Abstract
We report two cases of newborns who developed marked local edema after application of a eutectic mixture of local anesthetic (EMLA) topical anesthetic cream for neonatal male circumcision (NMC). Although local edema and erythema are known potential side effects of EMLA cream, a common anesthetic used for NMC, the loss of landmarks precluding safe NMC has not previously been reported, and is described here. Although we cannot recommend an alternate local anesthetic for neonates with this reaction to EMLA, based on a review of the published data we think that serious systemic adverse events related to EMLA are extremely rare.
Keywords: Male circumcision, neonatal, EMLA, Eutectic Mixture of Local Anesthetic, adverse events, edema
Background
Local anesthesia is routinely used during NMC. Methods of pain control studied for NMC include injected lidocaine for dorsal penile nerve block (DPNB) or ring block, topical lidocaine, topical EMLA (2.5% lidocaine and 2.5% prilocaine) cream, acetaminophen and sucrose/dextrose pacifiers. Many practitioners advocate a combination of interventions, such as injectable anesthesia together with sucrose pacifiers1. While mild to moderate blanching, erythema and edema2–4 are known potential side effects of EMLA cream, we do not know of cases in which the topically applied anesthesia resulted in loss of landmarks necessary for safe NMC.
Although clinical trials have concluded that DPNB is more effective for pain control than topical anesthetic creams during NMC1, the latter have some practical advantages. Topical anesthetic creams avoid pain of the injection, avoid potential complications from inadvertent intravascular injection, including overdose5, and eliminate potential nosocomial infections, such as methicillin-resistant Staphylococcus aureus, that have been reported with multiuse vials of injectable anesthetics6. They do not require new needles and syringes and reduce the need for sharps disposal, important in resource-limited settings7. Additionally, they can be applied safely by non-physician providers, a vital issue for settings where physicians’ time is limited8, 9.
Male circumcision has been demonstrated to reduce heterosexual acquisition of HIV in men by about 60% 10–12 and to reduce significantly the acquisition of human papilloma virus and herpes simplex virus13. The World Health Organization (WHO) recommends “that male circumcision should be recognized as an efficacious intervention for HIV risk reduction”14. WHO further states that, “Since neonatal circumcision is a less complicated and risky procedure than circumcision performed in young boys, adolescents or adults … countries should consider how to promote neonatal circumcision in a safe, culturally acceptable and sustainable manner”14. NMC is not, however, currently a routine practice in southern Africa. In keeping with the WHO guidelines, we are conducting a pilot study of the safety, feasibility and uptake of NMC in Botswana and, for the reasons noted above, we selected EMLA cream as the anesthetic of choice.
Cases Reports
Case 1
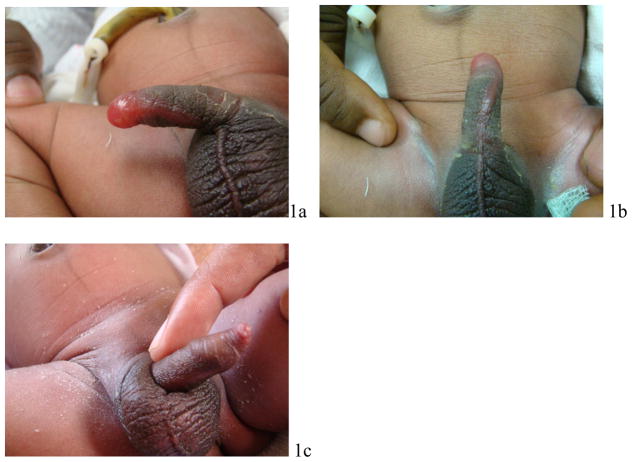
Infant 1 was born at estimated 39 weeks gestation, weighing 2.73 kg. On day 2 of life, his family requested circumcision. Initial examination of the infant by the nurse midwife revealed normal external male genitalia. As per protocol, approximately 1 gram of EMLA cream was applied to the penis and covered with an occlusive dressing. Approximately 2 hours later he was brought to the study physician for circumcision. On examination at that time the foreskin was edematous and no corona was discernable (Figures 1a and 1b). EMLA cream was removed and the newborn was not circumcised. He had no other local or systemic signs of reaction to the medication. He was discharged home in good condition. He was examined 96 hours later and found to have normal uncircumcised external male genitalia (Figure 1c).
Figure 1.
Infant 1: a) about 2 hours after application of EMLA, with localized bullous edema of distal foreskin with obliteration of normal anatomical landmarks; b) ventral aspect of penis showing spread of edema along median raphe. Note obliteration of the perimeter of the coronal sulcus that would usually be apparent beneath the foreskin: c) Follow-up 96 hours later reveals normal uncircumcised penis and discernable coronal sulcus.
Case 2
Infant 2 was born at estimated 41 weeks gestation, weighing 3.32 kg. On day 2 of life, his family requested circumcision. Initial examination of the infant by the nurse midwife revealed normal external male genitalia. Approximately 1 gram of EMLA cream was applied to the penis and covered with an occlusive dressing. Approximately 2 hours later he was brought to the study physician for circumcision. On examination at that time the foreskin was markedly edematous and no corona was discernable (Figure 2a). EMLA cream was removed and the newborn was not circumcised. He had no other local or systemic signs of reaction to the medication. He was examined 24 hours later and found to have normal uncircumcised external male genitalia (Figure 2b).
Figure 2.

Infant 2: a) about 2 hours after application of EMLA, with localized bullous edema of distal foreskin with obliteration of normal anatomical landmarks: b) Follow-up 24 hours later reveals normal uncircumcised penis.
The same tube of EMLA was used for the two infants and also for several other infants who did not experience any discernable reaction. In more than 450 NMCs performed at our site these are the only two such reactions we have seen. The typical reaction we have seen in our practice is mild edema of the distal foreskin (Figure 3).
Figure 3.

Typical reaction to EMLA with mild edema of the distal foreskin.
Discussion
Transient local reactions to EMLA cream are not uncommon2–4, 9, 15. One series reported erythema and mild blistering of the foreskin; this is the only published report we found (using PubMed keyword search “EMLA” and “circumcision”) in which EMLA cream precluded NMC16. We report here for the first time loss of anatomical landmarks secondary to marked local edema after the application of EMLA cream that precluded NMC. No other local or systemic adverse events were noted in these cases.
Previous clinical trials with EMLA cream for NMC have used doses ranging from 0.5 grams – 5.0 grams left under an occlusive dressing from 30 – 120 minutes or more9, 17–19. Although longer application time (2–3 hours) results in maximal anesthetic effect20, this must be weighed against the concern for local side effects and for systemic absorption of the anesthetic agents which increases with size of the application area, amount of cream applied and duration of application. The development of a local reaction to EMLA cream does not in itself indicate an increased risk for systemic toxicity.
The most common concern for systemic toxicity is methemoglobinemia, which has been reported after the use of some local anesthetic agents, such as injected prilocaine21. Methemoglobinemia is the oxidation of the iron moiety within the hemoglobin molecule that results in effective tissue hypoxia. This rare complication is of special concern in neonates, as they have a relative deficiency of the enzyme required to reduce methemoglobin (mtHB). Immature skin barrier and relatively high ratio of surface area to body weight are additional risk factors placing neonates at greater risk for systemic toxicity from mtHB-inducing agents. The manufacturer of EMLA cream, AstraZeneca, warns: “EMLA Cream should not be used in neonates with a gestational age less than 37 weeks nor in infants under the age of twelve months who are receiving treatment with [other] methemoglobin-inducing agents”. Of note: neither infant in this series had received any other potentially mtHB-inducing agents. The manufacturer also recommends that for children 0–3 months or < 5 kg, the dose not exceed 1 gram applied to more than 10 cm2 and application no more than 1 hour20.
Nonetheless, in published studies of EMLA cream for NMC in which serum levels of mtHB were measured, no infant was found to have toxic levels. One study found no difference in percentage of mtHB concentration in blood between infants who received EMLA cream (1 gram for 60–80 min) and those who received no anesthetic (mean mtHB concentration 1.3±0.6 % in EMLA cream group and 1.3±0.2 % in placebo group, P = 0.80)15. Although another study showed no significant difference in the mean mtHB concentration among infants who received EMLA cream (1.3%) (2 grams for at least 90 minutes) and those who received placebo (0.6%) or injected lidocaine for DPNB (0.7%) or injected lidocaine for ring block (0.4%), two infants in the EMLA cream group had mtHB levels of 2.4% and 4.5%, respectively, and no treatment was required22. A third study showed that although levels of methemoglobin increased from baseline in infants treated with EMLA cream prior to circumcision, they did not exceed normal values23.
Though we do not have the capacity to measure serum mtHB concentration at our facilities, the two newborns discussed here did not develop any pallor or cyanosis, findings known to develop as a result of methemoglobinemia when serum levels of mtHB exceed about 5%. For other clinical signs and symptoms of methemoglobinemia to become apparent, mtHB levels usually exceed 30%24. Searching PubMed “eutectic mixture of local anesthetic” or “EMLA” and “methemoglobinemia”, we find two case reports of infants developing methemoglobinemia after application of EMLA cream for NMC: in one case 3.5 grams were applied for an hour and in another case an unknown amount was applied for 3 hours. In both cases the infants were noted to have changes in skin color that prompted testing for methemoglobinemia. Both infants had methemoglobin levels of 16%. No treatment other than supplemental oxygen was required and no other sequelae were noted25, 26. A published, systematic review of EMLA cream in neonates, not limited to use for NMC, found no clinically significant cases of methemoglobinemia, with a maximum measured value of 16%27.
The question remains as to what local anesthetic would be safe to use in neonates experiencing local tissue edema secondary to EMLA cream. As both lidocaine and prilocaine have been independently reported to cause tissue edema, we do not feel comfortable recommending other preparations of prilocaine or lidocaine (either injectable or topical) in individuals who have had this reaction to EMLA cream. The US Food and Drug Administration recommends avoiding benzocaine in children less than 4 months of age28. Although tetracaine is an ester-type (while lidocaine and prilocaine are both amide-type) local anesthetic and this may suggest reduced risk of cross-reactivity29, tetracaine has not been studied in NMC so we cannot currently recommend its use. Infants with localized reactions to EMLA cream may have to wait until they are old enough to undergo the procedure under general anesthesia if so desired.
It is important to emphasize that NMC is an elective procedure and should only be undertaken when providers can ensure there are no unnecessary risks resulting from the conditions under which it is performed. Providers must be encouraged to evaluate each neonate carefully and be willing to abandon the procedure in circumstances such as the one described here.
Conclusions
Although EMLA cream has a number of advantages over injectable formulations and serious adverse reactions are extremely rare, providers should be aware that one potential side effect is marked edema resulting in the complete loss of anatomical landmarks necessary for safe NMC. It is not clear whether this is related to duration of application. We do not recommend re-challenge with EMLA cream.
Acknowledgments
Funding: Supported by NIH 5K23AI084579 from the National Institutes of Allergy and Infectious Diseases (Dr. Plank). The larger study was supported through the President’s Emergency Plan for AIDS Relief (PEPFAR) grant U2GPS000941-01, Programme No. 08-P0157. The content is solely the responsibility of the authors and does not necessarily represent the official views of PEPFAR or the National Institutes of Health. The study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Abbreviations
- DPNB
Dorsal penile nerve block
- EMLA
Eutectic mixture of local anesthetic
- mtHB
methemoglobin
- NMC
Neonatal male circumcision
- WHO
World Health Organization
Footnotes
Conflict of interest statement
None of the authors has any financial or personal relationships with other people or organisations that could inappropriately influence his or her work.
Ethical Approval
The study was approved by the Botswana Ministry of Health’s Health Research and Development Committee and by Partners Institutional Review Board (Brigham and Women’s Hospital). Written informed consent was obtained from the mothers for the procedure and for the photos.
The larger trial is registered at www.clinicaltrials.gov as NCT00971958.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebeca M. Plank, Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, United States. Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, United States. Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
David W. Kubiak, Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, United States. Dana-Farber Cancer Institute, Boston, United States.
Rasak Bamidele Abdullahi, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Nnamdi Ndubuka, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Maggie M. Nkgau, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Fredrick Dapaah-Siakwan, Scottish Livingstone Hospital, Molepolole, Botswana.
Kathleen M. Powis, Departments of Internal Medicine and Pediatrics, Massachusetts General Hospital, Boston, Unites States. Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, United States. Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Shahin Lockman, Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, United States. Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, Unites States. Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
References
- 1.Brady-Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database Syst Rev. 2004;(4):CD004217. doi: 10.1002/14651858.CD004217.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallen B, Carlsson P, Uppfeldt A. Clinical study of a lignocaine-prilocaine cream to relieve the pain of venepuncture. Br J Anaesth. 1985 Mar;57(3):326–328. doi: 10.1093/bja/57.3.326. [DOI] [PubMed] [Google Scholar]
- 3.Villada G, Zetlaoui J, Revuz J. Local blanching after epicutaneous application of EMLA cream. A double-blind randomized study among 50 healthy volunteers. Dermatologica. 1990;181(1):38–40. [PubMed] [Google Scholar]
- 4.Butler-O’Hara M, LeMoine C, Guillet R. Analgesia for neonatal circumcision: a randomized controlled trial of EMLA cream versus dorsal penile nerve block. Pediatrics. 1998 Apr;101(4):E5. doi: 10.1542/peds.101.4.e5. [DOI] [PubMed] [Google Scholar]
- 5.Rezvani M, Finkelstein Y, Verjee Z, Railton C, Koren G. Generalized seizures following topical lidocaine administration during circumcision: establishing causation. Paediatr Drugs. 2007;9(2):125–127. doi: 10.2165/00148581-200709020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DM, Bancroft E, Mascola L, Guevara R, Yasuda L. Risk factors for neonatal methicillin-resistant Staphylococcus aureus infection in a well-infant nursery. Infect Control Hosp Epidemiol. 2007 Apr;28(4):406–411. doi: 10.1086/513122. [DOI] [PubMed] [Google Scholar]
- 7.Drucker E, Alcabes PG, Marx PA. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet. 2001 Dec 8;358(9297):1989–1992. doi: 10.1016/S0140-6736(01)06967-7. [DOI] [PubMed] [Google Scholar]
- 8.Rosen M. Anesthesia for ritual circumcision in neonates. Paediatr Anaesth. 2010 Dec;20(12):1124–1127. doi: 10.1111/j.1460-9592.2010.03445.x. [DOI] [PubMed] [Google Scholar]
- 9.Russell CT, Chaseling J. Topical anaesthesia in neonatal circumcision: a study of 208 consecutive cases. Aust Fam Physician. 1996 Jan;(Suppl 1):S30–34. [PubMed] [Google Scholar]
- 10.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007 Feb 24;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 12.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 13.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009 Mar 26;360(13):1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. New Data on Male Circumcision and HIV Prevention: Policy and Programme Implications. 2007 http://www.who.int/hiv/mediacentre/MCrecommendations_en.pdf.
- 15.Taddio A, Stevens B, Craig K, et al. Efficacy and safety of lidocaine-prilocaine cream for pain during circumcision. N Engl J Med. 1997 Apr 24;336(17):1197–1201. doi: 10.1056/NEJM199704243361701. [DOI] [PubMed] [Google Scholar]
- 16.Holliday MA, Pinckert TL, Kiernan SC, Kunos I, Angelus P, Keszler M. Dorsal penile nerve block vs topical placebo for circumcision in low-birth-weight neonates. Arch Pediatr Adolesc Med. 1999 May;153(5):476–480. doi: 10.1001/archpedi.153.5.476. [DOI] [PubMed] [Google Scholar]
- 17.Benini F, Johnston CC, Faucher D, Aranda JV. Topical anesthesia during circumcision in newborn infants. JAMA. 1993 Aug 18;270(7):850–853. [PubMed] [Google Scholar]
- 18.Mohan CG, Risucci DA, Casimir M, Gulrajani-LaCorte M. Comparison of analgesics in ameliorating the pain of circumcision. J Perinatol. 1998 Jan-Feb;18(1):13–19. [PubMed] [Google Scholar]
- 19.Lehr VT, Cepeda E, Frattarelli DA, Thomas R, LaMothe J, Aranda JV. Lidocaine 4% cream compared with lidocaine 2.5% and prilocaine 2.5% or dorsal penile block for circumcision. Am J Perinatol. 2005 Jul;22(5):231–237. doi: 10.1055/s-2005-871655. [DOI] [PubMed] [Google Scholar]
- 20.AstraZeneca. [Accessed 1 August 2011];EMLA Cream (lidocaine 2.5% and prilocaine 2.5%) 2005 http://www1.astrazeneca-us.com/pi/EMLA.pdf.
- 21.Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009 Mar;108(3):837–845. doi: 10.1213/ane.0b013e318187c4b1. [DOI] [PubMed] [Google Scholar]
- 22.Lander J, Brady-Fryer B, Metcalfe JB, Nazarali S, Muttitt S. Comparison of ring block, dorsal penile nerve block, and topical anesthesia for neonatal circumcision: a randomized controlled trial. JAMA. 1997 Dec 24–31;278(24):2157–2162. [PubMed] [Google Scholar]
- 23.Law RM, Halpern S, Martins RF, Reich H, Innanen V, Ohlsson A. Measurement of methemoglobin after EMLA analgesia for newborn circumcision. Biol Neonate. 1996;70(4):213–217. doi: 10.1159/000244367. [DOI] [PubMed] [Google Scholar]
- 24.Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. 1996 Jun;14(6):394–405. doi: 10.2165/00002018-199614060-00005. [DOI] [PubMed] [Google Scholar]
- 25.Couper RT. Methaemoglobinaemia secondary to topical lignocaine/ prilocaine in a circumcised neonate. J Paediatr Child Health. 2000 Aug;36(4):406–407. doi: 10.1046/j.1440-1754.2000.00508.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar AR, Dunn N, Naqvi M. Methemoglobinemia associated with a prilocaine-lidocaine cream. Clin Pediatr (Phila) 1997 Apr;36(4):239–240. doi: 10.1177/000992289703600410. [DOI] [PubMed] [Google Scholar]
- 27.Taddio A, Ohlsson A, Einarson TR, Stevens B, Koren G. A systematic review of lidocaine-prilocaine cream (EMLA) in the treatment of acute pain in neonates. Pediatrics. 1998 Feb;101(2):E1. doi: 10.1542/peds.101.2.e1. [DOI] [PubMed] [Google Scholar]
- 28.FDA. FDA Drug Safety Communication: Reports of a rare, but serious and potentially fatal adverse effect with the use of over-the-counter (OTC) benzocaine gels and liquids applied to the gums or mouth. 2011 http://www.fda.gov/drugs/drugsafety/ucm250024.htm.
- 29.Gonzalez-Delgado P, Anton R, Soriano V, Zapater P, Niveiro E. Cross-reactivity among amide-type local anesthetics in a case of allergy to mepivacaine. J Investig Allergol Clin Immunol. 2006;16(5):311–313. [PubMed] [Google Scholar]