Abstract
Various radioligands have been used to characterize and quantify the platelet P2Y12 receptor, which share several weaknesses: (a) they are metabolically unstable and substrates for ectoenzymes, (b) they are agonists, and (c) they do not discriminate between P2Y1 and P2Y12. We used the [3H]PSB-0413 selective P2Y12 receptor antagonist radioligand to reevaluate the number of P2Y12 receptors in intact platelets and in membrane preparations. Studies in humans showed that: (1) [3H]PSB-0413 bound to 425 ± 50 sites/platelet (KD = 3.3 ± 0.6 nM), (2) 0.5 ± 0.2 pmol [3H]PSB-0413 bound to 1 mg protein of platelet membranes (KD = 6.5 ± 3.6 nM), and (3) competition studies confirmed the known features of P2Y12, with the expected rank order of potency: AR-C69931MX > 2MeSADP ≫ ADPβS > ADP, while the P2Y1 ligand MRS2179 and the P2X1 ligand α,β-Met-ATP did not displace [3H]PSB-0413 binding. Patients with severe P2Y12 deficiency displayed virtually no binding of [3H]PSB-0413 to intact platelets, while a patient with a dysfunctional P2Y12 receptor had normal binding. Studies in mice showed that: (1) [3H]PSB-0413 bound to 634 ± 87 sites/platelet (KD = 14 ± 4.5 nM) and (2) 0.7 pmol ± 0.3 [3H]PSB-0413 bound to 1 mg protein of platelet membranes (KD = 9.1 ± 5.3 nM). Clopidogrel and other thiol reagents like pCMBS or DTT abolished the binding both to intact platelets and membrane preparations. Therefore, [3H]PSB-0413 is an accurate and selective tool for radioligand binding studies aimed at quantifying P2Y12 receptors, to identify patients with P2Y12 deficiencies or quantify the effect of P2Y12 targeting drugs.
Keywords: P2Y12 receptor, Competitive antagonist, Radioligand, [3H]PBS-0413, Platelets, Nucleotide analog, AR-C67085MX
Introduction
Platelet activation by ADP plays a crucial role in hemostasis and thrombosis, and their so-called P2 receptors are potential targets for antithrombotic drugs. Two G protein-coupled ADP receptors, P2Y1 and P2Y12, selectively contribute to platelet aggregation. The P2Y1 receptor is responsible for ADP-induced shape change, weak and transient aggregation, while the P2Y12 receptor is responsible for the completion and amplification of the response to ADP and to all platelet agonists including thromboxane A2, thrombin, and collagen [1]. Due to its central role in the formation and stabilization of a thrombus, the P2Y12 receptor is a well-established target of drugs like the thienopyridines (ticlopidine, clopidogrel, prasugrel) and ticagrelor which proved to have potent antithrombotic efficacy both in clinical trials in humans and in experimental models of thrombosis [2,3]. One important point is to be able to quantify the number of receptors expressed on platelets in order to assess the inter-individual variability in the general population, to characterize patients with inherited deficiencies, and to monitor and study patients treated with P2Y12 targeting drugs [4].
Various radioligands have been used to characterize and quantify the platelet P2Y receptors such as, [14C]ADP [5,6], [3H]ADP [6,7], [3H]2-methylthio-ADP [8], [β-32P]2-methylthio-ADP [9,10], and [β-33P]2-methylthio-ADP [11], but they all share several weaknesses: (a) They are metabolically unstable and may be cleaved by a number of enzymes such as alkaline phosphatase and ectonucleotidases; (b) being agonists, they may complicate the quantification when intact, living cells are used and receptors are internalized upon activation; (c) they do not discriminate between P2Y1 and P2Y12 receptors. In the last decade, the only one possibility to selectively quantify P2Y12 receptors was to use the non-selective radiolabeled ligand 2-methylthio-ADP in the presence of a P2Y1 antagonist such as N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179) [12] or 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2500) [13]. Concerning P2Y1, recent studies used the very selective antagonist MRS2500 as a radioligand [14,15]. From these studies, it is thought that platelets express approximately 150 P2Y1 receptor copies/cell. Concerning P2Y12, based on earlier radioligand binding studies, the current idea is that individual platelets express around 450–1,000 copies. In the present study, we wished to more precisely quantify and characterize the P2Y12 receptors in intact platelets as well as in membrane fractions. To do that, we used the recently described [3H]PSB-0413 [16,17], which is a tritiated derivative of a selective antagonist of the P2Y12 receptor, the 2-propylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride (AR-C67085MX) compound. We also measured the binding to platelets from known patients, two with severe P2Y12 deficiency [6,18] and one with a dysfunctional P2Y12 receptor [19] and from clopidogrel treated mice.
Materials and methods
Subjects
For experiments on human platelets, blood was taken from ten healthy subjects and three patients with congenital defects of the platelet P2Y12 receptors. Two patients (A and B) displayed severe deficiency of the receptors, which was associated with a homozygous frameshift mutation resulting in premature truncation of the protein in patient A [6,20] or with heterozygous frameshift mutation resulting in premature truncation of the protein and haploinsufficiency in patient B [18,20]. The platelets from the third patient displayed abnormal P2Y12-mediated responses to ADP, but normal number of dysfunctional P2Y12 receptors, associated with Arg256 to Gln transition in one allele and a Arg265 to Trp transition in the other allele [19].
Chemicals
[3H]PSB-0413 was prepared by catalytic hydrogenation with tritium gas (GE, Healthcare, Buckinghamshire, UK) of the propargyl precursor PSB-0412 as described [16]. Apyrase was purified from potatoes as previously described [21]. AR-C69931MX (Cangrelor) was provided by the Medicines Company (Parsippany, NJ, USA). MRS2179 was purchased from TOCRIS (Bristol, UK). ADP was from Sigma-Aldrich Corp. (St. Louis, MO, USA). Clopidogrel was from Sanofi-Aventis (Sanofi-Aventis, France). All other drugs were from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Cell culture
Human astrocytoma 1321N1 cells (reference no. 80630402, European Collection of Cell Cultures, UK) stably expressing the P2Y1 or the P2Y12 receptor and subcloned were used for binding experiments and cultured as described previously [22]. For binding experiments, cells were removed by trypsinization and resuspended in PBS at a concentration of 2.107 cells/ml.
Preparation of washed platelet suspension
Human blood was collected from a forearm vein and mouse blood from the aorta under ether anesthesia acid–citrate–dextrose anticoagulant. Washed platelets from human or mouse blood were prepared as previously described [21]. Platelets were suspended in Tyrode’s buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 5.5 mM glucose, 5 mM Hepes, pH 7.3) containing 0.35 % human serum albumin and apyrase (0.02 U/ml). The platelet count was adjusted at a concentration of 600,000 platelets/μl and the suspension maintained at 37 °C until use.
Preparation of platelet membranes
Human platelet membranes were prepared essentially as described [23]. Briefly, washed platelets were resuspended in Tyrode’s buffer containing no Ca2+, in the presence of 2 mM EDTA and apyrase (0.02 U/ml) at room temperature. These platelets were loaded with glycerol by centrifugation through a 0–30 % (v/v) glycerol gradient and lysed in a hypotonic Tris buffer containing 2 mM EDTA and a cocktail of protease inhibitors. After lysis, the broken platelets were layered onto a 30 % (w/v) sucrose cushion and centrifuged for 4 h at 60,000×g. The floating membranes were removed carefully with a plastic pipette, washed and pelleted by centrifugation for 1 h at 100,000×g. Mouse platelet membranes were prepared by nitrogen cavitation as described [24]. The broken platelets (crude membranes) were centrifuged at 30,000×g for 1 h. Human plasma membrane pellets and mouse crude membrane pellets were resuspended in 50 mM Tris HCl, pH 7.5 containing 3 % (v/v) glycerol. Protein concentrations from human and mouse platelet membranes were determined using the BCA assay and adjusted at 1 mg/ml.
Platelet aggregation
Aggregation was measured at 37 °C by a turbidimetric method in a Carat TX4 aggregometer (Entec GmbH, Ilmenau, Germany). Platelets were activated by addition of 5 μM ADP in the absence or presence of 100 nM of [3H]PSB-0413 and human fibrinogen (0.8 mg/ml). The extent of aggregation was estimated quantitatively by measuring the maximum curve height above the baseline level.
Radioligand binding assay with human and mouse platelets
Binding experiments were performed with 200 μl of human or mouse washed platelets or with 20 μg of membrane proteins using [3H]PSB-0413 ranging from 0.030 to 50 nM (hot saturation). Non-specific binding was defined in the presence of 1 mM ADP. Intact platelets were incubated at 37 °C for 5 min while platelet membranes were incubated at 25 °C for 60 min. The reaction was stopped by washing the filters with 3 × 5 ml of ice-cold washing buffer (Tris HCl 50 mM pH 7.5, EDTA 1 mM, MgCl2 5 mM, NaCl 100 mM). Bound and free radioactivity was separated by filtration through Whatman GF/B glass fiber filters for intact platelets or through nitrocellulose filters for platelet membranes using a Brandel cell harvester. Filter-bound radioactivity was counted using a liquid scintillation counter. Assays were performed in triplicate in three independent experiments.
Data analysis
Data binding was analyzed with the program EBDA-LIGAND [25]. The dissociation constant (KD) of the radioligand and the inhibition constant for the drug (KI) were calculated using the GraphPad software package (GraphPad, San Diego, CA, USA). The results are presented as the mean ± SEM averaged from three or more independent experiments.
Results
[3H]PSB-0413 is a selective P2Y12 antagonist radioligand
Platelet aggregation induced by 5 μM ADP is completely inhibited by 100 nM of the selective antagonist radioligand [3H]PSB-0413 (Fig. 1a) while the P2Y1-dependent shape change, reflected by the initial decrease in light transmission upon addition of ADP, is preserved (Fig. 1b). The selectivity of [3H]PSB-0413 was further confirmed using P2Y1 and P2Y12 transfected 1321N1 cell lines. Binding only occurred in P2Y12 transfected cells, while no binding was observed in control or P2Y1 transfected cells (Fig. 1c). Furthermore, the binding of [3H]PSB-0413 was completely abolished in the presence of 10 μM of the P2Y12 selective antagonist AR-C69931MX (Fig. 1c).
Fig. 1.
Pharmacological characterization. a [3H]PSB-0413 was obtained by catalytic hydrogenation using tritium gas with a specific radioactivity of 1.85 to 3.7 TBq/mmol where 3H was fixed on the propyl moieties (black stars). b Washed platelets were activated with 5 μM ADP in the presence of human fibrinogen and in the absence or presence of 100 nM of [3H]PSB-0413. c 1321N1 cell lines transfected with P2Y12, P2Y1 receptors or control (empty vector) were incubated with 1 nM of [3H]PSB-0413 at 37 °C for 5 min. Binding was performed in the presence (closed square) or absence (open square) of 10 μM of the P2Y12 antagonist AR-C69931MX. Assays were performed in triplicate in three independent experiments
Binding properties of [3H]PSB-0413 to intact human platelets
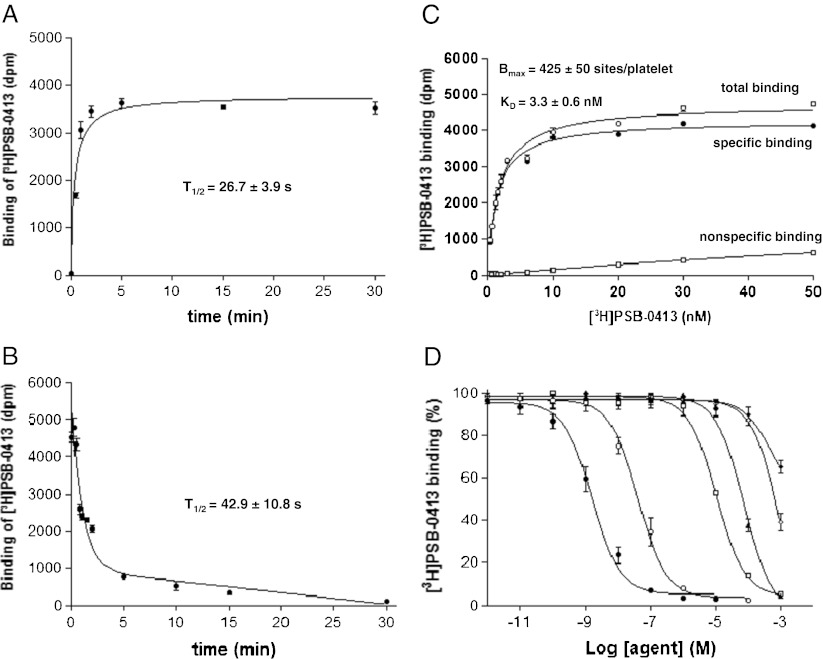
In a first series of experiments, we wanted to establish the general binding properties of [3H]PSB-0413 toward intact platelets. The kinetic of association was very fast (t1/2 of 26.7 ± 3.9 s), and the steady-state was reached within 5 min (Fig. 2a). After 15 min incubation, the dissociation was initiated by the addition of 1 mM ADP and the residual binding measured over time during 30 min (Fig. 2b). The kinetic of dissociation was also fast (t1/2 = 42.9 ± 10.8 s), and dissociation was completed after 30 min incubation. Saturation on intact human platelets is reached with concentration range of 30 nM [3H]PSB-0413, and under these conditions, non-specific binding in the presence of 1 mM of ADP was 7.5 % of total binding (Fig. 2c). Saturation curves generated on intact platelets from ten healthy volunteers revealed a single type of binding site with a high affinity (KD) of 3.3 ± 0.6 nM and number of binding sites (Bmax) of 425 ± 50 sites per platelet (Fig. 2c). Displacement experiments confirmed the known features of the P2Y12 receptor with the expected rank order of potency of various ligands: KI = 1.5 ± 0.26 nM (AR-C69931MX), 32.1 ± 3.03 nM (2MeSADP), 7.3 ± 1.76 μM (ADPβS), and 75 ± 29.3 μM (ADP), while the P2Y1 ligand MRS2179 and the P2X1 ligand α,β-Me-ATP at 100 μM did not displace the binding of [3H]PSB-0413 at concentrations below the millimolar range (Fig. 2d). Saturation experiments on intact platelets from two patients (A and B) with known severe P2Y12 deficiency [6,18] displayed virtually no binding, while one patient with dysfunctional P2Y12 (C) [19] displayed normal binding (Table 1).
Fig. 2.
Binding and pharmacological properties of [3H]PSB-0413 on human intact platelets. a Association kinetic of [3H]PSB-0413 binding on human intact platelets, measured at 37 °C over 30 min. b Dissociation kinetic was measured after [3H]PSB-0413 binding had reached a steady state (15 min) and was initiated by addition of 1 mM ADP. Association and dissociation curves are representative from three independent experiments. c Saturation experiment on human intact platelets from ten healthy subjects was achieved using different concentrations of [3H]PSB-0413 ranging from 0.05 to 50 nM (closed circle). Non-specific binding assessed in the presence of excess ADP (1 mM) and isotherm binding curves were calculated using the software EBDA-LIGAND in the hot-saturation mode. The saturation curve is representative of ten independent experiments. d Competition experiments were performed at 37 °C after a 5-min incubation with 5 nM of [3H]PSB-0413 in the presence of increasing concentration of: AR-C69931MX (closed circle), 2MeSADP (open circle), ADPβS (open square), ADP (closed triangle), α,β-Me-ATP (open diamond), and MRS2179 (closed diamond). Competition curves are the mean of three independent experiments
Table 1.
Binding studies of [3H]PSB-0413 on intact platelets from patients with selective inherited P2Y12 deficiencies
| Control | Patient A | Patient B | Patient C | |
|---|---|---|---|---|
| Bmax (sites/platelet) | 425 ± 50 | 0 | 0 | 420 |
| KD (nM) | 3.3 ± 0.6 | nc | nc | 3.6 |
Saturation experiments were performed on human intact platelets from two patients (A,B) with severe P2Y12 deficiency [6,18] and one patient with dysfunctional P2Y12 (C) [19] and on ten healthy donors as control. The number of binding sites (Bmax) and the affinity constant (KD) were calculated using EBDA-LIGAND software
nc not calculated
Effects of thiol reagents on [3H]PSB-0413 binding
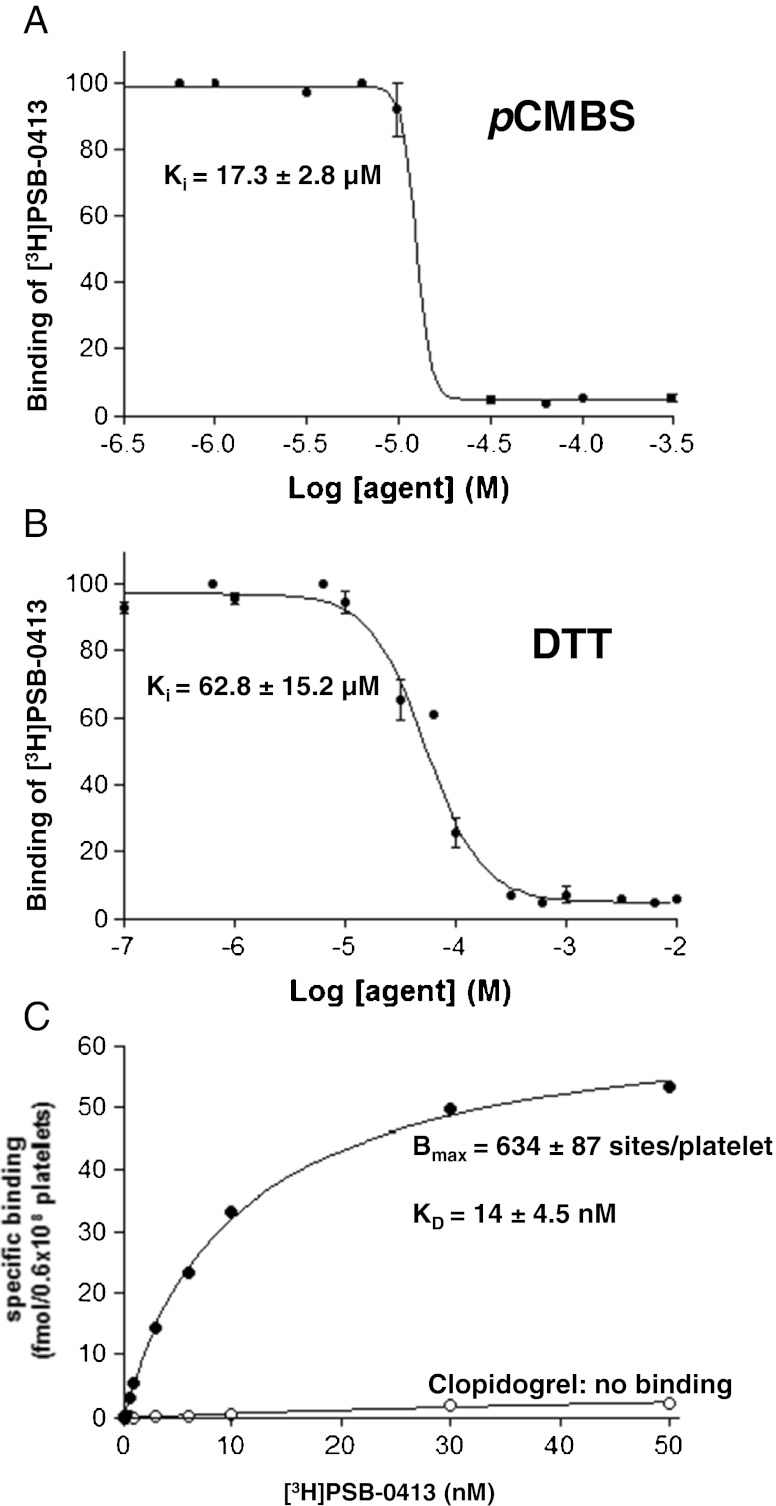
Clopidogrel, one of the most prescribed P2Y12 targeting drugs, is a prodrug of which the active liver metabolite is a thiol reagent which covalently binds to cysteine moieties of the P2Y12 receptor. Other thiol reagents were previously reported to target the P2Y12 receptor [4,9,26–28]. We thus wanted to check whether these compounds have an effect on the binding of [3H]PSB-0413. The reducing agents para-chloromercuribenzene sulfonic acid (pCMBS) or dithiothreitol (DTT) inhibited the binding of [3H]PSB-0413 on intact cells in a dose-dependent manner with KI of 17.3 ± 2.8 μM for p-CMBS (Fig. 3a) and a KI of 62.8 ± 15.2 μM for DTT (Fig. 3b). The evaluation of the effect of clopidogrel on [3H]PSB-0413 binding to intact platelets was performed in mice treated by clopidogrel 50 mg/kg, a dose that inhibits 100 % platelet aggregation by ADP [29]. Control mouse platelets display 643 ± 87 [3H]PSB-0413 binding sites/platelet with a KD = 14 ± 4.5 nM (Fig. 3c). The binding of [3H]PSB-0413 to platelets from clopidogrel treated mice was completely abolished.
Fig. 3.
Effects of thiol reagents on [3H]PSB-0413 binding to intact human and mouse platelets. a, b Inhibition experiments were performed by incubating 5 nM of [3H]PSB-0413 with intact platelets pretreated for 10 min with increasing concentrations of thiol reagents: apCMBS and b DTT; inhibition curves are the mean of three independent experiments. c Saturation experiment was achieved on intact platelets from control mice (closed circle) or from mice that had been treated with 50 mg/kg clopidogrel (open circle) using concentrations of [3H]PSB-0413 ranging from 0.05 to 50 nM. The saturation curve is representative from three independent experiments
Binding of [3H]PSB-0413 to platelet membranes
Saturation experiments showed that the radioligand bound to a single class of binding sites with a KD = 6.5 ± 3.6 nM and a Bmax = 0.5 ± 0.2 pmol/mg in human platelet membranes (Fig. 4a) and KD = 9.1 ± 5.3 nM and Bmax = 0.7 ± 0.3 pmol/mg in mouse platelet membranes (Fig. 4b). The binding of [3H]PSB-0413 to mouse platelet membrane preparations was completely abolished after clopidogrel treatment. Similarly the direct competitive P2Y12 antagonist AR-C69931MX inhibited the binding, while the P2Y1 ligand MRS2179 had only minimal effect at concentrations above 100 μM (Fig. 4c).
Fig. 4.
Binding and pharmacological properties of [3H]PSB-0413 on platelet membranes. Saturation experiments on a human or b mice platelet membrane preparations were achieved by incubating 20 μg of membrane proteins at 25 °C for 60 min with different concentrations of [3H]PSB-0413 ranging from 0.030 to 50 nM. c Binding on platelet membranes from control or clopidogrel (50 mg/kg) treated mice was achieved using 5 nM of [3H]PSB-0413 in the absence (white square) or presence of 1 μM AR-C69931MX (gray square) or 100 μM MRS2179 (black square). Assays were performed in triplicate in three independent experiments
Discussion
Reliable methodology for the quantification of P2Y12 receptor binding sites as well as their characterization is crucial for advancing our understanding of a variety of conditions. For example, there is a debate on whether the platelet P2Y12 receptor rapidly desensitizes and undergoes trafficking upon agonist activation [30–36]. Similarly, polymorphisms of the P2Y12 receptor have been proposed to be associated with a gain of function in terms of platelet activation and an increased risk of cardiovascular disease [37–41], but the relative densities and binding properties of the platelet P2Y12 receptor associated with these polymorphisms are not known. Finally, patients with inherited P2Y12 defects may present with total absence of receptor expression [6,18,42] while other express abnormal receptors with modified binding properties or normal binding properties and, likely, defective signal transduction [19,42], which require fine characterization [4,20,42]. The [3H]PSB-0413 selective P2Y12 radioligand was reported recently with only preliminary evaluation as a selective tool using membrane preparations [16] but not intact platelets from healthy control, patients with inherited P2Y12 defects, P2Y12 targeting drugs treated patients or animals. The data reported here establish [3H]PSB-0413 as a valuable new radioligand to investigate mechanistic aspects of the P2Y12 receptor in these and other conditions.
Not surprisingly, we found that clopidogrel completely inhibited the binding of [3H]PSB-0413 in intact mouse platelets, and it is not hazardous to speculate that it does the same in human platelets. However, we were happy to be able to measure the effect of clopidogrel in membrane preparations (Fig. 4c) as we were not able to do so in a previous study using [33P]2MeSADP and membrane preparations from clopidogrel treated rats [11]. In fact, reasoning that clopidogrel acts through an active metabolite that covalently binds the cysteine residues of the P2Y12 receptor, we thought to evaluate the impact of DTT itself on the binding of [3H]PSB-0413. As expected, DTT strongly inhibited the binding as did the chemical pCMBS which is a well-known inhibitor of ADP-induced platelet aggregation [9,26–28,43]. Since DTT was present in our previous membrane preparations to preserve them from oxidation, it is not surprising that we could not observe any difference between clopidogrel treated and control rats. We now have a clear explanation of the previous failure to measure the effect of clopidogrel on platelet membrane preparations.
Overall, the results presented here do not dramatically differ from previous studies using non-selective radioligands. However, new tools now exist to clearly distinguish and properly quantify the P2Y12 receptor in the one hand and the P2Y1 receptor on the other hand using [3H]PSB-0413 and the [32P]MRS2500 [14] or [125I]MRS2500 [15] radioligands, respectively. Such studies will be very helpful not only in the platelet field but also in any other cell type and tissues where these receptors display regulated expression [44] .
Conclusion
[3H]PSB-0413 is an accurate and selective tool for radioligand binding studies aimed at quantifying P2Y12 receptors, to identify patients or quantify the effect of P2Y12 targeting drugs.
Abbreviations
- AR-C67085MX
2-Propylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride
- AR-C69931MX (cangrelor)
N6-(2-Methylthioethyl)-2-(3,3,3-trifluoropropylthio)adenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride
- [3H]PSB-0413
[3H]2-Propylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride
- PSB-0412 (precursor of [3H]PSB-0413)
2-Propargylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride
- MRS2179
N6-Methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2500
2-Iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate)
- pCMBS
para-Chloromercuribenzene sulfonic acid
Footnotes
A. El-Tayeb is on leave from the University of Al-Azhar, Assiut, Egypt.
References
- 1.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 2.Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost. 2005;31(2):174–183. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo M. New P2Y(12) inhibitors. Circulation. 2010;121(1):171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M. Bleeding manifestations of congenital and drug-induced defects of the platelet P2Y12 receptor for adenosine diphosphate. Thromb Haemost. 2011;105(Suppl 1):S67–74. doi: 10.1160/THS10-11-0742. [DOI] [PubMed] [Google Scholar]
- 5.Lips JP, Sixma JJ, Schiphorst ME. Binding of adenosine diphosphate to human blood platelets and to isolated blood platelet membranes. Biochim Biophys Acta. 1980;628(4):451–467. doi: 10.1016/0304-4165(80)90394-3. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992;80(11):2787–2796. [PubMed] [Google Scholar]
- 7.Jefferson JR, Harmon JT, Jamieson GA. Identification of high-affinity (Kd 0.35 mumol/L) and low-affinity (Kd 7.9 mumol/L) platelet binding sites for ADP and competition by ADP analogues. Blood. 1988;71(1):110–116. [PubMed] [Google Scholar]
- 8.Savi P, Laplace MC, Maffrand JP, Herbert JM. Binding of [3H]-2-methylthio ADP to rat platelets—effect of clopidogrel and ticlopidine. J Pharmacol Exp Ther. 1994;269(2):772–777. [PubMed] [Google Scholar]
- 9.Macfarlane DE, Srivastava PC, Mills DC. 2-Methylthioadenosine[beta-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets. J Clin Invest. 1983;71(3):420–428. doi: 10.1172/JCI110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macfarlane DE. 2-Methylthioadenosine [beta-32P]diphosphate: synthesis and use as probe of platelet ADP receptors. Methods Enzymol. 1992;215:137–142. doi: 10.1016/0076-6879(92)15059-L. [DOI] [PubMed] [Google Scholar]
- 11.Gachet C, Cattaneo M, Ohlmann P, Hechler B, Lecchi A, Chevalier J, Cassel D, Mannucci PM, Cazenave JP. Purinoceptors on blood platelets: further pharmacological and clinical evidence to suggest the presence of two ADP receptors. Br J Haematol. 1995;91(2):434–444. doi: 10.1111/j.1365-2141.1995.tb05319.x. [DOI] [PubMed] [Google Scholar]
- 12.Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412(3):213–221. doi: 10.1016/S0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15(13–14):570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-Iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphat e ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147(5):459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohlmann P, de Castro S, Brown GG, Jr, Gachet C, Jacobson KA, Harden TK. Quantification of recombinant and platelet P2Y(1) receptors utilizing a [(125)I]-labeled high-affinity antagonist 2-iodo-N(6)-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([(125)I]MRS2500) Pharmacol Res. 2010;62(4):344–351. doi: 10.1016/j.phrs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Tayeb A, Griessmeier KJ, Muller CE. Synthesis and preliminary evaluation of [3H]PSB-0413, a selective antagonist radioligand for platelet P2Y12 receptors. Bioorg Med Chem Lett. 2005;15(24):5450–5452. doi: 10.1016/j.bmcl.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 17.Baqi Y, Atzler K, Kose M, Glanzel M, Muller CE. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem. 2009;52(12):3784–3793. doi: 10.1021/jm9003297. [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol. 2000;20(11):E101–106. doi: 10.1161/01.ATV.20.11.e101. [DOI] [PubMed] [Google Scholar]
- 19.Cattaneo M, Zighetti ML, Lombardi R, Martinez C, Lecchi A, Conley PB, Ware J, Ruggeri ZM. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci USA. 2003;100(4):1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattaneo M. The platelet P2Y receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011;117(7):2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- 21.Cazenave JP, Ohlmann P, Cassel D, Eckly A, Hechler B, Gachet C. Preparation of washed platelet suspensions from human and rodent blood. Methods in Mol Biol (Clifton NJ. 2004;272:13–28. doi: 10.1385/1-59259-782-3:013. [DOI] [PubMed] [Google Scholar]
- 22.Kauffenstein G, Hechler B, Cazenave JP, Gachet C. Adenine triphosphate nucleotides are antagonists at the P2Y receptor. J Thromb Haemost. 2004;2(11):1980–1988. doi: 10.1111/j.1538-7836.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 23.Barber AJ, Jamieson GA. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970;245(23):6357–6365. [PubMed] [Google Scholar]
- 24.Broekman MJ. Homogenization by nitrogen cavitation technique applied to platelet subcellular fractionation. Methods Enzymol. 1992;215:21–32. doi: 10.1016/0076-6879(92)15049-I. [DOI] [PubMed] [Google Scholar]
- 25.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 26.Cristalli G, Mills DC. Identification of a receptor for ADP on blood platelets by photoaffinity labelling. Biochem J. 1993;291(Pt 3):875–881. doi: 10.1042/bj2910875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood. 2003;101(10):3908–3914. doi: 10.1182/blood-2002-10-3027. [DOI] [PubMed] [Google Scholar]
- 28.Margaritis A, Priora R, Frosali S, Di Giuseppe D, Summa D, Coppo L, Di Stefano A, Di Simplicio P. The role of protein sulfhydryl groups and protein disulfides of the platelet surface in aggregation processes involving thiol exchange reactions. Pharmacol Res. 2011;63(1):77–84. doi: 10.1016/j.phrs.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99(3):466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 30.Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, Gachet C. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84(3):484–491. [PubMed] [Google Scholar]
- 31.Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Leon C, Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67(3):721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 32.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105(9):3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 33.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic (Copenhagen, Denmark) 2006;7(10):1420–1431. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with beta-arrestin-1 and −2. J Biol Chem. 2008;283(45):30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nisar S, Daly ME, Federici AB, Artoni A, Mumford AD, Watson SP, Mundell SJ. An intact PDZ motif is essential for correct P2Y12 purinoceptor traffic in human platelets. Blood. 2011;118(20):5641–5651. doi: 10.1182/blood-2011-02-336826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaff M, Receveur N, Bourdon C, Ohlmann P, Lanza F, Gachet C, Mangin P (2012) beta-Arrestin-1 participates in thrombosis and regulates integrin alphaIIb-beta3 signalling without affecting P2Y receptors desensitization and function. Thromb Haemost 107:735–748 [DOI] [PubMed]
- 37.Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108(8):989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 38.Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case–control study. Circulation. 2003;108(24):2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 39.Fontana P, Remones V, Reny JL, Aiach M, Gaussem P. P2Y1 gene polymorphism and ADP-induced platelet response. J Thromb Haemost. 2005;3(10):2349–2350. doi: 10.1111/j.1538-7836.2005.01483.x. [DOI] [PubMed] [Google Scholar]
- 40.Bierend A, Rau T, Maas R, Schwedhelm E, Boger RH. P2Y12 polymorphisms and antiplatelet effects of aspirin in patients with coronary artery disease. Br J Clin Pharmacol. 2008;65(4):540–547. doi: 10.1111/j.1365-2125.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009;133(3):341–345. doi: 10.1016/j.ijcard.2007.12.118. [DOI] [PubMed] [Google Scholar]
- 42.Cattaneo M. Molecular defects of the platelet P2 receptors. Purinergic signal. 2011;7(3):333–339. doi: 10.1007/s11302-011-9217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macfarlane DE. The effects of methyl mercury on platelets: induction of aggregation and release via activation of the prostaglandin synthesis pathway. Mol Pharmacol. 1981;19(3):470–476. [PubMed] [Google Scholar]
- 44.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]