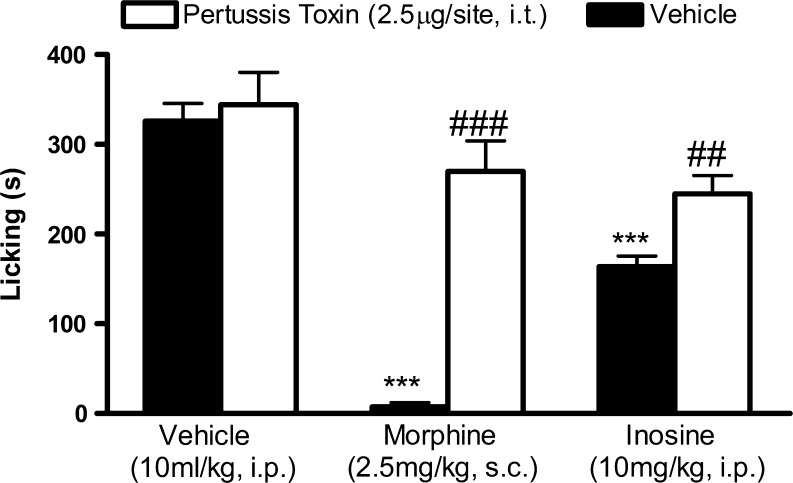
To perform the following experiments, we used inosine 10 mg/kg, i.p. The dose was selected based on our recently published study [11]. The results illustrated in Fig. 1 shows that the intrathecal administration of pertussis toxin, an inactivator of Gi/o protein (2.5 μg/site, i.t., for 7 consecutive days), resulted in complete inhibition of morphine (2.5 mg/kg, s.c.)-induced antinociception when assessed in formalin-induced nociception. Under the same conditions, pertussis toxin partially, but significantly, inhibited the antinociceptive effect promoted by inosine (10 mg/kg, i.p.).
Fig. 1.
Effect of pertussis toxin pretreatment on the antinociception induced by inosine. Mice were pretreated with intrathecal injection of pertussis toxin 7 days before intraperitoneal administration of inosine or morphine. Values are expressed as the mean + SEM. The asterisks denote significance levels when compared with the vehicle-treated group (one-way ANOVA followed by Newman–Keuls test). ***P < 0.001. ##P < 0.01; ###P < 0.001 indicates the significance levels when comparing the morphine treatments with their respective vehicle group
Involvement of potassium channels in the inosine antinociceptive effect
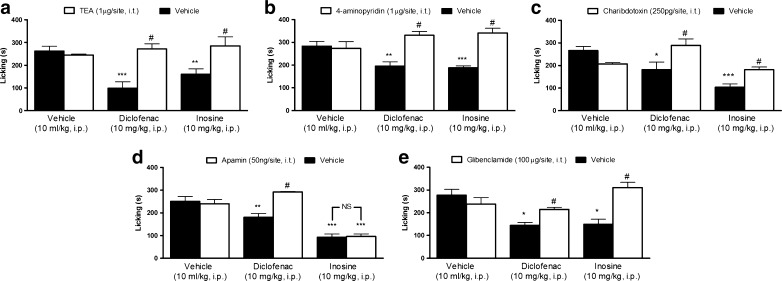
The results depicted in Fig. 2a–e show the effect of the K+ channel blockers in the antinociceptive effect promoted by inosine. Intrathecal pretreatment of mice with TEA (1 μg/site) and 4-aminopyridine (1 μg/site) significantly reversed the antinociceptive effect of inosine (Fig. 2a, b). Figure 2c, d shows that charybdotoxin (250 pg/site), but not apamin (50 ng/site), prevented the antinociception caused by inosine. Figure 2e shows that glibenclamide (100 μg/site) reversed the antinociceptive effect of inosine. However, pretreatment of mice with TEA, 4-aminopyridine, charybdotoxin, apamin, and glibenclamide prevented the antinociceptive action caused by diclofenac (10 mg/kg, i.p.) (see [23] and Fig. 2a–e). Moreover, when administered individually, none of the K+ channel blockers affected formalin-induced pain.
Fig. 2.
Effect of the voltage-dependent K+ channel blockers, tetraethylammonium (TEA, panel a) and 4-aminopyridine (4-AP, panel b); the large- and small- conductance Ca2+-activated K+ channel blockers, charybdotoxin (panel c) and apamin (panel d), and the ATP-sensitive K+ channel inhibitor, glibenclamide (panel e), on the antinociception induced by inosine. Mice were pretreated with an intrathecal injection of 4-AP, TEA, apamin, charybdotoxin, or glibenclamide 15 min before intraperitoneal administration of inosine or diclofenac. Values are expressed as the mean + SEM. The asterisks denote significance levels when compared with vehicle group (one-way ANOVA followed by Newman–Keuls test), *P < 0.05, **P < 0.01, and ***P < 0.001. #P < 0.01 indicates the significance levels when comparing the inosine or diclofenac treatments with their respective vehicle group
Involvement of calcium channels in the inosine antinociceptive effect
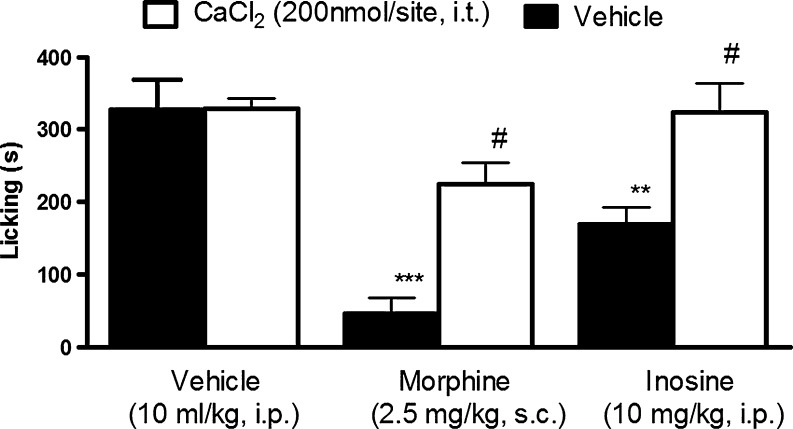
Pretreatment with CaCl2, (200 nmol/site, i.t.) prevented the antinociceptive effect of inosine and morphine on formalin-induced pain (Fig. 3). When administered individually, CaCl2 did not affect the nociception caused by formalin.
Fig. 3.
Effect of pretreatment with CaCl2 (200 nmol/site, i.t.) on the antinociception induced by inosine. Mice were pretreated with an intrathecal injection of CaCl2 15 min before intraperitoneal administration of inosine or subcutaneous administration of morphine. Values are expressed as the mean + SEM. The asterisks denote significance levels when compared with vehicle group (one-way ANOVA followed by Newman–Keuls test). **P < 0.01, ***P < 0.001. #P < 0.01 indicates the significance levels when comparing the inosine and morphine treatments with their respective vehicle group
Discussion
In our recent study, we demonstrated that inosine effectively reduces acetic acid-, glutamate-induced pain and the inflammatory phase of the formalin test through the activation of the A1 and A2A adenosine receptors [11]. The present study extends the knowledge of inosine actions and shows that pertussis toxin-sensitive Gi/0-protein coupled receptors, K+ channels and voltage-gated Ca2+ channels are involved in the antinociceptive effect of inosine.
Many clinical drugs, such as opioids, GABAergic agonists, and α-2 agonists among others, are ligands and acts via pertussis toxin-sensitive G-protein coupled receptors [24]. Of note, a ligand binding to its receptor activates the coupled G-protein, dissociating the inhibitory subunit αi from the initial trimer formed by the subunits αβγ. This dissociation between the subunits Gαi and Gβγ initiates an orchestrated cascade of intracellular events responsible for the antinociceptive effect of the ligand, like morphine for example [25–27].
Pertussis toxin inactivates the inhibitory Gi/0-protein via ribosylation of the α-subunit. The inactivated Gi/0 protein is not able to inhibit adenylate cyclase, the enzyme responsible for the elevation of intracellular cAMP. Thus, inactivation of the Gi/0 protein by pertussis toxin causes an increase in intracellular cAMP levels [28]. Therefore, the pertussis toxin is able to prevent activation of the intracellular cascade of events responsible for the antinociceptive effect of morphine or another ligand of pertussis toxin-sensitive G-protein coupled receptors.
In the current study, we found that pertussis toxin pretreatment was able to reverse morphine- (used as a positive control) and inosine-induced antinociception. These data suggest that inosine-induced antinociception is mediated by pertussis toxin-sensitive Gi/0-protein coupled receptors. Based on our previous data, we further suggest that the A1 adenosine receptor is the main G-protein coupled receptor responsible for transducing the antinociceptive effect of inosine. Activation of Gi/0-protein coupled receptors can promote the opening of some types of K+ channels. Agonists of G-protein coupled receptors such as opioid, adrenergic, serotoninergic, GABAergic, cannabinoid, and adenosinergic receptors are able to open K+ channels and consequently decrease nociception [29–32]. K+ channels are classified by their protein structures and their phylogenetic relationships; the four types include voltage-gated (Kv), calcium-activated (KCa), inward rectifier (Kir), and two-pore (K2P) K+ channels [29, 33]. Several classical analgesics and NSAIDs, such as tramadol, diclofenac, meloxicam, and ketorolac, also inhibit the inflammatory phase of the formalin test. In light of the classification of the K+ channels, the analgesic action of these drugs may be partially explained by the opening of distinct K+ channels [29, 34–36].
Several studies have demonstrated that activation of l-arginine-NO-cGMP-K+ channel pathway is involved in the antinociceptive effect of diclofenac and other NSAIDs [37–40]. l-arginine is used as a substrate for NOS in order to produce NO. NO, in turn, is able to activate the enzyme guanylate cyclase, stimulating the synthesis of cGMP. Finally, cGMP can interact with different targets, among them, K+ channels, promoting the opening these channels [for review see 41]. The opening of K+ channels leads to hyperpolarization of the cell membrane hindering the transmission of nociceptive stimulus along the CNS. Considering this signaling pathway, the opening of K+ channels represents the final step involved in the antinociceptive effect of diclofenac. Therefore, blockade of different types of K+ channels using different blockers can reduce the analgesic effect promoted by diclofenac, indicating that its analgesic effect depends on the opening of K+ channels.
The present investigation showed that the antinociceptive effect of inosine was blocked by pretreatment with intrathecal TEA and 4-aminopyridine, non-selective voltage-gated K+ channel inhibitors, suggesting that the opening of this kind of K+ channel is important for the antinociception induced by inosine. Moreover, the intrathecal administration of charybdotoxin, but not apamin, a small-conductance Ca2+-activated K+ channel blocker, prevented the antinociceptive effect produced by inosine, suggesting the participation of a large-conductance Ca2+-activated K+ channel in the effect caused by inosine. Charybdotoxin also inhibits intermediate-conductance Ca2+-activated K+ channels [42]. Therefore, the blockade of the inosine-induced antinociceptive effect by charybdotoxin suggests that inosine may induce its antinociceptive effect through the activation of intermediate- and large-conductance Ca2+-activated K+ channels. However, because it is possible that charybdotoxin also blocks voltage-gated K+ channels [43], we cannot unequivocally affirm that large-conductance Ca2+-activated K+ channels are involved in inosine-induced antinociception in the formalin test. Intrathecal administration of glibenclamide reversed the antinociceptive effect of inosine, suggesting that inosine activates ATP-sensitive K+ channels to reduce formalin-induced pain. Alone and at the concentrations used in this work, the K+ channel blockers (TEA, 4-aminopyridine, charybdotoxin, apamin, and glibenclamide) did not modify the pain threshold induced by formalin.
Notably, hyperpolarization resulting from the activation of K+ channel subtypes, especially the large-conductance Ca2+-activated K+ channel, reduces the amount of neurotransmitters released by shortening the duration of depolarization that allows Ca2+ influx through voltage-gated Ca2+ channels [44].
The analgesic action of some important drugs, like morphine and Prialt®, can be partially explained by the closing of voltage-gated Ca2+ channels (VGCCs) and subsequent reduction of Ca2+ influx. The VGCCs are divided into two categories: high-voltage-activated channels, including L-, N-, P/Q- and R-type Ca2+ channels that require strong depolarization for activation, and low-voltage-activated T-type Ca2+ channels that can be triggered by much milder depolarization. In the central nervous system, these channels carry out a variety of actions, regulating activity-dependent gene expression, synaptic transmission and neuronal excitability. The VGCCs most extensively studied in nociception are the N-, P/Q- and T-type Ca2+ channels [45–47].
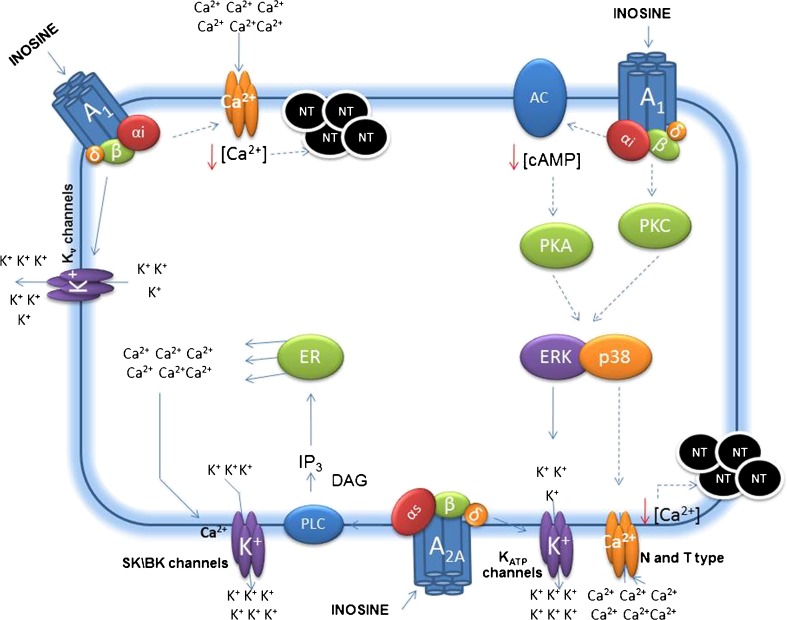
It is well established that an increase in intracellular Ca2+ has an important role in neurotransmitter release, cell membrane excitability, activation of intracellular proteins, and reduction of the pain threshold [48–50]. The concentration of intracellular Ca2+ is regulated by several mechanisms; one such mechanism is the influx of Ca2+ via VGCCs through the plasma membrane. The VGCC channels are inhibited by Gi/o-protein-coupled receptors. Consequently, intracellular Ca2+ levels are reduced and the pain threshold decreases [19]. Our results demonstrated that the pretreatment of mice with CaCl2 prevented the antinociceptive effect promoted by inosine, suggesting the participation of voltage-gated Ca2+ channels in the effect caused by inosine. Figure 4 shows the probable mechanism involved in the antinociceptive effect of inosine, from receptor activation through the modulation of K+ and Ca2+ channels.
Fig. 4.
Probable intracellular mechanisms involved in the inosine effect, from receptor interaction through modulation of ion channels. The continuous arrow indicates activation, and the dot arrow indicates inhibition. A1 (A1 Adenosine receptor); A2A (A2A Adenosine receptor); αi, αs, β, and δ (G-protein subunits); Kv channels (voltage-dependent potassium channels); SK/BK channels (small- and large-conductance Ca2+-activated potassium channels); KATP channels (ATP-sensitive potassium channels); N and T types (N and T voltage-dependent calcium channels); AC (adenylate cyclase); PLC (phospholipase C); cAMP (cyclic adenosine monophosphate); IP3 (inositol triphosphate); DAG (diacylglycerol); PKA (protein kinase A); PKC (protein kinase C); ERK and p38 (mitogen-activate protein kinases); ER (endoplasmic reticulum); K+ (potassium ion); Ca2+ (calcium ion); and NT (neurotransmitter)
Conclusion
Our results confirm and extend previous data that show that inosine exhibits a significant antinociceptive action during the inflammatory phase of formalin-induced pain in mice. Furthermore, this effect requires pertussis toxin Gi/0-protein coupled receptors, voltage-gated K+ channels, large-conductance Ca2+-activated K+ channels and ATP-sensitive K+ channels, but does not involve the opening of small-conductance Ca2+-activated K+ channels. Inosine-induced antinociception also involves the inhibition of voltage-gated Ca2+ channels.
Acknowledgements
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível superior (CAPES), Brazil. F.P. Nascimento is a Ph.D. student in Pharmacology; S. J. Macedo-Junior Msc. student in Pharmacology and M. Luiz-Cerutti Msc. student in Neuroscience. They thank CNPq and CAPES for financial support. Dr. A.R.S. Santos is a CNPq recognized researcher (1C category) and has additional financial support from CNPq.
References
- 1.Barankiewicz J, Cohen A. Purine nucleotide metabolism in resident and activated rat macrophages in vitro. Eur J Immunol. 1985;15:627–631. doi: 10.1002/eji.1830150618. [DOI] [PubMed] [Google Scholar]
- 2.Mabley JG, Pacher P, Liaudet L, Soriano FG, Haskó G, Marton A, Szabo C, Salzman AL. Inosine reduces inflammation and improves survival in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:138–144. doi: 10.1152/ajpgi.00060.2002. [DOI] [PubMed] [Google Scholar]
- 3.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/S0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 4.Hasko G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Aviado DM. Inosine: a naturally occurring cardiotonic agent. J Pharmacol. 1983;4:47–71. [PubMed] [Google Scholar]
- 6.Jones CE, Thomas JX, Jr, Devous MD, Norris CP, Smith EE. Positive inotropic response to inosine in the in situ canine heart. Am J Physiol. 1977;233:438–443. doi: 10.1152/ajpheart.1977.233.4.H438. [DOI] [PubMed] [Google Scholar]
- 7.Seesko RC, Zimmer GH. Hemodynamic effects of inosine in combination with positive and negative inotropic drugs: studies on rats in vivo. J Cardiovasc Pharmacol. 1990;16:249–256. doi: 10.1097/00005344-199008000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Soriano F, Liaudet L, Marton A, Haskó G, Batista Lorigados C, Deitch EA, Szabó C. Inosine improves gut permeability and vascular reactivity in endotoxic shock. Crit Care Med. 2001;29:703–708. doi: 10.1097/00003246-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Marton A, Pacher P, Murthy KG, Németh ZH, Haskó G, Szabó C. Anti-inflammatory effects of inosine in human monocytes, neutrophils and epithelial cells in vitro. Int J Mol Med. 2001;8:617–621. [PubMed] [Google Scholar]
- 11.Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ, Jr, Lima DAN, Almeida RC, Ostroski RM, Rodrigues ALS, Santos ARS. Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther. 2010;334:590–598. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson KA, Gao GZ. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/S0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 15.Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem. 2003;3:413–426. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- 17.North RA. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Reyes E. G protein-mediated inhibition of Cav3.2 T-type channels revisited. Mol Pharmacol. 2010;77:136–138. doi: 10.1124/mol.109.062133. [DOI] [PubMed] [Google Scholar]
- 19.Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/S0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 20.Hylden JL, Wilcox GL. Intrathecal morphine in mice. A new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 21.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 22.Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JBT, Santos ARS. Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur J Pharmacol. 2007;567:198–205. doi: 10.1016/j.ejphar.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz MI, Lozano-Cuenca J, Granados-Soto V, Castañeda-Hernández G. Additive interaction between peripheral and central mechanisms involved in the antinociceptive effect of diclofenac in the formalin test in rats. Pharmacol Biochem Behav. 2008;91:32–37. doi: 10.1016/j.pbb.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Hoehn K, Reid A, Sawynok J. Pertussis toxin inhibits antinociception produced by intrathecal injection of morphine, noradrenaline and baclofen. Eur J Pharmacol. 1988;146:65–72. doi: 10.1016/0014-2999(88)90487-6. [DOI] [PubMed] [Google Scholar]
- 25.Prather PL, Loh HH, Law PY. Interaction of delta-opioid receptors with multiple G proteins: a non-relationship between agonist potency to inhibit adenylyl cyclase and to activate G proteins. Mol Pharmacol. 1994;45:997–1003. [PubMed] [Google Scholar]
- 26.Smart D, Hirst RA, Hirota K, Grand DK, Lambert DG. The effects of recombinant rat mu-opioid receptor activation in CHO cells on phospholipase C, [Ca2+]i and adenylyl cyclase. Br J Pharmacol. 1997;120:1165–1171. doi: 10.1038/sj.bjp.0701012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues AR, Duarte ID. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolphin AC, Prestwich SA. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985;316:148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- 29.Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004;500:203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez JA, Gonoi T, Inagaki N, Katada T, Seino S. Modulation of reconstituted ATP-sensitive K+ channels by GTP-binding proteins in a mammalian cell line. J Physiol. 1998;507:315–324. doi: 10.1111/j.1469-7793.1998.315bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocaña M, Baeyens JM. Role of ATP-sensitive K+ channels in antinociception induced by R-PIA, an adenosine A1 receptor agonist. Naunyn Schmiedebergs Arch pharmacol. 1994;350:57–62. doi: 10.1007/BF00180011. [DOI] [PubMed] [Google Scholar]
- 32.Robles LI, Barrios M, Del Pozo E, Dordal A, Baeyens JM. Effects of K+ channel blockers and openers on antinociception induced by agonists of 5-HT1A receptors. Eur J Pharmacol. 1996;295:181–188. doi: 10.1016/0014-2999(95)00643-5. [DOI] [PubMed] [Google Scholar]
- 33.Gutman GA, Chandy KG, Aldeman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology: XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz MI, Castañeda-Hernández G, Granados-Soto V. Pharmacological evidence for the activation of Ca2+-activated K+ channels by meloxicam in the formalin test. Pharmacol Biochem Behav. 2005;81:725–731. doi: 10.1016/j.pbb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Yalcin I, Asku F. Involvement of potassium channels and nitric oxide in tramadol antinociception. Pharmacol Biochem Behav. 2005;80:69–75. doi: 10.1016/j.pbb.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz MI, Torres-López JE, Castañeda-Hernández G, Rosas R, Vidal-Cantú GC, Granados-Soto V. Pharmacological evidence for the activation of K+ channels by diclofenac. Eur J Pharmacol. 2002;438:85–91. doi: 10.1016/S0014-2999(02)01288-8. [DOI] [PubMed] [Google Scholar]
- 37.Lázaro-Ibáñez GG, Torres-López JE, Granados-Soto V. Participation of the nitric oxide-cyclic GMP-ATP-sensitive K+ channel pathway in the antinociceptive action of ketorolac. Eur J Pharmacol. 2001;426:39–44. doi: 10.1016/S0014-2999(01)01206-7. [DOI] [PubMed] [Google Scholar]
- 38.Alves D, Duarte I. Involvement of ATP-sensitive K+ channels in the peripheral antinociceptive effect induced by dipyrone. Eur J Pharmacol. 2002;444:47–52. doi: 10.1016/S0014-2999(02)01412-7. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz MI, Granados-Soto V, Castañeda-Hernández G. The NOcGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol Biochem Behav. 2003;76:187–195. doi: 10.1016/S0091-3057(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 40.Déciga-Campos M, López-Muñoz FJ. Participation of the l-arginine-nitric oxide-cyclic GMP-ATP-sensitive K+ channel cascade in the antinociceptive effect of rofecoxib. Eur J Pharmacol. 2004;484:193–199. doi: 10.1016/j.ejphar.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Soares AC, Duarte ID. Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw. Br J Pharmacol. 2001;134:127–131. doi: 10.1038/sj.bjp.0704224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price M, Lee SC, Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci. 1989;86:10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Possani LD, Selisko B, Gurrola GB. Structure and function of scorpion toxins affecting K+-channels. Perspect Drug Disc Des. 1999;15(16):15–40. doi: 10.1023/A:1017062613503. [DOI] [Google Scholar]
- 44.Hu H, Shao LR, Chavoshy S, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGivern JG, McDonough SI. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr Drug Targets CNS Neurol Disord. 2004;3:457–478. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- 46.Vanegas H, Schaible H. Effects of antagonists to high-threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain. 2000;85:9–18. doi: 10.1016/S0304-3959(99)00241-9. [DOI] [PubMed] [Google Scholar]
- 47.Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–S30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Prado WA. Involvement of calcium in pain and antinociception. Braz J Med Biol Res. 2001;34:449–461. doi: 10.1590/S0100-879X2001000400003. [DOI] [PubMed] [Google Scholar]
- 49.Cervero F, Laird JMA. Role of ions channels in mechanisms controlling gastrointestinal pain pathways. Curr Opin Pharmacol. 2003;3:608–612. doi: 10.1016/j.coph.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]