Abstract
The presence of P2X7 on erythroid cells is well established, but its physiological role remains unclear. The current study aimed to determine if P2X7 activation induces reactive oxygen species (ROS) formation in murine erythroleukaemia (MEL) cells, a commonly used erythroid cell line. ATP induced ROS formation in a time- and concentration-dependent fashion. The most potent P2X7 agonist, 2′(3′)-O-(4-benzoylbenzoyl)ATP, but not UTP or ADP, also induced ROS formation. The P2X7 antagonist, A-438079, impaired ATP-induced ROS formation. The ROS scavenger, N-acetyl-l-cysteine, and the ROS inhibitor, diphenyleneiodonium, also impaired P2X7-induced ROS formation, but use of enzyme-specific ROS inhibitors failed to identify the intracellular source of P2X7-induced ROS formation. P2X7-induced ROS formation was impaired partly by physiological concentrations of Ca2+ and Mg2+ and almost completely in cells in N-methyl-d-glucamine chloride medium. The p38 MAPK inhibitors SB202190 and SB203580, and the caspase inhibitor Z-VAD-FMK, but not N-acetyl-l-cysteine, impaired P2X7-induced MEL cell apoptosis. ATP also stimulated p38 MAPK and caspase activation, both of which could be impaired by A-438079. In conclusion, these findings indicate that P2X7 activation induces ROS formation in MEL cells and that this process may be involved in events downstream of P2X7 activation, other than apoptosis, in erythroid cells.
Keywords: P2X7, Extracellular ATP, Red blood cell, Reactive oxygen species, Apoptosis
Introduction
The P2X7 receptor is a trimeric ATP-gated cation channel that mediates the influx of extracellular Ca2+ and Na+, and efflux of intracellular K+, as well as the uptake of organic cations including N-methyl-d-glucamine+ (NMDG+) and fluorescent dyes such as ethidium+ [1]. P2X7 is expressed on haematopoietic, epithelial, bone and neuronal cells, where it functions in inflammation and immunity [2] and cellular homeostasis [3]. Due to these and other properties of P2X7 activation, this receptor plays important roles in health and disease [4]. The various biological roles attributed to P2X7 are the result of various signalling events downstream of P2X7 activation including reactive oxygen species (ROS) formation [5]. P2X7 activation induces ROS generation in a variety of cell types resulting in transcription factor activation [6], pro-inflammatory cytokine release [7, 8], microbial killing [9], cell death [10, 11] and autophagy [12]. However, it remains unknown if P2X7 activation can induce ROS formation in erythroid cells.
The presence of P2X7 on erythroid cells is well established, but its physiological role remains unclear. P2X7 is present on mature red blood cells (erythrocytes) [13, 14] where it participates in cation fluxes [13, 15–17], cell death [16, 18–20] and the release of lipid-derived mediators [21]. P2X7 is also present on erythroid precursors including murine erythroleukaemia (MEL) cells [22, 23]. Our group directly demonstrated that MEL cells express P2X7, and that activation of this receptor induces the uptake of organic cations, rapid phosphatidylserine exposure, microparticle release and apoptosis in these cells [23]. In the current study, we demonstrate that P2X7 activation also induces ROS formation in MEL cells, supporting a potential role for P2X7-induced ROS formation in intracellular signalling or oxidative stress in erythroid cells.
Materials and methods
Reagents
RPMI-1640 medium, l-glutamine, gentamicin and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were from Invitrogen (Grand Island, NJ). Foetal calf serum (FCS; heat inactivated before use) was from Lonza (Basel, Switzerland) or Bovogen Biologicals (East Kellior, Australia). ATP, 2′(3′)-O-(4-benzoylbenzoyl)ATP (BzATP), ADP, UTP, rotenone, allopurinol, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), EGTA and BAPTA-AM were from Sigma Chemical Co (St. Louis, MO). A-438079 was from Tocris Bioscience (Ellisville, MO). N-acetyl-l-cysteine (NAC) and 7-aminoactinomycin D (7AAD) were from Alexis Biochemicals (Lausen, Switzerland). Diphenyleneiodonium (DPI) was from Cayman Chemical Company (Ann Arbor, MI). Ethidium bromide was from Amresco (Solon, OH). Apocynin and SB202190 were from Calbiochem (San Diego, CA). Annexin-V-FLUOS and DNase I were from Roche Applied Science (Pensberg, Germany). SB203580 and Z-VAD-FMK were from Jena Bioscience (Jena, Germany) and BioVision (Mountain View, CA), respectively.
Cell culture
MEL cells were maintained in complete culture medium (RPMI-1640 medium containing 10 % FCS, 2 mM l-glutamine and 5 ng ml−1 gentamicin) as described [23].
ROS formation measurements
ATP-induced ROS formation was assessed in MEL cells loaded with H2DCFDA, which is converted to the fluorescent compound 2′,7′-dichlorofluorescein (DCF) upon oxidation by ROS. Briefly, MEL cells in NaCl medium (145 mM NaCl, 5 mM KCl, 5 mM d-glucose and 10 mM HEPES at pH 7.4; 1 × 106 cells ml−1) were incubated with 5 μM H2DCFDA for 5 min at 37 °C. Cells were centrifuged and washed once with NaCl medium. H2DCFDA-loaded cells in NaCl medium were incubated in the absence or presence of nucleotides (as indicated) at 37 °C. Incubations were stopped by addition of an equal volume of ice-cold MgCl2 medium (145 mM NaCl, 5 mM KCl, 20 mM MgCl2 and 10 mM HEPES at pH 7.4) and centrifugation. Cells were washed once with NaCl medium. The mean fluorescence intensity (MFI) of DCF fluorescence (ROS formation) was determined using a BD (San Diego, CA) LSR II flow cytometer and FlowJo software (Tree Star, Ashland, OR).
In some experiments, ATP-induced ROS formation was assessed in cells pre-incubated in the absence or presence of different compounds (as indicated) at 37 °C before the addition of ATP. In other experiments, ATP-induced ROS formation was assessed in cells in NaCl medium containing 2 mM CaCl2 and 1 mM MgCl2, KCl medium (150 mM KCl, 5 mM d-glucose and 10 mM HEPES at pH 7.4), N-methyl-d-glucamine chloride (NMDG Cl) medium (145 mM NMDG Cl, 5 mM KCl, 5 mM d-glucose and 10 mM HEPES at pH 7.4), complete culture medium or in NaCl medium containing either 1 mM CaCl2, 100 μM EGTA or 1 mM Mg2+. Since free Ca2+ or Mg2+ can lower the concentration of ATP4-, cells in the presence of 1 mM Ca2+ or 1 mM Mg2+ in some experiments were incubated with 1.54 or 1.74 mM ATP, respectively to provide equimolar ATP4− concentrations (415 μM) as calculated using the Bound and Determined Program [24].
Ethidium+ uptake measurements
P2X7-induced pore formation in MEL cells suspended in NaCl medium was assessed using a fixed-time ethidium+ uptake assay as described [23]. The MFI of ethidium+ uptake (pore formation) was determined by flow cytometry. In some experiments, ATP-induced ethidium+ uptake was assessed in cells pre-incubated with different compounds or incubated in different media as above (for ROS formation).
Apoptosis measurements
P2X7-induced apoptosis of MEL cells was performed as described [23]. Briefly, cells were pre-incubated in the absence or presence of different compounds (as indicated) at 37 °C, followed by incubation in the absence or presence of 1 mM ATP for 24 h. Following ATP incubation, the percentage of Annexin-V+/7AAD− (early apoptotic) and Annexin-V+/7AAD+ (late apoptotic) cells was determined by flow cytometry.
p38 MAPK activation measurements
MEL cells in complete culture medium in 24-well plates (5 × 105 cells ml−1 well−1) were pre-incubated in the absence or presence of 10 μM A-438079 for 15 min at 37 °C, followed by incubation in the absence or presence of 1 mM ATP for 5 min. Harvested cells were suspended in 100 μL ice-cold SDS sample buffer containing 5 % 2-mercaptoethanol, and sonicated in the presence of DNase I (10 μg ml−1) for 5 min. Proteins were separated by SDS-PAGE (4 % stacking and 10 % resolving gels) and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Immunoblotting of p38 MAPK and phospho-p38 MAPK was performed using the PhosphoPlus p38 MAPK (Thr180/Tyr182) Antibody Kit (Cell Signaling Technology, Danvers, MA) according to the manufacturer’s instructions.
Caspase activation measurements
Caspase activation was assessed using the CaspGLOW Fluorescein Active Caspase Staining Kit (BioVision) according to the manufacturer’s instructions. Briefly, MEL cells in complete culture medium in 24-well plates (5 × 105 cells ml−1 well−1) were pre-incubated in the absence or presence of 10 μM A-438079 for 15 min at 37 °C, followed by incubation in the absence or presence of 1 mM ATP for 6 h. Following ATP incubation, cells were incubated with fluorescein isothiocyanate (FITC)-VAD-FMK for 1 h at 37 °C; 7AAD was added during the final 5 min of incubation. Cells were harvested and washed twice with Wash Buffer. The percentage of FITC+/7AAD+ (caspase activated) cells was determined by flow cytometry.
Presentation of data and statistical analysis
Results are presented as means ± SD. Data were compared using one-way analysis of variance (using Tukey’s post-test) or (when indicated) the unpaired two-tailed Student’s t test using Prism 5 (GraphPad Software, San Diego, CA) with P < 0.05 considered significant.
Results
P2X7 activation induces ROS formation in MEL cells
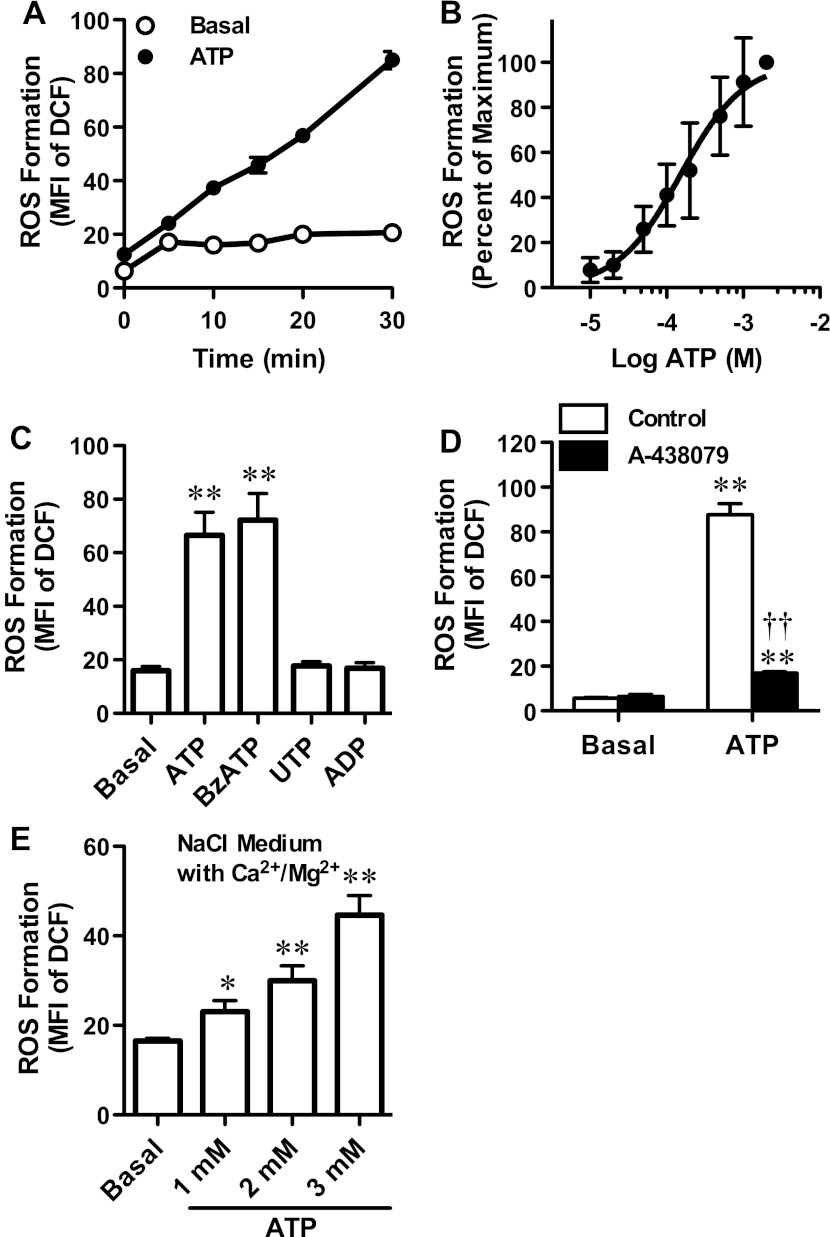
To determine whether P2X7 activation can induce ROS formation in MEL cells, cells were loaded with H2DCFDA, which is converted to the fluorescent compound DCF upon oxidation by ROS. Cells in NaCl medium (nominally free of Ca2+ and Mg2+) were then incubated in the absence or presence of ATP for up to 30 min and the amount of ROS formation determined by flow cytometry. ATP induced ROS formation in MEL cells in a time-dependent fashion (Fig. 1a). Moreover, ATP induced ROS formation in a concentration-dependent fashion, with a maximal response at 2 mM ATP and an EC50 of 151 μM (95 % confidence interval of 120–190 μM) (Fig. 1b). This is similar to the EC50 for ATP-induced ethidium+ uptake in MEL cells [23] and for cation fluxes mediated by recombinant murine P2X7 [25].
Fig. 1.
P2X7 activation induces ROS formation in MEL cells. H2DCFDA-loaded MEL cells in a–d NaCl medium or e NaCl medium (containing 2 mM Ca2+ and 1 mM Mg2+) were incubated at 37 °C in the absence (Basal) or presence of a 1 mM ATP for up to 30 min, b varying ATP concentrations for 15 min, c 1 mM ATP, UTP or ADP or 200 μM BzATP for 15 min, d 10 μM A-438079 for 15 min and then in the absence or presence of 1 mM ATP for 15 min or e 1, 2 or 3 mM ATP for 15 min. a–e Incubations were stopped by addition of MgCl2 medium and centrifugation, and the mean fluorescence intensity (MFI) of DCF (ROS formation) was determined by flow cytometry. ROS formation is expressed as a, c–e MFI or b percentage of maximum response compared with 2 mM ATP. Results are mean ± SD (a, c–en = 3 and bn = 6); where the SD is not visible, SD is ≤2.6; *P < 0.05 compared with corresponding basal; **P < 0.01 compared with corresponding basal; ††P < 0.01 compared with ATP alone (c, d ANOVA and e Student’s t test)
To determine further if the ATP-induced ROS formation in MEL cells was mediated by P2X7 activation, cells were incubated in the absence or presence of the most potent P2X7 agonist BzATP or the non-P2X7 agonists ADP or UTP [25] although it should be noted that BzATP also activates P2X1–P2X5 [26, 27]. ATP was included as a positive control. Both ATP and BzATP induced significant ROS formation in MEL cells compared with cells incubated in the absence of nucleotide (Fig. 1c). In contrast, ADP or UTP failed to induce ROS formation (Fig. 1c). Finally, to confirm that ATP-induced ROS formation was mediated by P2X7 activation, MEL cells were pre-incubated in the absence or presence of the P2X7 antagonist A-438079, before incubation in the absence or presence of ATP. As above (Fig. 1a–c), ATP induced significant ROS formation in MEL cells compared with cells incubated in the absence of ATP (Fig. 1d). Pre-incubation of MEL cells with 10 μM A-438079 impaired ATP-induced ROS formation by 87 ± 1 % (Fig. 1d). Collectively, these results indicate that extracellular ATP induces ROS formation in MEL cells by P2X7 activation.
The above studies were conducted with MEL cells suspended in NaCl medium nominally free of Ca2+ and Mg2+. Therefore, to assess if ATP could induce ROS formation in MEL cells in medium containing physiological concentrations of divalent cations, MEL cells were suspended in NaCl medium containing 2 mM Ca2+ and 1 mM Mg2+, and the ATP-induced ROS formation was assessed. Due to the known inhibitory actions of Ca2+ and Mg2+ on P2X7 [28, 29], cells were exposed to 1 mM ATP (as above), as well as 2 and 3 mM ATP. ATP induced ROS formation in MEL cells in NaCl medium (containing physiological concentrations of divalent cations) in a concentration-dependent fashion (Fig. 1e).
P2X7-induced ROS formation in MEL cells is impaired by NAC and DPI
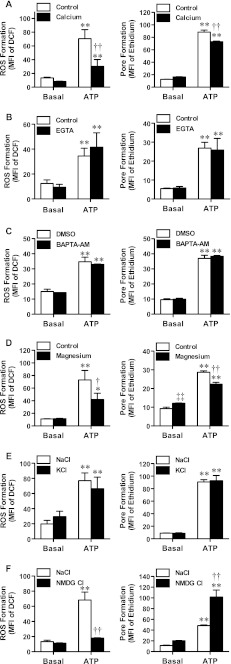
To confirm that P2X7 activation induced ROS formation in MEL cells, cells in NaCl medium (nominally free of Ca2+ and Mg2+) were pre-incubated in the absence or presence of the ROS scavenger NAC, or in the presence of DMSO diluent control or the broad-spectrum ROS inhibitor DPI before incubation in the absence or presence of ATP. As above (Fig. 1), ATP induced significant ROS formation in MEL cells compared with cells incubated in the absence of ATP (Fig. 2a, b). Pre-incubation of MEL cells with 10 mM NAC or 20 μM DPI impaired ATP-induced ROS formation by 70 ± 7 and 50 ± 15 %, respectively (Fig. 2a, b). To determine if NAC or DPI directly affected P2X7, ATP-induced ethidium+ uptake was measured in the absence or presence of each compound. Pre-incubation of MEL cells with NAC or DPI (as above) did not alter the amount of ATP-induced ethidium+ uptake (Fig. 2a, b).
Fig. 2.
P2X7-induced ROS formation in MEL cells is impaired by NAC and DPI. H2DCFDA-loaded MEL cells (left) or MEL cells (right) in NaCl medium were pre-incubated at 37 °C in the a, f absence (control) or presence of a 10 mM NAC for 30 min or f 1 mM l-NAME for 60 min or in the presence of b DMSO or 20 μM DPI for 30 min, c DMSO or 100 μM apocynin for 60 min, d DMSO or 5 μM rotenone for 60 min or e NaOH or 100 μM allopurinol for 30 min. a–f Cells were then incubated in the absence (basal) or presence of 1 mM ATP at 37 °C for 15 min; 25 μM ethidium+ was also present (right). Incubations were stopped by addition of MgCl2 medium and centrifugation, and the mean fluorescence intensity (MFI) of DCF (ROS formation; left) or ethidium+ uptake (pore formation; right) analysed by flow cytometry. Results are mean ± SD (n = 3); *P < 0.05 compared with corresponding basal; **P < 0.01 compared with corresponding basal; ††P < 0.01 compared with ATP without NAC or antagonist; ‡‡P < 0.01 compared with basal control
ROS can be generated from numerous sources within cells. Therefore, in an attempt to identify the intracellular source of ROS-generated downstream of P2X7 activation, MEL cells in NaCl medium (nominally free of Ca2+ and Mg2+) were pre-incubated in the presence of diluent control (as indicated), or apocynin, rotenone, allopurinol or l-NAME, which impair NADPH oxidase, mitochondrial complex I, xanthine oxidase or nitric oxide synthase, respectively, before incubation in the absence or presence of ATP. Pre-incubation times and antagonist concentrations were based on previous studies with murine macrophages [10, 11]. Again, ATP induced significant ROS formation in MEL cells compared with cells incubated in the absence of ATP (Fig. 2c–f). Pre-incubation of MEL cells with either 100 μM apocynin, 5 μM rotenone, 100 μM allopurinol or 1 mM l-NAME had no significant effect on ATP-induced ROS formation compared with cells incubated with both the respective diluent control and ATP (Fig. 2c–f). In the absence of ATP, ROS formation in the presence of apocynin, allopurinol or l-NAME was not significantly different to the respective diluent control (Fig. 2c, e, f). In contrast, in the absence of ATP, rotenone induced significant ROS formation compared with DMSO (Fig. 2d). Shorter (45 min) incubations with different concentrations of rotenone in the absence of ATP also induced ROS formation in MEL cells in concentration-dependent fashion, with significant ROS formation observed with a minimum of 0.5 μM rotenone and with an EC50 of >2 μM (results not shown). While 30 min pre-incubation of MEL cells with 0.1 μM rotenone potentiated ATP-induced ROS formation compared with cells incubated with both DMSO and ATP (results not shown). Pre-incubation of MEL cells with either apocynin, rotenone, allopurinol or l-NAME (as above) had no significant effect on ATP-induced ethidium+ uptake compared with cells incubated with both the respective diluent control and ATP (Fig. 2c–f).
P2X7-induced ROS formation is impaired in MEL cells in NaCl medium containing Ca2+ or Mg2+ or in NMDG Cl medium
P2X7 is a trimeric ATP-gated cation channel [1], therefore a series of experiments were undertaken to explore a possible role for divalent or monovalent cations in P2X7-induced ROS formation. For each experiment, cells in different media were compared with cells in NaCl medium (nominally free of Ca2+ and Mg2+). P2X7-induced ROS formation is dependent on Ca2+ influx in rat, but not murine, submandibular gland cells [30, 31]. Therefore, to determine if P2X7-induced ROS formation in MEL cells is dependent on Ca2+ influx, ATP-induced ROS formation was compared in cells in the absence or presence of additional Ca2+. ATP induced significant ROS formation in MEL cells in either the absence or presence of 1 mM Ca2+ compared with similarly treated cells incubated in the absence of ATP (Fig. 3a). Of note, ATP-induced ROS formation was significantly lower in the presence of Ca2+ compared with ATP-induced ROS formation in the absence of Ca2+ (Fig. 3a). This result paralleled observations with ATP-induced ethidium+ uptake, with ATP-induced ethidium+ uptake significantly lower in the presence of Ca2+ compared with ATP-induced ethidium+ uptake in the absence of Ca2+ (Fig. 3a).
Fig. 3.
P2X7-induced ROS formation is impaired in MEL cells in NaCl medium containing Ca2+ or Mg2+ or in NMDG Cl medium. H2DCFDA-loaded MEL cells (left) or MEL cells (right) in a, b and d NaCl medium in the absence (control) or presence of a 1 mM Ca2+, b 100 μM EGTA or d 1 mM Mg2+, c NaCl medium in the presence of DMSO or 10 μM BAPTA-AM (pre-incubated at 37 °C for 30 min), e NaCl or KCl medium or f NaCl or NMDG Cl medium were incubated in the absence (basal) or presence of 415 μM ATP4- (1, 1.54 or 1.74 mM ATP as explained in “Materials and methods”) at 37 °C for 15 min; 25 μM ethidium+ was also present (right). a–f Incubations were stopped by addition of MgCl2 medium and centrifugation, and the mean fluorescence intensity (MFI) of DCF (ROS formation; left) or ethidium+ uptake (pore formation; right) analysed by flow cytometry. Results are mean ± SD (n = 3); *P < 0.05 compared with corresponding basal; **P < 0.01 compared with corresponding basal; †P < 0.05 compared with ATP alone; ††P < 0.01 compared with ATP alone; ‡‡P < 0.01 compared with basal control
NaCl medium may contain nominal amounts of Ca2+. Therefore, to further exclude a role for Ca2+ influx in P2X7-induced ROS formation, ATP-induced ROS formation was compared in MEL cells in the absence or presence the Ca2+ chelator EGTA. ATP induced significant ROS formation in MEL cells in either the absence or presence of 100 μM EGTA compared with similarly treated cells incubated in the absence of ATP (Fig. 3b). Moreover, ATP-induced ethidium+ uptake was similar in MEL cells in either the absence or presence of EGTA (Fig. 3b) indicating that neither EGTA nor nominal amounts of extracellular Ca2+ had a direct effect on P2X7-induced pore formation.
Next, the potential role of intracellular Ca2+ increase in P2X7-induced ROS formation in MEL cells was examined by comparing ATP-induced ROS formation in MEL cells pre-incubated in the presence of DMSO or the intracellular Ca2+ chelator BAPTA-AM. ATP induced significant ROS formation in MEL cells in either the presence of DMSO or 10 μM BAPTA-AM compared with similarly treated cells incubated in the absence of ATP (Fig. 3c). Moreover, ATP-induced ethidium+ uptake was similar in MEL cells in either the presence of DMSO or BAPTA-AM (Fig. 3c) indicating that neither BAPTA-AM nor intracellular Ca2+ increase had a direct effect on P2X7-induced pore formation.
Since the current study predominately assessed P2X7-induced ROS formation in MEL cells in NaCl medium, which may contain nominal amounts of Mg2+, the possibility remained that this process was dependent on Mg2+ efflux. Therefore, ATP-induced ROS formation was compared in cells in the absence or presence of 1 mM Mg2+, a concentration similar to that of free intracellular Mg2+. ATP induced significant ROS formation in MEL cells in either the absence or presence of Mg2+ compared with similarly treated cells incubated in the absence of ATP (Fig. 3d). As above for Ca2+ (Fig. 3a), ATP-induced ROS formation was significantly lower in the presence of Mg2+ compared with ATP-induced ROS formation in the absence of Mg2+ (Fig. 3d). This result paralleled observations with ATP-induced ethidium+ uptake, with ATP-induced ethidium+ uptake significantly lower in the presence of Mg2+ compared with ATP-induced ethidium+ uptake in the absence of Mg2+ (Fig. 3d).
Both ROS formation and K+ efflux are involved in P2X7-induced interleukin-1β release from monocytes, although it remains to be determined if these upstream processes are linked [32]. Therefore, to determine if P2X7-induced ROS formation in MEL cells is dependent on K+ efflux, ATP-induced ROS formation was compared in cells in NaCl medium and KCl medium, which prevents the loss of intracellular K+. The amount of ATP-induced ROS formation in MEL cells in KCl medium was similar to ROS formation in cells in NaCl medium (Fig. 3e). To determine if the inability of high extracellular K+ to impair ATP-induced ROS formation was not due to altered P2X7 function in KCl medium, ATP-induced ethidium+ uptake was assessed in cells in both media. ATP-induced ethidium+ uptake was similar in MEL cells incubated in either medium (Fig. 3e).
The above results with KCl medium (Fig. 3e) indirectly exclude a role for Na+ influx in P2X7-induced ROS formation in MEL cells. It is well known however that high concentrations of extracellular K+ will depolarise cells and interfere with various transport systems. Therefore, ATP-induced ROS formation was compared in cells in NaCl medium and NMDG Cl medium, which is nominally free of Na+. Both media were nominally free of Ca2+ and Mg2+. In contrast to observations with KCl medium above (Fig. 3e), ATP-induced ROS formation was impaired by 88 ± 1 % in MEL cells in NMDG Cl medium compared with cells in NaCl medium (Fig. 3f). To determine if this inhibition was due to impaired P2X7 function in NMDG Cl medium, ATP-induced ethidium+ uptake was assessed in cells in both media. In contrast to ROS formation, ATP-induced ethidium+ uptake was approximately 2-fold greater in MEL cells incubated in NMDG Cl medium compared with cells in NaCl medium (Fig. 3f).
p38 MAPK and caspase activation, but not ROS formation, mediates P2X7-induced apoptosis of MEL cells
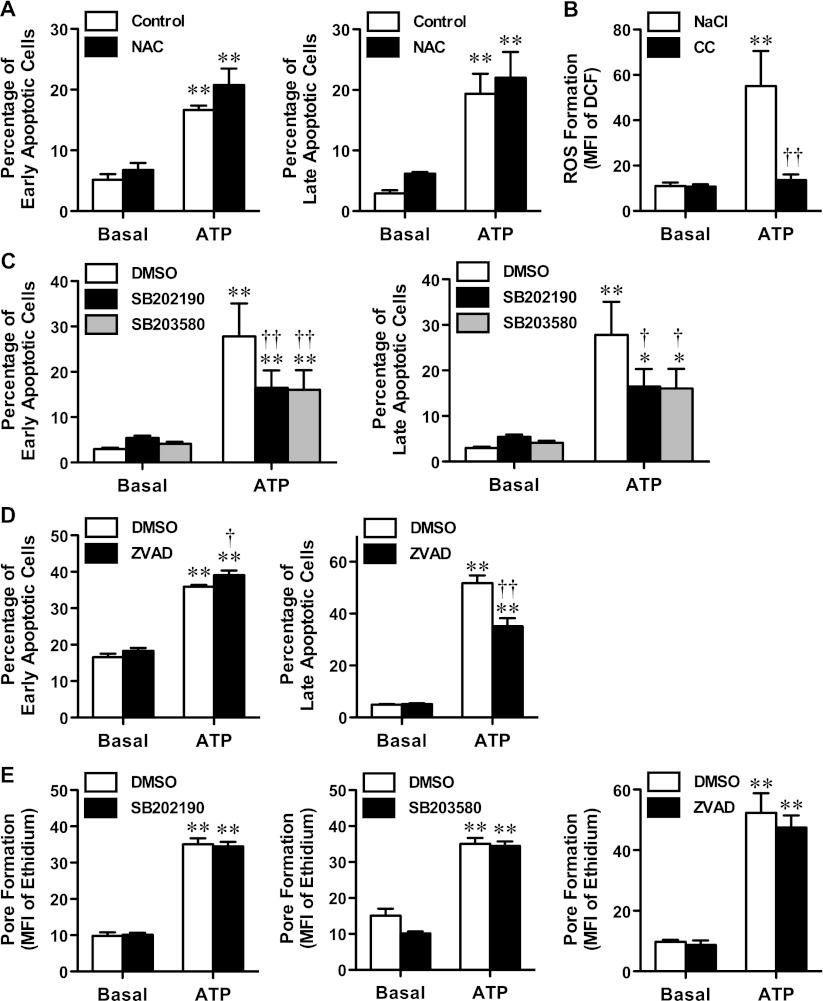
Our group previously demonstrated that P2X7 activation induces apoptosis of MEL cells [23]; however, the intracellular signalling pathways involved were not determined. ROS formation, as well as the p38 MAPK and caspase pathways are involved in P2X7-induced apoptosis of RAW264.7 macrophages [10]. Therefore, the roles of these pathways in P2X7-induced MEL cell apoptosis were examined. First, cells were pre-incubated in the absence or presence of NAC before incubation in the absence or presence of ATP. As previously observed [23], incubation of MEL cells with 1 mM ATP induced a significant increase in the percentage of Annexin-V+/7AAD− (early apoptotic) and Annexin-V+ /7AAD+ (late apoptotic) cells (Fig. 4a). Pre-incubation of MEL cells with 10 mM NAC failed to impair ATP-induced early or late apoptosis (Fig. 4a). Moreover, incubation of MEL cells in complete culture medium with 1 mM ATP (as used in the apoptosis assay) did not induce significant ROS formation despite 1 mM ATP causing significant ROS formation in MEL cells in NaCl medium (nominally free of Ca2+ and Mg2+) (Fig. 4b).
Fig. 4.
p38 MAPK and caspase activation, but not ROS formation, mediates P2X7-induced apoptosis in MEL cells. a, c and d MEL cells in complete culture medium were pre-incubated at 37 °C for 30 min a in the absence (control) or presence of 10 mM NAC, in the presence of c DMSO, 20 μM SB202190 or 20 μM SB203580, or d DMSO or 20 μM Z-VAD-FMK. Cells were then incubated in the absence (basal) or presence of 1 mM ATP at 37 °C for 24 h. Cells were harvested and labelled with Annexin-V-FLUOS and 7AAD, and the percentage of Annexin-V+/7AAD− (early apoptotic; left) or Annexin-V+/7AAD+ (late apoptotic; right) cells was analysed by flow cytometry. b H2DCFDA-loaded MEL cells in NaCl medium or complete culture medium (CC) were incubated in the absence (basal) or presence of 1 mM ATP at 37 °C for 15 min. Incubations were stopped by addition of MgCl2 medium and centrifugation, and the mean fluorescence intensity (MFI) of DCF (ROS formation) was determined by flow cytometry. e MEL cells in NaCl medium were pre-incubated at 37 °C for 30 min in the presence of DMSO or 20 μM SB202190 (left), DMSO or 20 μM SB203580 (centre) or DMSO or 20 μM Z-VAD-FMK (right). Ethidium+ (25 μM) was added and cells were incubated in the absence (basal) or presence of 1 mM ATP at 37 °C for 15 min. Incubations were stopped by addition of MgCl2 medium and centrifugation, and the MFI of ethidium+ uptake (pore formation) was analysed by flow cytometry. a–e Results are mean ± SD (n = 3); *P < 0.05 compared with corresponding basal; **P < 0.01 compared with corresponding basal; †P < 0.05 compared with ATP alone; ††P < 0.01 compared with ATP alone
Next, cells were pre-incubated in the presence of DMSO or the p38 MAPK inhibitors SB202190 or SB203580 before incubation in the absence or presence of ATP. Again ATP induced a significant increase in early and late apoptotic cells (Fig. 4c). Pre-incubation of MEL cells with 20 μM SB202190 significantly impaired ATP-induced early and late apoptosis by 56 ± 5 and 56 ± 9 %, respectively (Fig. 4c). Similarly, pre-incubation of MEL cells with 20 μM SB203580 impaired the amount of ATP-induced early and late apoptosis by 48 ± 17 and 52 ± 6 %, respectively (Fig. 4c).
Next, cells were pre-incubated in the presence of DMSO, or the broad-spectrum caspase inhibitor Z-VAD-FMK before incubation in the absence or presence of ATP. Again ATP induced a significant increase in early and late apoptotic cells (Fig. 4d). Pre-incubation of MEL cells with 20 μM Z-VAD-FMK significantly impaired the amount of ATP-induced late apoptotic cells by 36 ± 2 % (Fig. 4d). In contrast, pre-incubation of cells with Z-VAD-FMK failed to impair the amount of early apoptotic cells induced by ATP (Fig. 4d).
p38 MAPK and caspase inhibitors have been reported to impair P2X7-induced pore formation [33, 34]. To exclude the possibility that the above compounds impaired P2X7-induced apoptosis of MEL cells by directly inhibiting P2X7, cells were pre-incubated in the presence of DMSO, SB202190, SB203580 or Z-VAD-FMK (as above), and the P2X7-induced ethidium+ uptake assessed. ATP induced significant ethidium+ uptake into MEL cells compared with cells incubated in the absence of ATP (Fig. 4e). Pre-incubation of MEL cells with any of the compounds failed to alter the amount of ATP-induced ethidium+ uptake (Fig. 4e). Collectively, these results indicate that p38 MAPK and caspase activation, but not ROS formation, are involved in P2X7-induced apoptosis of MEL cells.
P2X7 activation induces p38 MAPK and caspase activation in MEL cells
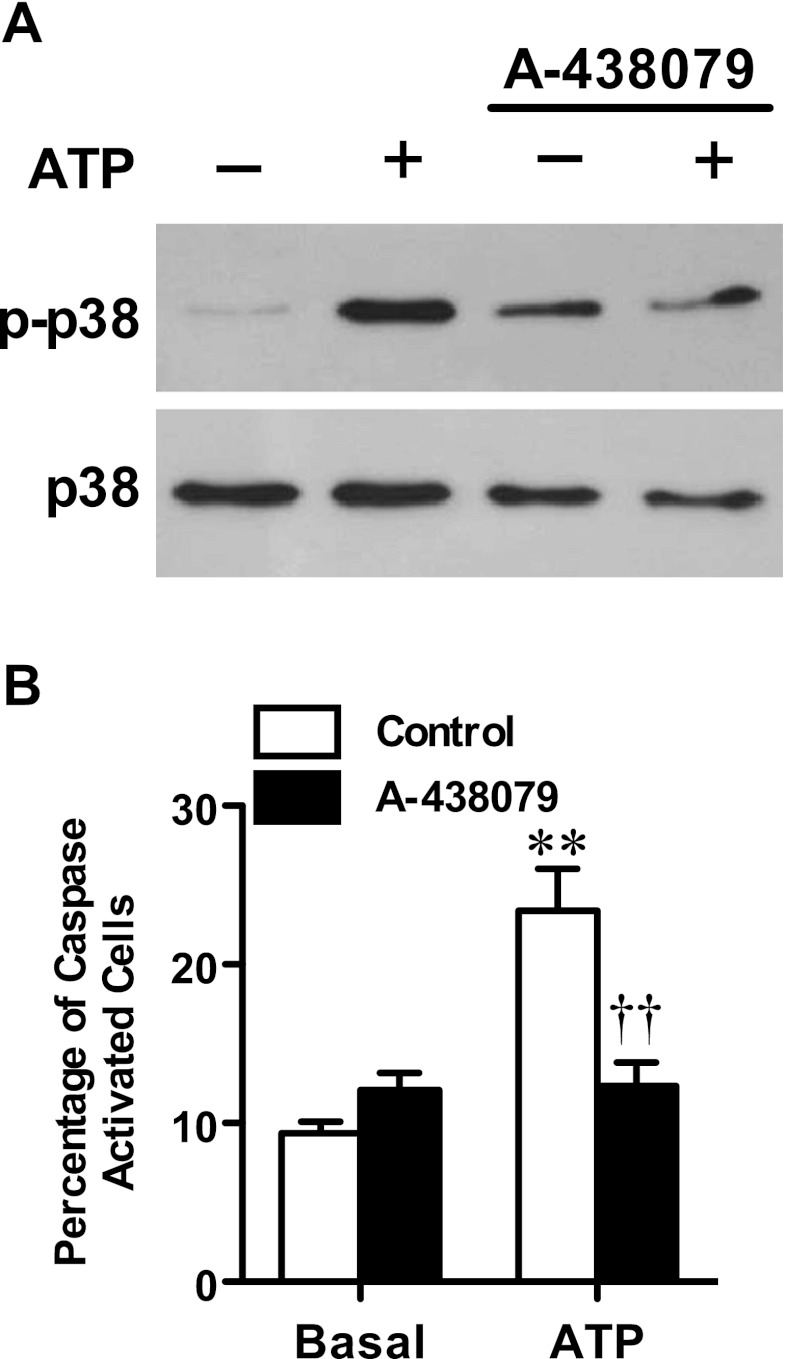
To support the role of p38 MAPK activation in the P2X7-induced apoptosis of MEL cells, ATP-induced p38 MAPK phosphorylation was examined by immunoblotting. Immunoblotting with an anti-phospho-p38 MAPK antibody revealed that incubation of cells with ATP for 5 min induced greater amounts of p38 MAPK activation compared with cells incubated in the absence of ATP (Fig. 5a). Moreover, pre-incubation of cells with the P2X7 antagonist A-438079 (10 μM) impaired the amount of ATP-induced p38 activation compared with ATP alone (Fig. 5a).
Fig. 5.
P2X7 activation induces p38 MAPK and caspase activation in MEL cells. a MEL cells in complete culture medium were pre-incubated in the absence or presence of 10 μM A-438079 at 37 °C for 15 min, and then in the absence or presence of 1 mM ATP at 37 °C for 5 min. Whole lysates of MEL cells were then separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with (top) anti-phospho-p38 (p-p38) and (bottom) anti-p38 (p38) antibodies. b MEL cells in complete culture medium were pre-incubated in the absence (control) or presence of 10 μM A-438079 at 37 °C for 15 min and then in the absence (basal) or presence of 1 mM ATP at 37 °C for 6 h. Cells were then incubated with FITC-VAD-FMK at 37 °C for 1 h and 7AAD for the final 5 min, and the percentage of FITC+/7AAD+ (caspase activated) cells was analysed by flow cytometry. Results are mean ± SD (n = 3); **P < 0.01 compared with corresponding basal; ††P < 0.01 compared with ATP alone
To support the role of caspase activation in the P2X7-induced apoptosis of MEL cells, ATP-induced caspase activity was examined using a FITC-conjugated caspase substrate and flow cytometry. MEL cells were incubated in the absence or presence of ATP for 6 h, and then for a further 1 h with FITC-VAD-FMK, and the percentage of FITC+/7AAD+ cells (caspase activation) was measured by flow cytometry. Incubation of cells with ATP resulted in a 2.6-fold increase in the relative percentage of cells with activated caspases compared with cells incubated in the absence of ATP (Fig. 5b). Pre-incubation of cells with 10 μM A-438079 impaired the amount of ATP-induced caspase activation by 95 ± 9 % compared with cells incubated with ATP alone (Fig. 5b). Collectively, these results indicate that P2X7 activation stimulates p38 MAPK and caspases and indirectly supports a role for these enzymes in the P2X7-induced apoptosis of MEL cells.
Discussion
The current study demonstrates that P2X7 activation results in the formation of ROS in MEL cells, an erythroid cell line. P2X7-induced ROS formation has been previously observed in myeloid cells [6–12, 35], glomerular mesangial cells [36] and submandibular gland cells [30, 31], but to the best of our knowledge, the current study is the first to demonstrate that P2X7 activation causes ROS formation in erythroid cells. It will be of future importance to determine if P2X7 induces ROS formation in primary erythroid cells. Moreover, it will be important to identify the intracellular source of ROS formation downstream of P2X7 activation in erythroid cells. In the current study, use of apocynin, rotenone, allopurinol and l-NAME, which impair NADPH oxidase, mitochondrial complex I, xanthine oxidase and nitric oxide synthase, respectively, failed to identify the intracellular source of P2X7-induced ROS formation in MEL cells; however the mitochondrial respiratory chain cannot be excluded as a source. Although rotenone is commonly used as an inhibitor of mitochondrial ROS formation, it has been widely reported to also induce mitochondrial ROS formation [37]. Consistent with the latter, rotenone repeatedly increased basal ROS formation in MEL cells, making it difficult to ascertain if mitochondria were the source of P2X7-induced ROS formation. Moreover, DPI can impair mitochondrial ROS formation, as well as NADPH oxidase ROS formation [38], supporting a possible role for mitochondria in P2X7-induced ROS formation. The failure of apocynin to impair P2X7-induced ROS formation excludes a role for NADPH oxidase in this process; however, further studies using gene knockdown approaches are required to support this conclusion. Nevertheless based on the current results, the mitochondrial respiratory chain or enzyme systems other than those examined in the current study remain the most likely sources of P2X7-induced ROS formation in erythroid cells.
The physiological role of P2X7-induced ROS formation in erythroid cells remains to be established. Accumulating evidence suggests that ROS are involved in cell homeostasis, proliferation, differentiation and death [39, 40]. Specifically in relation to erythroid cells, low amounts of ROS are involved in the differentiation of erythroid progenitors, through interactions with FoxO3 and other transcription factors [41]. Whereas, excess amounts of ROS may shorten the life-span of mature erythroid cells [41]. In the current study however, the presence of the ROS scavenger NAC failed to impair P2X7-induced apoptosis of MEL cells, suggesting that P2X7-induced ROS formation is more likely to be involved in signalling events related to erythroid cell homeostasis, proliferation or differentiation rather than erythroid cell death. An alternative, but not mutually exclusive, concept is that P2X7-induced ROS formation may result in phosphatidylserine exposure (in the absence of immediate cell death) and the subsequent removal of erythroid precursors from the circulation in certain disease states such as thalassaemia. In this regard, thalassaemic DNA-containing reticulocytes and erythrocytes have a higher content of ROS and exposed phosphatidylserine compared with DNA-free counterparts, leading the authors to postulate that oxidative stress-induced phosphatidylserine exposure is involved in the clearance of these cells from the circulation [42].
The current study demonstrates that Na+ influx but not K+ efflux may have a role in P2X7-induced ROS formation in MEL cells. NMDG Cl medium, which is nominally free of Na+, almost completely impaired P2X7-induced ROS formation; however, KCl medium, which is also nominally free of Na+, did not. These conflicting results may be explained by at least two, but not mutually exclusive, possibilities. First, since P2X7 activation can induce NMDG+ uptake [43], the possibility remains that P2X7-induced ROS formation is impaired by increased intracellular NMDG+ rather than through the absence of Na+ influx. Second, since high concentrations of extracellular K+ can depolarise cells and interfere with various transport systems, the possibility remains that under these (KCl medium) but not other conditions (NaCl medium) that Na+ influx is not essential for P2X7-induced ROS formation. Reports for a role for Na+ influx in P2X7-induced signalling events are few; however, others have shown that Na+ influx is involved in P2X7-induced rapid exposure of phosphatidylserine in murine thymocytes [44] and P2X7-induced mitochondrial membrane depolarisation in rat submandibular glands [45]. Thus, the current study may offer another example of the importance of Na+ influx in a P2X7-induced signalling event. In contrast, this study indicates that K+ efflux is not essential for P2X7-induced ROS formation in MEL cells. Both ROS formation and K+ efflux are involved in P2X7-induced interleukin-1β from monocytes although it remains unknown if these upstream processes are linked [32]. The absence of a role for K+ efflux in P2X7-induced ROS formation in MEL cells suggests that K+ efflux and ROS formation may be involved in distinct pathways in monocyte interleukin-1β release downstream of P2X7 activation.
The current study also demonstrates that Ca2+ influx or Mg+ efflux or an increase in intracellular Ca2+ is not essential for P2X7-induced ROS formation in MEL cells. Others have shown that P2X7-induced ROS formation is dependent on Ca2+ influx in rat, but not murine, submandibular gland cells [30, 31]. Thus, the current study with a murine erythroid cell line parallels those of murine submandibular gland cells. Of note however, 1 mM extracellular Ca2+ or Mg2+, but not nominal concentrations of extracellular Ca2+ (as observed with the use of EGTA), impaired both P2X7-induced ROS formation and pore formation in MEL cells despite the presence of equivalent ATP4− concentrations. This suggests that millimolar (or perhaps micromolar) concentrations of extracellular Ca2+ and Mg2+ may act as allosteric inhibitors of murine P2X7 as originally observed for rat P2X7 [46] and established by more recent studies of rat P2X7 [28, 29]. The current study also demonstrates that an increase in intracellular Ca2+ is not essential for P2X7-induced ROS formation (and pore formation) in MEL cells. To the best of our knowledge, the potential role of intracellular Ca2+ on P2X7-induced ROS formation in any cell type has not been reported although others have excluded a role for an increase in intracellular Ca2+ in ATP-induced organic cation uptake into RAW264.7 macrophages and HEK-293 cells transfected with rat P2X7 [47].
The current study confirms that P2X7 activation induces apoptosis of MEL cells, and demonstrates for the first time that this process involves activation of p38 MAPK and caspases, but not ROS formation. The involvement of p38 MAPK and caspase activation in P2X7-induced MEL cell apoptosis parallels observations with P2X7-induced apoptosis of RAW264.7 cells [10]. In contrast, this previous study also demonstrated a role for ROS formation in RAW264.7 cell apoptosis, which was not required for MEL cell apoptosis in the current study. This difference was not due to impaired scavenger action of NAC, as this compound impaired P2X7-induced ROS formation in MEL cells. Collectively, the above indicates that p38 MAPK and caspase activation and ROS formation are independent processes downstream of P2X7 activation in MEL cells. Although the current study demonstrated that caspase activation mediates the P2X7-induced apoptosis of MEL cells, the identity of the caspases involved remains unknown. Others have shown that high pressure-induced apoptosis of MEL cells involves both the extrinsic and intrinsic pathways mediated by caspase-8 and caspase-9, respectively, as well as the downstream activation of caspase-3 [48, 49]. Thus, it is likely that either or both caspase-8 and caspase-9, as well as caspase-3 are involved in P2X7-induced MEL cell apoptosis.
The physiological role of P2X7-induced apoptosis of erythroid cells, as well as other cell types remains poorly defined. Studies of P2X7-deficient mice suggest that the P2X7 is not involved in regulating cell numbers under normal, physiological conditions [50, 51], except in bone homeostasis [52] and the peripheral T cell compartment [53]. Nevertheless P2X7-induced apoptosis remains a well-described event [2, 3]. Therefore, it is likely that P2X7-induced apoptosis may play a role in removing damaged erythroid cells to prevent the development of anaemia or leukaemia [54] or to promote their rapid clearance from the circulation to prevent autoimmunity [55]. Alternatively, errant ATP release and subsequent P2X7-induced apoptosis of erythroid progenitors may be associated with the premature loss of these cells to cause anaemia [54]. This may be particular relevant to chemotherapy, which can cause the release of ATP from cells [56] and induce cytotoxicity in immature erythroid progenitors to cause anaemia in cancer patients [57].
Finally, the current study demonstrates that neither the ROS scavenger (NAC) nor any of the ROS inhibitors (DPI, apocynin, rotenone, allopurinol and l-NAME) had a direct effect on P2X7 itself. The inability of these compounds to impair P2X7-induced pore formation in MEL cells is consistent with similar findings in murine J774 cells and primary murine macrophages [11]. Thus, these observations validate the current and future use of these compounds to study ROS formation downstream of P2X7 activation and indicate that ROS formation does not modulate P2X7-induced pore formation. In contrast, P2X7 in some cell types or studies can be directly impaired by a number of enzyme inhibitors [33, 34, 58–60]. Of relevance to the current study, the p38 MAPK inhibitors SB202190 or SB203580 can impair P2X7-induced pore formation in human THP-1 myeloid cells, murine 2BH4 thymic epithelial cells and primary murine macrophages [33, 34]. Although others have demonstrated that these compounds impair ATP-induced pore formation mediated by human, but not rat or murine, recombinant P2X7 [61]. This latter finding is consistent with the absence of an inhibitory effect of SB202190 or SB203580 on P2X7-induced pore formation in MEL cells. The caspase-1 and caspase-3 inhibitors YVAD and DEVD, respectively, have also been shown to impair P2X7-induced pore formation in THP-1 cells [33]. However, the broad-spectrum caspase inhibitor Z-VAD-FMK failed to impair P2X7-induced pore formation in MEL cells.
In conclusion, these findings indicate that P2X7 activation induces ROS formation in MEL cells and that this process may be involved in events downstream of P2X7 activation, other than apoptosis, in erythroid cells. This study highlights the potential role of P2X7 activation in intracellular signalling pathways or oxidative stress in erythroid cells.
Acknowledgments
This work was supported by grants from Cure Cancer Australia and the University of Wollongong. We thank Heath Ecroyd (University of Wollongong) and Takuya Noguchi (University of Lausanne) for helpful advice and Aleta Pupovac, Rachael Bartlett (both University of Wollongong) and Iman Jalilian (University of New South Wales) for reviewing the manuscript. Technical support by the staff of the Illawarra Health and Medical Research Institute is also gratefully acknowledged.
References
- 1.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors—recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 3.Lenertz L, Gavala M, Zhu Y, Bertics P. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22–38. doi: 10.1007/s12026-011-8203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq. 2011;5:41–54. doi: 10.2174/187221511794839219. [DOI] [PubMed] [Google Scholar]
- 5.Hewinson J, Mackenzie AB. P2X7 receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans. 2007;35:1168–1170. doi: 10.1042/BST0351168. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz CM, Rinna A, Forman HJ, Ventura ALM, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewinson J, Moore SF, Glover C, Watts AG, MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1β processing in human monocytes. J Immunol. 2008;180:8410–8420. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- 9.Corrêa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 11.Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 12.Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, Takenouchi T, Kitani H, Kojima S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun. 2012;420:102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- 13.Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem. 2004;279:44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- 15.Parker JC, Snow RL. Influence of external ATP on permeability and metabolism of dog red blood cells. Am J Physiol. 1972;223:888–893. doi: 10.1152/ajplegacy.1972.223.4.888. [DOI] [PubMed] [Google Scholar]
- 16.Sluyter R, Shemon AN, Hughes WE, Stevenson RO, Georgiou JG, Eslick GD, Taylor RM, Wiley JS. Canine erythrocytes express the P2X7 receptor: greatly increased function compared with human erythrocytes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2090–R2098. doi: 10.1152/ajpregu.00166.2007. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson RO, Taylor RM, Wiley JS, Sluyter R. The P2X7 receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types. Purinergic Signal. 2009;5:385–394. doi: 10.1007/s11302-009-9163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skals M, Jorgensenb NR, Leipzigera J, Praetoriusa HA. α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skals M, Leipziger J, Praetorius H. Haemolysis induced by α-toxin from Staphylococcus aureus requires P2X receptor activation. Pflugers Arch. 2011;462:669–679. doi: 10.1007/s00424-011-1010-x. [DOI] [PubMed] [Google Scholar]
- 20.Sluyter R, Shemon AN, Wiley JS. P2X7 receptor activation causes phosphatidylserine exposure in human erythrocytes. Biochem Biophys Res Commun. 2007;355:169–173. doi: 10.1016/j.bbrc.2007.01.124. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chahwala SB, Cantley LC. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem. 1984;259:13717–13722. [PubMed] [Google Scholar]
- 23.Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJCGM, Wiley JS, Sluyter R. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta. 2010;1798:1797–1804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Marks PW, Maxfield FR. Preparation of solutions with free calcium concentration in the nanomolar range using 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. Anal Biochem. 1991;193:61–71. doi: 10.1016/0003-2697(91)90044-T. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk EC, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X7 receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/S0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 27.Bo XN, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- 28.Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem. 2007;101:17–26. doi: 10.1111/j.1471-4159.2006.04343.x. [DOI] [PubMed] [Google Scholar]
- 29.Yan Z, Khadra A, Sherman A, Stojilkovic S. Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol. 2011;138:437–452. doi: 10.1085/jgp.201110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontanils U, Seil M, Pochet S, El Ouaaliti M, Garcia-Marcos M, Dehaye JP, Marino A. Stimulation by P2X7 receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands. Biochim Biophys Acta. 2010;1800:1183–1191. doi: 10.1016/j.bbagen.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Seil M, Fontanils U, Etxebarria I, Pochet S, Garcia-Marcos M, Marino A, Dehaye J-P. Pharmacological evidence for the stimulation of NADPH oxidase by P2X7 receptors in mouse submandibular glands. Purinergic Signal. 2008;4:347–355. doi: 10.1007/s11302-008-9118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- 34.Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- 35.Lenertz L, Gavala M, Hill L, Bertics P. Cell signaling via the P2X7 nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal. 2009;5:175–187. doi: 10.1007/s11302-009-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada H, Tsukimoto M, Ikari A, Takagi K, Suketa Y. P2X7 receptor-induced generation of reactive oxygen species in rat mesangial cells. Drug Dev Res. 2003;59:112–117. doi: 10.1002/ddr.10204. [DOI] [Google Scholar]
- 37.Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, Willems PH. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431–1470. doi: 10.1089/ars.2009.2743. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Trush M. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 39.Valko M, Leibfritz DMJ, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dana M, Prus E, Fibach E. Thalassemic DNA-containing red blood cells are under oxidative stress. Anemia. 2012;2012:943974. doi: 10.1155/2012/943974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. N-methyl-d-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am J Physiol Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- 44.Courageot M-P, Lepine S, Hours M, Giraud F, Sulpice J-C. Involvement of sodium in ealry phophatidylserine exposure and phosolipid scrambling induced by P2X7 purinoceptor activation in thymocytes. J Biol Chem. 2004;279:21815–21823. doi: 10.1074/jbc.M401426200. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Marcos M, Fontanils U, Aguirre A, Pochet S, Dehaye JP, Marino A. Role of sodium in mitochondrial membrane depolarization induced by P2X7 receptor activation in submandibular glands. FEBS Lett. 2005;579:5407–5413. doi: 10.1016/j.febslet.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 46.Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/S0028-3908(97)00141-X. [DOI] [PubMed] [Google Scholar]
- 47.Cankurtaran-Sayar S, Sayar K, Ugur M. P2X7 receptor activates multiple selective dye-permeation pathways in RAW 264.7 and human embryonic kidney 293 cells. Mol Pharmacol. 2009;76:1323–1332. doi: 10.1124/mol.109.059923. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi T, Hashiguchi K, Katsuki S, Iwamoto W, Tsuruhara S, Terada S. Activation of the intrinsic and extrinsic pathways in high pressure-induced apoptosis of murine erythroleukemia cells. Cell Mol Biol Lett. 2008;13:49–57. doi: 10.2478/s11658-007-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Take J, Yamaguchi T, Mine N, Terada S. Caspase activation in high-pressure-induced apoptosis of murine erythroleukemia cells. Jpn J Physiol. 2001;51:193–199. doi: 10.2170/jjphysiol.51.193. [DOI] [PubMed] [Google Scholar]
- 50.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 51.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WSS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 53.Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral γδ cells. J Immunol. 2012;189:174–180. doi: 10.4049/jimmunol.1101582. [DOI] [PubMed] [Google Scholar]
- 54.Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18:1176–1199. doi: 10.1038/sj.leu.2403383. [DOI] [PubMed] [Google Scholar]
- 55.Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunol Rev. 2007;220:237–250. doi: 10.1111/j.1600-065X.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 56.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Seror C, Metivier D, Perfettini JL, Zitvogel L, Kroemer G. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–3728. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 57.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 58.Shemon AN, Sluyter R, Conigrave AD, Wiley JS. Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor. Br J Pharmacol. 2004;142:1015–1019. doi: 10.1038/sj.bjp.0705868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shemon AN, Sluyter R, Stokes L, Manley PW, Wiley JS. Inhibiton of the human P2X7 receptor by a novel protein tyrosine kinase antagonist. Biochem Biophys Res Commun. 2008;365:515–520. doi: 10.1016/j.bbrc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Shemon AN, Sluyter R, Wiley JS. Rottlerin inhibits P2X7 receptor stimulated phospholipase D activity in chronic lymphocytic leukaemia B-lymphocytes. Immunol Cell Biol. 2007;85:68–72. doi: 10.1038/sj.icb.7100005. [DOI] [PubMed] [Google Scholar]
- 61.Michel AD, Thompson KM, Simon J, Boyfield I, Fonfria E, Humphrey PPA. Species and response dependent differences in the effects of MAPK inhibitors on P2X7 receptor function. Br J Pharmacol. 2006;149:948–957. doi: 10.1038/sj.bjp.0706938. [DOI] [PMC free article] [PubMed] [Google Scholar]