Abstract
Aims
1) To evaluate the role of pre-existing weakness in working memory ability (WM) as a risk factor for early alcohol use as mediated by different forms of impulsivity. 2) To assess the adverse effects of progressive alcohol use on variations in WM over time.
Design, Setting and Participants
A community sample of 358 adolescents [48% males, Meanage(baseline) = 11.4± 0.87 years] from a longitudinal cohort design, assessed annually over four consecutive years with less than 6% attrition.
Measurements
Repeated assessments were conducted for the following key variables: WM (based on performance on four separate tasks), frequency of alcohol use (AU), and three forms of impulsivity, namely sensation seeking (SS), acting-without-thinking (AWT) and delay discounting (DD). Latent growth curve modeling procedures were used to identify individual trajectories of change for all key variables.
Findings
Weakness in WM (at baseline) significantly predicted both concurrent alcohol use and increased frequency of use over the four waves (p <.05). This effect was entirely mediated by two forms of impulsivity, AWT and DD, both of which were characterized by underlying weakness in WM. No individual variation was observed in the slopes of WM, which suggests that individual variations in alcohol use were not associated with changes in WM in our early adolescent sample.
Conclusions
Early adolescent alcohol use may be a consequence of (pre-existing) weaknesses in working memory (WM) rather than a cause of it. Efforts to reduce early alcohol use should consider the distinct roles of different impulsivity dimensions, in addition to WM, as potential targets of intervention.
Introduction
Alcohol use is increasingly common during adolescence (1,2), with a link between early onset and subsequent abuse/dependence (3,4). Studies in both animals (5) and humans (6,7) have found impairments in executive cognitive functions (ECFs) in adolescents exposed to large quantities of alcohol. The conclusion drawn from these studies is that alcohol adversely impacts the nascent adolescent brain, producing neurocognitive deficits that leave the adolescent even more vulnerable to its addictive properties (8). This hypothesis has however been tested in human adolescents mostly using cross-sectional designs (9–11). The only longitudinal evaluation that we are aware of (12) found very limited effects of alcohol use on neurocognitive functioning, and did not report on the baseline differences (if any) in the neurocognitive abilities of adolescents who had engaged in moderate/heavy drinking by follow-up vs. those who had not. This leaves open the possibility that at least some of the neurocognitive deficits observed in adolescent drinkers may have been present beforehand and may have increased the likelihood of early drinking in the first place (13). The current research sought to clarify this issue by examining the developmental associations between working memory ability (WM) – a fundamental form of ECF (14) and early alcohol use, in a community cohort of adolescents (ages 10–12 at baseline) assessed annually for four consecutive years.
WM is an important ECF to study because it is critical to various high-order cognitive functions, including planning and decision-making (14). WM impairments have been linked to both impulsive decision making (15,16) and risk-taking in early adolescents (17,18). Although WM continues to develop during adolescence (19), it can be challenged by the increasing activation of the mesolimbic dopamine system that elevates appetite for novel and exciting behaviors (20) and enhances impulsive drives. Adolescents with relatively weak WM may therefore be at greater risk for involvement in early and risky alcohol use (17,18).
While the specific role of WM as a predictor of adolescent drinking is less well understood, prior research suggests that pre-existing deficits in ECFs may increase risk for early alcohol use. For instance, Giancola et al. (21) found that pre-adolescent children from families with alcohol-use-disorders (AUD), who were at greater risk for later alcohol dependence, had lower ECF scores as compared to their counterparts without a family history of AUD, even prior to the onset of drinking. Similarly, Nigg et al. (22) reported that adolescents from families with AUD performed poorly on ECF measures (e.g. stop-signal task). In addition, they found that adolescents with poor response inhibition exhibited greater alcohol-related problems (23). These findings are however limited in that they are based on comparisons of high-risk adolescents with normal controls and are primarily cross-sectional. Furthermore, these studies do not explore the specific role of WM as a predictor of early and risky drinking.
Impulsivity or lack of self-control is a well-established risk-factor for alcohol use (24–26), which is also found to be associated with underlying weaknesses/deficits in WM (27,28). Considering impulsivity’s joint relationship with WM and early drinking, it can be argued (though not yet tested) that impulsive tendencies may serve as potential mediators of the influence of weak WM on early alcohol use (29). At the same time, it is important to emphasize that impulsivity is a multi-dimensional construct (30,31), with only some dimensions regarded as dysfunctional (32,33). In particular, sensation-seeking (SS) (30) is found to be positively associated with WM (18) and IQ (34). Therefore, SS does not necessarily represent a weakness in executive control as compared to other impulsivity dimensions such as acting-without-thinking (AWT) (35) and delay discounting (DD) (28). Not surprisingly, adolescents high in SS do experiment with alcohol, but they do not report as many drinking-related problems as adolescents high in AWT or DD (36). Given the opposing relationships shared by the different dimensions of impulsivity with WM, unless their pathways of influence are distinctly modeled, it may be difficult to ascertain their precise contribution to early and risky drinking.
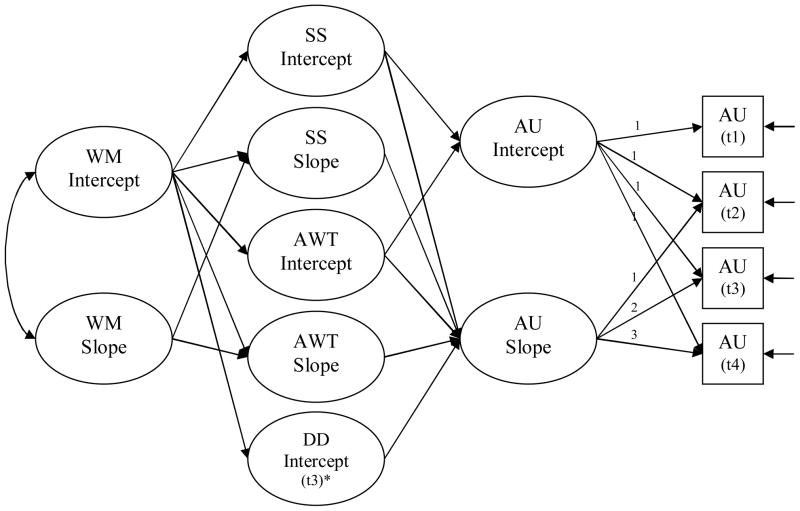
To design preventive interventions it is important to examine the longitudinal associations between WM, impulsivity and alcohol use during younger years. To that end, we tested a version of the model (see Figure 1) proposed by Giancola and Tarter (21) and de Wit (29). This model allows us to (i) explore the influence of WM on alcohol use longitudinally, and (ii) determine the distinct roles of SS, AWT and DD as mediators of this influence. Using latent growth curve modeling (LGCM), we examined: (1) whether individual differences in the initial level (intercept) and rate of change (slope) of WM predict individual differences in the intercept and slope of alcohol use (AU) either directly and/or as mediated by three forms of impulsivity; and (2) in a separate model, we tested whether individual differences in the intercept or slope of AU were associated with individual variations in the slope of WM.
Figure 1.
Hypothesized model with an illustration of a linear latent growth curve for the Alcohol Use outcome variable only.
Note. WM = Working Memory, SS = Sensation Seeking, AWT = Acting Without Thinking, DD = Delay Discounting, AU = Alcohol Use frequency.
*Delay discounting was only assessed during the last two follow-ups and therefore it was not possible to model trajectories of change in DD. Both fixed and random components were estimated for each of the repeated measure variables. The residual variances in intercepts and slopes corresponding to each endogenous key variable will be correlated (correlations not shown here for reasons of clarity). Although not shown for the sake of clarity, direct paths from WM to AU intercept and slope were also included in the model.
Method
Participants
A community sample of adolescents (N=358; Mage=11.4±0.87yrs) from the Philadelphia area was surveyed annually for four consecutive years with minimal (<6%) attrition. No statistically significant differences were found in terms of demographics or baseline scores of key variables between those who dropped out and those who were retained. Details about sample recruitment and data collection have been described previously (17). The study was approved by the IRB of the Children’s Hospital of Philadelphia. The sample comprised of 48% males. Fifty-five percent of youth self-identified as non-Hispanic White, followed by non-Hispanic Black (28%) and Hispanic (9%). Majority of the participants were classified as lower-middle class (Mean =47.0±15.8; reverse scored) using the Hollingshead Two-Factor Index of Social Status (37).
Measures
The key variables described below were assessed at each wave, except for DD, which was assessed only at the last two waves. Self-reports on sensitive topics (e.g., alcohol use) were obtained using audio computer-assisted self-interviewing (ACASI) (38).
Frequency of Alcohol Use (AU)
Participants were asked about ever and recent (past 30 days) alcohol use. Responses were used to create a new variable that was coded 0 (Never drank), 1 (Used, but not in past 30 days), 2 (Drank 1–2 days in past 30 days), and 3 (Drank more than 2 days in past 30 days).
Working Memory Ability (WM)
Four tasks were used to assess WM: Backward Digit Span (39), Corsi Block Tapping (40), Letter Two-back (41) and Spatial Working Memory (42). Details about the nature of these tasks and their administration procedures have been reported previously (17,18). Of the various measures typically used to assess spatial WM, we employed a self-directed computerized task in which participants searched for hidden tokens one at a time within sets of 4–8 randomly positioned boxes. WM skills are tapped as the participant while searching must hold in memory the locations already checked and as tokens are found, must remember and update information about their locations. Spatial WM performance was indexed using the between-search error score (42). All WM tasks were scored such that higher values implied better performance.
Sensation-seeking (SS)
Respondents’ level of agreement with four items representing the four dimensions of Zuckerman’s sensation-seeking scale (i.e., experience seeking, boredom susceptibility, thrill/adventure seeking, and disinhibition) (43,44) was assessed on a scale ranging from 1(strongly disagree) – 4(strongly agree). Responses were averaged to obtain a continuous score (range: 1 – 4) for each wave.
Acting-without-thinking (AWT)
A self-report measure including nine yes/no questions (e.g. “Do you usually do and say things without stopping to think?”) derived from the Junior Eysenck Impulsivity Scale (45) was used to assess AWT at each wave. Scores were averaged to obtain a continuous score (range: 0 – 1) for each wave.
Delay discounting (DD)
A monetary choice procedure adapted from Green et al. (46) was used to assess adolescents’ preference for immediate reward. Similar procedures have been shown to be valid with this age-group youth (47) and to be valid indicators of the ability to delay gratification (48). Respondents were asked in the context of payment for a job to identify an amount of money between $10 and $90 that, if received immediately, would be equivalent to receiving $100 six months later. Respondents are initially asked if they would accept a payment of $50 immediately in lieu of being paid $100 in six months. Those who accepted the $50 offer, were then asked if they would accept an amount lower than $50 in $10 decrements. The lowest amount they accepted was taken as their equivalent value. A comparable procedure with successively increasing values was used for those who did not accept $50. Scores on this variable ranged from 10 – 100, which were reverse-scored such that higher scores were indicative of greater delay discounting. Research comparing hypothetical with real rewards and delays indicates that this procedure produces comparable estimates of individual differences (49). Assessments of DD were conducted only at the last two assessments and exhibited considerable stability (r =.43, p<.001).
Covariates
Baseline assessments of the following covariates: age, pubertal development (50), sex, race-ethnicity (White, Black, Hispanic & others), socio-economic status, positive expectancies of alcohol and friends’ approval of alcohol use (51), as well as perceived parental monitoring (52) – were included in the model.
Analytic Strategy
LGCM was used to identify individual differences in intercepts and slopes of the trajectories of key variables (53). Since WM was assessed using four measures, each with a separate scale, a multiple-indicator LGCM strategy (54) was used to generate its intercept and slope. Unconditional growth curve models were estimated first to identify the change pattern in each of the key variables. We then tested the model presented in Figure 1. The effect of WM intercept and slope on the intercept and slope of AU was tested first. The potential mediators, i.e. the intercepts and slopes of SS, AWT, and DD, were added next. Finally, the covariates were added to the model. Confidence intervals for mediated effects were obtained using the bias-corrected bootstrap re-sampling method (55). Model fit was evaluated using multiple indices of global fit and an examination of residual diagnostics. The criteria for good model fit included a low chi-square (χ2) test-statistic, a root-mean-square-error-of-approximation (RMSEA) value less than 0.05, and values of the comparative fit index (CFI) and the Tucker-Lewis Index (TLI) greater than 0.90. The influence of AU intercept and slope on WM intercept and slope was tested in a separate model. All analyses were conducted in Mplus v6 using robust estimation procedures (56).
Results
Preliminary Analyses
Four latent WM factors were estimated corresponding to each wave of the study. Each factor had four indicators, i.e. the four WM tasks administered at each wave. The loading corresponding to corsi block tapping was fixed to one for each WM factor to identify the model and scale the factor. Correlations between the four WM indicators at each wave ranged from r = 0.16 – 0.38, while the correlations between repeated measurements of each of the four WM indicators ranged from r = 0.25 – 0.61. The loadings of each WM factor ranged from 0.40–0.60, with a reliability coefficient (rho) of 0.70 (57). The measurement invariance of the latent WM factor across all four waves was tested and found to be tenable, given that there was a non-significant drop in model fit when factor loadings corresponding to each repeated indicator were constrained to be equal across waves [χ2(dfdiff =9) =16.14, p=.07].
At each assessment, the correlations between SS and AWT ranged from .36–.44, between AWT and DD ranged from .14–.16, while SS and DD were virtually unrelated (r’s=.03–.04). Correlations across time for each of the impulsivity measures reflected a high degree of stability, SS (r =0.43–0.68), AWT (r =0.47–0.69), and DD (r =0.43). At each wave, reliability coefficients (rho) of SS ranged between 0.73–0.78, and those of AWT were between 0.75–0.80.
Based on the model fit statistics obtained from unconditional LGCM, it was determined that a linear model provided an acceptable fit to the underlying pattern of change in all key variables (Table 1). The mean slope of all key variables was significant and positive, indicating an increase over time (Table 1). There was also significant variance in intercepts and slopes, except for the slope of WM, suggesting that even though there was an increase in WM on average, [MSlope(SE)=0.50(.05), p <.001], there was no significant variation in the individual trajectories over time. It is also noteworthy that with the exception of SS, the intercepts and slopes of all key variables were uncorrelated, indicating that initial status was unrelated to subsequent rate of change.
Table 1.
Descriptives for repeated measures and unconditional Latent Growth Curve Modeling (LGCM) results for key variables
| Unconditional Linear LGCMs
|
|||||||
|---|---|---|---|---|---|---|---|
| Model Fit Indices | Unstandardized Estimates (SE)sig | ||||||
| Variable Names | Mean (SD) Waves 1,2, 3, and 4 | χ2 statistic (df), p value | RMSEA (95% CI) | CFI, TLI | Mean (int), Mean(slp) | Var (int), Var (slp) | Covar (int↔slp) |
| WM (Multiple Indicator LGCM) | −0.01(.83), 0.50(.83), 0.99(.73), 1.47(.81) | 108.22(92), p = 0.12 | 0.02 (.00, .04) | 0.99, 0.98 | 0†, 0.50(.05)* | 2.65(.43)*, .00(.00)S | −.12(.09) |
| SS (Range:1–4) | 2.14(.77), 2.21(.72), 2.27(.77), 2.39(.76) | 8.27 (6), p = 0.22 | 0.03 (.00, .08) | 0.99, 0.99 | 2.13(.04)*, 0.08(.01)* | .40(.04)*, .03(.01)* | −.04(.01)* |
| AWT (Range:0–1) | 0.34(.26), 0.39(.28), 0.40(.30), 0.41(.31) | 14.40 (8), p = 0.07 | 0.05 (.00, .08) | 0.98, 0.99 | 0.35(.01)*, 0.02(.01)* | 0.04(.004)*, .004(.001)* | 0.00(.002)†† |
| AU (Range:0–3) | 0.21(.49), 0.41(.67), 0.58(.77), 0.71(.83) | 4.84 (4), p = 0.30 | 0.02 (.00, .08) | 0.99, 0.99 | 0.21(.03)*, 0.17(.01)* | 0.19(.03)*, 0.04(.01)* | −.01(.01) |
Note.
Int = intercept, slp = slope, WM = Working Memory, SS = Sensation Seeking, AWT = Acting Without Thinking, DD = Delay Discounting, AU = Alcohol Use frequency.
p < .05;
Since WM model was estimated using multiple-indicator LGCM technique, the mean intercept value is fixed to zero for model identification purposes.
The variance of WM slope was non-significant and close to zero. Therefore to help model estimation its value was fixed to zero.
The 95% CI for the covariance between the intercept and slope of AWT are (−.002, .003).
Main Findings
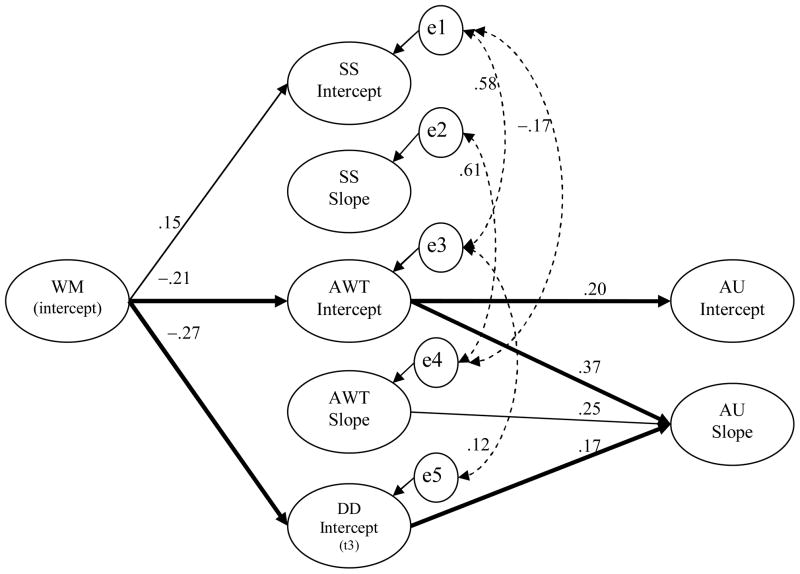
Testing the model in Figure 1, we found that WM at baseline significantly predicted both the intercept [β(95%CI)= −.02(−.036, −.001)] and slope of AU [β(95%CI)= −.02(−.05, −.01)]. The effect of WM intercept on the intercept of AU was mediated by the intercept of AWT, [β(95%CI)= −.02(−.036, −.001)] while the effect of WM intercept on the slope of AU was mediated by both the intercept of DD, [β(95%CI)= −.01(−.023, −.001)] and the intercept of AWT, [β(95%CI)= −.02(−.034, −.003)]. The rate of increase (i.e. slope) of AWT was positively related to the rate of progression in AU [β(SE) =.84(.31), p<.01].
The effect of WM on the intercept and slope of AU was entirely channeled through the mediated pathways described above (see bold lines in Figure 2), with no left over direct effect. Since the frequency of alcohol use at baseline was quite low, with nearly all adolescents (97%) reporting either a 0 or 1, the effect of WM intercept on the intercept of AU can be interpreted as WM’s association with alcohol use initiation. Although the intercept of WM was positively related to the intercept of SS, the net mediated effect on AU through the intercept of SS was not significant, [β(95%CI)= −.01(−.001,.03)]. This was mainly due to the weak association between the intercepts of SS and AU, β(SE)=.13(.08), p<.10.
Figure 2.
Final model showing only the significant (p<.05) relationships between key variables along with corresponding standardized coefficient values.
Note. WM = Working Memory, SS = Sensation Seeking, AWT = Acting Without Thinking, DD = Delay Discounting, AU = Alcohol Use frequency. Bold lines represent the three significant mediated effects [WM → IntAWT → IntAU; WM → IntAWT → SlpAU and WM → IntDD → SlpAU]. The effect of age, gender, pubertal development, race-ethnicity, SES, positive expectations of alcohol, friends’ approval of alcohol use, and perceived parental monitoring was covaried out (see Table 2). The residual errors of the intercepts of SS, AWT, and AU were allowed to correlate with the residual errors associated with their respective slopes. Dashed paths represent the significant correlations between residual errors (p<.10).
All covariates except parental monitoring were found to have significant associations with either the outcome or the mediating variables (see Table 2). Some of the residual errors were significantly correlated as shown in Figure 2. Overall, the model fit was excellent with an RMSEA (90%CI) =0.03 (0.02, 0.04), TLI =0.95, CFI =0.95, SRMR =0.04, even though the chi-square was significant, χ2(282) =378.9, p<.001.
Table 2.
Unstandardized and standardized coefficient estimates for significant effects on intercepts (int) and slopes (slp) of key variables.
| Path Represented | Unstandardized coefficient (SE) | p value |
|---|---|---|
| Key Variables effect on Mediating Variables | ||
| IntWM → IntSS | .07(.04) | =.05 |
| IntWM →IntAWT | −.04(.01) | <.05 |
| IntWM → IntDD | −.27(.11) | <.05 |
| Key Variables effect on Outcome Variables | ||
| IntAWT → IntAU | .40(.20) | <.05 |
| IntAWT → SlpAU | .41(.13) | =.001 |
| SlpAWT → SlpAU | .84(.31) | <.01 |
| IntDD → SlpAU | .04(.02) | <.05 |
| Influence of Covariates | ||
| Age → IntWM | .45(.10) | <.001 |
| Age → IntAWT | .04(.02) | <.05 |
| Age → SlpAU | .05(.02) | <.01 |
| Age → SlpSS | −.23(.11) | <.05 |
| Female → IntWM | −.44(.22) | <.05 |
| Female → IntAWT | −.07(.02) | <.001 |
| Female → IntSS | −.23(.06) | <.001 |
| Pubertal dev X Female → IntWM | .34(.15) | <.05 |
| Pubertal dev → IntAWT | .04(.01) | <.01 |
| Pubertal dev → IntSS | .16(.03) | <.001 |
| Black (Race-eth) → IntAWT | .08(.03) | <.01 |
| Black (Race-eth) → IntDD | .41(.18) | <.05 |
| Others (Race-eth) → SlpAU | −.11(.03) | =.001 |
| SES → IntWM | .02(.01) | <.001 |
| SES → IntDD | −.01(.00) | <.05 |
| Friends Approve → IntAU | .14(.05) | <.01 |
| Positive Expectancies → IntAU | .23(.06) | <.001 |
Note.
Int = Intercept, Slp = Slope, WM = Working Memory, SS = Sensation Seeking, AWT = Acting Without Thinking, DD = Delay Discounting, AU = Alcohol Use frequency.
White was the reference category for Race-Ethnicity covariate. All continuous covariates are scored such that higher scores imply higher values on that variable.
Since there was no individual variation in the slopes of WM, individual differences in drinking frequency observed in our early adolescent sample were unrelated to the developmental trajectories of WM.
Discussion
The present study shows that pre-existing weakness in WM predicted both concurrent alcohol use (intercept) as well as the increased frequency of use (slope) over a four-year follow-up period in a community-based sample of early adolescents. This effect was entirely channeled through two forms of impulsivity, AWT and DD, both of which were characterized by underlying weakness in WM. Individual differences in drinking frequency did not impact the developmental trajectories of WM in our early adolescent sample. In fact, the lack of variance in WM slopes suggests that baseline differences in WM among participants tended to remain stable over the study period.
As expected, SS was positively associated with WM (18). Although SS shared a weak positive association with alcohol use at baseline, it was not a significant mediator of the effect of WM on AU intercept. Furthermore, unlike the intercepts of AWT and DD, it did not independently predict the slope of AU. Thus, adolescents high in SS experimented with alcohol use initially but they did not progress in drinking frequency during the follow-up years. Overall, as adolescents aged, the influence of WM as mediated by the three forms of impulsivity was largely protective.
Our findings provide longitudinal evidence in support of models proposed by Tarter (21) and Nigg (22,23), wherein weaknesses in ECFs and associated forms of behavioral disinhibition are viewed as risk-factors for early alcohol use. In comparison to their cross-sectional research, our findings more clearly demonstrate the link between pre-existing WM weaknesses and increased frequency of alcohol use during early-mid adolescence and delineate the mediational pathways of impulsivity through which this effect is transmitted.
Consistent with past longitudinal research (12), we did not detect a detrimental influence of increased drinking frequency on WM in particular. This may be because our sample was too young to have engaged in heavy (and potentially harmful) drinking during the study period. Nevertheless, given that weakness in WM preceded (and predicted) increases in drinking frequency observed in our sample, it can be argued that neurocognitive weaknesses observed in older adolescent samples in previous research (9,12) may not have been entirely a consequence of their drinking behaviors. At least some of those weaknesses could have been present beforehand and may have been partly responsible for risky drinking in the first place. Longitudinal research spanning the course of adolescence is needed to test the possibility that weak WM leads to excessive alcohol consumption, which can further compromise WM abilities (58).
The unique role played by the different dimensions of impulsivity as mediators of the influence of WM on alcohol use is also noteworthy. The two dimensions (AWT and DD) that displayed negative associations with WM were also the ones linked to increased drinking frequency. The third dimension of SS, although shared a weak positive association with initial alcohol use, it was not related to an increase in drinking frequency. In fact, the intercept of SS was negatively correlated with the slope of AWT (59). This is consistent with other studies that have found SS to be linked with greater experimentation with alcohol, but not with increased risk for negative consequences (36). This uniqueness of SS may be explained by the fact that just like the other impulsivity dimensions, it is influenced by the ventral-striatal dopamine system (60), but unlike them, it does not share weakness in other dopamine pathways such as the dorsal nigrostriatal system, which subserves WM and flexible behavioral control (18,61). Past studies that have reported links between SS and alcohol use (62) may have detected spurious associations because of inadequate control for other potentially correlated forms of impulsivity like AWT. Future research would benefit from systematically accounting for the distinct, yet potentially overlapping forms of impulsivity in order to better understand their association with alcohol use. Similarly, interventions targeting SS to reduce early alcohol use (63) might more profitably focus on reducing the AWT and DD dimensions of impulsivity.
It is also important to highlight the lack of correlation observed between alcohol use at ages 10–12 and subsequent changes in drinking frequency. This finding seems to contradict retrospective research that finds early age of drinking onset to be a risk factor for AUD (4). One reason for this discrepancy could be that it is not necessarily early initiation that increases risk for later dependence, but instead the presence of other risk factors early on that predispose youth to later problems with alcohol (64). Another reason may be that it is too early in the lives of our cohort, given their low rates of alcohol use, to detect an association between early initiation and subsequent progression in drinking frequency. A follow-up at later ages may be needed to conclusively prove or disprove this association.
In terms of clinical implications, the results point to the usefulness of working memory training as a viable intervention strategy to reduce early alcohol use and enhance cognitive control over impulsive drives (65), thereby reducing risk for problematic alcohol use during later years. Interventions to improve WM have already been tested with some success (66–68) and can be delivered universally at young ages (69). Furthermore, given the importance of impulsivity as a contributor to early alcohol use, combining WM training with skill-building programs that enhance decision making and problem solving (70) may be especially effective in reducing impulsive behavior. The ability of WM interventions to reduce early and risky alcohol use through the potential reduction in dysfunctional forms of impulsivity seems promising and warrants further investigation.
Limitations
Although we controlled for important covariates such as parental monitoring and friends’ approval of alcohol use, we did not have information about parental drinking history. Nevertheless, most theories of early drug use suggest that parental influences are mediated by factors that were assessed in the present study, such as behavioral disinhibition and parental monitoring (71). Our study is also limited by the self-reported nature of our data which may have resulted in under-reporting of alcohol use, although our use of ACASI should have minimized this bias (38). Further, we focused on the frequency of drinking episodes and not on the quantity consumed per occasion because of the expectedly low rates of binge drinking in our early adolescent sample (<7% reported more than one episode). Nevertheless, it is likely that heavier use of alcohol, than reported by our sample, may adversely impact WM over time (72). It is also possible that increase in drinking frequency may have affected other brain functions, such as those sub-served by the hippocampus (73). Therefore, our null finding regarding the neurocognitive effects of drinking frequency is limited to WM in a community-based, early adolescent sample. Further, since DD was assessed only during the last two waves, it can be argued that its effect on the slope of AU is not necessarily causal. Similarly, the effect of WM on the intercept of AU, mediated through the intercept of AWT, is also cross-sectional in nature. Finally, because of the limited time-points of assessments of DD, we could not model its developmental trajectories. Also, the reliability of our DD measure was weaker in comparison to the WM latent factor and other impulsivity measures. However, given that this tendency is found to remain relatively stable (46,74) it seems likely that we were able to parse out its unique mediational effect.
To conclude, our results indicate that early adolescent alcohol use may be a consequence of (pre-existing) weaknesses in WM rather than a cause of it, as is often assumed. Furthermore, understanding the role of distinct forms of impulsivity as mediators of the influence of weak WM on early alcohol use is important for designing effective preventive interventions.
Footnotes
Declaration of Interest: The project described was supported by Grant Number R01DA018913 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002 May 1;97(5):517–31. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan SC, Duncan TE, Strycker LA. Alcohol use from ages 9 to 16: A cohort-sequential latent growth model. Drug and Alcohol Dependence. 2006 Jan 4;81(1):71–81. doi: 10.1016/j.drugalcdep.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000 May;157(5):745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 5.Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000 Nov;24(11):1712–23. [PubMed] [Google Scholar]
- 6.Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: Basic to clinical studies. Annals of the New York Academy of Sciences. 2004 Jun 1;1021(1):234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- 7.Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005 Jan;40(1):23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl. 2002 Mar;(14):71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- 9.Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research [Internet] doi: 10.1111/j.1530-0277.2011.01527.x. [cited 2011 Aug 7]; Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1530-0277.2011.01527.x/abstract. [DOI] [PMC free article] [PubMed]
- 10.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000 Feb;24(2):164–71. [PubMed] [Google Scholar]
- 11.Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, et al. Adolescent substance abuse: The effects of alcohol and marijuana on neuropsychological performance. Alcoholism: Clinical and Experimental Research. 2011 Jan 1;35(1):39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23(4):715–22. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008 Mar;32(3):375–85. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 15.Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- 16.Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–62. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- 17.Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009 Nov;47(13):2916–26. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Yang W, Hurt H. Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Developmental Science. 2011 Sep;14(5):1119–1133. doi: 10.1111/j.1467-7687.2011.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005 May 1;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- 20.Somerville LH, Casey B. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010 Apr;20(2):236–41. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999 May 1;10(3):203–205. [Google Scholar]
- 22.Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, et al. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004 May;113(2):302–14. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- 23.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006 Apr;45(4):468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 24.Littlefield AK, Sher KJ, Steinley D. Developmental trajectories of impulsivity and their association with alcohol use and related outcomes during emerging and young adulthood. Alcohol Clin Exp Res. 2010 Aug;34(8):1409–16. doi: 10.1111/j.1530-0277.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004 May;28(3):343–51. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, et al. Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Dev. 2006;77(4):1016–33. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitney P, Jameson T, Hinson JM. Impulsiveness and executive control of working memory. Personality and Individual Differences. 2004 Jul;37(2):417–28. [Google Scholar]
- 28.Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, et al. Individual differences in delay discounting. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 29.de Wit H, Richards JB. Dual determinants of drug use in humans: Reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- 30.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001 Mar;30(4):669–89. [Google Scholar]
- 31.Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16(2):124–31. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- 32.Dickman SJ. Functional and dysfunctional impulsivity: personality and cognitive correlates. J Pers Soc Psychol. 1990 Jan;58(1):95–102. doi: 10.1037//0022-3514.58.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Gullo MJ, Dawe S. Impulsivity and adolescent substance use: Rashly dismissed as “all-bad”? Neuroscience & Biobehavioral Reviews. 2008 Oct;32(8):1507–18. doi: 10.1016/j.neubiorev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Raine A, Reynolds C, Venables PH, Mednick SA. Stimulation seeking and intelligence: a prospective longitudinal study. J Pers Soc Psychol. 2002 Apr;82(4):663–74. [PubMed] [Google Scholar]
- 35.Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005 Jun 30;135(3):191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Magid V, MacLean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007 Oct;32(10):2046–61. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 38.Metzger DS, Koblin B, Turner C, Navaline H, Valenti F, Holte S, et al. Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. American Journal of Epidemiology. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D. Wechsler Intelligence Scale for Children®. 4. San Antonio, TX: The Psychological Corporation; 2003. (WISC®-IV) [Google Scholar]
- 40.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27(3):272–7. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 41.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2(3):221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 42.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–34. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 43.Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences. 2002;32(3):401–14. [Google Scholar]
- 44.Zuckerman M. Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology. 1971 Feb;36(1):45–52. [Google Scholar]
- 45.Kuo PH, Chih YC, Soong WT, Yang HJ, Chen WJ. Assessing personality features and their relations with behavioral problems in adolescents: tridimensional personality questionnaire and junior eysenck personality questionnaire. Comprehensive Psychiatry. 2004;45(1):20–8. doi: 10.1016/j.comppsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5(1):33–36. [Google Scholar]
- 47.Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychological Science. 2005 Dec 1;16(12):939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds B, Schiffbauer R. Delay of gratification and delay discounting: A unifying feedback model of delay-related impulsive behavior. Psychological Record. 2005;55(3):439. [Google Scholar]
- 49.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolescence. 1980 Jun;9(3):271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 51.Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: The role of affect evaluation and peer-group influence in adolescent drug use. Prev Sci. 2007;8(2):89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Stanton B, Feigelman S. Impact of perceived parental monitoring on adolescent risk behavior over 4 years. Journal of Adolescent Health. 2000 Jul;27(1):49–56. doi: 10.1016/s1054-139x(00)00092-6. [DOI] [PubMed] [Google Scholar]
- 53.Bollen KA, Curran PJ. Latent Curve Models: A Structural Equation Perspective. 1. Hoboken, NJ: John Wiley and Sons, Inc; 2006. [Google Scholar]
- 54.Chan D. The conceptualization and analysis of change over time: An integrative approach incorporating Longitudinal Mean and Covariance Structures Analysis (LMACS) and Multiple Indicator Latent Growth Modeling (MLGM) Organizational Research Methods. 1998 Oct;1(4):421–83. [Google Scholar]
- 55.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004 Jan 1;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muthén LK, Muthén BO. Mplus. Los Angeles, CA: 2007. [Google Scholar]
- 57.Raykov T. Evaluation of scale reliability for unidimensional measures using latent variable modeling. Measurement and Evaluation in Counseling and Development. 2009;42(3):223–232. [Google Scholar]
- 58.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Child Adolesc Subst Abuse. 2011 Jan 1;20(2):135–54. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prev Sci. 2010;11(3):319–30. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008 Aug;14(4):381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- 62.Hittner JB, Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addictive Behaviors. 2006 Aug;31(8):1383–401. doi: 10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Sargent JD, Tanski S, Stoolmiller M, Hanewinkel R. Using sensation seeking to target adolescents for substance use interventions. Addiction. 2010 Mar 1;105(3):506–14. doi: 10.1111/j.1360-0443.2009.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999 Jan;23(1):101–7. [PubMed] [Google Scholar]
- 65.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, et al. Computerized training of working memory in children with ADHD: A randomized, controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(2):177–86. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2010 Nov;18(1):46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 67.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences [Internet] 2011 Jun 13; doi: 10.1073/pnas.1103228108. [cited 2011 Jun 15]; Available from: http://www.pnas.org/content/early/2011/06/03/1103228108.abstract. [DOI] [PMC free article] [PubMed]
- 68.Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12(4):F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 69.Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Espada J, Griffin K, Pereira J, Orgilés M, García-Fernández J. Component Analysis of a School-Based Substance Use Prevention Program in Spain: Contributions of Problem Solving and Social Skills Training Content. Prevention Science. 2012;13(1):86–95. doi: 10.1007/s11121-011-0249-y. [DOI] [PubMed] [Google Scholar]
- 71.King KM, Chassin L. Mediating and moderated effects of adolescent behavioral undercontrol and parenting in the prediction of drug use disorders in emerging adulthood. Psychol Addict Behav. 2004 Sep;18(3):239–49. doi: 10.1037/0893-164X.18.3.239. [DOI] [PubMed] [Google Scholar]
- 72.Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25(1):127–42. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29(1):141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Experimental and Clinical Psychopharmacology. 2006;14(3):318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]