Abstract
Animal models have been invaluable in the efforts to better understand and ultimately treat patients suffering from leukemia. While important insights have been gleaned from these models, limitations must be acknowledged. In this review, we will highlight the various animal models of leukemia and describe their contributions to the improved understanding and treatment of these cancers.
Introduction
The leukemias represent a diverse group of diseases that lead to significant morbidity and mortality. In 2011, over 44,600 Americans were diagnosed with leukemia leading to 21,780 deaths [1]. Four major types account for 85% of all leukemias: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphocytic leukemia (CLL). These will be the focus of this review. CLL is the most common, but AML accounts for ~42% of all leukemia deaths [1]. As a result, AML is the most intensively studied leukemia and has the most diverse group of animal models. It is hard to overstate the impact that animal models have had on leukemia research. From the pioneering work of Dr Lloyd Law on the response of murine lymphoid leukemias, to antimetabolite agents in the 1940s and 1950s, to the discovery of the leukemic stem cell by Dr John Dick in the 1990s, animal models have been integral in our understanding of the biology of the leukemias.
Animal Models of AML
Introduction
In the last 40 years, our understanding of the molecular pathophysiology of AML has grown exponentially (reviewed in [2–4]). The disease presents with the proliferation of immature, clonal, myeloid precursors that leads to progressive marrow failure and ultimately death. Despite a better understanding of the genetics of AML, therapy for most patients has remained unchanged, and the overall 5-year survival rate is 30–40% [5]. This disappointing survival rate has inspired much work on the development of more relevant animal models for use in the discovery of new targets and the testing of new therapies. As a result, several studies have revealed novel insights made possible only by the use of animal models. Several notable examples include the role of the AML cell/bone marrow stroma interaction in resistance to chemotherapy [6], the ability of the immune system to interact with and target AML [7–8], the manipulation of the hematopoietic stem cell niche by AML [9], and the seminal observation that AML exists as a hierarchy of cells with only a rare population of leukemic stem cells [10]. This observation was the nidus for the cancer stem cell theory, now extended to many solid tumors as well (Reviewed in [11]).
Murine Models
Since 1930’s, murine models have been the most extensively used to study AML. The initial carcinogen-induced transplantable models gave way to the more modern transgenic, xenograft, and mosaic approaches. This section will review the major murine model types in AML.
Carcinogen-induced models
Among the earliest reported models of murine leukemia were the transplantable carcinogen-induced models used to test possible therapeutic agents (see Figure1). The use of antimetabolite agents was first tested in these models. They had the advantages of being able to propagate in vitro and then injected into large cohorts of recipients that would subsequently develop leukemia, allowing for trials of promising agents and schedules. They were used to great effect by Drs Skipper and Schabel to describe the kinetics of leukemogenesis and response to therapy that remain a major pillar of our current understanding of the disease (reviewed in [12]). One of the most widely used model is the L1210 cell line isolated by Dr Law by exposing DBA/2 mice to the carcinogen 3-methylcholantrene (3-MC)[13]. The resulting leukemia was harvested from a moribund animal and has been used experimentally for decades. Additional chemically induced murine leukemia cell lines include P388, P1534, and L5178Y, among others (reviewed in [14]). However, these models have several drawbacks. The strongest of these is the fact that the pathogenesis of this leukemia is not relevant to most human cases of AML, since few people develop leukemia after exposure to chemical agents. Indeed for the most part several of these cell lines were thought to be more closely related to lymphoid malignancies than AML. Furthermore, the genetic underpinnings of the disease are unknown, which limits the applicability of results using these models. Despite these drawbacks, these models have made significant contributions to the treatment of AML, most notably the supporting the use of cytarabine [15], which remains one of the most widely used agents to treat AML and ALL.
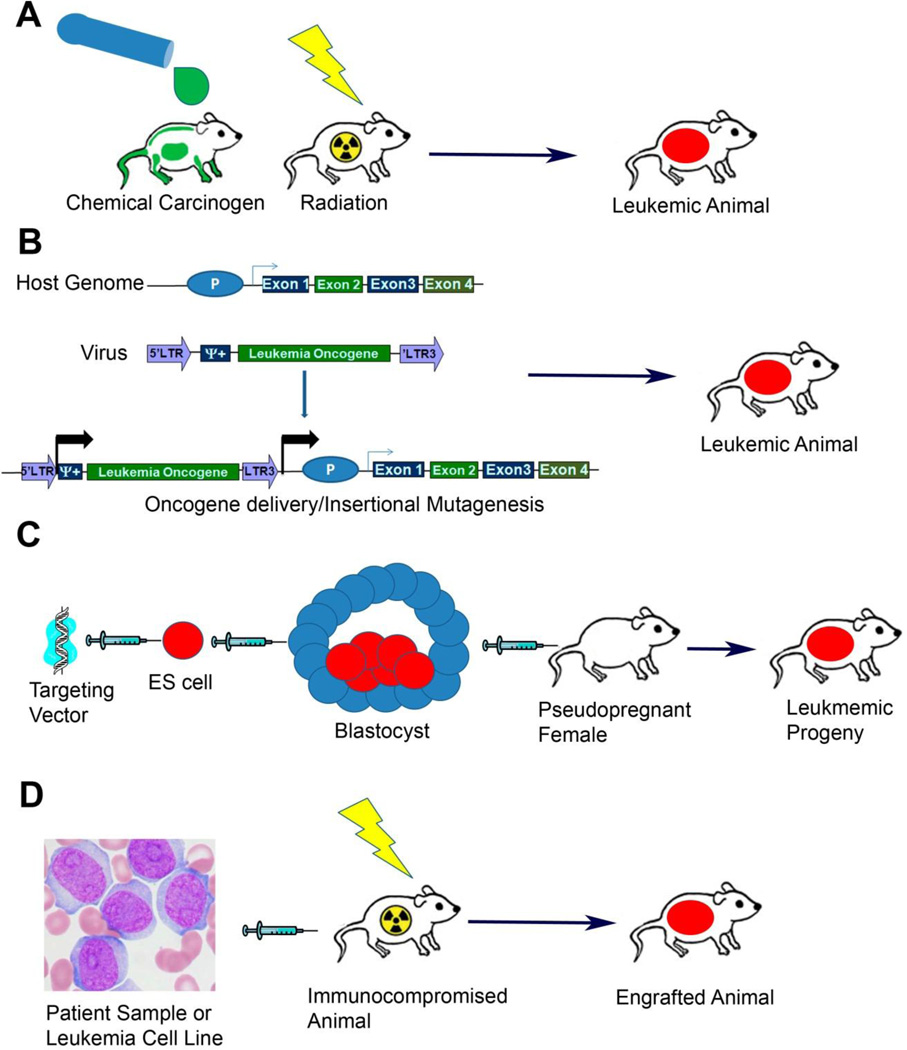
Figure 1.
Major techniques for generating animal models of leukemia. A) Carcinogen Induced Models. Animals from susceptible strains are exposed to carcinogenic chemicals or ionizing radiation. After exposure animals are followed for the development of leukemia. B) Mosaic, Transposon and Viral Induced Models. In mosaic models HSCs are harvested from donor mice and infected with viruses that encode an oncogene of interest. Viral integration into the host genome results in the delivery and expression of the oncogene. In addition to oncogene delivery the insertion of the virus can result in the aberrant expression of cellular proto-oncogenes genes (as depicted) or in disruption of a cellular tumor suppressor if integration occurs intragenically. Transposon mutagenesis results from similar consequences of transposon integration into the host genome. C) Transgenic Models. Targeting vector that has been engineered to express the transgene of interest is injected or electroporated into murine ES cells. ES cells that have integrated the vector are then injected into tetraploid blastocysts that are in turn then injected into pseudopregnant surrogate mothers. Alternatively vectors can be directly injected into fertilized zygotes and those zygotes then implanted in surrogate mothers. Offspring are then monitored for the development of leukemia. D) Xenograft Models. Primary patient samples or human cell lines can be directly injected into immunocompromised host animals. Host animals are frequently exposed to ionizing radiation prior to sample injection to suppress any residual immune function.
Viral and Transposon Models
One of the earliest viral models of AML is the erythroblastic AML caused by the Friend leukemia viruses, first reported in 1957 by Dr Charlotte Friend [16]. She reported the presence of a cell-free agent that could serially transmit an erythroblastic leukemia in susceptible strains of mice. This filterable agent was later found to be a combination of a replication-deficient spleen focus forming virus (SFFV) and a replication-competent Friend murine leukemia virus (MuLV) [17]. SFFV was identified as the cause of erythroblastic leukemia [18]; it acts in part by insertional mutagenesis (see Figure1). Integration of the viral genome in proximity to the Spi/PU.1 gene results in its overexpression, driven by the viral LTR, which contributes to the decreased differentiation of erythroblasts seen in the disease [19]. The MuLV has been used to discover novel genes involved in AML leukemogenesis in insertional mutagenesis screens [20]. Additional AML-inducing leukemia viruses have been isolated and utilized in insertional mutagenesis screens with transgenic models, including the MOL4070LTR virus [21–22]. Virally induced AML also has been used in the preclinical evaluation of possible therapeutics, most notably the spontaneous leukemia model in AKR mice as the result of an oncogenic RNA virus [23]. In addition to virally mediated insertional mutagenesis, transposon-based systems have also been developed, most notably the Sleeping Beauty system, which has been used to identify cooperating mutations [24–25]. These virally and transposon- induced models have contributed to the understanding of leukemogenesis and identification of active therapeutic agents. However, their relevance to the human disease is questionable, because no conclusive proof of an AML-inducing virus or transposon in humans has been reported.
Transgenic Models
AML is characterized by non-random, recurring karyotypic abnormalities known to affect prognosis, especially chromosomal translocations. These translocations can be pathognomonic for a particular subtype of AML, such as the t(15;17) seen in acute promyelocytic leukemia (APL), or can occur in a variety of leukemias, such as the Philadelphia chromosome seen in CML, ALL and rarely AML.
The earliest transgenic models of AML engineered mice to express the fusion proteins generated by these translocations. In transgenic models, mice are created through the manipulation of embryonic stem (ES) cells. In the classic approach, DNA is directly injected into the pro-nucleus of fertilized zygotes which are then injected into pseudopregnant females (see Figure 1). This results in a non-targeted integration of the transgene and is reliant on expression of the transgene to generate the phenotype. In a more modern approach, DNA is electroporated into ES cells and integrants are selected for by expression of antibiotic resistance genes. Selected cells are then injected into tetraploid blastocysts, which are in turn implanted into pseudopregnant females. Offspring are then backcrossed with wild type mice to generate the homozygously transgenic mice. Vectors can now be generated that target specific sites in the genome through homologous recombination. An additional layer of complexity can be added with newer vectors that conditionally express genes in response to doxycycline (Tet on/off systems) or Cre recombinase (using the Lox/Cre systems). Once mice with these conditional alleles are generated, they can then be crossed to other transgenics that express the Tet transactivator or Cre recombinase in a tissue-specific manner, to generate tissue-specific expression of the transgene (reviewed in [26]). In addition to the expression of oncogenic fusion proteins, gain of function oncogenic alleles can be "knocked in" to their corresponding normal loci and tumor suppressors can be "knocked out" using these same approaches.
APL
Early models of APL were created by expression of the fusion protein generated by t(15;17), PML-RAR , under the control of various myeloid-specific promoters [27–29]. These models not only recapitulated the histologic phenotype of human APL, but also the remissions seen after treatment with all trans-retinoic acid (ATRA). In a transgenic model that expressed the PLZF-RAR fusion protein, found in patients with a rare APL variant containing t(11;17), ATRA treatment did not induce remission in mice, mimicking the clinical scenario [30]. Despite these successes, the model has several drawbacks. The resulting phenotype varies with expression of the inserted transgene. When different myeloid-specific promoters were used, some promoters yielded models with high penetrance and shorter latencies, while others yielded disease more closely resembling myeloproliferative neoplasms than an acute leukemia. Indeed, the model that expressed PML-RARα under the control of the CD11b promoter did not develop leukemia at all [27]. These models still leave in question whether genetic events in addition to t(15;17) are required to cause APL in humans.
AML1-ETO Leukemias
The AML1-ETO fusion protein is generated by the t(8;21) found predominantly in AML FAB class M2. Patients with AML who are positive for the translocation t(8;21) have a better prognosis and are generally responsive to chemotherapeutics [31–32]. AML-ETO fusion genes result in embryonic lethality when inserted into the endogenous AML1 promoter due to poor hematopoiesis, similar to AML1−/− knockout animals [33]. This makes standard knock-in models of AML1-ETO uninformative. To circumvent this limitation, Dr Zhang and colleagues generated an inducible transgenic model that expressed AML1-ETO under the control of the Tet repressor. Despite robust expression of the transgene in the marrow of mice, no leukemia developed [34]. They subsequently found that when mice expressed AML1-ETO (under the control of the myeloid-specific promoter of MRP8) were treated with the carcinogen, N-ethyl-N-nitrosourea, 55% of animals developed AML [35] while none of the control mice did. This finding is consistent with the view that AML1-ETO expression is necessary but insufficient for leukemogenesis. Consistent with this idea, other groups have found cooperation of AML1-ETO with mutations in tyrosine kinases such as TEL-PDGFR, using mosaic models that will be discussed below [36–37].
MLL Leukemias
The Mixed Lineage Leukemia (MLL) gene is located on chromosome 11 and is frequently involved in balanced translocations in the acute leukemias. It has over 40 known fusion partners in the acute leukemias and is often associated with chemoresistance and a poor prognosis [31–32, 38]. Roughly 5% of acute leukemia patients express MLL fusions [39–40], although that number increased to ~70% of patients with previous exposure to anthracyclines and topoisomerase II poisons. Knock-in models of MLL fusion proteins such as Mll-lacZ result in AML in some animals, but not others. LacZ has no known oncogenic role, so this suggests that MLL alone is sufficient to cause oncogenesis. In the t(9;11) scenario, AF9 partners with MLL. AF9 protein is homologous to the product of ENL from 19p13.3, which is another partner gene for MLL involved in the acute leukemias. Corral et al. used homologous recombination in ES cells to produce the Mll-AF9 fusion. The fusion protein they generated relies on endogenous MLL control elements to transcribe the fusion protein. The mice developed AML despite widespread activity of the MLL promoter [41]. A small subset of animals developed ALL, consistent with what is seen in humans [42]. The engineering of a reciprocal translocation between chromosomes 9 and 11 to generate the MLL-AF9 fusion gene was generated in one model through the use of LoxP sites at both the MLL and AF9 loci, but these mice did not develop leukemia [43]. The model did demonstrate the feasibility of using Lox-Cre systems to engineer translocations in mice. Another recurrent translocation involving the MLL gene occurs with t(11;19) and results in the generation of the MLL-ENL fusion protein. This fusion event has been modeled in transgenic animals using the engineered translocation approach [44]. In this study, mice were generated with LoxP sites in both the MLL and ENL genes and crossed to mice expressing Cre from the hematopoietic cell-specific promoter of the Lmo2 gene. The resulting mice developed an aggressive, fully penetrant AML, demonstrating that the engineered translocation approach can successfully model AML.
Gain-of-Function "Knocked In" Models
Several oncogenes have been implicated in the development of AML in humans, most prominently the Flt3 receptor tyrosine kinase. Flt3 mutations occur in 25% of patients with AML and confer a worse prognosis [45]. The most common Flt3 mutation involves the generation of an in-frame tandem duplication, the so called "Flt3 ITD" mutation. Flt3 ITD is a constitutively active form of Flt3 that causes ligand-independent signaling. A knock-in model of the Flt3 ITD was generated; the resulting mice developed a myeloproliferative neoplasm but not AML; consistent with other models that suggest that the Flt3 ITD can lead to enhanced proliferation but alone are insufficient for the development of AML [46]. Also consistent with this finding, when transgenic mice expressing the Flt3 ITD were crossed to transgenic animals expressing the NUP98- HOXD13 fusion protein found in myelodysplastic syndrome (MDS) and AML patients, the resulting progeny developed a highly penetrant and lethal AML [47].
The g protein Ras is a target of activating mutations in AML. K-ras is mutated in approximately 10–15% of AMLs and N-ras 20–25%. An inducible K-ras model has been developed driven by its endogenous promoter [48]. In this model, investigators generated a mutated K-ras allele downstream of a transcriptional stop sequence that was flanked with LoxP sites. This mouse was then crossed to a mouse that expresses Cre recombinase under the control of the interferon-inducible MX-1 promoter (MX1-Cre). Conditional expression of K-rasG12D leads to a fatal myeloproliferative disorder similar to that in Flt3 ITD models. This finding is consistent with the “two hit” hypothesis, in which two alterations are necessary to cause AML [2]. Interestingly, when a similar model was constructed using N-rasG12D, a much more indolent myeloproliferative response was induced and mice died from a spectrum of hematologic malignancies, demonstrating that different Ras alleles are not functionally equivalent [49].
The most common mutation in AML is in the neuclophosmin 1 gene, or NPM1. It has multiple cellular functions and shuttles between nuclear, nucleolar, and cytoplasmic locations. In AML, ~35% of patients have an NPM1 mutation that alters cellular localization, resulting in cytoplasmic distribution of the mutant protein only. The first model that knocked in the mutated form of NPM1 to the endogenous mouse locus resulted in a mild myeloproliferative disorder, but not AML. In a second model, the most common form of NPM1 mutation was inserted into the mouse loci flanked by LoxP sites; resulting mice were crossed to MX1-Cre mice. Upon Cre induction, about one-third of the double transgenic mice developed delayed-onset AML. Penetrance was increased and latency shortened using a transposon-based insertional mutagenesis approach. These models have clarified the role of mutations that result in oncogenic gain of function in the development of AML, and will likely continue to contribute to the increased understanding of the molecular pathogenesis of the disease.
Loss-of-Function "Knocked Out" Models
Several growth and survival pathways can be hyper-activated by the loss of negative regulators. One such example in AML is the PI3 kinase pathway, which is hyperactivated in 50–70% of AML patients [50]. The tumor suppressor PTEN is a negative regulator of this pathway. When transgenic mice were generated that contained LoxP sites flanking PTEN and these animals were crossed to a myeloid-specific Cre-expressing strain, 11 of 18 progeny developed AML. The resulting AML was monocytic in morphology and the PI3 kinase pathway was activated in multiple cases of extramedullary monocytic AML [51]. A negative regulator of the Ras pathway is the gene NF1, which stimulates the GTPase activity of the Ras proteins, leading to their inactivation. NF1 loss was engineered by the construction of NF1 loci flanked by LoxP sites when these animals were crossed with the inducible K-RasG12D model discussed above. When Cre was induced, an aggressive AML with high penetrance was produced [52]. These models demonstrate that the transgenic approach can be applied in conjunction with LoxP-Cre systems to model tumor suppressor loss in the generation of AML.
Mosaic Models
As discussed above, although murine transgenic models have contributed greatly to our understanding of AML, these models have significant drawbacks. The amount of time and effort required to move from vector construction, to screening of founder mice, to maintenance of a breeding colony can be prohibitive. An alternative approach to the transgenic model is to take hematopoietic stem cells (HSCs) from mice, manipulate them ex-vivo, and transplant them into autologous or syngeneic recipients (see Figure 1). This model allows the rapid generation of genetically defined leukemias, most commonly by retro- or lentivirus transduction of cells (reviewed in [26]).
Murine stem cell virus (MSCV)-based vectors are the most commonly used replication-deficient retrovirus in the generation of mosaic leukemia models. This rapid and cost-effective system has been used extensively in AML to complement studies done with transgenic animals. For example, investigators have retrovirally transfected and transduced the AML1-ETO fusion protein into HSCs to generate chimeric mice with molecular abnormalities akin to those in patients with this translocation [53]. Transplantation models, using bone marrow transduced with AML1-ETO and N-rasG12D into syngeneic recipients, showed cooperation leading to leukemia that resembled the M2 subtype of AML most commonly associated with AML1-ETO [31]. A mosaic AML model generated with MLL-ENL fusions and N-rasG12D resembled the M4-M5 subtype associated with MLL fusions. When leukemic mice were treated with cytarabine and anthracycline chemotherapy, responses to the two models differed significantly, with the MLL-ENL mice being poorly responsive while the AML-ETO mice showed high remission rates and even a few cures. These responses mirror the clinical situation and demonstrate that these models can recapitulate the differing prognostic effects of these translocations. In addition, the mosaic model has been used to model the prognostic effects of mutated tyrosine kinases. When employed to study response to standard AML chemotherapeutics, Flt3 ITD accelerated engraftment. This model was sensitive to cytarabine providing a reduction in tumor burden and survival benefit. Doxorubicin did not show the same efficacy, and there was the suggestion of net resistance when the two drugs are combined, as in standard AML induction therapy [54].
Xenograft Models
Even the most clinically aggressive sample of leukemia cells from a patient is unlikely to grow when placed in culture, and cell characteristics can change depending on the media used [55–56]. Additionally, culture systems cannot replicate the interactions between the leukemia cells and their stroma and immune cells. To circumvent these limitations, murine xenograft models have been developed to allow engraftment of primary patient samples and cell lines.
The first attempts at xenografting AML into immunocompromised mice utilized athymic nude mice that harbor a mutation in the Foxn1 gene, leading to an almost complete lack of functional T cells. Early experiments using primary patient samples did result in the generation of granulocytic sarcomas, but without bone marrow and other organ involvement [57]. In an attempt to improve marrow engraftment, severe combined immunodeficient (SCID) mice were tested for their ability to engraft AML patient samples. These mice have mutations in the Prkdc gene and thus lack functional T or B cells. Although samples injected intraperitoneally or implanted in kidney capsules had significant take rates, intravenous injection lead to very few samples engrafting in recipient animals [58]. In a landmark paper, Dr John Dick and colleagues injected primary patient samples into SCID mice that were then treated with human stem cell factor (SCF) and granulocyte-macrophage colony stimulating factor (GM-CSF). This resulted in a high take rate from intravenously injected patient peripheral blood or marrow; more importantly, only a subset of leukemia cells could successfully engraft [10]. This was the first evidence of a hierarchy of leukemia cells, with a minority population being responsible for the maintenance of the disease. Since then, mice with more complete immunocompromise have been developed. Non-obese diabetic (NOD)/SCID mice have no functional B or T cells and diminished NK cell and monocyte activity. They have a higher engraftment rate of primary patient samples than SCID mice [59]. Further immunocompromise has been established with NOD/SCID mice, which have deletions in the gene encoding the interleukin 2 receptor gamma chain (IL2Rγ) (socalled NSG mice). Finally, a transgenic mouse has been created on this platform that expresses human iL3, GM-CSF and SCF (NSG-tg). This mouse had significantly improved engraftment rates for primary patient samples over the standard NSG mouse [60]. While these models are limited in their ability to address leukemia immune cell interactions, they are valuable tools in assessing preclinical activity of promising agents and leukemia cell-stroma interactions.
Non-Mouse Models
Other animal models have been used to study AML, including rats. While spontaneous leukemias are rare in rats, many carcinogen- and radiation-induced models have been reported [61–64]. A myelomonocytic leukemia, L5222, was induced in 1967, 326 days after a single intravenous injection of ethylnitrosourea (200 mg/kg body weight) in a 3- month-old female BD IX rat. This leukemia was transplantable into BD IX rats and has been used in preclinical efficacy studies [65]. The APL model generated in Brown Norway rats (BNML) has been used in preclinical studies of chemotherapy and transplantation [66]. The leukemia was induced in a female BN rat by 9,10-dimethyl-1,2-benzanthracene [67], and has similar colony formation characteristics to human AML samples in colony assays [68]. Additionally, this model has made important contributions to the understanding of minimal residual disease in AML (reviewed in [69]). More recently, non-mammalian systems of AML have been reported. One example is a recent report of the transplantation of human AML samples into zebrafish embryos. In this study, investigators injected human AML cell lines and patient samples into zebrafish embryos 48 hours post-fertilization. They used between 50 and 200 cells and saw a reduction in leukemic burden when embryos injected with susceptible cells were treated with imatinib [70]. In another study, human APL and CML in myeloid blast crisis cell lines were injected into zebrafish embryos; embryos were then treated with imatinib or ATRA, and they showed a reduced leukemic burden [71].
Another model system used to study AML is the fruit fly, Drosophila melanogaster. Expression of the AML1-ETO fusion protein in Drosophila blood cells impaired the differentiation of blood cells that rely on RUNX1, similar to what is seen in patients [72]. These non-mammalian systems have the advantages of lower cost, and increased reproductive capacity in systems with well-understood genetics. They have the potential to make significant contributions in the understanding of the pathogenesis of AML.
Animal Models of ALL
ALL is the most common leukemia in children and also occurs in a significant number of adults. It is characterized by the aggressive proliferation of clonal lymphoblasts. ALL has several sub-types, including pre-B cell, T-cell, and mature B cell or Burkitt's. It can present primarily as a solid mass (lymphoblastic lymphomas) or with primary marrow involvement (lymphoblastic leukemias). Unlike AML, it is now estimated that over 90% of children diagnosed with ALL can be cured [73]. While this remarkable achievement is the result of many avenues of research, several important insights into ALL have been discovered using animal models. These insights include demonstration that Notch-1 activation leads to T-cell ALL in a murine transplant model [74], the finding that transgenic mice expressing the p190 version of the BCR-ABL fusion developed ALL [75], and the preclinical assessment of activity of antimetabolites [76].
Murine Models
As in AML, murine models of ALL utilize carcinogen-induced, viral-induced, transgenic, mosaic, and xenograft approaches. These models have contributed to the current understanding of ALL.
Carcinogen-Induced Models
As in AML, early work in murine models was based on the carcinogen-induced model. In addition to the important L1210 cell line discussed above, there are several other carcinogen-induced ALL lines, including the L5178Y cell line. This cell is a DBA/2-derived methylcholanthrene-induced T-cell line that has been used extensively in preclinical efficacy studies and mechanisms of resistance [77–79].
Viral Models
The leukemia-prone AKR strain of mice has been used to characterize the oncogenic properties of various viruses. As a result, a T cell ALL model has been derived from AKR mice infected with a recombinant retrovirus that targets thymocytes [80]. This model has been used to assess the effect of obesity on ALL progression [81]. Additionally, lymphoblastic leukemias and lymphomas that have occurred as a result of retroviral insertion mutagenesis have allowed the characterization of novel genes involved in lymphoid leukemogenesis by the characterization of common viral insertion sequences [82]. The robustness of the techniques was confirmed when a gene identified through the screen, Prdm14, was found to be overexpressed in human B and T cell ALL and could initiate ALL when overexpressed by viral transduction of murine bone marrow cells [83].
Transgenic Models
Several ALL transgenic models have been published. These models recapitulate B cell, T cell, and Burkitt's leukemia/lymphoblastic lymphomas.
BCR-ABL
The BCR-ABL fusion protein is the result of the translocation t(9;22)(q34;q11), also known as the Philadelphia chromosome. This translocation is characteristic of CML but is also found in B cell ALL. There are several isoforms of this fusion protein, the p210 form (found primarily in CML) and the p190 form (found primarily in ALL). Transgenic mice have been generated that expressed the p190 isoform from the BCR promoter, but they had embryonic lethality [75]. By contrast, transgenic mice that expressed the p190 BCR-ABL fusion from the metallothionein promoter are born alive, and 95% die from a pre-B cell leukemia/lymphoma after 35 to 200 days [84]. This model has been used to test activity of novel compounds [85], tyrosine kinase inhibitors [86], and the effect of obesity on ALL progression [81].
MLL Leukemias
As the name Mixed Lineage Leukemia implies MLL translocations are seen in both AML and ALL. The MLL-AF4 fusion protein is seen in patients with t(4;11) and it is strongly associated with B cell ALL. A transgenic mouse model was generated that expressed MLL-AF4 from the endogenous MLL promoter. The resulting mice demonstrated deregulated lymphoid and myeloid growth and, after a long latency, B cell lymphomas [87]. Another model was generated using an inverted AF4 allele targeted to an intron in the MLL gene; when exposed to Cre recombinase, inversion and generation of MLL-AF4 occurred. Mice were then crossed to various tissue-specific Cre expressing transgenic mice. When Cre was present, the mice developed B cell malignancies with varying latencies depending on the Cre promoter [88]. A transgenic model using the human MLL-AF4 fusion protein expressed from the MSCV viral LTR resulted in B cell neoplasms, and the latency could be shortened by crossing these animals to transgenic mice expressing K-rasG12D [89]. This result supports the multi-hit hypothesis in leukemogenesis.
Eµ-myc
Burkitt's lymphoma/leukemia is considered a subtype of ALL and is characterized by translocations that result in the MYC gene being expressed under the control of the immunoglobulin heavy chain or light chain promoters. As a result, the disease is characterized by a high proliferative rate and very aggressive behavior. Transgenic mice have been generated with the murine Myc gene under the control of the IgG heavy chain promoter mimicking the t(8;14) seen clinically. The resulting mice developed aggressive B cell lymphomas and leukemias, with 90% of animals dying within the first 5 months of life [90]. This model has been exploited to explore the role of cooperating mutations in lymphoma/ leukemogenesis [91], and in a seminal paper on how p53 loss or BCL2 overexpression affects chemotherapy response in vivo [92].
NOTCH1
Notch receptors are transmembrane receptors that, when activated by a ligand, are cleaved into extra- and intracellular portions. The intracellular portion (ICN) translocates to the nucleus, where it interacts with additional proteins, resulting in transcriptional activation of target genes (reviewed in [93]). NOTCH1 was first identified in the human T cell ALL-bearing translocation, t(7;9)(q34;q34.3). This translocation results in a fusion gene in which the TCR promoter drives expression of the 3’ end of the NOTCH1 locus, encoding only the ICN and resulting in constitutive NOTCH1 activation [94]. This translocation is found in only about 1% of T cell ALL. However, in a subsequent report, 50 to 60% of T cell ALL samples contained point mutations in NOTCH1, implicating this gene in the pathogenesis of most types of T cell ALL [95]. Several transgenic mouse models have been generated to express activated NOTCH1 alleles from T cell-specific promoters [96–98]. These mice have altered CD4, CD8 thymocyte development and develop T cell malignancies. A more recent model uses a transgenic mouse that expresses two common point mutations in NOTCH1 from its endogenous promoter in a conditional manner using a Lox-Stop-Lox cassette. These mice demonstrated accelerated development of T cell malignancies in a Sleeping Beauty transposon mutagenesis system [99].
Mosaic Models
As with AML, ex-vivo manipulation of HSCs has been used to generate mouse models of ALL. MSCV vectors engineered to express the p190 BCR-ABL fusion protein have been used extensively to generate Philadelphia chromosome- positive B cell ALL models. A few examples include a study of the effects of chemotherapy on the development of tyrosine kinase inhibitor (TKI) resistant BCR-ABL mutants [100], the role of BCL6 in resistance to TKIs [101], and the role of the tumor suppressor Arf in the development of B cell ALL [102]. MLL fusion oncogenes have also been used extensively to generate mosaic ALL models. Researchers combined the mosaic model with the xenograft model and using human HSC derived from cord blood, infected them with an MSCV-based MLL-ENL-expressing vector, and injected them into sublethally irradiated NOD/SCID mice. In 26 of 29 injected mice an aggressive B cell ALL developed, demonstrating that MLL-ENL could induce ALL in human cells [103]. In an additional study, the MLL-AF9 fusion could produce AML or ALL in human cord blood CD34+ cells depending on the recipient mouse (NSG vs NSG-tg) [104]. These studies have established the feasibility of generating ALL models from human cells propagated in immunocompromised mice, and have begun to unravel how MLL-rearranged leukemias can be either myeloid or lymphoid.
Xenograft Models
The same immunocompromised mouse strains described in the xenograft section in the AML models have been used to conduct preclinical efficacy studies in ALL samples. Additionally, they have extended the leukemic stem cell model to ALL through the observations that not all B or T-cell ALL cells can initiate leukemia in NOD/SCID or NSG mice [105–106].
Non-Mouse Models
A sub-line of inbred Sprague-Dawley rats have a high incidence of T cell malignancies and have been used in the preclinical evaluation of therapeutics [107–108]. A B-cell ALL has been derived from inbred guinea pigs and was serially transplantable into inbred recipients, though not into non-inbred animals [109]. There are also non-mammalian ALL models, including a transgenic zebrafish model based on expression of the TELAML1 fusion protein found in patients with pre-B cell ALL with t(12;21)(p13;q22). Researchers expressed the transgene in either a ubiquitous fashion (using a modified - actin promoter) or in a lymphoid-restricted manner (using the Rag2 promoter). Only zebrafish that expressed the transgene ubiquitously developed ALL after a long latency [110]. A chemical mutagenesis screen using transgenic zebrafish that express enhanced green fluorescent protein in a T cell-specific manner yielded multiple lines that have a heritable T-cell ALL [111]. Another T cell ALL model was generated in zebrafish using the murine c-myc gene expressed under the control of the zebrafish Rag2 promoter. The transgenic fish developed a T cell ALL with a short latency [112]. In an elegant model, Dr Look and colleagues used a transgenic zebrafish that contained a conditional Cre allele driven by the heat shock 70 promoter, and crossed it with a transgenic model that contained murine c-myc driven by the Rag2 promoter containing an intervening Lox-stop-DS Red-Lox cassette. The resulting progeny could be placed in 37°C water to induce Cre expression. Excision of the stop cassette could be followed by fluorescence imaging to detect the loss of DS Red and induction of EGFP expression. These animals developed an EGFP-expressing T cell lymphoblastic lymphoma (LBL) that progressed to leukemia after heat shock treatment [113]. This model was later used to investigate the genetic events that lead to the conversion of LBL to ALL [114]. The zebrafish has well established genetics, low cost, high reproductive rates making it an ideal system to carryout genetic screens. Contributions from these models are only just beginning.
Animal Models of CML
CML is unique among the leukemias in that there is a single oncogenic event that drives the disease – the BCR-ABL fusion protein generated by the Philadelphia chromosome. This was the first known somatic chromosomal abnormality directly associated with a malignancy, and provided the first evidence that cancer was a genetic disease [115]. The disease is characterized by the abnormal proliferation and accumulation of mature and maturing myeloid cells. It has three distinct clinical phases, a chronic phase, an accelerated phase, and a blast phase. In the past, CML could only be cured by allogeneic stem cell transplantation, but in more recent years treatment has been revolutionized by the advent of BCR-ABL kinase inhibitors like imatinib, nilotinib, and dasatinib (reviewed in [116]).
Animal models have played a unique role in CML studies. Dr Van Etten showed that the p210 isoform of the BCR-ABL protein directly caused CML by using a mosaic mouse model. He used a retroviral vector that expressed the p210 protein and infected mouse HSCs. When the infected cells were transplanted into syngenic recipients, the animals developed a myeloproliferative disorder that strongly resembled human CML [117]. These models have been used extensively since then to evaluate many aspects of CML, including the transforming potential of the various isoforms of BCR-ABL [118], the genetics of progression of the disease [36] and characterization of CML stem cells [119].
CML has also been modeled using transgenic mice. In one model, the p210 isoform was expressed from a tetracycline-inducible protomer and crossed to a transgenic mouse that expressed the tet-transactivator from the CD34 promoter, ensuring p210 expression in the presence of doxycycline in the HSC compartment. The resulting mice developed a myeloproliferative disease with thrombocytosis [120]. This is opposed to the B cell ALL they saw when p210 was expressed in pre-B cells [121], demonstrating that the phenotype of BCR-ABL-induced leukemias depends at least in part on the cell of origin. In addition to mosaic and transgenic models, xenograft studies using CML patient samples have been conducted. In a recent example, persistence of a CML stem cell population in patients in cytogenetic remission was demonstrated by engraftment into NSG mice [122]. This study provides proof of the persistence of these leukemic stem cells even in patients who have received TKI therapy for years. Primary patient samples and cell lines established from CML patients in various phases have been engrafted into athymic mice [123], SCID mice [124], and NOD/SCID mice [125]. The use of mosaic, transgenic, and xenograft models has provided invaluable insights into the molecular pathology of this leukemia.
Animal Models of CLL
CLL is the most common of all the leukemias and, unlike CML, the disease is genetically heterogenous. It is a disease of the elderly and is characterized by the clonal proliferation of immune incompetent CD5+ mature B lymphocytes. The disease is considered indolent, with median survival of low stage disease over a decade, although a subset of patients will have a more fulminant course [126]. The disease can occasionally transform into a more aggressive diffuse large B cell lymphoma or, more rarely, into Hodgkin's lymphoma via a process called a Richter's transformation. To date, several animal models of CLL have been described. As with the other leukemias, mice are the most widely used model.
One of the earliest CLL mouse models is the spontaneously occurring New Zealand Black (NZB) mouse model. NZB mice develop a clonal proliferation of aneuploid CD5+ B lymphocytes. The disease can be serially transplanted and will occasionally become a large cell lymphoma consistent with a Richter's transformation seen clinically [127]. A mutation in the microRNA-16 locus was found in these mice; also seen in human CLL cells, it is thought to contribute to the development of CLL [128]. In addition to this spontaneous model several transgenic models have been developed. In the TCL1 transgenic model the human TCL1 gene was cloned downstream of the E promoter resulting in B cell-restricted high-level expression. The TCL1 gene has elevated expression in a number of B cell malignancies, including CLL [129]. The resulting mice develop a clonal or oligo-clonal proliferation of CD5+ B lymphocytes in later life, mimicking the human disease [130]. Additional insights into the molecular pathology of CLL were gained by the TRAF2DN/Bcl-2 double transgenic model. Both Bcl-2 and tumor necrosis factor receptor associated factors (TRAFs) are overexpressed in B cell malignancies, including CLL [131–132]. In this model, investigators crossed transgenic animals that expressed human BCL2 from the IgG heavy chain promoter with those that expressed a truncated form of TRAF2. The resulting progeny developed a progressive accumulation of clonal CD-expressing B cells, leading to shortened survival [133]. As with the other leukemias, propagation of human CLL samples in immunocompromised mice has been reported. A human-derived fludarabine-resistant CLL cell line, WSUCLL, can cause tumors when injected subcutaneously in SCID mice [134]. This model has been used for preclinical testing [135]. Nude mice have also served as recipients of human CLL cell lines [136]. Finally, NOD/SCID animals have been used to engraft primary patient samples [137]. This model has been used to study the effects of known CLL prognostic markers on sample engraftment; samples with adverse factors engrafted more aggressively in this model [138]. The initial successes of this model make it highly attractive for future preclinical efficacy studies of novel agents.
Summary and Future Directions
Animal models of leukemia have been indispensible in the study of these devastating diseases. A compelling argument can be made that our understanding of leukemias, more than any other malignancy, have benefited from these model systems. Much of the current understanding and therapeutic approaches were first tested or discovered using these models. In the future, one can easily imagine a synergy between the various models for the further development of novel therapeutic targets. The transgenic and mosaic models have a clear utility in preclinical efficacy testing, as well as exploring stroma-immune cell interactions. Xenograft models have the unique advantage of being able to explore human cell specific biology in an in vivo, albeit immunocompromised environment. Newer, non-mammalian systems can bring to bear large-scale genetic screens to uncover critical molecular pathways that can serve as the next generation of therapeutic targets to be evaluated in mosaic, transgenic, and xenograft models and moved into clinical trials to improve the lives of our patients with leukemias.
Acknowledgements
The authors would like to acknowledge Karen Klein for help editing the manuscript. Support provided by the Doug Coley Foundation for Leukemia Research, the MacKay Foundation for Cancer Research, the Leight Endowed Research Fund and the Frances P Tutwiler Fund.
Bibliography
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2005:137–142. doi: 10.1182/asheducation-2005.1.137. [DOI] [PubMed] [Google Scholar]
- 4.Lowenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program. 2008;2008:1–11. doi: 10.1182/asheducation-2008.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 11.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 12.Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883–1897. [PubMed] [Google Scholar]
- 13.Law LW, Taormina V, Boyle PJ. Response of acute lymphocytic leukemias to the purine antagonist 6-mercaptopurine. Ann N Y Acad Sci. 1954;60:244–250. doi: 10.1111/j.1749-6632.1954.tb40015.x. [DOI] [PubMed] [Google Scholar]
- 14.McCormack E, Bruserud O, Gjertsen BT. Animal models of acute myelogenous leukaemia - development, application and future perspectives. Leukemia. 2005;19:687–706. doi: 10.1038/sj.leu.2403670. [DOI] [PubMed] [Google Scholar]
- 15.Skipper HE, Schabel FM, Jr, Wilcox WS. Experimental evaluation of potential anticancer agents. XXI. Scheduling of arabinosylcytosine to take advantage of its S-phase specificity against leukemia cells. Cancer Chemother Rep. 1967;51:125–165. [PubMed] [Google Scholar]
- 16.Friend C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957;105:307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linemeyer DL, Menke JG, Ruscetti SK, Evans LH, Scolnick EM. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982;43:223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff L, Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985;228:1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- 19.Back J, Dierich A, Bronn C, Kastner P, Chan S. PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood. 2004;103:3615–3623. doi: 10.1182/blood-2003-11-4089. [DOI] [PubMed] [Google Scholar]
- 20.Erkeland SJ, Valkhof M, Heijmans-Antonissen C, et al. Large-scale identification of disease genes involved in acute myeloid leukemia. J Virol. 2004;78:1971–1980. doi: 10.1128/JVI.78.4.1971-1980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caudell D, Harper DP, Novak RL, et al. Retroviral insertional mutagenesis identifies Zeb2 activation as a novel leukemogenic collaborating event in CALM-AF10 transgenic mice. Blood. 2010;115:1194–1203. doi: 10.1182/blood-2009-04-216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slape C, Hartung H, Lin YW, Bies J, Wolff L, Aplan PD. Retroviral insertional mutagenesis identifies genes that collaborate with NUP98-HOXD13 during leukemic transformation. Cancer Res. 2007;67:5148–5155. doi: 10.1158/0008-5472.CAN-07-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skipper HE, Schabel FM, Jr, Trader MW, Laster WR., Jr Response to therapy of spontaneous, first passage, and long passage lines of AK leukemia. Cancer Chemother Rep. 1969;53:345–366. [PubMed] [Google Scholar]
- 24.Vassiliou GS, Cooper JL, Rad R, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43:470–475. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier LS, Adams DJ, Hackett CS, et al. Whole-body sleeping beauty mutagenesis can cause penetrant leukemia/lymphoma and rare high-grade glioma without associated embryonic lethality. Cancer Res. 2009;69:8429–8437. doi: 10.1158/0008-5472.CAN-09-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyer J, Kwong LN, Lowe SW, Chin L. Non-germline genetically engineered mouse models for translational cancer research. Nat Rev Cancer. 2010;10:470–480. doi: 10.1038/nrc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Early E, Moore MA, Kakizuka A, et al. Transgenic expression of PML/RARalpha impairs myelopoiesis. Proc Natl Acad Sci U S A. 1996;93:7900–7904. doi: 10.1073/pnas.93.15.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown D, Kogan S, Lagasse E, et al. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grisolano JL, Wesselschmidt RL, Pelicci PG, Ley TJ. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RAR alpha under control of cathepsin G regulatory sequences. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- 30.He LZ, Guidez F, Tribioli C, et al. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 31.Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML-10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 33.Okuda T, Cai Z, Yang S, et al. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91:3134–3143. [PubMed] [Google Scholar]
- 34.Rhoades KL, Hetherington CJ, Harakawa N, et al. Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model. Blood. 2000;96:2108–2115. [PubMed] [Google Scholar]
- 35.Yuan Y, Zhou L, Miyamoto T, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci U S A. 2001;98:10398–10403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dash AB, Williams IR, Kutok JL, et al. A murine model of CML blast crisis induced by cooperation between BCR/ABL and NUP98/HOXA9. Proc Natl Acad Sci U S A. 2002;99:7622–7627. doi: 10.1073/pnas.102583199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grisolano JL, O'Neal J, Cain J, Tomasson MH. An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci U S A. 2003;100:9506–9511. doi: 10.1073/pnas.1531730100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavau C, Du C, Thirman M, Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J. 2000;19:4655–4664. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 40.Daser A, Rabbitts TH. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 2004;18:965–974. doi: 10.1101/gad.1195504. [DOI] [PubMed] [Google Scholar]
- 41.Corral J, Lavenir I, Impey H, et al. An Mll-AF9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell. 1996;85:853–861. doi: 10.1016/s0092-8674(00)81269-6. [DOI] [PubMed] [Google Scholar]
- 42.Strissel PL, Strick R, Tomek RJ, Roe BA, Rowley JD, Zeleznik-Le NJ. DNA structural properties of AF9 are similar to MLL and could act as recombination hot spots resulting in MLL/AF9 translocations and leukemogenesis. Hum Mol Genet. 2000;9:1671–1679. doi: 10.1093/hmg/9.11.1671. [DOI] [PubMed] [Google Scholar]
- 43.Collins EC, Pannell R, Simpson EM, Forster A, Rabbitts TH. Inter-chromosomal recombination of Mll and Af9 genes mediated by cre-loxP in mouse development. EMBO Rep. 2000;1:127–132. doi: 10.1093/embo-reports/kvd021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forster A, Pannell R, Drynan LF, et al. Engineering de novo reciprocal chromosomal translocations associated with Mll to replicate primary events of human cancer. Cancer Cell. 2003;3:449–458. doi: 10.1016/s1535-6108(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 45.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Piloto O, Nguyen HB, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111:3849–3858. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenblatt S, Li L, Slape C, et al. Knock-in of a FLT3/ITD mutation cooperates with a NUP98-HOXD13 fusion to generate acute myeloid leukemia in a mouse model. Blood. 2012 doi: 10.1182/blood-2011-10-382283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan IT, Gilliland DG. Oncogenic K-ras in mouse models of myeloproliferative disease and acute myeloid leukemia. Cell Cycle. 2004;3:536–537. doi: 10.4161/cc.3.5.828. [DOI] [PubMed] [Google Scholar]
- 49.MacKenzie KL, Dolnikov A, Millington M, Shounan Y, Symonds G. Mutant N-ras induces myeloproliferative disorders and apoptosis in bone marrow repopulated mice. Blood. 1999;93:2043–2056. [PubMed] [Google Scholar]
- 50.Martelli AM, Nyakern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 51.Yu H, Li Y, Gao C, et al. Relevant mouse model for human monocytic leukemia through Cre/lox-controlled myeloid-specific deletion of PTEN. Leukemia. 2010;24:1077–1080. doi: 10.1038/leu.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cutts BA, Sjogren AK, Andersson KM, et al. Nf1 deficiency cooperates with oncogenic K-RAS to induce acute myeloid leukemia in mice. Blood. 2009;114:3629–3632. doi: 10.1182/blood-2009-02-205146. [DOI] [PubMed] [Google Scholar]
- 53.de Guzman CG, Warren AJ, Zhang Z, et al. Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation. Mol Cell Biol. 2002;22:5506–5517. doi: 10.1128/MCB.22.15.5506-5517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pardee TS, Zuber J, Lowe SW. Flt3-ITD alters chemotherapy response in vitro and in vivo in a p53-dependent manner. Exp Hematol. 2011;39:473–485. e474. doi: 10.1016/j.exphem.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruserud O, Tore Gjertsen B, Brustugun OT, et al. Effects of interleukin 10 on blast cells derived from patients with acute myelogenous leukemia. Leukemia. 1995;9:1910–1920. [PubMed] [Google Scholar]
- 56.Bruserud O, Gjertsen BT, von Volkman HL. In vitro culture of human acute myelogenous leukemia (AML) cells in serum-free media: studies of native AML blasts and AML cell lines. J Hematother Stem Cell Res. 2000;9:923–932. doi: 10.1089/152581600750062372. [DOI] [PubMed] [Google Scholar]
- 57.Nara N, Miyamoto T. Direct and serial transplantation of human acute myeloid leukaemia into nude mice. Br J Cancer. 1982;45:778–782. doi: 10.1038/bjc.1982.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawyers CL, Gishizky ML, Quan S, Golde DW, Witte ON. Propagation of human blastic myeloid leukemias in the SCID mouse. Blood. 1992;79:2089–2098. [PubMed] [Google Scholar]
- 59.Ailles LE, Gerhard B, Kawagoe H, Hogge DE. Growth characteristics of acute myelogenous leukemia progenitors that initiate malignant hematopoiesis in nonobese diabetic/severe combined immunodeficient mice. Blood. 1999;94:1761–1772. [PubMed] [Google Scholar]
- 60.Wunderlich M, Chou FS, Link KA, et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svejda J, Kossey P, Hlavayova E, Svec F. Histological picture of the transplantable rat leukaemia induced by x-irradiation and methylcholanthrene. Neoplasma. 1958;5:123–131. [PubMed] [Google Scholar]
- 62.Moriuchi T, Oikawa T, Kodama T, Yamaguchi H, Kobayashi H. Establishment and characterization of a transplantable rat myelomonocytic leukemia. Cancer Res. 1983;43:5478–5483. [PubMed] [Google Scholar]
- 63.Ivankovic S, Zeller WJ. [Leukemia L 5222 of the rat strain BD IX. An ethylnitrosourea-induced monocytic-myeloic transplantable form for cytochemical and chemotherapeutic studies] Blut. 1974;28:288–292. doi: 10.1007/BF01631649. [DOI] [PubMed] [Google Scholar]
- 64.Pearson JW, Chaparas SD, Torgersen JA, Perk K, Chirigos MA, Sher NA. The effect of drug therapy against a histologically defined rat leukemia. Cancer Res. 1974;34:355–361. [PubMed] [Google Scholar]
- 65.Zeller WJ, Ivankovic S, Schmahl D. Chemotherapy of the transplantable acute leukemia L5222 in rats. Cancer Res. 1975;35:1168–1174. [PubMed] [Google Scholar]
- 66.Hagenbeek A, Martens AC. Efficacy of high-dose cyclophosphamide in combination with total-body irradiation in the treatment of acute myelocytic leukemia: studies in a relevant rat model. Cancer Res. 1983;43:408–412. [PubMed] [Google Scholar]
- 67.Hagenbeek A, Martens AC. The pathogenesis of a rat model for human acute myelocytic leukemia. Haematologica. 1980;65:293–308. [PubMed] [Google Scholar]
- 68.van Bekkum DW, van Oosterom P, Dicke KA. In vitro colony formation of transplantable rat leukemias in comparison with human acute myeloid leukemia. Cancer Res. 1976;36:941–946. [PubMed] [Google Scholar]
- 69.Martens AC, van Bekkum DW, Hagenbeek A. Minimal residual disease in leukemia: studies in an animal model for acute myelocytic leukemia (BNML) Int J Cell Cloning. 1990;8:27–38. doi: 10.1002/stem.5530080105. [DOI] [PubMed] [Google Scholar]
- 70.Pruvot B, Jacquel A, Droin N, et al. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica. 2011;96:612–616. doi: 10.3324/haematol.2010.031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corkery DP, Dellaire G, Berman JN. Leukaemia xenotransplantation in zebrafish- -chemotherapy response assay in vivo. Br J Haematol. 2011;153:786–789. doi: 10.1111/j.1365-2141.2011.08661.x. [DOI] [PubMed] [Google Scholar]
- 72.Osman D, Gobert V, Ponthan F, Heidenreich O, Haenlin M, Waltzer L. A Drosophila model identifies calpains as modulators of the human leukemogenic fusion protein AML1-ETO. Proc Natl Acad Sci U S A. 2009;106:12043–12048. doi: 10.1073/pnas.0902449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunger SP, Lu X, Devidas M, et al. Improved Survival for Children and Adolescents With Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report From the Children's Oncology Group. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pear WS, Aster JC, Scott ML, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 76.Law LW, Dunn TB, et al. Observations on the effect of a folic-acid antagonist on transplantable lymphoid leukemias in mice. J Natl Cancer Inst. 1949;10:179–192. [PubMed] [Google Scholar]
- 77.Trainer DL, Wheelock EF. Characterization of L5178Y cell phenotypes isolated during progression of the tumor-dormant state in DBA2 mice. Cancer Res. 1984;44:2897–2906. [PubMed] [Google Scholar]
- 78.Nishimura T, Muto K, Tanaka N. Drug sensitivity of an adriamycin-resistant mutant subline of mouse lymphoblastoma L5178Y cells. J Antibiot (Tokyo) 1978;31:493–495. doi: 10.7164/antibiotics.31.493. [DOI] [PubMed] [Google Scholar]
- 79.Nishimura T, Suzuki H, Muto K, Tanaka N. Mechanism of adriamycin resistance in a subline of mouse lymphoblastoma L5178Y cells. J Antibiot (Tokyo) 1979;32:518–522. doi: 10.7164/antibiotics.32.518. [DOI] [PubMed] [Google Scholar]
- 80.Cloyd MW, Hartley JW, Rowe WP. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980;151:542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yun JP, Behan JW, Heisterkamp N, et al. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev Res (Phila) 2010;3:1259–1264. doi: 10.1158/1940-6207.CAPR-10-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiser KC, Liu B, Hansen GM, et al. Retroviral insertions in the VISION database identify molecular pathways in mouse lymphoid leukemia and lymphoma. Mamm Genome. 2007;18:709–722. doi: 10.1007/s00335-007-9060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dettman EJ, Simko SJ, Ayanga B, et al. Prdm14 initiates lymphoblastic leukemia after expanding a population of cells resembling common lymphoid progenitors. Oncogene. 2011;30:2859–2873. doi: 10.1038/onc.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groffen J, Voncken JW, Kaartinen V, Morris C, Heisterkamp N. Ph-positive leukemia: a transgenic mouse model. Leuk Lymphoma. 1993;11(Suppl 1):19–24. doi: 10.3109/10428199309047857. [DOI] [PubMed] [Google Scholar]
- 85.Reichert A, Heisterkamp N, Daley GQ, Groffen J. Treatment of Bcr/Abl-positive acute lymphoblastic leukemia in P190 transgenic mice with the farnesyl transferase inhibitor SCH66336. Blood. 2001;97:1399–1403. doi: 10.1182/blood.v97.5.1399. [DOI] [PubMed] [Google Scholar]
- 86.Kaur P, Feldhahn N, Zhang B, et al. Nilotinib treatment in mouse models of P190 Bcr/Abl lymphoblastic leukemia. Mol Cancer. 2007;6:67. doi: 10.1186/1476-4598-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen W, Li Q, Hudson WA, Kumar A, Kirchhof N, Kersey JH. A murine Mll-AF4 knock-in model results in lymphoid and myeloid deregulation and hematologic malignancy. Blood. 2006;108:669–677. doi: 10.1182/blood-2005-08-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Metzler M, Forster A, Pannell R, et al. A conditional model of MLL-AF4 B-cell tumourigenesis using invertor technology. Oncogene. 2006;25:3093–3103. doi: 10.1038/sj.onc.1209636. [DOI] [PubMed] [Google Scholar]
- 89.Tamai H, Miyake K, Takatori M, et al. Activated K-Ras protein accelerates human MLL/AF4-induced leukemo-lymphomogenicity in a transgenic mouse model. Leukemia. 2011;25:888–891. doi: 10.1038/leu.2011.15. [DOI] [PubMed] [Google Scholar]
- 90.Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 93.Grabher C, von Boehmer H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6:347–359. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- 94.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 95.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 96.Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity. 2000;13:73–84. doi: 10.1016/s1074-7613(00)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fowlkes BJ, Robey EA. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol. 2002;169:1817–1821. doi: 10.4049/jimmunol.169.4.1817. [DOI] [PubMed] [Google Scholar]
- 98.Priceputu E, Bouallaga I, Zhang Y, et al. Structurally distinct ligand-binding or ligand-independent Notch1 mutants are leukemogenic but affect thymocyte development, apoptosis, and metastasis differently. J Immunol. 2006;177:2153–2166. doi: 10.4049/jimmunol.177.4.2153. [DOI] [PubMed] [Google Scholar]
- 99.Berquam-Vrieze KE, Swing DA, Tessarollo L, Dupuy AJ. Characterization of transgenic mice expressing cancer-associated variants of human NOTCH1. Genesis. 2012;50:112–118. doi: 10.1002/dvg.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boulos N, Mulder HL, Calabrese CR, et al. Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117:3585–3595. doi: 10.1182/blood-2010-08-301267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duy C, Hurtz C, Shojaee S, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473:384–388. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang PY, Young F, Chen CY, et al. The biologic properties of leukemias arising from BCR/ABL-mediated transformation vary as a function of developmental origin and activity of the p19ARF gene. Blood. 2008;112:4184–4192. doi: 10.1182/blood-2008-02-142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 104.Wei J, Wunderlich M, Fox C, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.le Viseur C, Hotfilder M, Bomken S, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiu PP, Jiang H, Dick JE. Leukemia-initiating cells in human T-lymphoblastic leukemia exhibit glucocorticoid resistance. Blood. 2010;116:5268–5279. doi: 10.1182/blood-2010-06-292300. [DOI] [PubMed] [Google Scholar]
- 107.Otova B, Sladka M, Panczak A, Marinov I. Biological characteristics of spontaneous transplantable T-cell lymphomas in inbred Sprague-Dawley/cub rats. Transplant Proc. 1997;29:1754–1755. doi: 10.1016/s0041-1345(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 108.Otova B, Vaclavikova R, Danielova V, et al. Effects of paclitaxel, docetaxel and their combinations on subcutaneous lymphomas in inbred Sprague-Dawley/Cub rats. Eur J Pharm Sci. 2006;29:442–450. doi: 10.1016/j.ejps.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Congdon CC, Lorenz E. Leukemia in guinea-pigs. Am J Pathol. 1954;30:337–359. [PMC free article] [PubMed] [Google Scholar]
- 110.Sabaawy HE, Azuma M, Embree LJ, Tsai HJ, Starost MF, Hickstein DD. TELAML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frazer JK, Meeker ND, Rudner L, et al. Heritable T-cell malignancy models established in a zebrafish phenotypic screen. Leukemia. 2009;23:1825–1835. doi: 10.1038/leu.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Langenau DM, Traver D, Ferrando AA, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 113.Feng H, Langenau DM, Madge JA, et al. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br J Haematol. 2007;138:169–175. doi: 10.1111/j.1365-2141.2007.06625.x. [DOI] [PubMed] [Google Scholar]
- 114.Feng H, Stachura DL, White RM, et al. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. 2010;18:353–366. doi: 10.1016/j.ccr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nowell PC, Hungerford DA. Chromosome studies in human leukemia. IV. Myeloproliferative syndrome and other atypical myeloid disorders. J Natl Cancer Inst. 1962;29:911–931. [PubMed] [Google Scholar]
- 116.Goldman JM. Initial treatment for patients with CML. Hematology Am Soc Hematol Educ Program. 2009:453–460. doi: 10.1182/asheducation-2009.1.453. [DOI] [PubMed] [Google Scholar]
- 117.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 118.Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H, Li H, Xi HS, Li S. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huettner CS, Koschmieder S, Iwasaki H, et al. Inducible expression of BCR/ABL using human CD34 regulatory elements results in a megakaryocytic myeloproliferative syndrome. Blood. 2003;102:3363–3370. doi: 10.1182/blood-2003-03-0768. [DOI] [PubMed] [Google Scholar]
- 121.Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 122.Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118:5565–5572. doi: 10.1182/blood-2010-12-327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lozzio BB, Lozzi CB, Machado E. Human myelogenous (Ph+) leukemia cell line: transplantation into athymic mice. J Natl Cancer Inst. 1976;56:627–629. doi: 10.1093/jnci/56.3.627. [DOI] [PubMed] [Google Scholar]
- 124.Skorski T, Nieborowska-Skorska M, Nicolaides NC, et al. Suppression of Philadelphia1 leukemia cell growth in mice by BCR-ABL antisense oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1994;91:4504–4508. doi: 10.1073/pnas.91.10.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dazzi F, Hasserjian R, Gordon MY, et al. Normal and chronic phase CML hematopoietic cells repopulate NOD/SCID bone marrow with different kinetics and cell lineage representation. Hematol J. 2000;1:307–315. doi: 10.1038/sj.thj.6200051. [DOI] [PubMed] [Google Scholar]
- 126.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 127.Phillips JA, Mehta K, Fernandez C, Raveche ES. The NZB mouse as a model for chronic lymphocytic leukemia. Cancer Res. 1992;52:437–443. [PubMed] [Google Scholar]
- 128.Raveche ES, Salerno E, Scaglione BJ, et al. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007;109:5079–5086. doi: 10.1182/blood-2007-02-071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Narducci MG, Pescarmona E, Lazzeri C, et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res. 2000;60:2095–2100. [PubMed] [Google Scholar]
- 130.Bichi R, Shinton SA, Martin ES, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aguilar-Santelises M, Rottenberg ME, Lewin N, Mellstedt H, Jondal M. Bcl-2, Bax and p53 expression in B-CLL in relation to in vitro survival and clinical progression. Int J Cancer. 1996;69:114–119. doi: 10.1002/(SICI)1097-0215(19960422)69:2<114::AID-IJC8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 132.Munzert G, Kirchner D, Stobbe H, et al. Tumor necrosis factor receptorassociated factor 1 gene overexpression in B-cell chronic lymphocytic leukemia: analysis of NF-kappa B/Rel-regulated inhibitors of apoptosis. Blood. 2002;100:3749–3756. doi: 10.1182/blood.V100.10.3749. [DOI] [PubMed] [Google Scholar]
- 133.Zapata JM, Krajewska M, Morse HC, 3rd, Choi Y, Reed JC. TNF receptorassociated factor (TRAF) domain and Bcl-2 cooperate to induce small B cell lymphoma/chronic lymphocytic leukemia in transgenic mice. Proc Natl Acad Sci U S A. 2004;101:16600–16605. doi: 10.1073/pnas.0407541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mohammad RM, Mohamed AN, Hamdan MY, et al. Establishment of a human B-CLL xenograft model: utility as a preclinical therapeutic model. Leukemia. 1996;10:130–137. [PubMed] [Google Scholar]
- 135.Mohammad RM, Limvarapuss C, Hamdy N, et al. Treatment of a de novo fludarabine resistant-CLL xenograft model with bryostatin 1 followed by fludarabine. Int J Oncol. 1999;14:945–950. doi: 10.3892/ijo.14.5.945. [DOI] [PubMed] [Google Scholar]
- 136.Loisel S, Ster KL, Quintin-Roue I, et al. Establishment of a novel human B-CLLlike xenograft model in nude mouse. Leuk Res. 2005;29:1347–1352. doi: 10.1016/j.leukres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 137.Durig J, Ebeling P, Grabellus F, et al. A novel nonobese diabetic/severe combined immunodeficient xenograft model for chronic lymphocytic leukemia reflects important clinical characteristics of the disease. Cancer Res. 2007;67:8653–8661. doi: 10.1158/0008-5472.CAN-07-1198. [DOI] [PubMed] [Google Scholar]
- 138.Aydin S, Grabellus F, Eisele L, et al. Investigating the role of CD38 and functionally related molecular risk factors in the CLL NOD/SCID xenograft model. Eur J Haematol. 2011;87:10–19. doi: 10.1111/j.1600-0609.2011.01626.x. [DOI] [PubMed] [Google Scholar]