Abstract
Borrelia burgdorferi antibodies preferentially present in cerebrospinal fluid (CSF) were examined by differentially probing a B. burgdorferi expression library with CSF and sera from patients with neurologic Lyme disease. Several phage clones selectively reacted with CSF, and these genes were then expressed in recombinant form and used to detect specific antibody in an enzyme-linked immunosorbent assay. Decorin-binding protein B (BBA25) and BBA50 (hypothetical protein) elicited immunoglobulin G (IgG) or IgM detectable in CSF—but not sera—of patients, demonstrating preferential antibody production during neuroborreliosis.
Infection with Borrelia burgdorferi, the agent of Lyme disease, commonly results in clinical symptoms involving the nervous system, including facial nerve palsy, aseptic meningitis, and radiculitis, among other manifestations (3). During infection, B. burgdorferi invades the central nervous system (CNS), and this contributes, at least in part, to the pathogenesis of the disease. Patients with neurologic Lyme disease mount a strong humoral response against the spirochete, with detectable antibodies to B. burgdorferi in both sera and cerebrospinal fluid (CSF) (3). In particular, increased ratios of intrathecal antibody production in CSF versus that in serum have been used to document CNS disease with B. burgdorferi, but its absence does not exclude infection (3, 13, 15, 16, 23). In addition, the B. burgdorferi outer surface protein A (OspA) antigen has been detected in CSF during Lyme disease, while immunoglobulin M (IgM) directed against OspA and OspC has been identified in both sera and CSF of patients with neurologic infection (2-5, 25, 26).
B. burgdorferi preferentially expresses different genes throughout its life cycle, and these gene products may facilitate pathogen survival (6, 17, 19, 27). Within both the arthropod vector and the vertebrate host, B. burgdorferi gene expression appears to vary based on specific location (7, 20). Spirochete gene expression in the gut of a flat tick is different from gene expression in the salivary gland of a fed tick (7, 19, 20). Similarly, B. burgdorferi expresses different genes in diverse tissues in the vertebrate host, including the skin and deeper internal organs, such as joints and the heart (8, 9). We hypothesized, therefore, that spirochete gene expression in the CNS is different from that in other tissues, particularly since B. burgdorferi may require specific ligands to invade and persist within this sequestered compartment (10).
Differential immunoscreening of a B. burgdorferi genomic expression library has been used to identify spirochete genes preferentially expressed by B. burgdorferi under different conditions (27). The B. burgdorferi library was probed with sera from B. burgdorferi-infected mice and sera from mice hyperimmunized with B. burgdorferi lysates. This identified a series of spirochete genes preferentially expressed in vivo (27). Differential immunoscreening was then used to help delineate groups of B. burgdorferi genes induced or repressed by spirochetes within engorged ticks (19). We have now used CSF and sera from patients with neurologic Lyme disease to probe a B. burgdorferi expression library in order to characterize spirochete gene products that elicit antibodies preferentially expressed within the nervous system.
Differential immunoscreening of a B. burgdorferi expression library to identify antigens recognized by antibodies in CSF.
A B. burgdorferi genomic DNA expression library was screened (27) to identify spirochete genes encoding antigens that were preferentially recognized by antibodies in the CSF of patients with neurologic Lyme disease. Initially, CSF and sera were separately pooled from several individuals with Lyme-related aseptic meningitis and used to differentially probe the library. The patients had 2 to 3 weeks of symptoms, including headache, fever, and a stiff neck, and B. burgdorferi antibodies in sera and CSF by enzyme-linked immunosorbent assay (ELISA). Plaques that strongly reacted with the CSF, but that did not exhibit significant binding with sera, were selected for further examination. Of the over 10,000 plaques (representing at least three complete copies of the B. burgdorferi genome) that were probed, 6 demonstrated substantial reactivity with CSF and little reactivity with sera. Of these six phage clones, three identical clones contained all or part of the bba23 (conserved hypothetical protein), bba24 (decorin-binding protein A), and bba25 (decorin-binding protein B) genes, two identical clones encoded all or part of bba50 (hypothetical protein) and bba51 (hypothetical protein), and one clone had the bba35 (hypothetical protein) gene. These data suggest that one or more of these genes within each plaque encode antigens that elicit antibodies which are more prominent in the CSF as opposed to serum of patients with neurologic Lyme disease.
To determine which, if any, of these genes encoded antigens that elicit antibodies commonly present in CSF, the proteins were first expressed in recombinant form using described techniques (18). BBA23, BBA50, and BBA51 were purified as fusion proteins with maltose-binding protein; BBA24, BBA25, and BBA35 were synthesized as fusion proteins with glutathione transferase. Two different expression systems were used, because three of the antigens (BBA24, BBA25, and BBA35) could not be purified when expressed as fusions with maltose-binding proteins, due to poor solubility.
CSF and serum IgG and IgM responses to recombinant antigens.
The recombinant B. burgdorferi antigens were probed simultaneously in sera and CSF from a cohort of patients with well-documented neurologic Lyme disease. The mean age of the patients was 44 years with a range of 21 to 67 years. Five individuals had meningitis, and three of these persons had concomitant facial nerve palsy. Five patients had facial nerve palsy, and one of these persons had bilateral facial nerve involvement. Three individuals had acute radiculoneuritis. The remainder had unifocal or multifocal erythema migrans with severe headaches. Patients had an abnormal CSF and/or positive immunoblots or ELISA for B. burgdorferi. The samples were obtained within 2 weeks of the initial presentation and assessment.
IgM and IgG ELISAs were performed to determine the antibody profile to these specific antigens in the CSF and sera of individuals with neurologic Lyme disease, patients that represent the diverse manifestations of neuroborreliosis (Fig. 1). The ELISA was a solid-phase noncompetitive method similar to that used for the detection of antibodies to other recombinant B. burgdorferi antigens (18). In all the assays, reactivity to recombinant maltose-binding protein and glutathione transferase served as controls to determine background reactivity to the fusion partners. This reactivity was negligible and, therefore, subtracted from the reading recorded against the fusion proteins. In addition, CSF and sera from eight normal individuals were tested as controls. These control samples showed no significant reactivity to any of the recombinant antigens (Fig. 1).
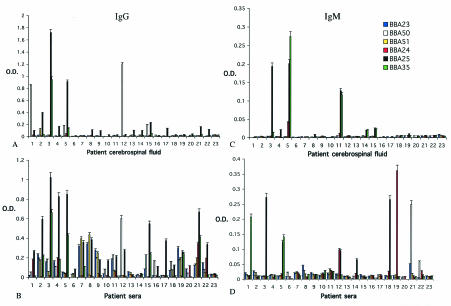
FIG. 1.
Antibodies in the sera and CSF of patients with neurological manifestations of Lyme disease that bind recombinant BBA23, -24, -25, -35, -50, and -51. (A) IgG in CSF; (B) IgG in sera; (C) IgM in CSF; (D) IgM in sera. Lanes 1 to 22 represent individuals with neurologic Lyme disease. Lane 23 (control) is pooled sera from eight normal healthy persons.
Thirteen of 22 patients with neurologic Lyme disease (individuals 1 to 5, 8, 9, 13 to 15, 17, 21, and 22) developed CSF IgG to BBA25 (optical density ≥ 0.1, which is more than 3 standard deviations above control, up to an optical density of 1.7), implying that BBA25 (decorin-binding protein B) is an important antigen during neurologic infection (Fig. 1A). This is particularly interesting since BBA25 has recently been shown to facilitate the attachment of spirochetes to glial cells, suggesting a role in CNS pathogenesis (10). IgG against BBA23 and BBA24 was not detected in the CSF of patients with neurologic Lyme disease (Fig. 1A), while IgG against BBA50 was detected in four persons (numbers 1, 5, 12, and 15) and IgG against BBA51 (individual number 2) and BBA35 (individual number 5) were each present in one individual. Patients, therefore, had IgG to varied antigens, with the most prominent response directed against BBA25.
IgG directed against these six antigens was generally easier to detect in the serum, rather than CSF, of patients with neurologic Lyme disease (Fig. 1B). BBA25 IgG was detected in 19 of 22 patients, BBA23 IgG was evident in 9 individuals, and BBA24 IgG was apparent in 4 persons. BBA50 IgG was present in 10 persons, BBA51 IgG was found in 6 individuals, and BBA35 IgG was present in 8 patients. Of note, the IgG response to BBA50 was higher in the CSF than in the sera of patients 5 and 8; patient 1 had detectable BBA50 IgG in the CSF without the presence of detectable BBA50 IgG in the serum at the same time. These data suggest that BBA50 IgG was generated intrathecally in specific instances.
IgM was not expected to be present in the CSF, due to the relatively large size of this antibody, unless a true intrathecal response was occurring. IgM directed towards BBA25 was evident in the CSF of three patients (numbers 3, 5, and 11), and IgM against BBA35 was apparent in one individual (number 11) (Fig. 1C). Serum IgM against BBA25 was present in three persons (numbers 3, 5, and 18), while BBA35 IgM was present in two persons (numbers 1 and 5), BBA24 IgM was evident in two individuals (numbers 12 and 19), and BBA50 IgM was evident in one individual (number 21) (Fig. 1D). Surprisingly, patient 11 had detectable IgM against BBA25 and BBA35 in CSF, but not in serum (Fig. 1C and D). These data suggest that de novo synthesis of specific IgM may occur in the CNS.
CNS infection with B. burgdorferi.
Assessment of the CSF humoral response can aid in the diagnosis of neurologic Lyme disease. Antibody profiles in the serum and CSF often have similar profiles. When the blood-brain barrier becomes increasingly permeable due to bacterial infiltration of the CNS, antibodies in serum more easily diffuse into the CSF. B. burgdorferi infection, however, usually causes aseptic meningitis and other CNS disease, without the infiltration of large numbers of lymphocytes and polymorphonuclear cells into CSF, and therefore the permeability of the blood-brain barrier is not altered as substantially as in purulent bacterial meningitis. This allows identification of intrathecal antibody responses that are more specific for CNS infection.
These data provide the first description of differential immunoscreening to identify B. burgdorferi antibodies that may be preferentially expressed in the CNS. Antibody production may not always be an accurate reflection of gene expression, as it can be limited by differences in the antigenicity of proteins, cross-reactivity of antibodies among members of paralogous gene families, and variations in the temporal development and decline of antibody production. B. burgdorferi microarrays and RNA from spirochetes in human tissues and fluid in the CNS are the most logical methods of examining environmental (1, 21, 22, 24) and tissue-specific spirochete gene expression. Obtaining enough B. burgdorferi RNA from human tissues for array analysis remains a challenge. Nevertheless, the present study demonstrates that B. burgdorferi-specific antibodies against selected B. burgdorferi antigens may sometimes be present in the CNS. BBA25, the most commonly recognized antigen in this study, plays an important role in spirochete pathogenesis (11, 12) and has recently been shown to mediate the adherence of spirochetes to glial cells (10). Moreover, a recent report has demonstrated that BBA25 antibodies are prominent in European cases of neuroborreliosis (14). Interestingly, the identified genes are all present on linear plasmid 54, suggesting that this plasmid may encode neurotropic spirochete virulence determinants. The potential of this avenue of investigation is likely to be even greater as different strains of B. burgdorferi are used to explore the CSF antibody response in a similar system. An understanding of preferential CSF antibody expression may lead to new insights into the pathogenesis of and the ability to diagnose and possibly to prognosticate the neurological manifestations of Lyme disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI 49200, RR010710, and NS034092). E. Fikrig is the recipient of a Clinical-Scientist Award in Translational Research from the Burroughs Wellcome Fund. R. A. Flavell is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyle, P. K., Z. Deng, S. E. Schutzer, A. L. Belman, J. Benach, L. B. Krupp, and B. Luft. 1993. Detection of Borrelia burgdorferi antigens in cerebrospinal fluid. Neurology 43:1093-1098. [DOI] [PubMed] [Google Scholar]
- 3.Coyle, P. K., and S. E. Schutzer. 2002. Neurologic aspects of Lyme disease. Med. Clin. North Am. 86:261-284. [DOI] [PubMed] [Google Scholar]
- 4.Coyle, P. K., S. E. Schutzer, A. L. Belman, L. B. Krupp, and M. G. Golightly. 1990. Cerebrospinal fluid immune complexes in patients exposed to Borrelia burgdorferi: detection of Borrelia-specific and -nonspecific complexes. Ann. Neurol. 28:739-744. [DOI] [PubMed] [Google Scholar]
- 5.Coyle, P. K., S. E. Schutzer, Z. Deng, L. B. Krupp, A. L. Belman, J. L. Benach, and B. J. Luft. 1995. Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 45:2010-2015. [DOI] [PubMed] [Google Scholar]
- 6.de Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 9.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, J. R., N. Parveen, L. Magoun, and J. M. Leong. 2003. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 100:7307-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen, K., and A. M. Lebech. 1991. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi-specific immunoglobulin G, A, and M. Ann. Neurol. 30:197-205. [DOI] [PubMed] [Google Scholar]
- 14.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, M. Peltomaa, H. I. Huppertz, and P. Lahdenne. 2002. Cloning of the gene encoding the decorin-binding protein B (DbpB) in Borrelia burgdorferi sensu lato and characterisation of the antibody responses to DbpB in Lyme borreliosis. J. Med. Microbiol. 51:641-648. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser, R., and S. Rauer. 1998. Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur. J. Clin. Microbiol. Infect. Dis. 17:159-166. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser, R., and S. Rauer. 1999. Serodiagnosis of neuroborreliosis: comparison of reliability of three confirmatory assays. Infection 27:177-182. [DOI] [PubMed] [Google Scholar]
- 17.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasimhan, S., F. Santiago, R. A. Koski, B. Brei, J. F. Anderson, D. Fish, and E. Fikrig. 2002. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J. Bacteriol. 184:3122-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojaimi, C., C. Brooks, D. Akins, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katonah, J. Radolf, M. Caimano, J. Skare, K. Swingle, S. Sims, and I. Schwartz. 2002. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol. 358:165-177. [DOI] [PubMed] [Google Scholar]
- 22.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picha, D., L. Moravcova, E. Zdarsky, and J. Benes. 2000. Clinical comparison of immunoblot and antibody index for detection of intrathecal synthesis of specific antibodies in Lyme neuroborreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:805-806. [DOI] [PubMed] [Google Scholar]
- 24.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutzer, S. E., P. K. Coyle, L. B. Krupp, Z. Deng, A. L. Belman, R. Dattwyler, and B. J. Luft. 1997. Simultaneous expression of Borrelia OspA and OspC and IgM response in cerebrospinal fluid in early neurologic Lyme disease. J. Clin. Investig. 100:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutzer, S. E., P. K. Coyle, P. Reid, and B. Holland. 1999. Borrelia burgdorferi-specific immune complexes in acute Lyme disease. JAMA 282:1942-1946. [DOI] [PubMed] [Google Scholar]
- 27.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]