Abstract
Human β-1,4-galactosyltransferase (β-1,4-GalT) V was shown to be involved in the biosynthesis of N-glycans, O-glycans and lactosylceramide (Lac-Cer) by in vitro studies. To determine its substrate specificity, enzymatic activity and its products were analyzed using mouse embryonic fibroblast (MEF) cells from β-1,4-GalT V (B4galt5)-mutant mice. Analysis of expression levels of the β-1,4-GalT I-VI genes revealed that the expression of the β-1,4-GalT V gene in B4galt5+/−- and B4galt5−/−-derived MEF cells are a half and null when compared to that of B4galt5+/+-derived MEF cells without altering the expression levels of other β-1,4-GalT genes. These MEF cells showed no apparent difference in their growth. When β-1,4-GalT activities were determined towards GlcNAcβ-S-pNP, no significant difference in its specific activity was obtained among B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells. No significant differences were obtained in structures and amounts of N-glycans and lectin bindings to membrane glycoproteins among B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells. However, when cell homogenates were incubated with glucosylcer-amide in the presence of UDP-[3H]Gal, Lac-Cer synthase activity in B4galt5+/−- and B4galt5−/−-derived MEF cells decreased to 41% and 11% of that of B4galt5+/+-derived MEF cells. Consistent with this, amounts of Lac-Cer and its derivative GM3 in B4galt5−/−-derived MEF cells decreased remarkably when compared with those of B4galt5+/+ derived MEF cells. These results indicate that murine β-1,4-GalT V is involved in Lac-Cer biosynthesis.
Keywords: B4galt5−/− mice, MEF cells, β-1, 4-GalT V, Lactosylceramide
Introduction
It is well known that glycans attached to proteins and lipids at cell surfaces are involved in cell-to-cell and cell-to-matrix interactions, thus inducing embryonic development, growth and differentiation [2–6]. In fact, such biological events are often hampered by impairments of glycosylation of proteins and lipids [7–16 and reviewed in 17]. The β-1,4-galactosylation of N- and O-glycans is particularly important for biological events since many functional carbohydrate antigens such as polysialic acids, HNK-1 antigen, sialyl Lewis X antigen, and Lewis X and Y antigens are expressed soley on the galactose (Gal) residues [reviewed in 18]. On the other hand, the β-1,4-galactosylation of glucosylceramide (Glc-Cer) opens diverse biosynthetic pathways of glycosphin-golipids (GSLs) such as ganglio-, lacto-, neolacto-, globo- and isoglobo-series [reviewed in 19]. There are six β-1,4-galactosyltransferases (β-1,4-GalTs) I, II, III, IV, V and VI [reviewed in 20], which are supposed to be involved in the biosyntheses of N- and O-glycans attached to proteins [21, 22] and of glycans attached to sphingolipids [23–25]. However, their exact acceptor specificities have not been established. Expression of individual human β-1,4-GalT cDNAs in Sf-9 cells, which lack a β-1,4-GalT activity and express N-acetylglucosamine (GlcNAc)-terminating N-glycans [26], resulted in the galactosylation of proteins as revealed by lectin blot analysis using Ricinus communis agglutinin-I (RCA-I), which interacts with oligosaccharides terminated with the Galβ1→4GlcNAc/Glc group [27], suggesting that all β-1,4-GalTs can galactosylate N-glycans in Sf-9 cells [28].
We isolated the β-1,4-GalT V cDNAs from human breast cancer cells [21] and mouse brain [29], and showed it to be involved in the biosyntheses of N-glycans, O-glycans, and lactosylceramide (Lac-Cer) by in vitro studies [21, 22, 28, 30]. In the previous study, we analyzed the phenotypes of the β-1,4-GalT V-mutant (B4galt5−/−) mice raised by a gene trap method, and showed that B4galt5−/− mice can grow up to E10.5 [14]. However, due to limited amounts of proteins available from B4galt5−/−-mouse embryos for biochemical studies, analyses of acceptor specificities of β-1,4-GalT V and structures of their glycans attached to proteins and sphingolipids were hardly conducted. In the present study, we isolated fibroblast cells from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-mouse embryos for elucidation of acceptor specificity of β-1,4-GalT V and their structures of N-glycans attached to proteins and of glycans attached to sphingolipids. The results showed that murine β-1,4-GalT V is involved in Lac-Cer biosynthesis like human β-1,4-GalT V as we reported by in vitro study 10 years ago [30].
Materials and methods
Chemicals
Glucosylceramide (Glc-Cer), Lac-Cer, GM3, sphingomyelin (SM) and UDP-Gal were purchased from Sigma-Aldrich Inc. (St. Louis, MO). UDP-[3H]Gal (20 Ci/mmol) was from American Radiolabeled Chemicals Inc. (St. Louis, MO). Horseradish peroxidase (HRP)-conjugated concanavalin A (Con A), RCA-I and peanut agglutinin (PNA), and biotinylated Maackia amurensis agglutinin (MAA) were from Seikagaku Kogyo Co. (Tokyo). Biotinylated Sambucus nigra agglutinin (SNA) and HRP-conjugated streptavidin were from Vector Laboratories Inc. (Burlingame, CA) and from ZYMED Laboratory (South San Francisco, CA), respectively. TSK gel Amide-80 column and Cosmosil 5C18-P column were from Tosoh Co. (Tokyo) and Nacalai Tesque Inc. (Kyoto), respectively.
Isolation of mouse embryonic fibroblast (MEF) cells
B4galt5−/−-mouse embryos were obtained as described previously [14]. MEF cells were isolated from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-mouse embryos at E10.5, and cultured in Dulbecco’s modified Eagle’s medium containing 15% fetal calf serum, 50 units/ml penicillin and 50 μg/ml streptomycin at 37°C under a 5% CO2 condition. The genotypes of the MEF cells were determined by PCR according to the method described previously [14]. All experiments were conducted according to institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments.
RT-PCR analysis
The expression of the mouse β-1,4-GalT I-VI genes was analyzed by reverse transcription-polymerase chain reaction (RT-PCR) using a Quick Master Mix (Toyobo Co., Ltd., Osaka) according to the manufacturer’s instructions. In brief, total RNA preparations were obtained from MEF cells using a Sepasol RNA I total RNA isolation reagent (Nacalai Tesque Inc., Kyoto). RT-PCR analysis was conducted using total RNAs and oligonucleotide primers specific to the mouse β-1,4-GalT I-VI genes. Their nucleotide sequences of the forward and reverse primers for the β-1,4-GalT I-VI genes and elongation factor (EF) 1α gene used are described in Table 1. Conditions for RT-PCR were as follows: one cycle at 90°C for 30 s; 60°C for 30 min; 94°C for 1 min, and then 26 cycles at 94°C for 30 s, 60°C for 30 s and 72°C for 1 min. The PCR products were analyzed by agarose gel electrophoresis as described previously [14] Fig. 1, 2.
Table 1.
Oligonucleotides used for RT-PCR analysis
| Gene | Primers | Sequences (5′ to 3′) |
|---|---|---|
| β-1,4-GalT I | B4galt1-F | GCCATCATCATCCCATTCCGTAACC |
| B4galt1-R | TGTCGTCATCTTCTCCTCCCCAACC | |
| β-1,4-GalT II | B4galt2-F | TCCCACAGCTCCAGTCATTCC |
| B4galt2-R | AACGGTACAGATTGCGGTCAT | |
| β-1,4-GalT III | B4galt3-F | CCAGAAAACGACCATAACCTGT |
| B4galt3-R | AGTTCATTCCATCTTGTGTCCA | |
| β-1,4-GalT IV | B4galt4-F | AACCCACCTTATCACCTCTCCT |
| B4galt4-R | GAATACGAAGCAGTCCCAGTTC | |
| β-1,4-GalT V | B4galt5-F | ACTTGGATTGGGATTGTCTGAT |
| B4galt5-R | CGCAGAGTAGTTCAGGTTGTTG | |
| β-1,4-GalT VI | B4galt6-F | CATCAGCTCTTCTCCAAGGACT |
| B4galt6-R | ACTCTGTTCCAAAGGTCATCGT | |
| elongation factor 1α | EF1α-F | CCATGAAGCTTTGAGTGAAGCTCT |
| EF1α-R | TAGCCTTCTGAGCTTTCTGGGCAG |
Fig. 1.
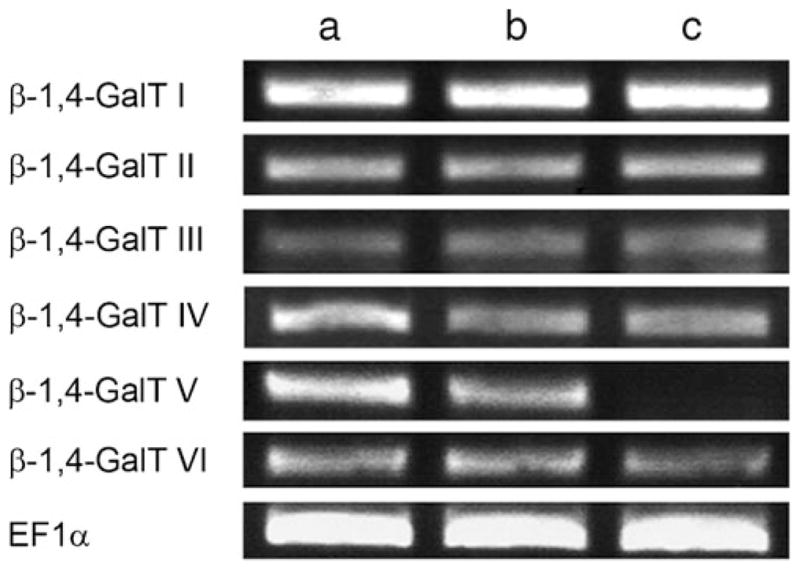
Relative expression levels of the β-1,4-GalT I-VI genes among B4galt5+/+(a)-, B4galt5+/− (b)- and B4galt5−/− (c)-derived MEF cells. RT-PCR analysis was carried out using total RNAs from each cell preparations and oligonucleotide primers specific to the β-1,4-GalT gene to be analyzed (shown in Table 1). PCR products were detected in a 2% agarose gel by staining with ethidium bromide. EF1α was used as an internal control. The analysis was conducted three times, and identical results were obtained
Fig. 2.
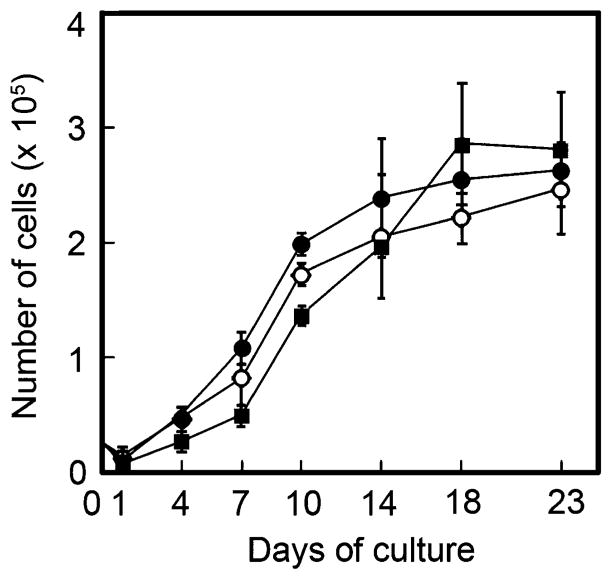
Proliferation kinetics of MEF cells. Values represent means with S.D. Closed circles, open circles and closed squares indicate B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells, respectively
Characterization of PA-oligosaccharides from MEF cells by two-dimensional mapping analysis
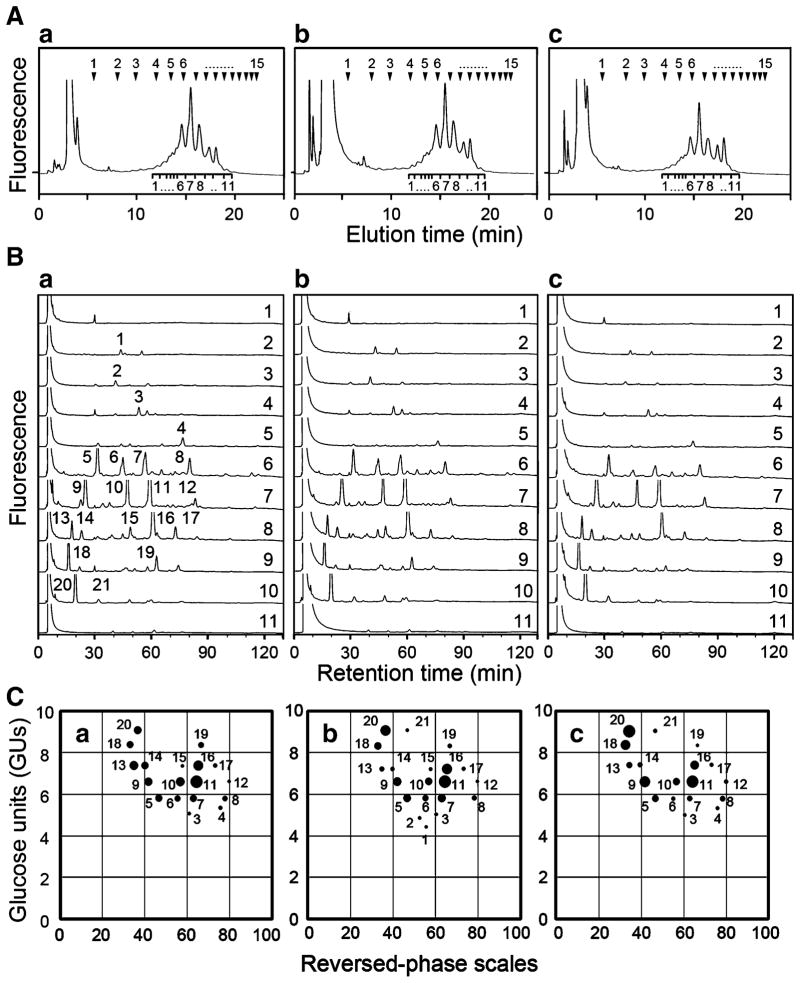
Membrane protein samples (2 mg) prepared from MEF cells were dried thoroughly over P2O5 in vacuo, and subjected to hydrazinolysis at 100°C for 10 h as described previously [31]. After N-acetylation, liberated oligosaccharides were reduced with dimethylamino borane in the presence of 2-aminopyridine (PA) to obtain pyridylaminated (PA)-oligosaccharides [32]. After desialylation of PA-oligosaccharides with 25 mM HCl solution at 80°C for 1 h, PA-oligosaccharides were subjected to two-dimensional mapping analysis as described previously [33]. In brief, PA-oligosaccharides were separated by size-fractionation high performance liquid chromatography (HPLC) on a TSK gel Amide-80 column with ammonium formate/acetonitrile gradient solution. Elution positions of PA-isomalto-oligosaccharides with 1–15 glucose units (GUs) were shown at the top of each panels in Fig. 3A. Then, oligosaccharides separated into 11 fractions were further separated by reversed-phase HPLC on a Cosmosil 5C18-P column with triethylamine acetate/1-butanol gradient solution. In order to characterize individual oligosaccharides separated by two-dimensional mapping analysis, retention times of individual oligosaccharide peaks separated into 21 components on a Cosmosil 5C18-P column as shown in Fig. 3B were converted to reversed-phase scales. Thus, a given oligosaccharide from these two columns can obtain a set of GU-value and reversed-phase scale, which affords a unique co-ordinate on the two-dimensional map. Relative peak areas of individual PA-oligosaccharides were calculated by taking a maximum peak area of one of PA-oligosaccharides in each sample as 100%, and then relative amounts of other PA-oligosaccharides were expressed as dot-sizes. In the present study, peaks 11 were maximal in all three samples, and their areas were taken as 100%.
Fig. 3.
Characterization of PA-oligosaccharides by two-dimensional mapping method. A Size-fractionation HPLC profiles of PA-oligosaccharides derived from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells. PA-oligosaccharides were separated into 11 fractions by size-fractionation HPLC. Arrowheads at the top of panels indicate elution positions of PA-isomalto-oligosaccharides with 1–15 GUs. Panels a, b, and c indicate elution patterns of PA-oligosaccharides from B4galt5+/+-, B4galt5+/−-, and B4galt5−/−-derived MEF cells, respectively. B Reversed-phase HPLC profiles of PA-oligosaccharides in 11 fractions shown in (A). Panels a, b, and c indicate retardation patterns of individual PA-oligosaccharides from B4galt5+/+-, B4galt5+/−-, and B4galt5−/−-derived MEF cells, respectively. PA-oligosaccharides with significant amounts were separated into 21 peaks, and they were numbered in panel a as a representative. C Two-dimensional map of PA-oligosaccharides. The elution positions of each PA-oligosaccharide on size-fractionation and reversed-phase columns were expressed in GUs and reversed-phase scales, respectively, and plotted on the map. The relative amounts of individual PA-oligosaccharides were expressed as dot-sizes. Panels a, b and c indicate plots on the maps from B4galt5+/+-, B4galt5+/−-, and B4galt5−/−-derived MEF cells, respectively. Dot numbers are identical among panels a, b and c, and to those in panel a of (B)
Assays of β-1,4-GalT activities
MEF cells harvested were washed with 10 mM phosphate buffered saline (pH 7.4) (PBS) by three times and suspended in 100 mM 2-(N-morpholino)ethansulfonic acid (MES) buffer (pH 7.0) containing 1.25% Triton X-100. They were sonicated and cell homogenates were used for transferase assays as an enzyme source. β-1,4-GalT assays were performed as described previously [21]. The reaction mixture contained 100 mM MES buffer (pH 7.0) containing 4 mM 5′-AMP, 250 μM UDP-[3H]Gal, 1 mM GlcNAcβ-S-pNP, 20 mM MnCl2 and enzyme preparation in a total volume of 50 μl. After incubation at 37°C for 1 h, product was isolated by using Sep-Pak C18 cartridges (Waters Corp., Milford, MA) and radioactivity incorporated into product was determined. Assay for Lac-Cer synthase activity was performed as described previously [30]. The reaction mixture contained 100 mM MES buffer (pH 7.0) containing 4 mM 5′-AMP, 250 μM UDP-[3H]Gal, 1 mM Glc-Cer, 20 mM MnCl2, and enzyme preparation in a total volume of 50 μl. After incubation, glycolipid was extracted from the reaction mixture with chloroform:methanol (2:1, v/v) by three times and then subjected to thin-layer chromatography (TLC) together with Lac-Cer, Glc-Cer and SM as standards in a developing solvent containing chloroform:methanol:water (60:35:8, v/v). Glycolipids were detected with an anthrone-thiourea reagent by heating the plate at 120°C. The areas corresponding to Lac-Cer in each sample were excised, and glycolipid was extracted. Radioactivities incorporated were determined by liquid scintillation counter.
Analyses of glycoproteins and glycolipids
MEF cells were washed with PBS by three times. Membrane proteins prepared from cells were subjected to SDS-polyacrylamide gel electrophoresis, and proteins were transferred to polyvinylidene difluoride filters. The blotted filters were incubated with SNA, MAA, Con A, RCA-I, L-PHA and PNA according to the method described previously [34]. Protein concentrations of cell pellets were determined with a BCA kit (Pierce Co., Rockford, IL) using bovine serum albumin as a standard, and glycolipids were analyzed by the method described previously [35]. In brief, total lipid fraction was extracted from cells with chloroform:methanol:water (1:2:0.8, v/v), and centrifuged at 12,000 rpm for 5 min. The supernatant containing lipids was dried by centrifugation using a SpeedVac concentrator (Savant Instruments Inc., Farmingdale, NY). Lipid samples were subjected to mild alkaline hydrolysis with 0.1 M NaOH/methanol solution at 40°C for 1 h, and then neutralized with 1 M acetic acid/methanol solution. For neutral glycolipid analysis, lipid samples whose amounts equivalent to 700 μg of cellular proteins were subjected to TLC using a high-performance TLC silica gel 60 plate (Merck KGaA, Darmstadt, Germany). The plate was developed in a solvent containing chloroform:methanol: water (60:25:4, v/v), and stained with an anthrone-thiourea reagent by heating at 120°C for detecting neutral glycolipids. In the case of gangliosides, lipid samples whose amounts equivalent to 350 μg of cellular proteins were subjected to TLC as described before. The plate was developed in a solvent containing chloroform:methanol:0.5% CaCl2 (11:9:2, v/v), and stained with a resorcinol-HCl reagent by heating at 120°C for detecting gangliosides. Ratios of individual GSL components were determined by densitometric analysis. Furthermore, neutral glycolipid fractions were subjected to electrospray ionization linear-ion-trap mass spectrometric analysis as described previously [36].
Results
Expression of β-1,4-GalT I-VI genes in MEF cells
The expression of the β-1,4-GalT V gene among B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells was analyzed by RT-PCR. The results showed that the expression of the β-1,4-GalT V gene in B4galt5−/−-derived MEF cells is null, and the expression level of the β-1,4-GalT V gene in B4galt5+/−-derived MEF cells is about a half of that of B4galt5+/+-derived MEF cells (Fig. 1-β-1,4-GalT V). As ablation of the N-acetylglucosaminyltransferase Va gene in mice affected the expression of several other glycosyltransferase genes [37], the expression levels of the β-1,4-GalT I, II, III, IV and VI genes were examined by RT-PCR. The results showed that no significant changes in their expression levels are observed among three samples (Fig. 1-β-1,4-GalTs I, II, III, IV and VI, respectively).
Proliferation kinetics of MEF cells
In order to examine whether or not growth of the MEF cells changes by the gene-ablation, proliferation kinetics of cells were studied by determining cell numbers of B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells with a Coulter counter. The results showed that proliferation kinetics of the MEF cells are relatively similar among three samples (Fig. 2), indicating that growth of B4galt5−/−-derived MEF cells is not affected by the gene-ablation.
Structural analysis of N-glycans from MEF cells by two-dimensional mapping method
Our previous studies showed that β-1,4-GalT V can be involved in the galactosylation of N-glycans in addition to the galactosylation of Glc-Cer [21, 28, 30]. Therefore, it was important to characterize structures of N-glycans, particularly galactosylated complex-type oligosaccharides. To conduct this, N-glycans were released from membrane protein samples of B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells by hydrazinolysis, and then pyridylaminated. Then, they were treated with mild acid to convert acidic PA-oligosaccharides to neutral ones [38], and subjected to HPLC using two different columns [33]. Size-fractionation column chromatography using an amide column showed that PA-oligosaccharides from three samples are separated into 11 fractions as indicated below individual peaks in Fig. 3A panels a, b and c, respectively, and amounts of individual peaks are relatively constant among three samples (Fig. 3A). PA-oligosaccharides in individual peaks obtained in Fig. 3A were further separated by reversed-phase HPLC, and similar retardation profiles of PA-oligosaccharides were obtained from three samples (Fig. 3B panels a, b and c, respectively). The positions of individual PA-oligosaccharides fractionated on each column were plotted as dots whose sizes were proportional to their amounts on the two-dimensional map as determined by two-dimensional mapping method [33]. In this analysis, dots 6, 7 and 8 represent mono-galactosylated bi-antennary complex-type oligosaccharides, dots 10, 11 and 12 di-galactosylated bi-antennary ones, and dots 16 and 17 tri-galactosylated tri-antennary ones. Since sizes of most dots described above were relatively constant among three samples (Fig. 3C), amounts of individual galactosylated complex-type oligosaccharides are not changed significantly by the ablation of the β-1,4-GalT V gene in mouse. Dots 1, 2, 3 and 4 represent ungalactosylated bi-antennary complex-type and hybrid-type oligosaccharides. Dots 1 and 2 were detected in B4galt5+/−-derived MEF cells (Fig. 3C panel b) but not in B4galt5+/+- and B4galt5−/−-derived MEF cells (Fig. 3C panels a and c). Dot 21 represents mono-glucosylated high mannose-type oligosaccharides, and was detected in B4galt5+/−- and B4galt5−/−-derived MEF cells (Fig. 3C panels b and c) but not in B4galt5+/+-derived MEF cells (Fig. 3C panel a). These minor changes of N-glycan components among three samples could be due to slight differences in their genetic backgrounds. Furthermore, the sizes of dots 18 and 20 in the B4galt5−/−-derived MEF cell sample (Fig. 3C panel c) appeared larger than those of the B4galt5+/−- and B4galt5+/+-derived MEF cell samples (Fig. 3C panels a and b). Since dots 18 and 20 are oligosaccharides having the Man8GlcNAc2 and Man9GlcNAc2 structures, respectively, the defect of β-1,4-GalT V may affect the biosynthetic pathways of N-glycans in B4galt5−/−-derived MEF cells or these differences could be also due to differences in genetic backgrounds among these animals used.
Lectin blot analysis of membrane glycoprotein samples from MEF cells
The two-dimensional mapping method showed that apparent components of N-glycans are similar among B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells. To examine further changes in the galactosylation of N-glycans from B4galt5−/−-derived MEF cells, membrane glycoprotein samples prepared from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells were subjected to lectin blot analysis using SNA and MAA lectins that bind sialylated oligosaccharides since most sialylation occurs to penultimate galactose residues. Membrane glycoprotein samples were subjected to SDS-polyacrylamide gel electrophoresis, and proteins were transferred to polyvinylidene difluoride filters. When the blotted filters were stained with Coomassie Brilliant Blue (CBB), three samples showed similar protein components (Fig. 4-CBB). No significant changes in binding of glycoprotein samples to SNA, which interacts with oligosaccharides terminated with the Neu5Acα2→6Gal residue [39] (Fig. 4-SNA) and to MAA, which interacts with oligosaccharides terminated with the Neu5Acα2→3Gal residue [40] (Fig. 4-MAA), were observed among three samples, indicating no significant change in the sialylation, which reflects no change of the galactosylation of N-glycans in B4galt5−/−-derived MEF cells when compared with B4galt5+/+- and B4galt5+/−-derived MEF cells. Furthermore, no significant changes in bindings of glycoprotein samples to Con A, which interacts mainly with high mannose-type oligosaccharides [41] (Fig. 4-Con A), to RCA-I, which interacts with oligosaccharides terminated with the Galβ1→4GlcNAc/Glc group [27] (Fig. 4-RCA-I), and to L-PHA, which interacts with highly branched N-glycans [42] (Fig. 4-L-PHA), were obtained from three samples. These results indicate that the ablation of the β-1,4-GalT V gene does not affect the apparent glycosylation patterns including the gal-actosylation of N-glycans of the MEF cells.
Fig. 4.

Lectin blot analysis of membrane glycoprotein samples from MEF cells. The blots containing samples from B4galt5+/+-, B4galt5+/−-and B4galt5−/−-derived MEF cells were incubated with CBB, SNA, MAA, Con A, RCA-I, L-PHA and PNA. Lanes a, b and c indicate samples from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells, respectively. Three experiments were conducted, and identical results were obtained
Since our previous study also showed that human β-1,4-GalT V can galactosylate O-glycans [22], we initially examined whether or not O-glycans are expressed on MEF cells by lectin blot analysis using PNA which interacts with the Galβ1→3GalNAc group on O-glycans [43]. The results showed that a few protein bands with relatively higher molecular weight react weakly with PNA in all samples from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells (Fig. 4-PNA), indicating that MEF cells produce only a small amount of O-glycans when compared with that of N-glycans, and it is hard to examine changes if any in the galactosylation of O-glycans among three genetically different samples.
Determination of β-1,4-GalT activities towards GlcNAcβ-S-pNP and Glc-Cer
Our previous studies showed that recombinant human β-1,4-GalT V possesses an activity towards GlcNAcβ-S-pNP [21], and also a relatively higher activity towards Glc-Cer than human β-1,4-GalT VI [30]. When cell homogenates as an enzyme source were incubated with GlcNAcβ-S-pNP in the presence of UDP-[3H]Gal, 5.1±0.7, 4.6±0.2 and 6.0± 0.5 nmol Gal-transferred/mg protein/h were obtained from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells, respectively, (Fig. 5A), indicating no significant difference in the activity among three samples. However, when they were incubated with Glc-Cer in the presence of UDP-[3H]Gal, as few as 5.5±1.5 pmol Gal-transferred/mg protein/h was obtained from B4galt5−/−-derived MEF cells while 46.2±4.6 pmol Gal-transferred/mg protein/h was obtained from B4galt5+/+-derived MEF cells and 19.2± 1.5 pmol Gal-transferred/mg protein/h was obtained from B4galt5+/−-derived MEF cells, which is roughly half of that of B4galt5+/+-derived MEF cells (Fig. 5B). These results indicate that β-1,4-GalT V is involved in Lac-Cer synthesis and that the residual activity found in B4galt5−/−-derived MEF cells could be due to β-1,4-GalT VI, originally isolated as a Lac-Cer synthase [25], as expressed in the MEF cells (Fig. 1-β-1,4-GalT VI lane c).
Fig. 5.
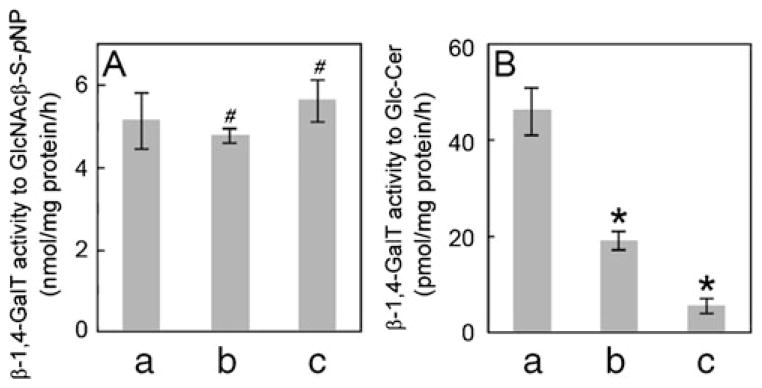
β-1,4-GalT activities in MEF cells. β-1,4-GalT activities toward GlcNAcβ-S-pNP (panel A) and Glc-Cer (panel B) were determined using homogenates prepared from B4galt5+/+ (a)-, B4galt5+/− (b)- and B4galt5−/− (c)-derived MEF cells. Values represent means with S.D. of three independent assays. p values were obtained by Student’s t-test (#, no significant difference against control, and *, p<0.01 against control)
Determination of glycolipid components in MEF cells
To examine whether or not glycolipid components changed by the ablation of the β-1,4-GalT V gene, total lipid fractions were prepared from B4galt5+/+-, B4galt5+/−- and B4galt5−/−-derived MEF cells, and subjected to TLC analysis. When neutral glycolipids on TLC plates were detected with an anthrone-thiourea reagent, amounts of Lac-Cer in B4galt5+/−- and B4galt5−/−-derived MEF cells decreased to 41% and 7%, respectively, of that of B4galt5+/+derived MEF cells as determined by densitometric analysis (Fig. 6A). However, no significant accumulation of Glc-Cer was detected in samples from B4galt5+/−- and B4galt5−/−-derived MEF cells when compared with that from B4galt5+/+-derived MEF cells (Fig. 6A). Similar results were obtained by electrospray ionization linear-ion-trap mass spectrometric analysis (data not shown). There were three major bands detected below Lac-Cer on a TLC plate in all samples (Fig. 6A), and positions of two of them coincided with those of authentic SM, indicating that they are SM, but the other component was unidentified.
Fig. 6.
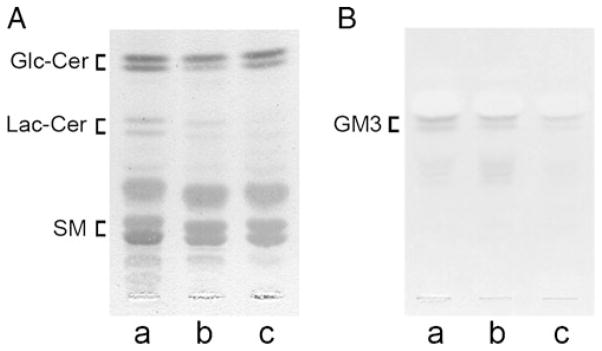
Separation of GSL components of MEF cells. Lipids were extracted from B4galt5+/+ (a)-, B4galt5+/− (b)- and B4galt5−/− (c)-derived MEF cells, and subjected to TLC in a developing solvent containing chloroform:methanol:water (65:25:4, v/v) for detecting neutral glycolipids with an anthrone-thiourea reagent (panel A), and in a developing solvent containing chloroform:methanol:0.5% CaCl2 (11:9:2, v/v) for detecting gangliosides with a resorcinol-HCl reagent (panel B), respectively. The ratios of GSL components were determined by densitometric analysis. Three independent experiments were conducted, and identical results were obtained. Positions of Glc-Cer, Lac-Cer, and SM or GM3 migrated are indicated at the left side of plate
Since Lac-Cer is a precursor molecule for gangliosides [19], it was of interest to investigate whether or not components of gangliosides also changed by the ablation of the β-1,4-GalT V gene. Acidic glycolipid fractions were prepared from three samples and subjected to TLC analysis. When gangliosides on TLC plates were detected with a resorcinol-HCl reagent, amounts of GM3, which is sialylated Lac-Cer, in samples from B4galt5+/−- and B4galt5−/−-derived MEF cells decreased to 42% and 12%, respectively, of that from B4galt5+/+-derived MEF cells as determined by densitometric analysis (Fig. 6B). These results strongly indicate that murine β-1,4-GalT V is involved in the biosynthesis of Lac-Cer.
Discussion
The present study shows that murine β-1,4-GalT V is involved in Lac-Cer biosynthesis and appears not to be involved in the galactosylation of N-glycans. About 90% of Lac-Cer synthase activity was lost in B4galt5−/−-derived MEF cells when compared with B4galt5+/+-derived MEF cells. The residual 10% of the activity found in B4galt5−/−-derived MEF cells could be due to activityies of β-1,4-GalT VI expressed, which was initially isolated from rat brain as a Lac-Cer synthase [25], and/or of β-1,4-GalTs I and III expressed, which also possess a Lac-Cer synthase activity as shown by in vitro studies [23, 44]. Accordingly, amounts of Lac-Cer and its derivative gangliosides, mainly GM3, in the cells decreased significantly in B4galt5−/−-derived MEF cells when compared with B4galt5+/+- and B4galt5+/−-derived MEF cells, which is another strong evidence indicating that murine β-1,4-GalT V is substantially involved in the biosynthesis of Lac-Cer in the cells. In further support of this, human β-1,4-GalT V had a much higher specific activity of Lac-Cer synthesis than human β-1,4-GalT VI [30]. However, enzymes that are involved in Lac-Cer biosynthesis may differ among tissues and cell types since the gene expression patterns of the β-1,4-GalTs I, III, V and VI are different among species, tissues, cell types and developmental stages [20, 21, 23–25, 29].
Although human β-1,4-GalT V can galactosylate N-glycans in Sf-9 cells when its cDNA was transfected [28], we failed to detect significant changes in ratios between galactosylated and ungalactosylated N-glycans in B4galt5−/−-derived MEF cells by the two-dimensional mapping method [33] when compared to those of B4galt5+/+- and B4galt5+/−-derived MEF cells. Since O-glycans were not expressed in MEF cells in sufficient amounts for their structural analysis, the involvement of murine β-1,4-GalT V in O-glycan biosynthesis was not established in the present study. These results indicate that murine β-1,4-GalT V is not involved in the galactosylation of N-glycans in mammalian cells although human β-1,4-GalT V can galactosylate N- and O-glycans in Sf-9 cells and in vitro, respectively [22, 28]. This discrepancy could come from the different localization of β-1,4-GalT V in Golgi-apparatus between Sf-9 cells and MEF cells, but not from a species difference between human and mouse since both enzymes share 95% identity at an amino acid level [29]. In this regard, we are currently investigating the fine localization of β-1,4-GalT V in Golgi-apparatus using mouse tissues by immunocytochemical method.
Our previous study showed that the growth of B4galt5−/−-mouse embryos is severely retarded, and they are eliminated by E10.5, while B4galt5+/− mice are born without any developmental defect [14]. However, little is known about the cause of death of B4galt5−/−-mouse embryos. Lactosylcer-amide plays a pivotal role as a precursor of nearly all major GSLs [19]. Previous studies showed that GSLs regulate signal transduction for cell proliferation, adhesion and migration, and by external stimuli [45–47]. GSLs clustered at cell surfaces interact with transmembrane proteins and cytoplasmic factors such as integrins, cadherin, growth factor receptors, tetraspanins, and non-receptor-type cytoplasmic protein kinases, and form glycosynaptic microdomains, which are supposed to control GSL-dependent/modulated cell adhesion, growth and motility of cells [48–50] through their associating proteins to bind to focal adhesion kinases, and cytoskeleton proteins including vinculin, talin and paxillin [reviewed in 51]. Quite interestingly, the fates of vinculin, talin, paxillin and pp125 focal adhesion kinase-knockout mice are similar, and they are eliminated by E8.5-E10.5 [52–55], at which stage B4galt5−/− mice also die [14].
The Glc-Cer synthase and β-1,4-GalT V (Lac-Cer synthase)-knockout mice are embryonic lethal [11, 14], while gene-knockout mice of ganglioside synthases and of globoseries GSL synthase are viable although they show impaired regeneration upon neural lesion, decreased nerve conduction velocity, age-associated axonal degeneration, and lethal audiogenic seizures [56–61]. This indicates that Lac-Cer itself is important for the survival and activity of the embryos. In fact, Lac-Cer has been shown to activate an oxygen-sensitive signaling pathway involving superoxides, p21 RasGTP loading, and PI3 kinase/Akt activation, which ultimately contributes to changes in cell adhesion, migration and angiogenesis [reviewed in 19]. Therefore, the lack of Lac-Cer is a major reason why B4galt5−/− mice die at a mid-gestation stage as we reported previously [14].
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (09240104, 12680708 and 22370048) from the Ministry of Education, Science, Culture and Sports of Japan, and the Research Promotion Fund from the Japan Science Technology Agency (2007–2009) to KF.
Abbreviations
- β-1
4-GalT, β-1,4-galactosyltransferase
- B4galt5
β-1,4-GalT V gene
- CBB
Coomassie Brilliant Blue
- Con A
Concanavalin A
- Gal
Galactose
- Glc-Cer
glucosylceramide
- GlcNAcβ-S-pNP
p-nitrophenyl-N-acetyl-1-thio-β-D-glucosaminide
- GU
Glucose unit
- GSL
Glycosphingolipid
- HPLC
High performance liquid chromatography
- Lac-Cer
Lactosylceramide
- MAA
Maackia amurensis agglutinin
- MEF
Mouse embryonic fibroblast
- MES
2-(N-morpholino)ethansulfonic acid
- Neu5Ac
N-acetylneuraminic acid
- PA
Pyridylamine
- PBS
10 mM phosphate-buffered saline (pH 7.4)
- PNA
Peanut agglutinin
- RCA-I
Ricinus communis agglutinin-I
- RT-PCR
Reverse transcription-polymerase chain reaction
- SNA
Sambucus nigra agglutinin
- TLC
Thin-layer chromatography
Footnotes
Ganglioside nomenclature used in the present study is that of Svennerholm [1].
Contributor Information
Tadahiro Kumagai, Laboratory of Glycobiology, Department of Bioengineering, Nagaoka University of Technology, Nagaoka, Niigata 940-2188, Japan.
Takeshi Sato, Laboratory of Glycobiology, Department of Bioengineering, Nagaoka University of Technology, Nagaoka, Niigata 940-2188, Japan.
Shunji Natsuka, Department of Biology, Faculty of Science, Niigata University, Nishi-ku, Niigata 950-2181, Japan.
Yukito Kobayashi, Laboratory of Glycobiology, Department of Bioengineering, Nagaoka University of Technology, Nagaoka, Niigata 940-2188, Japan.
Dapeng Zhou, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Tadashi Shinkai, Department of Cell Biology, Tokyo Metropolitan Institute of Gerontology, Itabashi-ku, Tokyo 173-0015, Japan.
Satoru Hayakawa, Department of Cell Biology, Tokyo Metropolitan Institute of Gerontology, Itabashi-ku, Tokyo 173-0015, Japan.
Kiyoshi Furukawa, Email: furukawa@vos.nagaokaut.ac.jp, Laboratory of Glycobiology, Department of Bioengineering, Nagaoka University of Technology, Nagaoka, Niigata 940-2188, Japan.
References
- 1.Svennerholm L. Designation and schematic structure of ganglio-sides and allied glycosphingolipids. Prog Brain Res. 1994;101:11–14. doi: 10.1016/S0079-6123(08)61935-4. [DOI] [PubMed] [Google Scholar]
- 2.Surani MA. Glycoprotein synthesis and inhibition of glycosylation by tunicamycin in preimplantation mouse embryos and compaction and trophoblast adhesion. Cell. 1979;18:217–227. doi: 10.1016/0092-8674(79)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Gooi HC, Feizi T, Kapadia A, Knowles BB, Solter D, Evans MJ. Stage-specific embryonic antigen involves α1→3 fucosylated type 2 blood group chains. Nature. 1981;292:156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- 4.Schnaar RL. Glycosphingolipids in cell surface recognition. Glycobiology. 1991;1:477–485. doi: 10.1093/glycob/1.5.477. [DOI] [PubMed] [Google Scholar]
- 5.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomura KH, Kobayashi R, Hirabayashi Y, Fujisue-Sasaki M, Mizuguchi S, Nomura K. Involvement of blood group-B-active trisaccharides in Ca2+-dependent cell-to-cell adhesion in the Xenopus blastula. Dev Genes Evol. 1998;208:9–18. doi: 10.1007/s004270050148. [DOI] [PubMed] [Google Scholar]
- 7.Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994;13:2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci USA. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asano M, Furukawa K, Kido M, Matsumoto S, Umesaki Y, Kochibe N, Iwakura Y. Growth retardation and early death of β-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 1997;16:1850–1857. doi: 10.1093/emboj/16.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Q, Hasty P, Shur BD. Targeted mutation in β1, 4-galactosyltransferase leads to pituitary insufficiency and neonatal lethality. Dev Biol. 1997;181:257–267. doi: 10.1006/dbio.1996.8444. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, Proia RL. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96:9142–9147. doi: 10.1073/pnas.96.16.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 2007;282:15578–15588. doi: 10.1074/jbc.M607434200. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai T, Tanaka M, Yokoyama M, Sato T, Shinkai T, Furukawa K. Early letharity of β-1,4-galactosyltransferase V-mutant mice by growth retardation. Biochem Biophys Res Commun. 2009;379:456–459. doi: 10.1016/j.bbrc.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara T, Sugihara K, Kizuka Y, Oka S, Asano M. Learning/memory impairment and reduced expression of the HNK-1 carbohydrate in β4-galactosyltransferase-II-deficient mice. J Biol Chem. 2009;284:12550–12561. doi: 10.1074/jbc.M809188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biellmann F, Hulsmeier AJ, Zhou D, Cinelli P, Hennet T. The Lc3-synthase gene B3gnt5 is essential to pre-implantation development of the murine embryo. BMC Dev Biol. 2008;8:109. doi: 10.1186/1471-213X-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa K, Takamiya K, Okada M, Inoue M, Fukumoto S, Furukawa K. Novel functions of complex carbohydrates elucidated by the mutant mice of glycosyltransferase genes. Biochim Biophys Acta. 2001;1525:1–12. doi: 10.1016/s0304-4165(00)00185-9. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa K, Kobata A. Cell surface carbohydrates-their involvement in cell adhesion. In: Ogura H, Hasegawa A, Suami T, editors. Carbohydrates Synthetic Methods and Application in Medicinal Chemistry. Kodansha; Tokyo: 1992. pp. 369–384. [Google Scholar]
- 19.Chatterjee S, Pandey A. The Yin and Yang of lactosylceramide metabolism: implications in cell function. Biochim Biophys Acta. 2008;1780:370–382. doi: 10.1016/j.bbagen.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa K, Sato T. β-1,4-galactosylation of N-glycans is a complex process. Biochim Biophys Acta. 1999;1473:54–66. doi: 10.1016/s0304-4165(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Furukawa K, Bakker H, Van den Eijnden DH, Van Die I. Molecular cloning of a human cDNA encoding β-1,4-galactosyltransferase with 37% identity to mammalian UDP-Gal: GlcNAc β-1,4-galactosyltransferase. Proc Natl Acad Sci USA. 1998;95:472–477. doi: 10.1073/pnas.95.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Die I, Van Tetering A, Schiphorst WECM, Sato T, Furukawa K, Van den Eijnden DH. The acceptor substrate specificity of human β4-galactosyltransferase V indicates its potential function in O-glycosylation. FEBS Lett. 1999;450:52–56. doi: 10.1016/s0014-5793(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 23.Almeida R, Amado M, David L, Levery SB, Holmes EH, Merkx G, van Kessel AG, Rygaard E, Hassan H, Bennett E, Clausen H. A family of human β4-galactosyltransferases. J Biol Chem. 1997;272:31979–31991. doi: 10.1074/jbc.272.51.31979. [DOI] [PubMed] [Google Scholar]
- 24.Schwientek T, Almeida R, Levery SB, Holmes EH, Bennett E, Clausen H. Cloning of a novel member of the UDP-galactose:β-N-acetylglucosamine β1,4-galactosyltransferase family, β4Gal-T4, involved in glycosphingolipid biosynthesis. J Biol Chem. 1998;273:29331–29340. doi: 10.1074/jbc.273.45.29331. [DOI] [PubMed] [Google Scholar]
- 25.Nomura T, Takizawa M, Aoki J, Arai H, Inoue K, Wakisaka E, Yoshizuka N, Imokawa G, Dohmae N, Takio K, Hattori M, Matsuo N. Purification, cDNA cloning, and expression of UDP-Gal:glucosylceramide β-1,4-galactosyltransferase from rat brain. J Biol Chem. 1998;273:13570–13577. doi: 10.1074/jbc.273.22.13570. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimi Y, Sato T, Ikekita M, Guo S, Furukawa K. Presence of monoantennary complex-type and hybrid-type oligo-saccharides terminated with β-N-acetylglucosamine in lepidopteran insect Sf-9 cells. Res Commun Biochem Cell Molec Biol. 2000;4:163–170. [Google Scholar]
- 27.Baenziger JU, Fiete D. Structural determination of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- 28.Guo S, Sato T, Shirane K, Furukawa K. Galactosylation of N-linked oligosaccharides by human β-1,4-galactosyltransferases I, II, III, IV, V, and VI expressed in Sf-9 cells. Glycobiology. 2001;11:813–820. doi: 10.1093/glycob/11.10.813. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura N, Yamakawa N, Sato T, Tojo H, Tachi C, Furukawa K. Differential gene expression of β-1,4-galactosyltransferases I, II and V during mouse brain development. J Neurochem. 2001;76:29–38. doi: 10.1046/j.1471-4159.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, Guo S, Furukawa K. Involvement of recombinant human β-1,4 galactosyltransferase V in lactosylceramide biosynthesis. Res Commun Biochem Cell Molec Biol. 2000;4:3–10. [Google Scholar]
- 31.Takasaki S, Mizuochi T, Kobata A. Hydrazinolysis of asparaginelinked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- 32.Hase S, Ikenaka T, Matsushima Y. Structural analysis of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem Biophys Res Commun. 1978;85:257–263. doi: 10.1016/s0006-291x(78)80037-0. [DOI] [PubMed] [Google Scholar]
- 33.Yanagida K, Ogawa H, Omichi K, Hase S. Introduction of new scale into reversed-phase high-performance liquid chromatography of pyridylamino sugar chains for structural assignment. J Chromatogr. 1998;800:187–198. doi: 10.1016/s0021-9673(97)01109-6. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Furukawa K, Greenwalt DE, Kobata A. Most bovine milk fat globule membrane glycoproteins contain asparagine linked sugar chain with GalNAcβ1→4GlcNAc groups. J Biochem (Tokyo) 1993;117:890–900. doi: 10.1093/oxfordjournals.jbchem.a124273. [DOI] [PubMed] [Google Scholar]
- 35.Ando S, Waki H, Kon K. New solvent system for high-performance thin-layer chromatography and high-performance liquid chromatography of gangliosides. J Chromatogr. 1987;405:125–134. doi: 10.1016/s0021-9673(01)81754-4. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Furukawa K, Hsu FF, Aldercreutz D, Weadge J, Palcic MM, Wang PG, Levery SB, Zhou D. Immunologic glycoshingolipidomics and NKT cell development in mouse thymus. J Proteome Res. 2009;8:2740–2751. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo HB, Nairn A, Harris K, Randolph M, Alvarez-Manilla G, Moremen Pierce M. Loss of expression of N-acetylglucosaminyltransferase Va results in altered gene expression of glycosyltransferases and galectins. FEBS Lett. 2008;582:527–535. doi: 10.1016/j.febslet.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natsuka S, Adachi J, Kawaguchi M, Ichikawa A, Ikura K. Method for purification of fluorescence-labeled oligosaccharides by pyridylamination. Biosci Biotechnol Biochem. 2002;66:1174–1175. doi: 10.1271/bbb.66.1174. [DOI] [PubMed] [Google Scholar]
- 39.Shibuya N, Goldstein IJ, Broekaert WF, Nshimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra) bark lectin recognizes the Neu5Ac(α2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 40.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 41.Ogata S, Muramatsu T, Kobata A. Fractionation of glycopeptides by affinity column chromatography on concanavalin A-Sepharose. J Biochem (Tokyo) 1975;78:687–696. doi: 10.1093/oxfordjournals.jbchem.a130956. [DOI] [PubMed] [Google Scholar]
- 42.Merkle RK, Cummings RD. Lectin affinity chromatography of glycoproteins. Methods Enzymol. 1987;138:232–259. doi: 10.1016/0076-6879(87)38020-6. [DOI] [PubMed] [Google Scholar]
- 43.Lotan R, Skutelsky E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea) J Biol Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- 44.Nakazawa K, Furukawa K, Narimatsu H, Kobata A. Kinetic study of human β-1,4-galactosyltransferase expressed in E. coli. J Biochem (Tokyo) 1993;113:747–753. doi: 10.1093/oxfordjournals.jbchem.a124115. [DOI] [PubMed] [Google Scholar]
- 45.Regina-Todeschini A, Hakomori S. Functional role of glyco-sphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta. 2008;1780:421–433. doi: 10.1016/j.bbagen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281:10230–10232. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami Y, Kawakami K, Steelant WF, Ono M, Baek RC, Handa K, Withers DA, Hakomori S. Tetraspanin CD9 is a “proteolipid,” and its interaction with α3 integrin in microdomain is promoted by GM3 ganglioside, leading to inhibition of laminin-5-dependent cell motility. J Biol Chem. 2002;277:34349–34358. doi: 10.1074/jbc.M200771200. [DOI] [PubMed] [Google Scholar]
- 49.Mitsuzuka K, Handa K, Satoh M, Arai Y, Hakomori S. A specific microdomain (“glycosynapse 3”) controls phenotypic conversion and reversion of bladder cancer cells through GM3-mediated interaction of α3β1 integrin with CD9. J Biol Chem. 2005;280:35545–35553. doi: 10.1074/jbc.M505630200. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SJ, Nakayama K, Hikita T, Handa K, Hakomori S. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci USA. 2005;50:18987–18991. doi: 10.1073/pnas.0609281103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 53.Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, Fässler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 54.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 56.Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Furukawa K, Aizawa S. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. Mice lacking complex ganglio-sides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96:7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawai H, Allende ML, Wada R, Kono M, Sango K, Deng C, Miyakawa T, Crawley JN, Werth N, Bierfreund U, Sandhoff K, Proia RL. Mice expressing only monosialogan-glioside GM3 exhibit lethal audiogenic seizures. J Biol Chem. 2001;276:6885–6888. doi: 10.1074/jbc.C000847200. [DOI] [PubMed] [Google Scholar]
- 59.Okada M, Itoh M, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S, Furukawa K. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J Biol Chem. 2002;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 60.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281:10230–10232. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 61.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Gröne H. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]