Abstract
Purpose
Depressive symptoms and inadequate social support are well-known independent predictors of increased mortality and morbidity in heart failure (HF). However, it is unclear how depressive symptoms and social support interact to influence quality of life. Thus, the purpose of this study was to determine the nature of the relationships (direct, mediator, and moderator) among depressive symptoms, social support, and quality of life in patients with HF.
Methods
We performed a secondary data analysis that included 362 patients with HF who completed measures of depressive symptoms (the Beck Depression Inventory-II), perceived social support (the Multidimensional Scale of Perceived Social Support), and quality of life (the Minnesota Living with Heart Failure Questionnaire) instruments. The direct, mediator, and moderator effects of both depressive symptoms and social support on quality of life were tested using multiple regressions and 2×2 ANCOVA.
Results
Less social support and greater depressive symptoms independently predicted poorer quality of life. The relationship between social support and quality of life was mediated by depressive symptoms. Neither social support nor depressive symptoms moderated quality of life.
Conclusion
Promotion of social support will improve quality of life only when depressive symptoms are also effectively managed.
Keywords: Quality of life, Depressive symptoms, Perceived social support, Mediator, Moderator, Heart failure
Introduction
The prevalence of heart failure (HF) is increasing in the United States. Currently 5.8 million Americans have a diagnosis of HF and this includes one of every 100 individuals over 66 years of age [1]. Despite advances in management, mortality and morbidity rates of patients with HF remain high. In prior studies, patients with HF self-reported that their quality of life was significantly more impaired when compared with patients diagnosed with other serious chronic medical disorders (i.e., chronic bronchitis or arthritis)[2]. Therefore, improving quality of life has been recognized as one of the major treatment goals for patients with HF.
Positive social support is associated with improved quality of life [3] and better outcomes in patients with coronary heart disease [4]. Reinforcing and increasing social support has been suggested to be an effective intervention that may improve quality of life, as well as reduce mortality and morbidity, in patients with HF [5; 6]. For example, the presence of a spouse provided significant support when compared to those without, as unmarried patients with HF were at 2.1 – 3.8 times higher risk of readmission or death compared to married patients [5; 7]. Social support may contribute to positive health outcomes by two possible pathways [8]. First, social support may have a direct and main effect on health-related outcomes, regardless of individual stress level or the presence of a stressful event. Second, social support may have a buffering effect, as it protects individuals from the harmful outcomes of a stressful environment or event. Thus, social support may be a mediating or intervening variable which reduces the effects of stress. Because the role of social support has been measured using a variety of instruments, and results varied across prior studies, there is no consistent evidence to definitively support either pathway.
Depressive symptoms are the most prevalent psychological symptom identified by patients with HF and one in every five patients have diagnosed clinical depression [9]. Depressive symptoms of patients with HF are well-known to be associated with declining physical functional status [9]. Depressive symptoms are also an independent predictor of poorer quality of life in patients with HF [10]. The most serious impact of presence of depression or depressive symptoms is the association with frequent hospital readmission and greater mortality. Patients with HF and depressive symptoms have a 3-times higher risk of hospital admission and 2-times higher risk of death at 1 year follow up, compared to those without depressive symptoms [9]. Screening and treatment of depressive symptoms is an essential component in the management of HF [11] and pharmacological and non-pharmacological treatments have been tested to improve outcomes of patients with HF.
The direct or buffer model of social support may explain why a greater degree of social support is beneficial in improving quality of life in patients with HF. However, there is insufficient evidence about the association of social support to quality of life in the presence of concurrent depressive symptoms. It is also unclear whether depressive symptoms decrease quality of life in patients who perceive greater support. Investigators have not studied the combination of depressive symptoms and perceived social support in studies predicting quality of life in patients with HF. Thus, it is not clear whether depressive symptoms and perceived social support are independent predictors of quality of life, and whether either depressive symptoms or perceived social support act as a mediator in the association with quality of life. It is also unknown whether the relationship between social support and quality of life varies with different levels of depressive symptoms, which would demonstrate a moderator effect. Therefore, the present study examined the nature of the relationships among depressive symptoms, perceived social support, and quality of life in patients with HF. Specific aims of the study were: 1) to test the direct (main) effects of both social support and depressive symptoms on quality of life; 2) to test the moderator effect of both social support and depressive symptoms on quality of life; and 3) to test the mediator effect of both social support and depressive symptoms on quality of life, while controlling for age, gender, New York Heart Association (NYHA) class, and self-reported functional status.
Methods
Study design and sample
This study is a secondary data analysis from larger studies[5; 12; 13] that used a descriptive, comparative, correlational design and included 362 patients with HF. Patients were eligible if they had a confirmed diagnosis of chronic heart failure, no history of acute myocardial infarction within 3 months, and no terminal illness including cancer or end-stage liver or renal disease. All patients were receiving stable doses of HF medications. We recruited eligible patients from outpatient clinics at a major academic medical center and a private hospital in central Kentucky between January 2002 and December 2008. A total of 2358 patients were screened during this time; 1346 patients were eligible, but only 490 patients (36%) participated. Three hundred fifty three patients (26.2%) intentionally declined to participate and 503 additional patients could not participate for various reasons including scheduling meeting with eligible participants, missing appointments, long distance, and family issues. Of the 490 participants who participated, we analyzed data from the 362 patients who completed self-reports of perceived social support, depressive symptoms, functional status and quality of life at the baseline assessment time.
Measures
Perceived social support
Social support was assessed using the Multidimensional Scale of Perceived Social Support (MSPSS) [14; 15]. The MSPSS assesses perceived social support adequacy from family, friends, or significant others. The MSPSS consists of 12-items that are rated on a 7-point Likert scale. Each item is rated from 1 (very strongly disagree) to 7 (very strongly agree). The total score is the sum of the 12 items and ranges from 12 to 84. Higher scores indicate a higher level of perceived social support. Construct validity and reliability have been reported [14; 15]. Cronbach’s alpha coefficients previously ranged from .85 to .90 [14; 15], and reliability in this study was high with a Cronbach’s alpha of .94. The MSPSS has been previously used by patients with HF [16].
Depressive symptoms
Depressive symptoms were assessed by the Beck Depression Inventory-II (BDI-II) [17]. The BDI-II has 21 items and each item is rated on a 4-point Likert scale ranging from 0 to 3. The total score is the sum of scores for the 21 items and total scores range from 0 to 63. Higher total scores indicate greater depressive symptoms. Individuals who score greater than 13 are considered to have at least mild depressive symptoms [17]. The BDI-II has been used in patients with HF [18]. In this study, the reliability of the BDI-II was acceptable, as Cronbach’s alpha coefficient was .89.
Quality of life
Quality of life was assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ)[19; 20]. The MLHFQ is a disease-specific measure of quality of life in patients with HF, which assesses patient perception of the degree to which HF and its treatment influences physical symptoms (i.e., shortness breath, fatigue, peripheral edema, and difficulty sleeping), physical and social functions (i.e., walking, climbing stairs, household work, need to rest, working to earn a living, going places away from home, recreational activities, sexual activities, eating and mental and emotional functions of concentration, memory, loss of self-control, and being a burden to others), and psychological components of living (i.e., anxiety and depression). The MLHFQ consists of 21 items which are rated on a 6-point Likert scale ranging from 0 (no effect) to 5 (very much). The total score is the sum of the 21 items and the possible total score ranges from 0 to 105. Higher scores reflect worse quality of life. Construct validity has been reported in several studies [19; 21]. Adequate reliability of the MLHFQ, indicated by Cronbach’s alpha of .73 to .93, has been reported [19; 22] and Cronbach’s alpha in this study was .94. This instrument has been used exclusively in HF research [23; 24].
Demographic and clinical variables
Demographic (i.e., age, gender, education, marital status, and ethnicity) and clinical characteristics (i.e. left ventricular ejection fraction, comorbidities, prescribed medications, New York Heart Association [NYHA] class) were obtained using a structured questionnaire and brief interview with patients and a medical records review. The NYHA classification data were collected by the trained nurse researchers. All involved nurse researchers received specific training about measurement of NYHA class to ensure accurate data; training continued until each data collector was able to attain a 100% agreement with expert evaluations of 15 sample cases. We also assessed self-reported functional status using the Duke Activity Status Index (DASI) [25], as functional status has previously been identified as a confounding variable in studies of quality of life [26]. The DASI is 12-item self-assessment of the functional capabilities of cardiovascular patients related to common activities of daily living [25]. Patients were asked to rate the difficulty of each activity using three response categories (i.e., ‘not done because of health reason’, ‘done with difficulty’, and ‘done without difficulty’). The total score ranges from 0 to 58.2 based on different weights for each activity in the DASI, and the higher scores indicate better functional status. The DASI has been used to assess functional status in patients with coronary heart disease and HF [27; 28]. Cronbach’s alpha coefficient has been reported to be from .81 to .89 with construct validity of this measure supported [27; 28]. Coefficient alpha as an indicator of internal consistency of reliability was .84 for the unweighted items of the DASI in this study.
Procedures
Approval for each study was obtained from the University of Kentucky Institutional Review Board. Potential participants were referred to the study by primary physicians or nurse practitioners, or were identified by medical records review performed by trained research nurses. Eligible patients were contacted and recruited from outpatient clinics during a face-to-face interview. When eligible patients agreed to participate, they completed a questionnaire packet either at home or in the General Clinical Research Center. Trained research nurses assessed NYHA class and obtained other clinical and demographic data during a brief interview, the use of a structured questionnaire and by review of medical records.
Data analysis
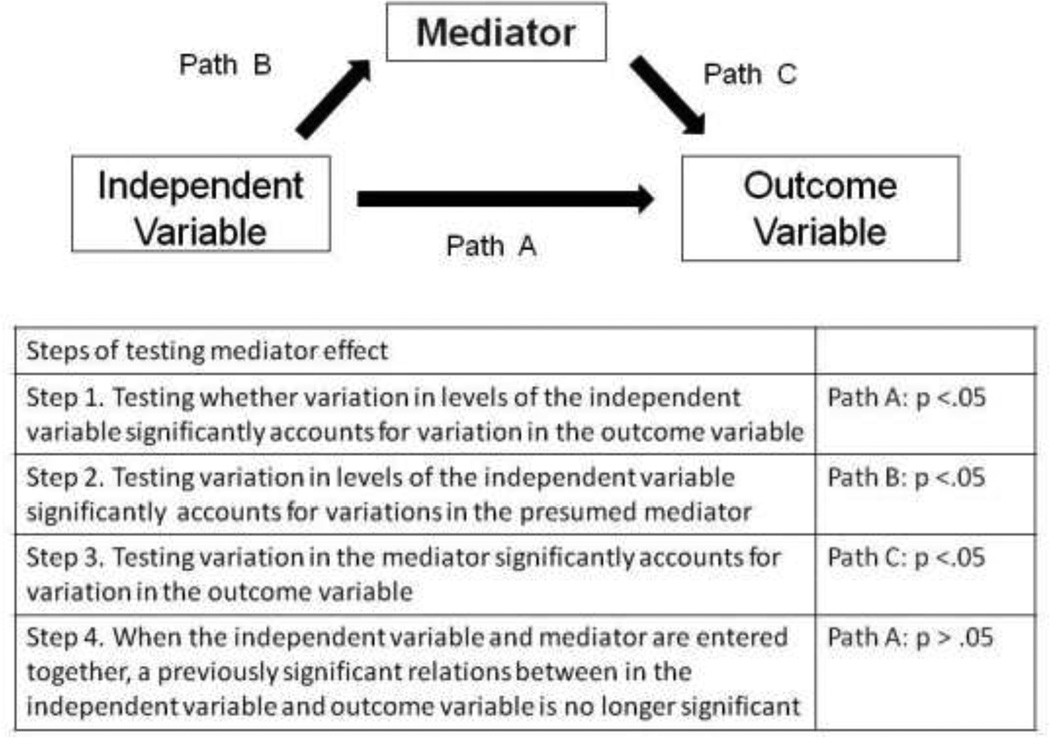
Descriptive statistics (frequency, mean, standard deviation, median, range) were used to describe patient demographic and clinical characteristics. Direct effects of each predictor (i.e., depressive symptoms and perceived social support) on quality of life were tested using separate multiple regressions, controlling for age, gender, NYHA class, and self-reported functional status (DASI). The steps to examine mediator and moderator effects were guided by Baron and Kenny [29]. Mediator effects for both depressive symptoms and perceived social support on quality of life were examined using a series of multiple regressions, while controlling for age, gender, NYHA class, and self-reported functional status. As shown in Figure 1, when there are significant relationships between an independent variable (either depressive symptoms or perceived social support) and an outcome variable (Step 1), between an independent variable and a potential mediator (Step 2), and between the potential mediator and the outcome variable (Step 3), a mediator effect is supported when the significant relationship between the independent variable and the outcome variable (quality of life) becomes less significant or non-significant when both the independent variable and mediator are entered in the regression model (Step 4).
Figure 1. Steps for testing a mediator effect.
Moderator effects were determined using 2 × 2 ANCOVA, after controlling for age, gender, NYHA class, and self-reported functional status. A significant interaction effect of depressive symptoms and perceived social support on quality of life would indicate a moderator effect according to Baron and Kenny.37 Prior to moderator testing, patients were grouped into those with and without depressive symptoms using a standard cut point of 13 on the BDI-II [17]. Patients were also grouped into high and low social support groups prior to analysis using the mean score of the MSPSS as the cut point (M = 71.5). The mean score was used because there is no standard cut point for the MSPSS. An a priori significance level of 0.05 was used for all analyses.
Results
Patient characteristics
Of the 362 patients, participants were primarily Caucasian (77.6%), men (68%) aged 60.6 ± 11.5 years (Table 1). More than half of participants were married (57%), and nearly half were classified as NYHA class III or IV (52.2%), with an average left ventricular ejection fraction of 34.4 ± 14.7%. Based on the scores of the BDI-II, one-third (30.1%) of these patients reported depressive symptoms, and of these depressed patients (BDI-II > 9), 39.7% were prescribed antidepressants. One fourth of all patients (24.6%) were taking antidepressant medications at time of participation, and of the antidepressant users, 54% still reported depressive symptoms. Main (direct) effect of perceived social support and depressive symptoms
Table 1.
Characteristics of patients with HF (N = 362)
| Characteristics | Mean ± SD or N (%) |
|---|---|
| Age, years | 60.6 ± 11.5 |
| Education, years | 13.3 ± 3.4 |
| Gender, male | 247 (68.2) |
| Left ventricular ejection fraction, % | 34.4 ± 14.7 |
| Marital status | |
| Married/co-habitants | 207 (57.2) |
| Single | 34 (9.4) |
| Widowed/ Divorced | 111 (33.5) |
| Ethnicity | |
| Caucasian | 281 (77.6) |
| African-American | 74 (20.4) |
| Others | 7 (2.1) |
| History of Hypertension | 258 (71.3) |
| History of Diabetes Mellitus | 156 (43.1) |
| NYHA class | |
| I | 29 (8.0) |
| II | 144 (39.8) |
| III | 150 (41.4) |
| IV | 39 (10.8) |
| Medication prescribed | |
| ACE Inhibitors | 258 (71.3) |
| Beta blockers | 318 (87.8) |
| Diuretics | 269 (74.3) |
| Digoxin | 99 (27.3) |
| Antidepressant | 89 (24.6) |
NYHA= New York Heart Association
All variables were moderately correlated with each other (r = −.056 to r = .687) without multicollinearity (r > 0.7) among independent variables (Table 2), which demonstrated the assumptions for the use of multiple regression were met. When age, gender, NYHA class, and self-reported functional status were controlled, both decreased social support (sβ = −.132; P < .001) and the presence of depressive symptoms (sβ = −.467; P < .001) were independently associated with poorer quality of life (Table 3).
Table 2.
Inter-correlations of variables included in regression analyses
| Age | Functional status |
Depressive symptoms |
Perceived social support |
Mean ± Standard deviation |
|
|---|---|---|---|---|---|
| Functional status (DASI) | −.056 | ||||
| Depressive symptoms (BDI-II) | −.231** | −.410** | 11.0 ± 8.9 | ||
| Perceived social support (MSPSS) | .195** | .181** | −.369** | 66.3 ± 17.9 | |
| Quality of life (MLHFQ) | −.207** | −.621** | .687** | −.285** | 40.4 ± 24.6 |
p = .05;
p < .001
Table 3.
Direct effect of predictors on quality of life
| variables | Standard β | p-value | R2 change | R2 for full model | |
|---|---|---|---|---|---|
| Model 1 | Age | −.245 | <.001 | .480** | |
| Gender | −.087 | .024 | |||
| NYHA | .203 | <.001 | |||
| Functional status | −.541 | <.001 | |||
| Perceived social support | −.132 | <.001 | .016** | .496* | |
| Model 2 | Age | −.245 | <.001 | .480** | |
| Gender | −.087 | .024 | |||
| NYHA | .203 | <.001 | |||
| Functional status | −.541 | <.001 | |||
| Depressive symptoms | .467 | <.001 | .163** | .643** | |
p = .05;
p < .001;
Model 1: Perceived social support is a predictor of quality of life; Model 2: Depressive symptoms are a predictor of quality of life.
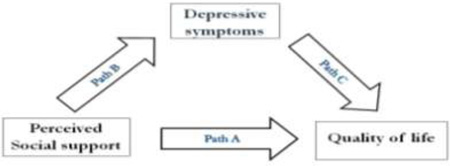
Mediator effect of depressive symptoms
Four multiple regressions in series identified a significant mediator effect for depressive symptoms (Table 4) with quality of life. Perceived social support predicted quality of life (sβ = −.132, P = .001) and depressive symptoms (sβ = −.262, P < .001). Depressive symptoms also predicted quality of life (sβ = .467, P < .001). However, the significant relationship between perceived social support and quality of life disappeared (sβ = −.010, P = .769) when depressive symptoms were entered with perceived social support, while controlling for age, gender, NYHA class, and functional status. Therefore, depressive symptoms exhibited a significant mediator effect in the relationship between perceived social support and quality of life.
Table 4.
Mediator effect of depressive symptoms on quality of life
 | ||||||
|---|---|---|---|---|---|---|
| Predictor | Outcome | Standard β | p-value | R2 for Model 1 a |
R2 for Model 2 b |
|
| Step 1 | Perceived social support | Quality of life | −.132 | .001 | .480** | .496* |
| Step 2 | Perceived social support | Depressive symptoms | −.262 | <.001 | .252** | .315** |
| Step 3 | Depressive symptoms | Quality of life | .467 | <.001 | .480** | .643** |
| Step 4 | Depressive symptoms Perceived social support |
Quality of life | .464 −.010 |
<.001 .769 |
.480** | .643** |
Model 1 includes only control variables (i.e., age, gender, NYHA class, and DASI functional status)
Model 2 includes both control variables (i.e., age, gender, NYHA class, and DASI functional status) and predictors
p < .01;
p < .001
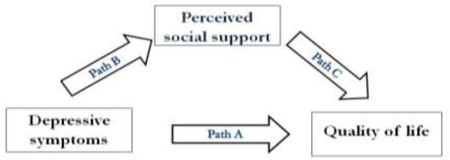
Mediator effect of perceived social support
Four multiple regressions in series were used to test the mediator effect of perceived social support (Table 5). Depressive symptoms predicted quality of life (s = −.467, P < .001) and perceived social support (s = −.324, P < .001). Perceived social support also predicted quality of life (s = −.132, P = .001). However, the significant relationship between depressive symptoms and quality of life remained when perceived social support was controlled (s = .464, P < .001). Thus, perceived social support did not mediate the relationship between depressive symptoms and quality of life, and both variables were independent predictors of quality of life.
Table 5.
Mediator effect of perceived social support on quality of life
 | ||||||
|---|---|---|---|---|---|---|
| Predictor | Outcome | Standard β | p-value | R2 for Model 1 a |
R2 for Model 2 b |
|
| Step 1 | Depressive symptoms | Quality of life | .467 | <.001 | .480** | .643** |
| Step 2 | Depressive symptoms | Perceived social support | −.324 | <.001 | .075** | .153** |
| Step 3 | Perceived social support | Quality of life | −.132 | .001 | .480** | .496* |
| Step 4 | Depressive symptoms Perceived social support |
Quality of life | .464 −.010 |
<.001 .769 |
.480** | .643** |
Model 1 includes only control variables (i.e., age, gender, NYHA class, and DASI functional status)
Model 2 includes both control variables (i.e., age, gender, NYHA class, and DASI functional status) and predictors
p < .01;
p < .001
Moderator effect
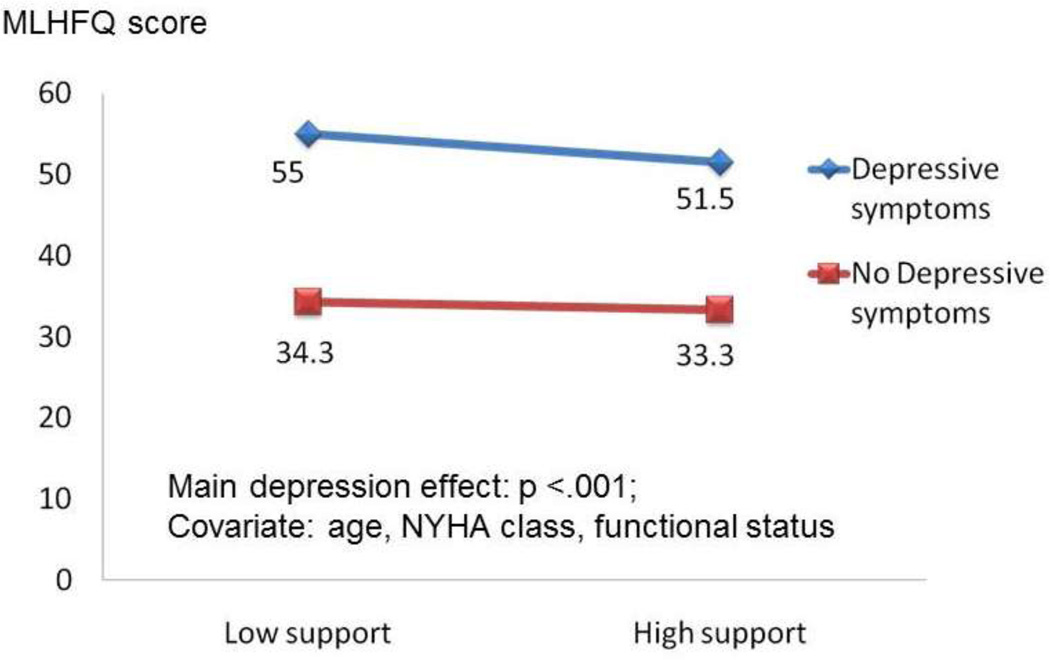
There was no significant interaction effect between depressive symptoms and perceived social support when age, gender, NYHA class, and functional status were controlled (Table 6). There was a main effect between depressive symptoms and quality of life (Figure 2). Thus, there was no moderator effect of depressive symptoms for either perceived social support or quality of life.
Table 6.
Moderator effects of perceived social support and depressive symptoms using 2×2 ANCOVA with quality of life as the outcome
| 2×2 ANCOVA | Mean square | F value | p-value |
|---|---|---|---|
| Covariate: Age | 4108.1 | 16.5 | < .001 |
| Gender | 1894.6 | 7.6 | .006 |
| NYHA | 2981.7 | 12.0 | .001 |
| Functional status | 28114.9 | 113.1 | < .001 |
| Perceived social support | 377.6 | 1.5 | .219 |
| Depressive symptoms | 23163.2 | 93.1 | < .001 |
| Perceived social support X Depressive symptoms | 121.9 | .49 | .484 |
| Model: F = 2.2; p=.084 | |||
Figure 2. Moderator effect by 2 × 2 ANCOVA.
Discussion
We explored the direct, mediator, and moderator effects of two predictors of quality of life, perceived social support and depressive symptoms, in patients with HF. We found that when each predictor was examined individually, perceived social support and depressive symptoms were independent predictors of quality of life in patients with HF. These findings are generally consistent with previous studies, in that higher perceived social support was associated with better quality of life [3; 30]; and severe depressive symptoms were associated with poorer quality of life in patients with HF.[10; 31] Depressive symptoms are a well-known predictor of poorer quality of life [10].
The compelling finding in this study is that depressive symptoms mediated the relationship between perceived social support and quality of life in patients with HF; neither depressive symptoms, nor perceived social support, exhibited a moderator effect on quality of life. This result indicated that perceived social support affected quality of life through its relationship with depressive symptoms. These findings suggested that interventions to increase quality of life by improvement of social support would be successful only when depressive symptoms were also effectively treated.
Depressive symptoms are a known predictor of both poorer quality of life and greater morbidity and mortality in patients with HF [9; 32], as well as, patients with coronary heart disease [33; 34]. Pharmacological interventions, including selective serotonin reuptake inhibitors, have been shown to be effective interventions for the improvement of depressive symptoms in patients with HF [35; 36]. Nonpharmacological interventions, including cognitive behavioral therapy, have also been successful in the improvement of depressive symptoms in patients with HF [37; 38]. However, there is a paucity of research about the use of social support as an intervention in patients with HF.
To date, only two research groups [39; 40] have investigated the effectiveness of a social support intervention for improved health outcomes in patients with HF. Riegel and colleagues [39] examined whether a peer support program improved self-management, perceived social support and confidence of patients with HF. The peer support program was a mentoring program; whereby, weekly phone calls were provided by trained mentors who were also patients with HF. Although this intervention effectively improved self-care, it failed to increase levels of perceived social support. Dunbar and colleagues [40] reported early results of an ongoing study examining the effects of a family partnership education program, which focused on reinforcing family support for patients with HF. These investigators reported that patients who received self-management education within the family partnership program became more adherent to a low sodium diet, compared with those who received self-management education alone. Thus, this study provided evidence that social support was important to improved outcomes in patients with HF.
Currently, there is limited evidence about the most effective types of social support and the intensity or dose of social support required to improve quality of life and clinical outcomes like mortality and rehospitalization rate, particularly in patients with depressive symptoms. There is also a lack of clarity about the most effective route of delivery for social support interventions. The use of technology like the phone and internet may be useful strategies for effective delivery. However, based on our findings, any social support intervention must also effectively address depressive symptoms to ensure optimal improvement in outcomes.
To date, only one intervention study has focused on improving both social support and depressive symptoms in patients with cardiovascular disease. In the Enhancing Recovery in Coronary Heart Disease (ENRICHD) trial [41], a cognitive behavioral therapy intervention was used to improve perceived social support and depressive symptoms in patients after acute myocardial infarction (n = 2481) [41]. This cognitive behavioral therapy intervention produced modest improvement in quality of life in patients with concurrent lower perceived social support and depressive symptoms after acute myocardial infarction [41]. However, the intervention failed to improve mortality and morbidity outcomes at an average follow of 29 months [42]. It is unknown whether this non-pharmacological intervention is effective in the improvement of outcomes of patients with HF. Thus, further investigation is necessary to determine whether a cognitive behavioral therapy intervention could improve outcomes of patients with HF.
Limitations of the present study include the use of cross-sectional data; thus, causality is not determinable. Also, the measure of perceived social support we used did not evaluate either the quality or quantity of social support provided to these patients, as there are currently no instruments capable of capturing the multidimensional nature of social support. Because the availability of support persons may be variable depending on individual patient situations and disease progression, longitudinal, prospective investigations of the long term effects of varied levels of both perceived social support and depressive symptoms on quality of life, as well as mortality and morbidity outcomes, are needed. Another limitation in this study would be that the measure of quality of life (i.e., the MLHFQ) includes an item about depressed feeling. Thus, depressive symptoms might be a major contributor to the explained variance in quality of life in this study. However, the MLHFQ is a disease specific quality of life measure and major components of this 21-item instrument are physical symptoms and physical/social function related to their HF experience. Using a generic quality of life measure would be another option, but most generic quality of life measures also contain emotional distress items. The last limitation in this study is low participation rate that may affect generalizability of study findings. It has been reported that refusal rate is as high as 23% and study enrollment is typically very difficult in the HF population [43]. Out study also has similar intentional refusal rate among eligible participants.
Conclusions
This is the first study to demonstrate the mediation effect of depressive symptoms on the relationship between perceived social support and quality of life in patients with HF. This study is robust, because we controlled known confounding factors, including age, gender, NYHA class and self-reported functional status in examining mediator and moderator effects using hierarchical multiple regressions and ANCOVA. Health care providers should regularly assess for depressive symptoms, as well as the adequacy of patient perceived social support, and determine the primary source of this support. Interventions to improve quality of life in this vulnerable group of patients must include an appreciation of the mediation effect of depressive symptoms on the association between perceived social support and quality of life to ensure optimal patient outcomes are achieved.
Acknowledgement
The authors would like to thank all staff in the Research and Intervention for Cardiopulmonary Health (RICH) Heart Program and all participants in the RICH Heart Program.
Grant/Financial support:
NIH/NINR 3R01 009280 (Moser, D. K., PI)
NINR 5R01 008567 (Lennie, T. A., PI)
AACN Phillips Medical Research Award (Moser, D.K., PI; Chung, M.L., co-PI) University of Kentucky Center for Clinical and Translational Science (NIH M01RR02602)
This work was supported in part by a Center grant to the University of Kentucky, College of Nursing from NIH, NINR, 1P20NR010679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett SJ, Perkins SM, Lane KA, Deer M, Brater DC, Murray MD. Social support and health-related quality of life in chronic heart failure patients. Qual Life Res. 2001;10(8):671–682. doi: 10.1023/a:1013815825500. [DOI] [PubMed] [Google Scholar]
- 4.Lett HS, Blumenthal JA, Babyak MA, Catellier DJ, Carney RM, Berkman LF, Burg MM, Mitchell P, Jaffe AS, Schneiderman N. Social support and prognosis in patients at increased psychosocial risk recovering from myocardial infarction. Health Psychol. 2007;26(4):418–427. doi: 10.1037/0278-6133.26.4.418. [DOI] [PubMed] [Google Scholar]
- 5.Chung ML, Lennie TA, Riegel B, Wu JR, Dekker RL, Moser DK. Marital status as an independent predictor of event-free survival of patients with heart failure. Am J Crit Care. 2009;18(6):562–570. doi: 10.4037/ajcc2009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23(3):258–265. doi: 10.1097/01.JCN.0000305093.20012.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79(12):1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 9.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Müller-Tasch T, Peters-Klimm F, Schellberg D, Holzapfel N, Barth A, Jäger J, Szecsenyi J, Herzog W. Depression Is a Major Determinant of Quality of Life in Patients With Chronic Systolic Heart Failure in General Practice. Journal of Cardiac Failure. 2007;13(10):818–824. doi: 10.1016/j.cardfail.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and Coronary Heart Disease: Recommendations for Screening, Referral, and Treatment: A Science Advisory From the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Psychiatric Association. 2008;Vol. 118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 12.Song EK, Moser DK, Frazier SK, Heo S, Chung ML, Lennie TA. Depressive symptoms affect the relationship of N-terminal pro B-type natriuretic peptide to cardiac event-free survival in patients with heart failure. J Card Fail. 2010;16(7):572–578. doi: 10.1016/j.cardfail.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JR, Chung M, Lennie TA, Hall LA, Moser DK. Testing the psychometric properties of the Medication Adherence Scale in patients with heart failure. Heart Lung. 2008;37(5):334–343. doi: 10.1016/j.hrtlng.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52(1):30. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 15.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55(3–4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 16.Wu JR, Moser DK, Chung ML, Lennie TA. Predictors of medication adherence using a multidimensional adherence model in patients with heart failure. J Card Fail. 2008;14(7):603–614. doi: 10.1016/j.cardfail.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 18.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O'Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154(1):102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124(4):1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71(12):1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 21.Bennet SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Deer M, Murray MD. Discriminant properties of commonly used quality of life measures in heart failure. Qual Life Res. 2002;11(4):349–359. doi: 10.1023/a:1015547713061. [DOI] [PubMed] [Google Scholar]
- 22.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing a published model of health-related quality of life in heart failure. J Card Fail. 2005;11(5):372–379. doi: 10.1016/j.cardfail.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: testing dyadic dynamics using Actor-Partner Interdependence Model. J Psychosom Res. 2009;67(1):29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150(5):984. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 26.Newsom JT, Schulz R. Social support as a mediator in the relation between functional status and quality of life in older adults. Psychol Aging. 1996;11(1):34–44. doi: 10.1037/0882-7974.11.1.34. [DOI] [PubMed] [Google Scholar]
- 27.Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI) Eur Heart J. 1997;18(3):414–419. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]
- 28.Parissis JT, Nikolaou M, Farmakis D, Bistola V, Paraskevaidis IA, Adamopoulos S, Filippatos G, Kremastinos DT. Clinical and prognostic implications of self-rating depression scales and plasma B-type natriuretic peptide in hospitalised patients with chronic heart failure. Heart. 2008;94(5):585–589. doi: 10.1136/hrt.2007.117390. [DOI] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 30.Bennett SJ, Baker SL, Huster GA. Quality of life in women with heart failure. Health Care Women Int. 1998;19(3):217–229. doi: 10.1080/073993398246386. [DOI] [PubMed] [Google Scholar]
- 31.de Leon CF, Grady KL, Eaton C, Rucker-Whitaker C, Janssen I, Calvin J, Powell LH. Quality of life in a diverse population of patients with heart failure: BASELINE FINDINGS FROM THE HEART FAILURE ADHERENCE AND RETENTION TRIAL (HART) J Cardiopulm Rehabil Prev. 2009;29(3):171–178. doi: 10.1097/HCR.0b013e31819a0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, Blazing MA, Davenport C, Califf RM, Krishnan RR, O'Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 33.Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102(15):1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 34.Barefoot JC, Brummett BH, Helms MJ, Mark DB, Siegler IC, Williams RB. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med. 2000;62(6):790–795. doi: 10.1097/00006842-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb SS, Kop WJ, Thomas SA, Katzen S, Vesely MR, Greenberg N, Marshall J, Cines M, Minshall S. A double-blind placebo-controlled pilot study of controlled-release paroxetine on depression and quality of life in chronic heart failure. Am Heart J. 2007;153(5):868–873. doi: 10.1016/j.ahj.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Lesperance F, Frasure-Smith N, Laliberte MA, White M, Lafontaine S, Calderone A, Talajic M, Rouleau JL. An open-label study of nefazodone treatment of major depression in patients with congestive heart failure. Can J Psychiatry. 2003;48(10):695–701. doi: 10.1177/070674370304801009. [DOI] [PubMed] [Google Scholar]
- 37.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119–131. doi: 10.1016/j.jpsychores.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostis JB, Rosen RC, Cosgrove NM, Shindler DM, Wilson AC. Nonpharmacologic therapy improves functional and emotional status in congestive heart failure. Chest. 1994;106(4):996–1001. doi: 10.1378/chest.106.4.996. [DOI] [PubMed] [Google Scholar]
- 39.Riegel B, Carlson B. Is individual peer support a promising intervention for persons with heart failure? J Cardiovasc Nurs. 2004;19(3):174–183. doi: 10.1097/00005082-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Dunbar SB, Clark PC, Deaton C, Smith AL, De AK, O'Brien MC. Family education and support interventions in heart failure: a pilot study. Nurs Res. 2005;54(3):158–166. doi: 10.1097/00006199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Mendes de Leon CF, Czajkowski SM, Freedland KE, Bang H, Powell LH, Wu C, Burg MM, DiLillo V, Ironson G, Krumholz HM, Mitchell P, Blumenthal JA. The effect of a psychosocial intervention and quality of life after acute myocardial infarction: the Enhancing Recovery in Coronary Heart Disease (ENRICHD) clinical trial. J Cardiopulm Rehabil. 2006;26(1):9–13. doi: 10.1097/00008483-200601000-00002. quiz 14–15. [DOI] [PubMed] [Google Scholar]
- 42.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. Jama. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 43.Pressler SJ, Subramanian U, Shaw RM, Meyer LE, Stoudemire K, Gradus-Pizlo I. Research in patients with heart failure: challenges in recruitment. Am J Crit Care. 2008;17(3):198–203. [PubMed] [Google Scholar]