Abstract
The P2X4 receptor is an ATP-gated ion channel expressed in neurons, endothelia and immune cells. Plasma membrane expression of P2X4 is regulated by dynamin-dependent endocytosis, and this study identifies a Rab5-dependent pathway of receptor internalisation. Expression of Rab5 constructs altered the distribution of P2X4 in HEK-293 cells, and both constitutive internalisation and agonist-induced desensitisation of P2X4 were increased by co-expression of wild-type Rab5 or constitutively active Rab5 (Q79L). Expression of inactive dynamin K44A and Rab5 S34N constructs abolished agonist-induced desensitisation, suggesting internalisation as the underlying mechanism. Blocking P2X4 internalisation in this way also abolished potentiation of ATP-induced currents by the allosteric modulator ivermectin. This suggests that the dynamin-Rab5 internalisation pathway is essential for the ivermectin potentiation effect. In agreement with this hypothesis, the co-expression of wild-type dynamin, wild-type Rab5 or active Rab5 (Q79L) could increase the potentiation of the ATP-induced P2X4 response by ivermectin. These findings highlight Rab5 GTPase as a key regulator of P2X4 receptor cell surface expression and internalisation.
Keywords: P2X4 receptor, Dynamin, Rab5, Ivermectin
Introduction
P2X receptors are a family of seven non-selective cation channels (P2X1-7) gated by extracellular ATP [1, 2]. The P2X4 receptor (P2X4) is widely expressed with highest levels in neurons, endothelia, epithelia and both macrophages and microglia. Studies using the P2X4 knockout mice have postulated a role for this receptor in endothelial vascular remodelling [3], in long-term potentiation in the hippocampus [4] and in neuropathic pain [5]. Ivermectin is an allosteric modulator of P2X4 [6] in addition to other ligand-gated ion channels including mammalian α7-nicotinic receptors, GABAA receptors and glycine receptors [7–9]. It is thought that ivermectin either increases the number of cell surface P2X4 to increase current amplitude [9] or interacts with the transmembrane regions of P2X4 causing rearrangements which stabilise the channel in the membrane [10].
Plasma membrane expression of P2X4 is controlled by the AP-2/clathrin-dependent endocytic pathway through a tyrosine-based internalisation motif in the C-terminus [9, 11]. Constitutive cycling of membrane proteins is a mechanism employed by cells to rapidly regulate surface expression of receptors and channels [12]. This is particularly important in the central nervous system where modulation of trafficking of ionotropic receptors underlies long-term potentiation and depression of synaptic activity. In macrophages, the surface expression of the P2X4 is kept low with ATP-induced P2X4-dependent inward currents ~1–2 mean current density (pA/pF) despite high levels of intracellular protein [13], and P2X4 may be sequestered in intracellular compartments such as lysosomes until required at the cell surface [13, 14].
Endocytosis through the clathrin pathway is typically dependent on the activity of dynamin GTPase which functions to promote membrane invagination and the scission of vesicles from the plasma membrane into the endocytic pathway [15]. Rab proteins are a family of Ras-like small GTP-binding proteins playing important roles in membrane transport and fusion. Rab5 is thought to play a role in sequestering ligands into clathrin-coated pits and to be involved in homotypic endosome fusion [16, 17]. Early endosomes are highly dynamic and comprise at least two populations of vesicles involved in sorting of cargo proteins to different cellular compartments including recycling versus degradation pathways [18]. Rab5 is known to play a role in regulating endocytosis of G-protein-coupled receptors such as EGF and insulin receptors [19, 20] and the Kv1.5 channel [21] and drives the NMDA receptor-mediated internalisation of AMPA receptors in neurons [22].
This study investigated constitutive and agonist-induced internalisation pathways for P2X4 expressed in mammalian HEK-293 cells to determine key regulators of plasma membrane expression. Dynamin plays a key role in both constitutive and agonist-induced internalisations, whereas Rab5 plays a small but significant role in P2X4 internalisation. However, this Rab5-dependent pathway is critical for potentiation of P2X4 responses by ivermectin. Therefore, Rab5 is a key protein involved in regulating the trafficking of the P2X4.
Methods
Cells
HEK-293 cells were maintained in DMEM/F12 medium (Invitrogen, San Diego, CA, USA) containing 10 % foetal calf serum (FCS) and 2 mM l-glutamine (Invitrogen, San Diego, CA, USA) at 37 °C in a humidified 5 % CO2 incubator. Confluent cells were transiently transfected in 35-mm Petri dishes with 0.1 μg rat P2X4-green fluorescent protein (GFP) in pShuttle vector together with 1 μg of wild-type or dominant negative K44A HA-tagged dynamin GTPase using Lipofectamine 2000 (Invitrogen, San Diego, CA, USA) as per manufacturer’s instructions. Myc-tagged wild-type Rab5, constitutively active Q79L Rab5 or inactive S34N Rab5 constructs were a kind gift from Professor Elizabeth Smythe, University of Sheffield, UK. Post-transfection (24 h) cells were plated onto 13-mm glass coverslips for electrophysiology and immunofluorescence experiments.
NR8383 rat alveolar macrophage cell line were cultured as described previously [13]. Briefly, NR8383 cells were maintained in F12K media (Invitrogen, San Diego, CA, USA) supplemented with 15 % FCS at 37 °C in a humidified 5 % CO2 incubator and were subcultured by scraping.
Electrophysiology
Whole-cell patch-clamp recordings were performed at room temperature using a HEKA EPC9 amplifier and Pulse acquisition software (HEKA, Lambrecht, Germany). Agonist and the allosteric modulator ivermectin (3 μM) were delivered using the RSC-160 fast-flow system (Bio-Logic Science Instruments, France). Unless otherwise stated, internal/external solutions were NaCl 145 mM, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) 10 mM, EGTA 10 mM/NaCl 145 mM, KCl 2 mM, CaCl2 2 mM, MgCl2 1 mM, HEPES 10 mM and glucose 13 mM; pH 7.3, osmolarity 300–310 mOsmol/l. Holding potential was −60 mV.
Immunofluorescence and confocal microscopy
HEK-293 cells transfected with P2X4-GFP plus Rab5 or dynamin constructs were grown on 13-mm glass coverslips. Cells were fixed with 2 % paraformaldehyde solution at 4 °C for 20 min. Excess fixative was removed by washing three times with phosphate-buffered saline (PBS). Cells were permeabilised using 0.1 % saponin (Sigma, St. Louis, MO, USA) in PBS containing 5 % FCS, and primary antibody was diluted in this buffer for staining. Anti-myc antibody (Covance Inc., Princeton, New Jersey, USA) was used at 1:500 dilution, and anti-HA antibody (Sigma, St. Louis, MO, USA), at 1:5,000 dilution. Staining with primary antibodies was performed for 1 h at room temperature. Cells were then washed three times in PBS (5 min per wash). Cells were incubated with anti-mouse IgG Alexa 594 (Molecular Probes, Invitrogen, San Diego, CA, USA) at 1:100 dilution in 0.1 % saponin/PBS/FCS for 1 h at room temperature. Excess antibody was removed by three washes in PBS. Coverslips were mounted onto glass slides with Prolong Gold antifade (Molecular Probes, Invitrogen, San Diego, CA, USA). Images were acquired on a Zeiss LSM 510 Meta confocal microscope using a × 63 oil-immersion objective.
Surface biotinylation and western blotting
NR8383 cells (5 × 107) were plated into 60-mm Petri dishes in complete media 24 h prior to stimulation. Cells were treated with vehicle or ivermectin (3 μM) for 5 min and washed once with PBS. An aliquot of 4 ml of 0.5 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce, Perbio Science UK, Ltd.) was added to each sample for 30 min at room temperature on a rotating wheel. Glycine was added to quench the reaction, and cells were centrifuged and lysed in 2 % Triton X-100 lysis buffer containing complete protease inhibitors (Roche, UK). Protein was measured using Bio-Rad Protein Assay (BioRad, Hercules, CA, USA), and equal amounts of protein were incubated with streptavidin beads (Pierce, Perbio Science UK, Ltd.) overnight at 4 °C to pull down all biotinylated proteins. Beads were washed four times and boiled in gel sample buffer to release biotinylated proteins. Equal amounts of biotinylated protein were loaded per lane and electrophoresed on 8 % SDS-PAGE gels. Protein was transferred to PVDF membranes (Sigma, St. Louis, MO, USA) and blocked overnight at 4 °C with 5 % non-fat milk. Rabbit anti-P2X4R C-terminal antibody (Alomone Labs, Jerusalem, Israel) and rabbit anti-β-actin (Abcam, Cambridge, UK) were both used at 1:2,000 dilution. Anti-Rab5 (BD Pharmingen, San Diego, CA, USA) was used at 1:250 dilution. HRP-conjugated goat anti-rabbit and anti-mouse secondary antibodies (Dako, Denmark) were used at 1:2,000 dilution. Western blots were developed using ECL plus kit (GE Healthcare, Amersham, Buckinghamshire, UK) and Kodak Bio-Max MS film (Sigma, St. Louis, MO, USA).
Results
Dynamin controls both constitutive and agonist-induced internalisations of P2X4
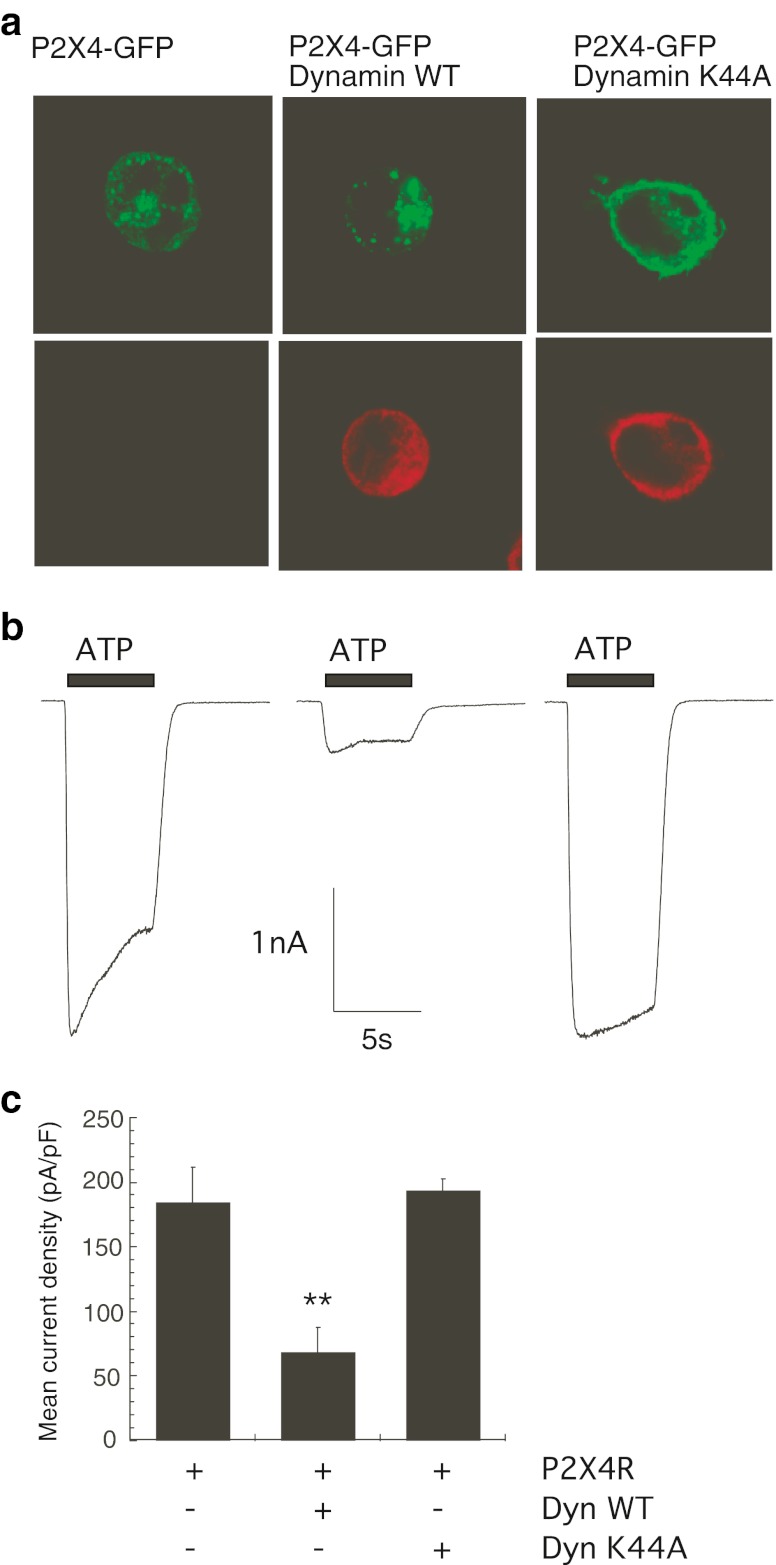
It has previously been reported that the constitutive internalisation of P2X4 heterologously expressed in neurons is dependent on dynamin [23] and that receptor internalisation is mediated via the AP-2/clathrin-dependent pathway in the Xenopus oocyte expression system [11]. The dependence of P2X4 plasma membrane localisation on dynamin GTPase activity was assessed by studying the distribution of green fluorescent protein-tagged P2X4 transiently transfected into HEK-293 cells when either wild-type dynamin (Dyn-WT) or the dominant negative mutant dynamin K44A GTPase (Dyn-K44A) was co-expressed. Confocal images in Fig. 1a show that the distribution of P2X4-GFP in HEK-293 cells was predominantly intracellular in punctate structures, and that co-expression with Dyn-K44A changed this distribution to a more uniform plasma membrane localisation similar to that previously reported in neurons [23]. Co-expression of Dyn-WT with P2X4-GFP increased the punctate nature of the receptor distribution and reduced the plasma membrane localisation of the receptor (Fig. 1a). To quantitate this effect of dynamin co-expression on P2X4 plasma membrane localisation, ATP-induced inward currents were recorded from HEK-293 cells expressing P2X4-GFP alone or in combination with dynamin constructs, and representative electrophysiological recordings are shown in Fig. 1b. The normalised mean current density in cells expressing P2X4-GFP alone was 173.8 ± 14.0 pA/pF (n = 19 cells), whereas co-expression of Dyn-WT significantly reduced the mean current density of the ATP-induced response to 68.1 ± 8.8 pA/pF (n = 11 cells, p < 0.0001) (Fig. 1c). Thus, over-expression of dynamin reduces surface expression of P2X4 by approximately 60 % in these cells. Despite a dramatic alteration in distribution of the GFP-tagged P2X4 when Dyn-K44A was co-expressed, there was only a slight increase in the mean current density of the ATP-induced response (193.3 ± 19.8 pA/pF, n = 9 cells compared to control 173.8 ± 14.0 pA/pF, n = 19 cells), which was not statistically significant. Increasing the expression of Dyn-WT can therefore limit the expression of the P2X4 at the cell surface of HEK-293 cells, suggesting that dynamin controls constitutive internalisation of the P2X4.
Fig. 1.
Expression of wild-type or dominant negative dynamin GTPase alters the cellular distribution of P2X4. GFP-tagged rat P2X4 receptors were transiently transfected into HEK-293 cells in the absence or presence of wild-type dynamin-HA or K44A dynamin-HA. a Confocal images showing the distribution of P2X4-GFP fluorescence in single cells (top panels) and localisation of dynamin-HA proteins using an anti-HA antibody (bottom panels). Cells were stained with anti-HA primary antibody followed by anti-mouse IgG TRITC secondary antibody. Images were acquired using a Zeiss LSM510 confocal microscope and a × 63 oil-immersion objective and are representative of three separate transfection experiments. b Representative inward currents in response to 10 μM ATP in cells expressing P2X4-GFP alone, P2X4-GFP plus wild-type dynamin or P2X4-GFP plus dynamin K44A mutant. Electrophysiological recordings were performed 24–48 h post-transfection. ATP was applied for 5 s as shown by the black bar. cBar chart shows that co-expression of Dyn-WT significantly reduced the ATP-induced P2X4 mean current density (pA/pF) compared to P2X4-GFP receptors alone (*p < 0.0001; n = 9–19 cells). Error bars represent SEM
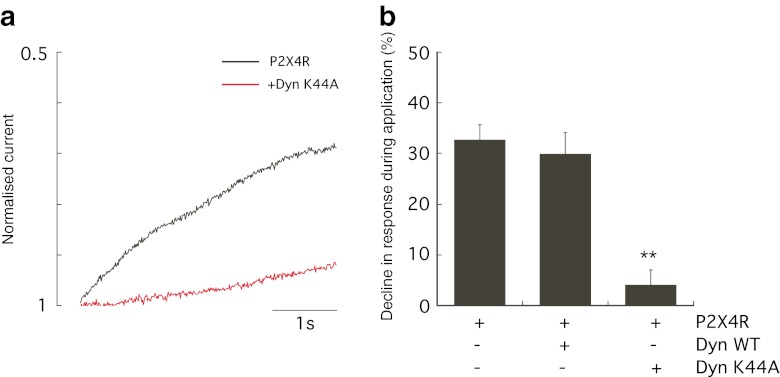
Desensitisation during the ATP-induced inward current mediated by the P2X4 is a known characteristic feature of this receptor determining the time course and magnitude of ATP action. Desensitisation was calculated as the percentage decline in the response during agonist application by measuring current amplitude 4 s after the peak of the response as described by Fountain and North [24]. The co-expression of Dyn-K44A markedly reduced desensitisation of the ATP-induced P2X4 response (Fig. 2a). Typically, the ATP-induced P2X4 response declined by an average of 32.7 ± 2.9 % (n = 16 cells), but co-expression of Dyn-K44A abolished the decline in response to 4.1 ± 2.1 % (n = 9 cells, p < 0.0001; Fig. 2b). Desensitisation during the agonist application was not affected by co-expression with Dyn-WT (mean decline in response 30.0 ± 4.1 %, n = 11 cells; Fig. 2b). This suggests that P2X4 desensitisation is mediated by a dynamin-dependent agonist-induced internalisation.
Fig. 2.
Dynamin controls agonist-induced internalisation of P2X4. a Desensitisation during the ATP-induced P2X4 response was measured as the percentage decline in inward current after 4 s of ATP application. Representative normalised current is shown for P2X4-GFP alone (black trace) showing desensitisation and P2X4-GFP plus Dyn-K44A (red trace) showing minimal desensitisation. b Normal P2X4 responses declined by an average of 32.7 % which was significantly reduced in the presence of Dyn-K44A mutant to 4.1 % (**p < 0.0001; n = 9–16 cells). Error bars represent SEM
Expression of Rab5 mutants alter the distribution and internalisation of P2X4
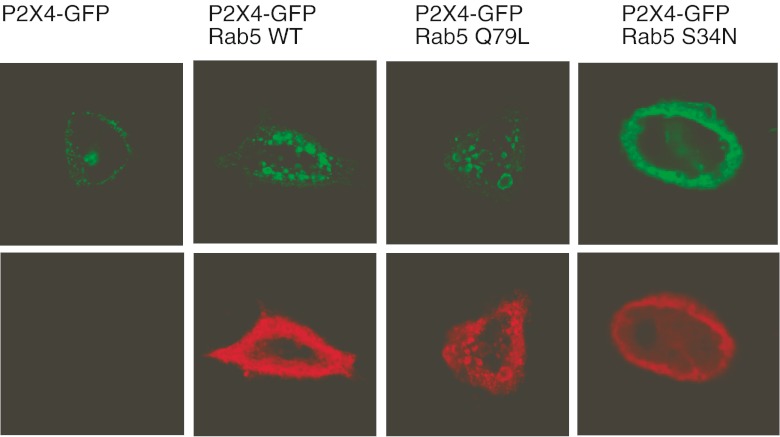
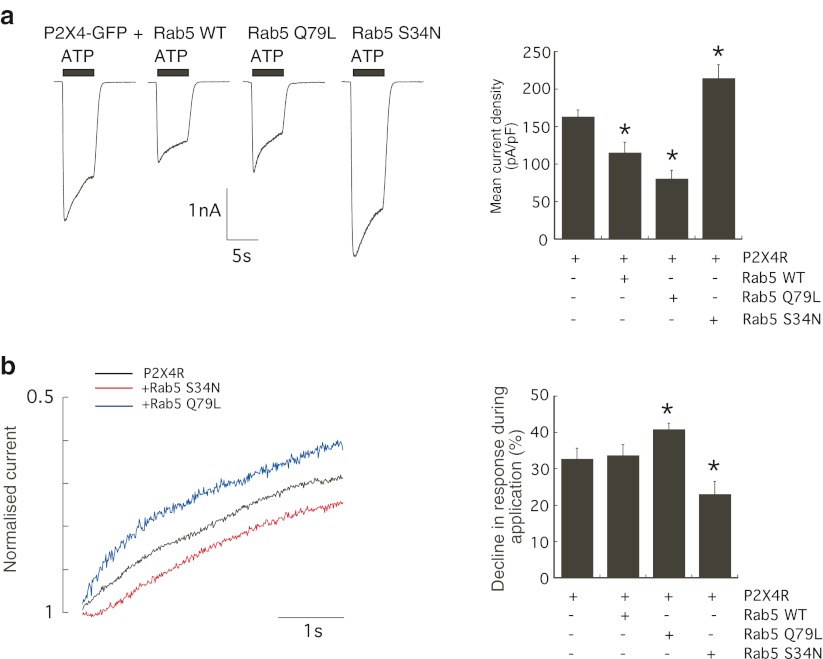
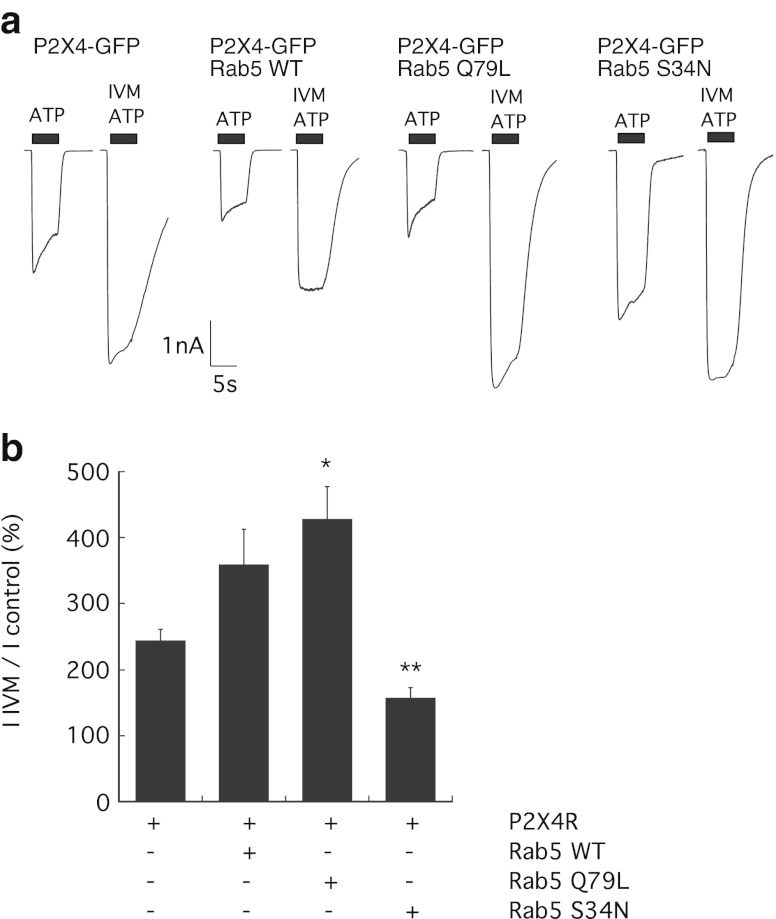
Rab5 is a rate-limiting enzyme involved in clathrin-dependent endocytosis pathways responsible for trafficking proteins from the plasma membrane to early endosomes. Two mutant GTPases, a constitutively active GTP-bound Rab5 enzyme (Rab5 Q79L) and an inactive GDP-bound GTPase (Rab5 S34N), were used to determine whether Rab5 could regulate P2X4 surface expression. The effect of co-expression of wild-type Rab5 (Rab5 WT), Rab5 Q79L or Rab5 S34N on the distribution of GFP-tagged P2X4 in HEK-293 cells was assessed. Figure 3 shows that the expression of Rab5 WT or Rab5 Q79L formed an enlarged endosomal compartment containing GFP fluorescence and therefore containing P2X4. An increase in Rab5 expression (Rab5 WT) or Rab5 activity (Rab5 Q79L) increased the endosomal localisation of P2X4 (Fig. 3). Rab5 S34N was found to alter the intracellular distribution of P2X4-GFP similar to the dominant negative Dyn-K44A mutant, showing a less punctate, more uniform surface localisation (Fig. 3). Next, ATP-induced inward currents were recorded to investigate to what extent Rab5 affected constitutive and agonist-induced internalisation pathways of P2X4. Figure 4 shows that the expression of Rab5 WT or Rab5 Q79L significantly reduced the mean current density of P2X4 (P2X4-GFP alone 163.0 ± 9.43 pA/pF, n = 19 cells; Rab5 WT 115.4 ± 13.9 pA/pF, n = 15 cells; Rab5 Q79L 80.6 ± 11.2 pA/pF, n = 17 cells; p < 0.01). Co-expression of Rab5 WT or Rab5 Q79L increased constitutive internalisation of the P2X4 by approximately 30 and 50 %, respectively. Conversely, co-expression of Rab5 S34N increased ATP-induced P2X4 responses by approximately 30 % (mean current density 214.6 ± 17.8 pA/pF, n = 11 cells; p < 0.05).
Fig. 3.
Expression of Rab5 induces an enlarged endosomal compartment and a redistribution of P2X4. GFP-tagged rat P2X4 was transiently transfected into HEK-293 cells in the absence or presence of myc-tagged wild-type Rab5, constitutively active Rab5 (Q79L) or inactive Rab5 (S34N). Top panels are confocal images showing the distribution of GFP fluorescence in single cells, and bottom panels show localisation of Rab5-myc proteins using an anti-myc antibody. Cells were grown on 13-mm glass coverslips, fixed and permeabilised and stained with anti-myc primary antibody (1:5,000 dilution), followed by anti-mouse IgG TRITC secondary antibody (1:100 dilution). Images were acquired using a Zeiss LSM510 confocal microscope and a × 63 oil-immersion objective and are representative of three separate transfection experiments. Expression of Rab5 WT or Rab5 Q79L induces an enlarged endosomal compartment that is stained with GFP and anti-myc
Fig. 4.
Rab5 plays a role in constitutive and agonist-induced internalisations of P2X4. a Representative inward currents in response to 10 μM ATP in cells expressing P2X4-GFP alone, P2X4-GFP plus Rab5 WT, P2X4-GFP plus Rab5 Q79L or P2X4-GFP plus Rab5 S34N. Electrophysiological recordings were performed 24–48 h post-transfection. Holding potential was −60 mV, and ATP was applied for 5 s as shown by the black bar. Normalised peak amplitudes (pA/pF) of ATP-induced P2X4 responses in cells expressing P2X4 alone (n = 19) or in combination with Rab5 WT (n = 15 cells), constitutively active Rab5 Q79L (n = 17 cells) or inactive Rab5 S34N mutant (n = 11 cells). Co-expression of Rab5 WT or active Rab5 Q79L significantly reduced the ATP-induced P2X4 response compared to P2X4-GFP receptors alone (p < 0.01). b Representative normalised current is shown for P2X4-GFP alone (black trace) showing typical desensitisation, P2X4-GFP plus Rab5 Q79L (blue trace) showing increased desensitisation and P2X4-GFP plus Rab5 S34N (red trace) showing reduced desensitisation. Desensitisation during the ATP-induced response was measured as percentage decline in response over 4 s. ATP-induced P2X4 responses typically decline by an average of 32.7 % which was increased in the presence of active Rab5 Q79L to 40.9 % and was reduced in the presence of Rab5 S34N mutant to an average of 23.1 % (*p < 0.05). Error bars represent SEM
Desensitisation or agonist-induced internalisation was measured as percentage decline in peak amplitude of the response, and Fig. 4b shows that the co-expression of Rab5 Q79L and Rab5 S34N altered the extent of desensitisation. Rab5 Q79L increased the decline in the response (average percentage decline 40.9 ± 1.7 %, n = 16 cells compared to 32.7 ± 2.9 %, n = 16 cells for control P2X4-GFP alone; p < 0.05), whereas Rab5 S34N slowed the decline in the response to an average of 23.1 ± 3.35 % (n = 9 cells, p < 0.05). This suggested that a Rab5-dependent component plays a role in agonist-induced internalisation of P2X4 but is not the major regulator of agonist-induced dynamin-dependent internalisation.
Ivermectin potentiation of P2X4 requires internalisation
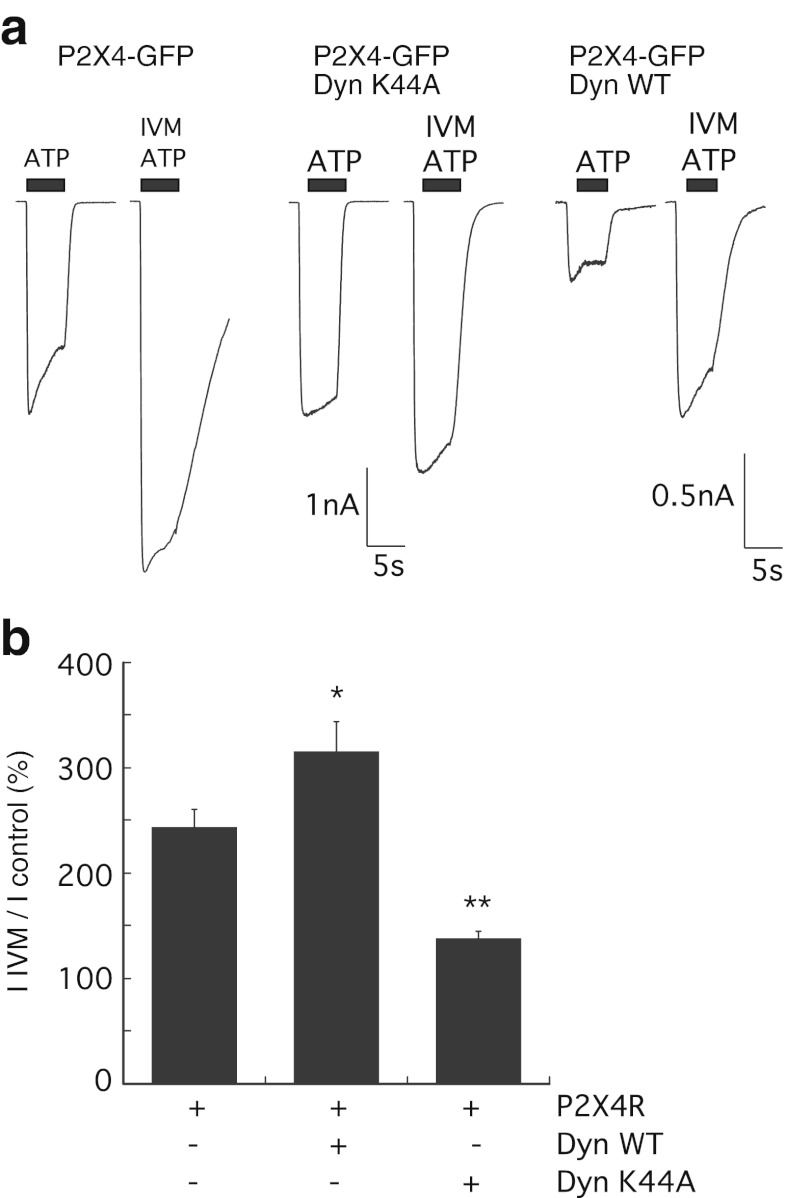
It has previously been suggested that clathrin-dependent endocytosis is required for potentiation of P2X4 responses by the allosteric modulator ivermectin (IVM) [9]. The requirement for endocytosis pathways in potentiation was investigated using co-expression of dynamin and Rab5 mutants which affect constitutive and agonist-induced internalisations to varying degrees. The IIVM/Icontrol (peak amplitude of ATP response after ivermectin/peak amplitude of ATP response before ivermectin) was measured as a percentage. Co-expression of Dyn-K44A significantly reduced ivermectin potentiation to 138 ± 7 % (n = 8 cells, p < 0.0001) compared to 244 ± 17 % (n = 16 cells) in cells expressing P2X4-GFP alone, a reduction of approximately 50 %. Conversely, co-expression of Dyn-WT increased the magnitude of ivermectin potentiation to 316 ± 27 % (n = 10 cells, p < 0.05) (Fig. 5). Similarly, co-expression of Rab5 S34N with P2X4 reduced the potentiation by ivermectin to 158 ± 14 % (n = 10 cells, p = 0.0008), and Rab5 WT or Rab5 Q79L increased the potentiation by ivermectin to 359.3 ± 5.4 % (n = 14 cells) and 429 ± 4.9 % (n = 16 cells, p = 0.0024), respectively (Fig. 6). Together, these data show that internalisation through the Rab5-dependent pathway is required for potentiation of P2X4 by ivermectin.
Fig. 5.
Dynamin K44A mutant reduces potentiation of P2X4 responses by ivermectin. a Representative inward currents in response to 10 μM ATP in cells expressing P2X4-GFP alone, P2X4-GFP plus Dyn-WT or P2X4-GFP plus Dyn-K44A. Following the first application of ATP (indicated by the black bar) for 5 s, the allosteric modulator ivermectin (3 μM) was applied for 1 min in standard extracellular solution. bIIVM/Icontrol was calculated as a percentage increase from normalised peak amplitudes (pA/pF) of the ATP-induced P2X4 responses in the presence and absence of ivermectin in cells expressing P2X4 alone (n = 16) or in combination with Dyn-WT (n = 10 cells) or Dyn-K44A mutant (n = 8 cells; *p < 0.05; **p < 0.0001). Error bars represent SEM
Fig. 6.
Rab5 activity is required for potentiation of P2X4 by ivermectin. a Representative inward currents in response to 10 μM ATP in cells expressing P2X4-GFP alone, P2X4-GFP plus Rab5 WT, Rab5 Q79L, or Rab5 S34N. Following the first application of ATP (indicated by the black bar) for 5 s, the allosteric modulator ivermectin (3 μM) was applied for 1 min in standard extracellular solution. bIIVM/Icontrol was calculated as a percentage increase from normalised peak amplitudes (pA/pF) of the ATP-induced P2X4 responses in the presence and absence of ivermectin in cells expressing P2X4 alone (n = 16) or in combination with Rab5 WT (n = 14 cells), Rab5 Q79L (n = 16 cells) or Rab5 S34N mutant (n = 10 cells; *p < 0.05; **p < 0.01). Error bars represent SEM
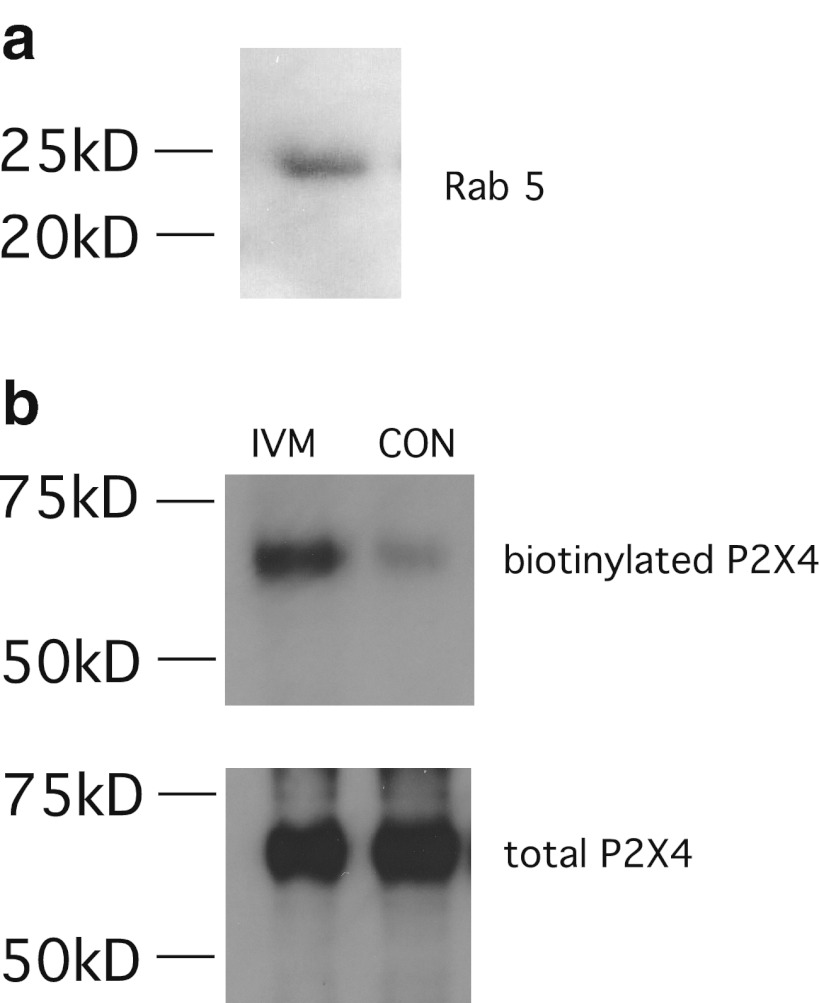
In macrophages, P2X4 receptor cell surface expression is kept low (~1 pA/pF) despite high levels of protein expression [13], and ivermectin potentiates P2X4 responses by approximately fivefold [13, 14]. It is known that Rab5 and dynamin GTPases are expressed in macrophages [25, 26], and Fig. 7a confirms the expression of Rab5 protein in NR8383 cells. There are no suitable antibodies available to the ectodomain of P2X4; therefore, to show that ivermectin affects internalisation and trafficking of P2X4 receptors in macrophages, surface biotinylation was used. Figure 7b demonstrates increased surface expression of endogenous P2X4 receptors in the presence of ivermectin for 3 min.
Fig. 7.
Ivermectin increases surface P2X4 receptors in NR8383 macrophages. a Expression of endogenous Rab5 protein in NR8383 macrophages by western blotting. b Treatment with 3 μM ivermectin increases the surface biotinylated fraction of endogenous P2X4 receptors compared to control cells (CON). Representative blots from two separate experiments are shown
Discussion
In this study, previous work showing that endocytosis of the P2X4 was dynamin dependent has been confirmed, and Rab5 has been identified as a key regulator of P2X4 surface expression. The internalisation of P2X4 is thought to be mediated via clathrin-dependent endocytosis through an AP-2 subunit interaction with a non-canonical tyrosine-based motif in the C-terminus [11]. Much of the previous work on the trafficking of P2X4 has been performed in a Xenopus oocyte expression system rather than in mammalian cells [9, 11]. Here, a GFP-tagged P2X4 was expressed in mammalian HEK-293 cells to identify proteins important in the regulation of cell surface expression and endocytosis of P2X4. Dynamin GTPase is essential for the pinching off of vesicles from the plasma membrane into the endocytic recycling and degradation pathways. The K44A mutation in dynamin abolishes the GTPase activity of dynamin and blocks clathrin-dependent endocytosis [27]. The co-expression of Dyn-K44A with P2X4 altered the cellular distribution of P2X4, although no significant increase in mean current density of the ATP-induced inward current was found (Fig. 1). This is likely to be because P2X4 is already expressed at a high receptor density (160–180 pA/pF) in HEK-293 cells possibly due to the reduced lysosomal localisation of P2X4 in this cell line [14]. Bobanovic et al. showed that Dyn-K44A could increase the functional expression of P2X4 in neurons; however, the mean current density of P2X4-GFP was much lower in these cells (20–60 pA/pF [23]) than what has been reported here for HEK-293 cells. Co-expression of the Dyn-WT construct reduced the mean current density of ATP-induced P2X4 responses by approximately 60 %, suggesting that less P2X4 was present at the cell surface to respond to ATP. It has been demonstrated that macrophages express a small number of P2X4 at the cell surface [13, 14, 28] with a normalised mean current density of only 1–2 pA/pF [13]. Macrophages are known to have high rates of endocytosis [29] and rapidly turn over their repertoire of cell surface receptors. Expression of dynamin 2 in macrophages [25] may contribute to the limited surface expression of P2X4. Rab5 is a rate-limiting enzyme responsible for trafficking proteins from the plasma membrane to early endosomes during clathrin-dependent endocytosis [17], and over-expression of WT Rab5 or constitutively active Rab5 (Q79L) reduced the number of P2X4 available at the surface for activation by ATP by approximately 30 % (Fig. 4). Therefore, by increasing expression or activation of Rab5, the cell could regulate surface P2X4 density through modulation of endocytosis. It is known that macrophages express an abundance of Rab5 where, in addition to endocytosis, Rab5 plays a role in phagocytosis and regulates phagosome maturation [26].
In addition to the regulation of constitutive internalisation of P2X4 and, therefore, availability of surface receptors for agonist binding, dynamin also controls agonist-induced desensitisation of P2X4. Desensitisation was measured as described in Fountain and North [24], and it was found that desensitisation was almost completely abolished when dynamin K44A was co-expressed with P2X4 (Fig. 2) and was slowed by co-expression with Rab5 S34N (Fig. 4), suggesting that internalisation through the clathrin-dependent endocytic pathway is responsible for desensitisation. Fountain and North identified a C-terminal lysine residue, K373 and an adjacent tyrosine residue Y374 which, when mutated to alanine, both accelerated desensitisation [24]. These residues are close to the predicted internalisation motif YEQGL [11] and may affect the binding of endocytic proteins to this C-terminal region to allow faster internalisation. Desensitisation is important for determining the duration and, therefore, magnitude of the P2X4-induced signal, and this may contribute to downstream signalling events associated with P2X4 activation such as calcium influx, MAPK activation and secretion of brain-derived neurotrophic factor (BDNF) and prostaglandin E2 (PGE2) [5, 30]. P2X4 is highly permeable to calcium [31], and the extent of desensitisation will regulate the spatial and temporal signalling aspects of both global and local calcium increases. An increase in expression or activity of Rab5 could increase agonist-induced internalisation, and this may be one mechanism by which cells can reduce signalling through P2X4.
Despite the differences seen in regulation of constitutive and agonist-induced internalisations of P2X4 by dynamin and Rab5, the effect of ivermectin potentiation of P2X4 responses were similar. Co-expression of Dyn-WT, Rab5-WT or active Rab5 significantly increased the potentiation by ivermectin, whereas inactive dynamin or Rab5 almost abolished ivermectin potentiation. Toulmé et al. have shown that ivermectin can increase the fraction of P2X4 associated with the plasma membrane in a Xenopus oocyte system and that disruption of clathrin-dependent endocytosis with membrane proximal dominant negative AP-2 or Eps15 proteins abolished potentiation by ivermectin [9]. The presented hypothesis suggested that internalisation of P2X4 was required for the increased ATP-induced response measured following treatment of the cell with ivermectin. Conversely, Silberberg et al. found that ivermectin did not increase the surface biotinylated fraction of P2X4 in the Xenopus oocyte system, thus challenging the hypothesis that endocytosis is required for ivermectin potentiation. Instead, Silberberg et al. proposed that ivermectin stabilises the receptor protein in the membrane in the open channel conformation [10]. The results with dynamin and Rab5 constructs would seem to favour the first hypothesis, but it is possible that the two hypotheses do complement each other. The fraction of P2X4 in the rapidly recycling endosomal pool likely dictates the number of P2X4 at the cell surface available for agonist binding. This study shows that altering the expression of key regulatory endocytic proteins can modulate cell surface expression by altering P2X4 turnover. The binding of ivermectin to P2X4 transmembrane regions may stabilise the protein in the membrane and prevent constitutive internalisation. Due to the continual insertion of P2X4-containing vesicles from the endosomal pool, this, in combination with blocked internalisation of ivermectin-bound P2X4, would increase the total number of P2X4 available for binding ATP, resulting in an increased response. Furthermore, surface biotinylation experiments in NR8383 macrophages demonstrate that ivermectin does increase the amount of P2X4 protein at the cell surface (Fig. 7).
P2X4 has recently been implicated in the pathology of neuropathic pain due to induction of BDNF secretion from spinal microglial cells [5] and inflammation-induced pain via secretion of the inflammatory mediator PGE2 [30]. The cell surface expression of P2X4 appears to be tightly regulated in macrophages and microglia through both constitutive cycling and lysosomal targeting, and it has been shown that upregulation of P2X4 at the plasma membrane could be achieved by lysosomal exocytosis, phagocytosis of foreign particles or by altering endocytosis [13, 14]. This study provides new information about the regulation of constitutive and agonist-induced internalisation pathways of P2X4 and highlights Rab5 as a key protein controlling P2X4 trafficking.
Acknowledgments
The author gratefully acknowledges the support of Professor Annmarie Surprenant during this project and would like to thank Elizabeth Martin and Weihong Ma for their help with cell culture and transfections at the University of Sheffield, Jeremy Sanderson (University of Sheffield) for helping with confocal microscopy and Professor Elizabeth Smythe (University of Sheffield) for the Rab5 constructs.
References
- 1.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 4.Sim JA. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26(35):9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulmann L, Hatcher J, Hughes J, Chaumont S, Green P, Conquet F, Buell G, Reeve A, Chessell I, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci. 1999;19(17):7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson GR, Wafford KA, Smith AV, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 2000;295(3):1051–1060. [PubMed] [Google Scholar]
- 8.Shan Q. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276(16):12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- 9.Toulmé E, Soto F, Garret M, Boué-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69(2):576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- 10.Silberberg S, Li M, Swartz K. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54(2):263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Royle SJ, Qureshi OS, Bobanović LK, Evans PR, Owen DJ, Murrell-Lagnado RD. Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci. 2005;118:3073–3080. doi: 10.1242/jcs.02451. [DOI] [PubMed] [Google Scholar]
- 12.Royle SJ, Murrell-Lagnado RD. Constitutive cycling: a general mechanism to regulate cell surface proteins. Bioessays. 2002;25(1):39–46. doi: 10.1002/bies.10200. [DOI] [PubMed] [Google Scholar]
- 13.Stokes L, Surprenant A. Dynamic regulation of the P2X 4receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol. 2009;39(4):986–995. doi: 10.1002/eji.200838818. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi OS, Paramasivam A, Yu JCH, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120(21):3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 15.Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5(7):463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 16.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 17.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70(5):715–728. doi: 10.1016/0092-8674(92)90306-W. [DOI] [PubMed] [Google Scholar]
- 18.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124(5):997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinneen J. Expression of dominant negative rab5 in HeLa cells regulates endocytic trafficking distal from the plasma membrane. Exp Cell Res. 2004;294(2):509–522. doi: 10.1016/j.yexcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Hunker CM, Kruk I, Hall J, Giambini H, Veisaga ML, Barbieri MA. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Arch Biochem Biophys. 2006;449(1–2):130–142. doi: 10.1016/j.abb.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Zadeh AD, Xu H, Loewen ME, Noble GP, Steele DF, Fedida D. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol. 2008;586:4793–4813. doi: 10.1113/jphysiol.2008.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45(1):81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22(12):4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281(22):15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- 25.Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190(12):1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitano M, Nakaya M, Nakamura T, Nagata S, Matsuda M. Imaging of Rab5 activity identifies essential regulators for phagosome maturation. Nature. 2008;453(7192):241–245. doi: 10.1038/nature06857. [DOI] [PubMed] [Google Scholar]
- 27.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122(3):565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140(3):567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pataki G, Czopf L, Jilling T, Marczin N, Catravas J, Matalon S. Regulation of fluid-phase endocytosis in alveolar macrophages. Am J Physiology. 1995;269(4 Pt 1):L520–L526. doi: 10.1152/ajplung.1995.269.4.L520. [DOI] [PubMed] [Google Scholar]
- 30.Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29(14):2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stühmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA. 1996;93(8):3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]