Abstract
The concordance of the prevalence of human papillomavirus (HPV) DNA in 188 sex workers in five different locations was investigated. HPV was found in 43.6% of the women, and its prevalence at genital sites was similar. Prevalence was highest among women aged 20 years or younger but declined thereafter in specimens from all anogenital sites.
The risk of acquiring anogenital human papillomavirus (HPV) infection is associated mainly with early sexual experience, number of lifetime sexual partners, and sexual contact with highly promiscuous partners (3, 10, 16).
Persistent infection with HPV has been identified as the most important cause of cervical cancer (2, 15, 25). HPV, particularly the high-risk type 16 HPV, has also been implicated in the development of other genital cancers, in particular, cancers of the vagina, vulva, penis, and anus, as well as in the development of neoplastic lesions of the oral cavity (21, 22).
However, the prevalence of HPV infection in these locations from the same subject has not yet been reported. We analyzed samples collected from these five sites in a high-risk population.
One hundred eighty-eight women who practiced prostitution and attended a dermatology and sexually transmitted disease clinic in Oviedo (Spain) for regular checkups were enrolled in this study. Personal interviews were carried out, and medical histories were recorded.
After informed consent was obtained, all women had a gynecologic examination where cellular exfoliates from the cervix, vagina, and vulva and anal mucus were collected.
HPV detection and genotyping were performed with an in-house PCR as previously described (4, 6, 19). One cervical sample and 21 anal samples were not suitable for the study because of repeated β-globin PCR failure. One hundred sixty-six women had samples suitable for HPV testing at all five sites. HPV DNA was detected in at least one location in 72 (43.3%) women, 65 (39.1%) of which were positive in one or more anogenital locations.
HPV DNA prevalence was 27.8% in cervical cells, 26.1% in vaginal specimens, 22.9% in vulval specimens, 15% in anal specimens, and 7.9% in oral mucosae (Table 1). HPV 16 was the most frequent type found at anogenital sites, followed by HPV 18; HPV 6 was the most frequent type in oral cells (33.3%). Almost all women with detectable HPV in cervical cells were also HPV positive in their vaginas (94.2%) (Table 2). The kappa statistic for HPV agreement between prevalences in the cervix and the other locations was 0.932 for vaginal specimens (P < 0.0001), 0.508 for vulval specimens (P < 0.0001), 0.41 for anal specimens (P < 0.001), and 0.72 for oral mucus specimens (P = 0.191).
TABLE 1.
Distribution of HPV type among oral and anogenital specimens of sex workersa
| Location (no. of samples tested) | No. of subjects positive for indicated HPV (% of no. of subjects positive for any HPV)
|
No. (%) of subjects with multiple infections | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any HPV | HPV 16 | HPV 18 | HPV 31 | HPV 33 | HPV 39 | HPV 11 | HPV 6 | HPV X | ||
| Cervix (187) | 52 (27.8) | 19 (36.5) | 7 (13.4) | 7 (3.4) | 2 (3.8) | 6 (11.5) | 4 (7.7) | 3 (5.7) | 17 (32.7) | 10 (19.0) |
| Vagina (188) | 49 (26.1) | 19 (38.5) | 7 (14.2) | 6 (12.2) | 2 (4.0) | 6 (12.2) | 4 (8.1) | 2 (4.0) | 15 (31.0) | 9 (18.0) |
| Vulva (188) | 43 (22.9) | 11 (25.5) | 9 (20.9) | 5 (11.6) | 2 (4.6) | 4 (9.3) | 4 (9.3) | 4 (9.3) | 15 (35.0) | 4 (9.0) |
| Anus (167) | 25 (15.0) | 8 (32.0) | 4 (16.0) | 1 (4.0) | 2 (8.0) | 2 (8.0) | 2 (8.0) | 8 (8.0) | 1 (4.0) | 2 (8.0) |
| Oral cavity (188) | 15 (7.9) | 4 (26.0) | 1 (6.6) | 5 (33.0) | 5 (33.0) | 1 (7.0) | ||||
Subjects with multiple infections are included in each specific-type column as well as in the multiple-infection column. HPV X subjects are subjects whose samples were HPV positive but whose HPV type could not be detected by our probe set.
TABLE 2.
Concordance of type-specific detection of HPV DNA in specimens from the cervix and all other sites tested among sex workers in Spain
| HPV type(s) | % of sex workers whose specimens were HPV DNA positive at the indicated sites
|
|||
|---|---|---|---|---|
| Cervix and vaginaa | Cervix, vagina, and vulvaa | Cervix, vagina, vulva, and anusb | Cervix and oral cavitya | |
| Any | 94.2 | 57.6 | 40.4 | 12.7 |
| HPV 6 | 66.6 | 66.6 | 66.6 | |
| HPV 11 | 50.0 | 25.0 | 50.0 | |
| HPV 16 | 100 | 52.6 | 43.7 | 5.0 |
| HPV 18 | 100 | 100 | 66.7 | |
| HPV 31 | 85.7 | 57.1 | ||
| HPV 33 | 50.0 | 50.0 | 50.0 | |
| HPV 39 | 100 | 50.0 | 16.6 | |
| Multiple | 90 | 70.0 | 20.0 | |
The concordance of HPV DNA detection among specimens from the cervix, vulva, vagina, and oral mucosa was analyzed for 187 women.
The concordance of HPV DNA detection between specimens from the cervix and anus was analyzed for 167 women.
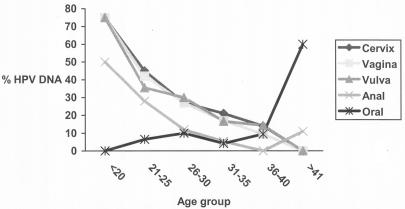
HPV prevalence was higher in 20-year-old or younger women than in women over 20 years old and decreased with age for anogenital sites tested. HPV detection in buccal cells was not age related (Fig. 1).
FIG. 1.
HPV DNA detection by age group in cervical, vaginal, vulval, anal, and oral mucosal cells.
Cervical abnormalities (categorized as cervical intraepithelial neoplasm grade I [CIN I] abnormalities) were identified in 10 women (5.3% of study subjects). In these cases, HPV DNA was detected in 70% of cervical and 60% of vagina or vulva cell samples (Table 3).
TABLE 3.
HPV DNA detection and cervical cytology diagnosis among sex workers
| Cervical cytology | Total no. of subjects | No. (%) of subjects positive for HPV at indicated site
|
||
|---|---|---|---|---|
| Cervix | Vagina | Vulva | ||
| Normal | 97 | 25 (25.7) | 24 (24.7) | 23 (23.7) |
| Inflammatory | 80 | 20 (25.0) | 19 (23.7) | 14 (17.5) |
| CIN I | 10 | 7 (70.0) | 6 (60.0) | 6 (60.0) |
The overall HPV DNA prevalence at the gynecological sites of 39.4% was quite similar to the prevalence reported for other populations of similar age and sexual behavior. A previous study in Denmark (17) reported a similar HPV prevalence (47%) among prostitutes, representing an almost threefold-increased prevalence compared to that in the general population of that country. In our study, HPV prevalence among sex workers was 10-fold higher than that observed in an age-matched sample of the general population (24). These differences are consistent with the low HPV infection rate in the general population of Spain (9).
Age-related profiles of HPV prevalence were remarkably similar for all genital sites and were not modified when the number of years the subject worked as a prostitute was taken into account, as has also been shown for other populations where only cervical samples were analyzed (3, 18). It has been suggested that the decreasing number of sexual partners with age may explain the age-related pattern of HPV prevalence. However, even among highly promiscuous women, there is a marked decline of HPV prevalence with an increase in age, which may be due to a constant systemic production of HPV antibodies contributing to the local response against new HPV infections. Our data are in agreement with the reduced risk of subsequent HPV infections observed in a follow-up study (13, 14).
Over 80% of HPV infections detected were of high-risk types in at least one of the genital sites explored, while a predominance of low-risk HPV types was observed in oral cavities, suggesting different affinities of HPV types for genital sites and oral mucosae. It is unlikely that this difference between HPV types in oral and genital sites can be explained by the untyped samples in our study. In a previous work it was shown that the majority of HPVs were high-risk types (8).
Levels of detection and genotype distributions of HPV DNA were almost identical for cervical and vaginal samples (94%), which further confirms the results of previous studies suggesting that vaginal collection can be used as an alternative to cervical sampling (11, 23).
The rate of HPV DNA detection was slightly lower in vulva cells, but it was still identified in 6 out of 10 women with CIN I cervical lesions. HPV is particularly involved in those tumors of the vulva that develop in women younger than 65 years (1, 5). Cancer of the vulva has been reported to appear at an early age in human immunodeficiency virus-infected patients (12). We did not perform morphological diagnosis of the exfoliated cells of the vulva, but the high prevalence of high-risk HPV types in this population suggests the adequacy of HPV detection in highly promiscuous women.
We identified anal HPV infection in 15% of sex workers, 72% of which had high-risk HPV types and 76% of which also had a type-specific HPV infection of the cervix. We have not been able to identify whether HPV infection in anal mucosae was related to different sexual practices. Previous studies have shown that anal infection may also occur in women without direct sexual transmission, especially if they have a concomitant infection at another close location (20, 26). Preneoplastic anal lesions have been reported to occur among women coinfected with HPV and human immunodeficiency virus (7).
We identified among highly promiscuous women a high prevalence of high-risk HPV types in all anogenital areas explored. The age-associated pattern of the infection in highly exposed women suggests an acquired immune response to HPV.
Further evaluations of larger groups are needed in order to assess the usefulness of anogenital cancer screening in these high-risk populations.
Acknowledgments
Our sponsor was the Fondo de Investigacion Sanitaria (grant number FIS 98/0646). Silvia de Sanjose was a visiting scientist at the National Cancer Institute, Bethesda, Md., during the writing of the manuscript.
We thank the late Virgilio Palacio and Mar Cuesta, who carried out all the personal interviews. We also thank Vincenzo Cirigliano for discussion and help in this work.
REFERENCES
- 1.Al-Ghamdi, A., D. Freedman, D. Miller, C. Poh, M. Rosin, L. Zhang, and B. Gilks. 2002. Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Eur. J. Gynecol. Oncol. 84:94-101. [DOI] [PubMed] [Google Scholar]
- 2.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. M. H. Peto, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. [DOI] [PubMed] [Google Scholar]
- 3.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadas, M. P., F. Martinez, S. de Sanjose, I. Valls, B. Lloveras, F. X. Bosch, and K. Shah. 1998. Detection of human papillomavirus DNA by PCR in high-risk women. Validation of a protocol. Enferm. Infecc. Microbiol. Clin. 16:400-403. [PubMed] [Google Scholar]
- 5.Canavan, T. P., and D. Cohen. 2002. Vulvar cancer. Am. Fam. Physician 66:1269-1274. [PubMed] [Google Scholar]
- 6.Castle, P. E., M. Schiffman, P. E. Gravitt, H. Kendall, S. Fishman, H. Dong, A. Hildeshein, R. Herrero, M. C. Bratti, M. E. Sherman, A. Lorincz, J. E. Schussler, and R. D. Burk. 2002. Comparisons of HPV DNA detection by MY09/11 PCR methods. J. Med. Virol. 68:417-423. [DOI] [PubMed] [Google Scholar]
- 7.de Sanjose, S., and J. M. Palefsky. 2000. Cervical and anal HPV infections in HIV positive women and men. Virus Res. 89:201-211. [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose, S., F. X. Bosch, P. Davis, M. P. Cañadas, B. Lloveras, J. Kornegay, Lorincz, V. Palacio, and G. Capella. 2000. Detection of HPV in cervical scrapes among women at high risk of infection: comparison of three HPV assays, p. 219. In Proceedings of the 18th International Papillomavirus Conference. ICO, Barcelona, Spain.
- 9.de Sanjose, S., R. Almirall, B. Lloveras, R. Font, M. Diaz, N. Muñoz, I. Català, J. L. M. Meijer, P. J. F. Snijders, R. Herrero, and F. X. Bosch. 2003. Cervical human papillomavirus infection in the female population in Barcelona, Spain. Sex. Transm. Dis. 30:788-793. [DOI] [PubMed] [Google Scholar]
- 10.Dillner, J., C. J. Meijer, G. von Krogh, and S. Horenblas. 2000. Epidemiology of human papillomavirus infection. Scand. J. Urol. Nephrol. Suppl. 205:194-200. [PubMed] [Google Scholar]
- 11.Finan, R. R., N. Irani-Hakime, H. Tamim, and W. Y. Almawi. 2000. Validity of vaginal testing in detecting human papillomavirus (HPV) genotypes. J. Clin. Virol. 19:163-168. [DOI] [PubMed] [Google Scholar]
- 12.Giaquinto, C., A. Del Mistro, A. De Rossi, R. Bertorelle, V. Giacomet, E. Ruga, and D. Minucci. 2000. Vulvar carcinoma in a 12-year-old girl with vertically acquired human immunodeficiency virus infection. Pediatrics 106:E57. [DOI] [PubMed] [Google Scholar]
- 13.Ho, G. Y. F., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 14.Ho, G. Y. F., Y. Studentsov, C. B. Hall, R. Bierman, L. Berdsley, M. Lempa, and R. D. Burk. 2002. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J. Infect. Dis. 186:737-742. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer. 1995. IARC Monogr. Eval. Carcinog. Risks Hum. 64:1-378. [Google Scholar]
- 16.Kjaer, S. K., E. M. de Villiers, C. Dahl, G. Engholm, J. E. Bock, B. F. Vestergaard, E. Lynge, and O. M. Jensen. 1991. Case-control study of risk factors for cervical neoplasia in Denmark. I. Role of the “male factor” in women with one lifetime sexual partner. Int. J. Cancer 22:39-44. [DOI] [PubMed] [Google Scholar]
- 17.Kjaer, S. K., E. I. Svare, A. M. Worm, J. M. Walboomers, C. J. Meijer, and A. J. van den Brule. 2000. Human papillomavirus infection in Danish female sex workers. Decreasing prevalence with age despite continuously high sexual activity. Sex. Transm. Dis. 8:438-445. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer, S. K., A. J. van den Brule, G. Paull, E. I. Svare, M. E. Sheramn, B. L. Thomsen, M. Suntum, J. E. Bock, P. A. Poll, and C. J. L. M. Meijer. 2002. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 325:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manos, M. M., Y. Ting, D. K. Wright, A. S. Lewis, T. R. Broker, and S. M. Wolinsky. 1989. Use of polymerase chain reaction amplification for the detection of genital human papillomavirus. Cancer Cells 7:209-214. [Google Scholar]
- 20.Moscicki, A. B., S. J. Durako, J. Houser, Y. Ma, D. A. Murphy, T. M. Darragh, S. Farhat, and C. Wilson. 2003. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS 17:311-320. [DOI] [PubMed] [Google Scholar]
- 21.Ringstrom, E., E. Peters, M. Hasegawa, M. Posner, M. Liu, and K. T. Kelsey. 2002. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin. Cancer Res. 8:3187-3192. [PubMed] [Google Scholar]
- 22.Schwartz, S. M., J. R. Daling, D. R. Doody, G. C. Wipf, J. J. Carter, M. M. Madeleine, E. J. Mao, E. D. Fitzgibbons, S. Huang, A. M. Beckmann, J. K. McDougall, and D. A. Galloway. 1998. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J. Natl. Cancer Inst. 90:1626-1636. [DOI] [PubMed] [Google Scholar]
- 23.Shah, K. V., R. W. Daniel, M. K. Tennant, N. Shah, K. T. McKee, Jr., C. A Gaydos, J. C. Gaydos, and A. Rompalo. 2001. Diagnosis of human papillomavirus infection by dry vaginal swabs in military women. Sex. Transm. Infect. 77:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touzé, A., S. de Sanjose, P. Coursaget, M. R. Almirall, V. Palacio, C. J. L. Meijer, J. Kornegay, and F. J. Bosch. 2001. Prevalence of anti-human papillomavirus type 16, 18, 31, and 58 virus-like particles in women in the general population and in prostitutes. J. Clin. Microbiol. 39:344-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 26.Willians, A. B., T. M. Darragh, K. Vranizan, C. Ochia, A. R. Moss, and J. M. Palefsky. 1994. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in immunodeficiency virus-infected women. Obstet. Gynecol. 83:205-211. [PubMed] [Google Scholar]