Abstract
Background
Bladder urothelial carcinoma (UrCa) proclaims high rates of mortality and morbidity. Identifying novel molecular prognostic factors and targets of therapy is crucial. mTOR pathway plays a pivotal role in establishing cell shape, migration and proliferation.
Design
TMA were constructed from 132 cystectomies (1994-2002). IHC was performed for Pten, c-Myc, p27, phosAkt, phosS6, and 4E-BP1. Markers were evaluated for pattern, percent and intensity of staining.
Results
Mean length of F/U was 62.6 months (1-182). Disease progression, overall survival (OS) and disease specific survival (DSS) rates were 42%, 60% and 68%, respectively.
Pten showed loss of expression in 35% of UrCa. All markers showed lower expression in invasive UrCa compared to benign urothelium with the exception of 4E-BP1. Pten, p27, phosAkt, phosS6 and 4E-BP1 expression correlated with pathologic stage (pT; p<0.03). Pten, 4E-BP1, and phosAkt expression correlated with divergent aggressive histology and invasion.
PhosS6 expression inversely predicted OS (p=0.01), DSS (p=0.001) and progression (p=0.05). c-Myc expression inversely predicted progression (p=0.01).
In a multivariate analysis model that included: TNM stage grouping, divergent aggressive histology, concomitant CIS, phosS6 and c-Myc expression; phosS6 was an independent predictor of DSS (p=0.03; HR: -.19) while c-Myc was an independent predictor of progression (p=0.02; HR:-.38). In a second model substituting organ confined disease and lymph node status for TNM stage grouping, phosS6 and c-Myc remained independent predictors of DSS (p=0.03; HR: -.21) and progression (p=0.03; HR:-.34), respectively.
Conclusions
We found an overall downregulation of mTOR pathway in UrCa. PhosS6 independently predicted DSS and c-Myc independently predicted progression.
Keywords: mTOR, Pten, Akt, phos S6, 4E-BP1, c-Myc, p27, urothelial carcinoma, bladder
Introduction
Despite recent multi-disciplinary advances in its treatment, urothelial carcinoma of the bladder (UrCa) continues to carry unacceptably high rates of mortality and morbidity with 10-years survival rate of 40-50% 1. Variable biologic behavior in UrCa appears to be related to differences in oncogenic pathways alterations. While superficial papillary neoplasms are driven by gain-of-function mutations in oncogenes such as H-RAS and FGFR3, flat carcinoma in situ and muscle-invasive tumors usually carry loss-of-function mutations, affecting tumor suppressor genes such as p53 and phosphatase and tensine homologue (PTEN) 2. Improvements in understanding UrCa oncogenesis have fueled a current search for biological markers which can be targeted for therapy or bare prognostic significance.
Inactivation of PTEN tumor suppressor gene triggers the phosphatidil inositil-3 kinase (PI3K)-protein kinase B (AKT) pathway that leads to Akt phosphorylation and activation (phos Akt). AKT plays a central role in orchestrating interaction between different growth regulating pathways and represents a major feedback control point 3, 4. Phos Akt promotes cell cycle progression through p27kip1 (p27) depletion 5, cell proliferation through c-Myc up-regulation 6, and protein translation through mTOR activation via its main downstream effectors: phosphorylated S6 protein (phos S6) and eukaryotic translation initiation factor 4E-binding protein-1 (4E-BP1) 7.
The current study evaluates the expression status and reciprocal interplay of six of the above biomarkers (Pten, phos Akt, phos S6, 4E-BP1, p27 and c-Myc) aiming to be the first to evaluate mTOR pathway status as it relates to outcome in a well characterized uniform cohort of UrCa treated by cystectomy.
Materials and Methods
Patient Cohort and Tissue Microarray Construction
We retrieved 144 cystectomy specimens performed at The Johns Hopkins Hospital between 1994 and 2002. All sections were reviewed for confirmation of original diagnoses and staged, according to the 2002 AJCC-TNM classification 8, by two urologic pathologists (RA and GJN). Paraffin blocks were available in 132 cases, including 120 pure UrCa and 12 UrCa with divergent aggressive histology. The latter included 2 tumors with extensive squamous differentiation, 5 with sarcomatoid features (carcinosarcoma), 1 micropapillary urothelial carcinoma and 1 urothelial carcinoma with plasmacytoid features and 3 undifferentiated carcinomas. TMAs were constructed using a Beecher Instrument (Silver Spring-MD) as previously described by Fedor et al 9. Triplicate tumor samples and paired benign urothelium were spotted from each specimen. Each TMA spot was further categorized as invasive UrCa, high grade non-invasive papillary UrCa or carcinoma in situ (CIS).
Clinico-pathological Data
All pertinent clinico-pathological data were retrieved from electronic medical records. These included patient demographics and preoperative information such as diagnostic procedure, pre-cystectomy treatment and clinical stage. Follow up data on disease progression, post-operative chemotherapy and/or radiotherapy, disease specific survival and overall survival were also obtained (Table 1). Since all patients underwent cystectomy with curative intent, pelvic recurrence as well as recurrent metastatic disease were considered progression events. Three cases that recurred in urinary tract locations other than bladder (eg. renal pelvis) were excluded from the progression and survival analyses. Both tumor pathological stage (pT) and TNM satge grouping were analyzed during statistical analysis.
Table 1.
Demographic and clinicopathological characteristics of 132 cystectomy patients
| Characteristic | N (%) |
|---|---|
| Age (years)at cystectomy | |
| <60 | 47 (35.6) |
| 60-70 | 42 (31.8) |
| >70 | 43 (32.6) |
| Ethnicity | |
| African American | 10 (7) |
| Caucasian | 116 (88) |
| other | 6 (5) |
| Gender | |
| Female | 26 (20) |
| Male | 106 (80) |
| Pathologic Stage at cystectomy | |
| pTa/pTis | 12 (9) |
| pT1 | 17 (13) |
| pT2 | 38 (29) |
| pT3/pT4 | 65 (49) |
| TMA spot sampling | |
| Non-invasive papillary/carcinoma in situ component | 27 (20) |
| Invasive component | 105 (80) |
| Pre-operative treatment | 56 (42) |
| Radiotherapy or systemic chemotherapy | 6 (4) |
| Intravesical therapy | 50 (38) |
Immunohistochemistry
Standard immunohistochemistry (IHC) analysis was performed for mTOR pathway members: Pten, phos Akt, phos S6, and 4E-BP1. IHC analysis was also performed for AKT regulated markers c-Myc and p27.
Immunostaining was performed on formalin fixed paraffin embedded tissue sections using Bond max- Leica autostainer (Leica Microsystems, Bannockburn, IL). Sections were deparaffinized, rehydrated and subjected to heat induced antigen retrieval with a buffer solution using a steamer. Sections were then incubated with appropriate primary antibody. Following the application of a secondary polyclonal rabbit antibody (except for c-Myc, for which we used a CSA kit), slides were developed using 3-3′-diaminobenzidine chromogen and counterstained with hematoxylin. Table 2 lists all pertinent markers information including: vendor, clone, dilution, pre-treatment and incubation conditions and detection kit. TMA spots with artifactual folds or lacking tissue target representation were omitted from further analysis. The latter accounted for any variability in number of total evaluable spots/cases among markers. Tumor and benign TMA spots stained with each marker were evaluated for pattern of staining (nuclear vs cytoplasmic), extent (percent of positive cells) and intensity (0 to 3+ score). A final H-score was generated for each marker as the sum of the products of each intensity category X extent of immunoexpression. H-scores were used during statistical analyses for all markers. In addition, Pten analysis was performed using extent scores. During multivariate analysis, a cut-off was used for phos S6 expression based on mean tumor H-score (H-score ≥ 27) and for c-MYC expression based on the 90th percentile tumor H-score (H-score ≥ 15).
Table 2.
Summary of antibodies used in the immunohistochemical analysis of mTOR pathway.
Pten: phosphatase and tensine homologue; p27: p27kip; phos Akt: phoporylated Akt; phos S6: phosphorylated S6 protein; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein-1.
| Vendor | Clone | Pre-treatment | Dilution | Incubation | Detection | |
|---|---|---|---|---|---|---|
| Pten | Cell Signaling | D4.3 | EDTA 45 min | 1:50 | overnight 4C | PowerVision |
| phos Akt | Cell Signaling | 736E11 | EDTA 45 min | 1:50 | 45 min room T | PowerVision |
| phos S6 | Cell Signaling | polyclonal | EDTA 45 min | 1:200 | overnight 4C | Envision |
| 4E-BP1 | ProSci | polyclonal | citrate 25 min | 1:250 | 45 min room T | Envision |
| c-Myc | Epitomics | Y69 | EDTA 45 min | 1:300 | overnight 4C | CSA kit |
| p27 | Transduction lab | 57 | citrate 25 min | 1:4k | 45 min room T | PowerVision |
Statistic Analysis
Findings were analyzed using the Stata 9.2 (StataCorp; college Station TX) software package. Equality of population medians among groups was tested using Kruskal-Wallis test for non-parametric one-way analysis of variance by ranks. ANOVA analysis of variance was used when comparing categorical variables. Pairwise correlation coefficients were calculated to test relationship among variables. A Cox regression model was used during multivariate analysis.
Results
Patients Cohort
Mean patient age at cystectomy was 68 y; M:F ratio was 4:1 and mean length of follow-up was 62.6 months (1-182). In our cohort, disease progression rate was 42% (25% local recurrence and 17% distant metastases). Overall survival (OS) and disease specific survival (DSS) rates were 60% and 68%, respectively.
Fifty (38%) patients received pre-operative intravesical therapy. Two patients received mitomycin and the remaining 48 were treated with BCG. Six patients (4%) received neoadjuvant systemic chemotherapy and or neoadjuvant radiotherapy.
The TMA sampling was limited to non invasive UrCa in 27 cases. These included 12 pTa/pTis cystectomies and 15 (11%) higher stage lesions where the invasive component was no longer present in the TMA spot.
Biomarkers Expression Status in Relation to Clinicopathological Parameters
Expression levels of all six mTOR pathway related biomarkers are summarized in table 3 and depicted in figure 1.
Table 3.
Summary of all six mTOR related biomarkers expression (mean H-score) in the categorized pathological parameters.
pT: pathological stage; Pten: phosphatase and tensine homologue; p27: p27kip; phos Akt: phoporylated Akt; phos S6: phosphorylated S6 protein; 4 EBP-1: eukaryotic translation initiation factor 4E-binding protein-1. NS: not significant.
| Marker | Benign vs malignant urothelium | Mean H-score | TMA spot histology | Mean H-score | UrCa Histology | Mean H-score | pT | Mean H-score | TNM stage group | Mean H-score |
|---|---|---|---|---|---|---|---|---|---|---|
| Pten* | Benign | 100 | Non-invasive | 148 | Pure UrCa | 74 | pT0 | 195 | 0a/0is | 195 |
| pT1 | 73 | I | 61 | |||||||
| Malignant (p=0.0000) |
42 | Invasive (p=0.0004) |
61 | Aggressive histology (p=0.009) |
34 | pT2 | 79 | II | 80 | |
| pT3/4 | 47 | III/IV | 52 | |||||||
| phos Akt | Benign | 17 | Non-invasive | 12 | Pure UrCa | 7 | pT0 | 23 | 0a/0is | 23 |
| pT1 | 7 | I | 8 | |||||||
| Malignant (p=0.0000) |
7 | Invasive (p=0.03) |
6 | Aggressive histology (p=0.04) |
3 | pT2 | 6 | II | 7 | |
| pT3/4 | 6 | III/IV | 5 | |||||||
| phos S6 | Benign | 117 | Non-invasive | 43 | Pure UrCa | 28 | pT0 | 25 | 0a/0is | 25 |
| pT1 | 84 | I | 75 | |||||||
| Malignant (p=0.0000) |
27 | Invasive (p=0.1) |
25 | Aggressive histology (p=NS) |
10 | pT2 | 19 | II | 22 | |
| pT3/4 | 19 | III/IV | 21 | |||||||
| 4E-BP1 | Benign | 9 | Non-invasive | 8 | Pure UrCa | 65 | pT0 | 10 | 0a/0is | 10 |
| pT1 | 39 | I | 42 | |||||||
| Malignant (p=0.0000) |
32 | Invasive (p=0.0055) |
37 | Aggressive histology (p=0.004) |
29 | pT2 | 25 | II | 27 | |
| pT3/4 | 37 | III/IV | 33 | |||||||
| c-Myc | Benign | 36 | Non-invasive | 24 | Pure UrCa | 2 | pT0 | 5 | 0a/0is | 5 |
| pT1 | 14 | I | 14 | |||||||
| Malignant (p=0.0000) |
7 | Invasive (p= 0.002) |
5 | Aggressive histology (p=NS) |
8 | pT2 | 7 | II | 9 | |
| pT3/4 | 6 | III/IV | 6 | |||||||
| p27 | Benign | 69 | Non-invasive | 76 | Pure UrCa | 53 | pT0 | 63 | 0a/0is | 63 |
| pT1 | 93 | I | 97 | |||||||
| Malignant (p=0.0029) |
51 | Invasive (p= NS) |
47 | Aggressive histology (p=0.04) |
24 | pT2 | 58 | II | 65 | |
| pT3/4 | 36 | III/IV | 36 |
Figure 1.
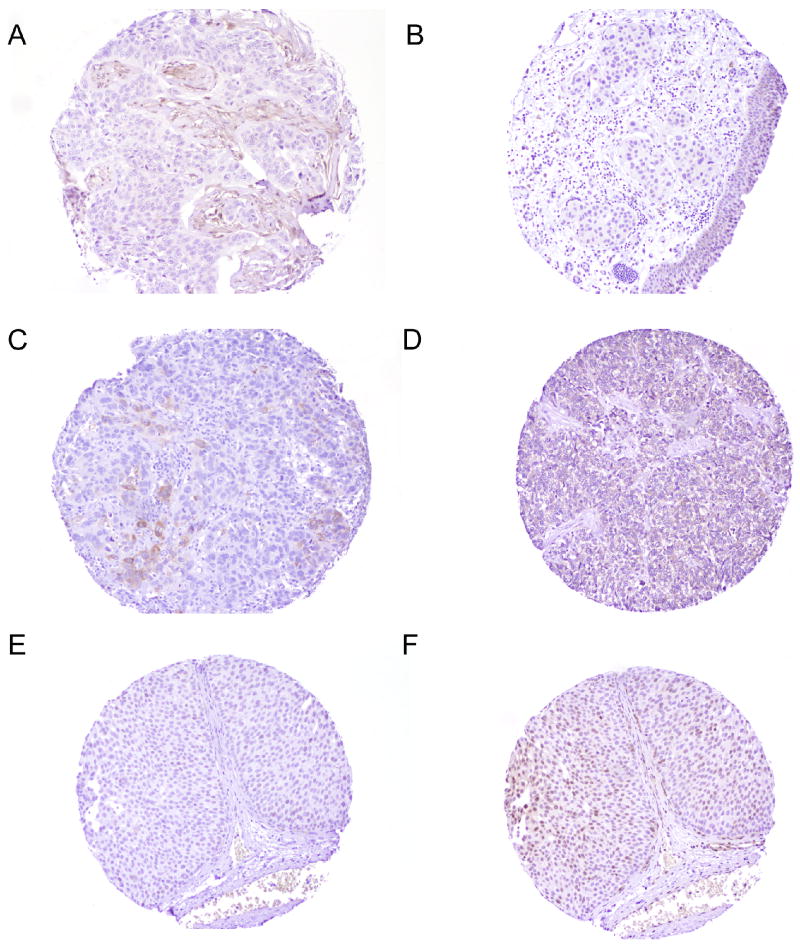
a: Tissue microarray spot showing lack of Pten immunohistochemical expression in the invasive urothelial carcinoma in contrast to adjacent Pten positive fibroblasts (200× magnification). b: Tissue microarray spot showing loss of phos Akt immunohistochemical expression in the invasive urothelial carcinoma compared to positive staining in the benign overlying urothelium (200× magnification). c: Tissue microarray spot showing focal strong (3+) cytoplasmic phos S6 immunohistochemical expression in invasive urothelial carcinoma (200× magnification). d: Tissue microarray spot showing diffuse moderate (2+) cytoplasmic 4E-BP1 immunohistochemical expression in invasive urothelial carcinoma (200× magnification). e: Tissue microarray spot showing focal weak to moderate (1+ to 2+) nuclear c-Myc immunohistochemical expression in non-invasive urothelial carcinoma (200× magnification). f: Tissue microarray spot showing multifocal moderate (2+) nuclear p27 and focal cytoplasmic p27 immunohistochemical expression in non-invasive urothelial carcinoma (200× magnification).
Pten
Nuclear and or cytoplasmic Pten expression was evaluated in 119 tumors. Pten expression levels were significantly lower in UrCa compared to benign urothelium (H-score: p=0.0001; extent: p=0.0000). Complete lack of Pten expression was seen in 50/119 (42%) UrCa. Additional 9 (7.5%) tumors showed low Pten expression levels (extent <20%). All corresponding benign urothelium showed high Pten expression (extent >50%).
On univariate analysis, significantly lower Pten expression extent were seen in tumor TMA spots with invasion compared to non invasive spots (p=0.0004) and in tumors of divergent aggressive histology compared to pure UrCa (p=0.009). Pten expression showed an inverse correlation with pT and TNM stage grouping (p=0.0001 and p=0.0004, respectively).
phos Akt
Nuclear phos Akt was evaluated in 113 tumors. Phos Akt expression was significantly lower in UrCa compared to benign urothelium (p=0.0000). Fifty (44%) tumors were negative for phos Akt while additional 20 (18%) UrCa showed low phos Akt expression (H-score ≤5).
On univariate analysis, lower levels of phos Akt expression significantly correlated with presence of invasion at TMA spot (p=0.03) and divergent aggressive histology (p=0.04). Phos Akt expression inversely correlated with pT (p= 0.0316). Non-invasive UrCa (pTa/pTis) had significantly higher levels of phos Akt compared to invasive (pT1+) UrCa (p=0.004).
Phos S6
Cytoplasmic phos S6 was evaluated in 114 UrCa. Fourty-two (37%) tumors were negative for phos S6 and additional 10 (9%) tumors showed low phos S6 expression ((H-score ≤5). Phos S6 expression was significantly lower in UrCa compared to benign urothelium (p=0.0000).
On univariate analysis, phos S6 expression did not correlate with presence of invasion at the TMA spot or with divergent aggressive histology (p=NS). However, phos S6 expression inversely correlated with pT stage (p=0.02). Non-muscle invasive UrCa (pT<2) had higher phos S6 levels compared to pT ≥2 (p=0.0030).
4 EBP-1
Cytoplasmic 4E-BP1 was evaluated in 114 UrCa. Thirty-seven (32%) tumors were negative for 4E-BP1. We found significantly higher levels of 4E-BP1 expression in UrCa compared to benign urothelium (p=0.0000).
On univariate analysis, significantly lower 4E-BP1 expression levels were seen in tumor TMA spots with invasion compared to non invasive spots (p=0.005) and in tumors of divergent aggressive histology compared to pure UrCa (p=0.004). 4E-BP1 expression did not correlated with overall pT or TNM stage grouping (p=NS). A trend toward lower expression levels in non-invasive UrCa (pTa/pTis) was found compared to pT ≥1 (p=0.07).
p27
Nuclear p27 was evaluated in 115 tumors. One hundred and three out of 115 (90%) tumors were positive for the marker. p27 expression levels were significantly lower in UrCa compared to benign urothelium (p=0.002).
On univariate analysis, tumors with divergent aggressive histology had significantly lower levels of p27 (p=0.04). p27 showed an inverse correlation with pT and TNM stage grouping (p=0.04 and p=0.003, respectively).
c-Myc
Nuclear c-Myc was evaluated in 114 tumors. 77/114 (67.5%) UrCa were negative for c-Myc and additional 16 (14%) tumors had low levels (H-score ≤ 5) of c-Myc expression. C-Myc expression was significantly lower in UrCa compared to benign urothelium (p=0.0000).
On univariate analysis, lower c-Myc expression correlated with presence of invasion at TMA spot (p=0.002).
Correlation among tested biomarkers
A weak but statistically significant positive correlation was present between phos Akt and Pten UrCa expression levels (cc=0.19; p=0.03) as well as between phos Akt and p27 (cc=0.25; p=0.008) UrCa expression levels. Pten and p27 UrCa expression also showed positive correlation (c=0.29; p=0.001). UrCa phos S6 expression showed strong positive correlation with UrCa c-Myc expression (c=0.42; p=0.000) and only weak positive correlation with p27 (c=0.20; p=0.03) UrCa expression levels.
Univariate and Multivariate Outcome Analyses
Clinicopathological parameters
On univariate analysis, TNM stage grouping and presence of divergent aggressive histology significantly predicted DSS, OS and disease progression. Concomitant CIS predicted overall survival (p=0.008). pT stage alone was of borderline significance in predicting outcome (Table 4). When pT stage was categorized as organ confined (pT<3) vs non-organ confined (pT≥3), organ confined status significantly predicted all 3 outcome parameters (DSS, OS and disease progression). Presence of lymphovascular invasion (LVI) as well as nodal status were also predictive of outcome (see table 4). The ratio of number of positive nodes to total number of examined nodes (positive node density) was only of borderline significance in predicting disease progression (p=0.07) and DSS (p=0.06). Preoperative therapy, including neoadjuvant chemo or radiation did not correlate with any outcome parameter (p=NS).
Table 4.
Results of univariate analysis of clinicopathological parameters and the expression levels of six mTOR related biomarkers in relations to disease specific survival, overall survival and disease progression. Pten: phosphatase and tensine homologue; p27: p27kip; phos Akt: phoporylated Akt; phos S6: phosphorylated S6 protein; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein-1. NS: not significant.
| Univariate Analysis | |||
|---|---|---|---|
| Outcome | |||
| DSS | OS | Progression | |
| Clinicopathological Parameters | |||
| pT | p=0.080 | p=0.083 | p=0.061 |
| TNM stage grouping | p=0.007 | p=0.024 | p=0.039 |
| Organ confined disease status | p=0.016 | p=0.021 | p=0.010 |
| Lymph node status | p=0.050 | p=NS | p=0.010 |
| Lymphovascular invasion | p=0.020 | p=0.002 | p=0.060 |
| Divergent aggressive histology | p=0.005 | p=0.029 | p=0.031 |
| Concomitant CIS | p=NS | p=0.008 | p=NS |
| mTOR Markers | |||
| Pten | p=NS | p=NS | p=NS |
| Phos Akt | p=NS | p=NS | p=NS |
| Phos S6 | p=0.001 | p=0.01 | p=0.05 |
| 4 EBP-1 | p=NS | p=NS | p=NS |
| c-Myc | p=NS | p=NS | p=0.01 |
| P27 | p=NS | p=NS | p=NS |
To evaluate the prognostic role of the above clinicopathologic parameters before factoring in the input of mTOR pathway markers, two multivariate models were adopted. The first included TNM stage grouping, presence of concomitant CIS and aggressive histology, TNM stage grouping remained an independent predictor of DSS, OS and disease progression (p=0.001; p=0.001; p=0.007 respectively) while concomitant CIS remained a predictor of OS (p=0.04) in this first model. In a second clinicopathologic parameter model that substituted organ confined status and lymph node status for TNM stage grouping, only nodal status remained an independent predictor of disease progression (p=0.03).
Clinicopathological parameters and mTOR related biomarkers expression
Among all six tested biomarkers, phos S6 was a significant predictor of DSS (p=0.001), OS (p=0.01) and disease progression (p=0.05) while c-Myc expression was a significant predictor of disease progression (p=0.01) but not of DSS or OS (p=NS) on univariate analysis (see Table 4).
In the first of two multivariate analysis models that included: TNM stage grouping, presence of divergent aggressive histology, concomitant CIS, phosS6 and c-Myc expression, phosS6 was an independent predictor of DSS (p=0.03; HR: -.19) while c-Myc was an independent predictor of progression (p=0.02; HR:-.38). In the second multivariate analysis model we substituted organ confined disease status and lymph node status for TNM stage grouping. phosS6 remained an independent predictor of DSS (p=0.03; HR: -.21) amd c-Myc remained an independent predictor of progression (p=0.03; HR:-.34).
Discussion
mTOR pathway is a key regulator of protein translation and cell proliferation that has been shown to be up-regulated in several solid malignancies including thyroid carcinoma 10, small cell carcinoma of lung 11, gastrointestinal tumors 12, 13 and clear cell renal cell carcinoma 14. The main downstream effectors of mTOR pathway (phos S6 and 4E-BP1) have been shown to be independent predictors of prognosis in several types of solid tumors including renal cell 14, ovarian 15, liver 16-19 and mammary carcinomas 20.
While the search for prognostic biomarkers in UrCa initially focused on cell cycle regulators, p53 and retinoblastoma (RB) alterations 21, 22, more recent efforts have also included tyrosine kinase receptors, mTOR and microenvironment interaction pathways 23 as potential prognosticators. Several studies have addressed the prognostic potential of individual mTOR pathway members in bladder cancer 24-26. Comprehensive simultaneous assessment of key members of PI3K- mTOR pathway and its downstream affected biomarkers, in relation to clinicopathologic parameters and outcome, in a well characterized cystectomy cohort is needed.
PTEN loss leading to activation of AKT/mTOR axis has been reported in other genitourinary tract tumors such as prostate adenocarcinoma27, 28, renal cell carcinoma29 and upper urinary tract UrCa30. These findings offered a rational for the limited but promising response to mTOR inhibitors therapy in such settings. However, Yoo et al 31, using a mouse model that conditionally deletes PTEN in urogential epithelium, found AKT/mTOR pathway highly activated in prostate tumors, but not in bladder epithelium. Moreover, PTEN knock-out mice were shown to develop urothelial carcinomas in the upper urinary tract, but not in the bladder 30. In contrast, synchronous deletions of both PTEN and p53 tumor suppressor genes have been shown to lead to the development of invasive bladder cancer in mouse models 32.
Our findings of frequent loss of Pten (42%) and phos Akt (44%) expression in UrCa are in line with prior studies. Pten and phos Akt loss of expression has been previously reported in up to 50% and 70% of UrCa, respectively, while lower Pten expression has been linked to invasive behavior 24-26. In our cohort, both Pten and phos Akt expression levels inversely correlated with pT stage and aggressive divergent histology and their loss was more likely to be seen in invasive compared to non-invasive tumor component within a given TMA spot. Our findings of lack of correlation of either Pten or phos Akt tumor expression with disease outcome have also been previously illustrated by Harris et al. and others 26, 33. The generally lower levels of mTOR pathway members in UrCa with divergent histology suggests downregulation of mTOR pathway in less differentiated urothelial tumors.
The positive but weak correlation between Pten and phos Akt expression is intriguing and may suggest a non-conditional association between the loss of inhibitory effect of PTEN tumor suppressor gene and phos Akt expression (Akt activation) in UrCa. Bose et al 34 found no correlation between Pten and phos Akt expression in mammary carcinoma, despite finding both markers to be altered, suggesting that Akt activation is not always directly linked to PTEN loss.
AKT regulates its downstream target mTOR which in turns operates through two distinct complexes: mTORC1 and mTORC2. mTORC2 directly activates AKT in a feedback fashion while mTORC1 pathway regulates cell growth through its main downstream effectors: ribosomal S6 kinase and 4E-BP1. Activation of mTORC1 is thought to stimulate translation through phosphorilation of S6 and inhibition of 4E-BP1. 35. Our findings of lower phos S6 and higher 4E-BP1 expression levels in UrCa compared to benign urothelium is consistent with the lower activation of AKT found in our UrCa cohort resulting in an overall downregulation of mTORC1 downstream events. The inverse correlation between phos S6 expression and tumor pT stage in our cohort is in keeping with our finding of a favorable prognostic effect of higher phos S6 levels in UrCa but is in contrast with prior findings of unfavorable prognostic effect of Phos S6 expression in other solid tumors 19.
Our finding of downregulation of phos S6 in more aggressive UrCa tumors could theoretically be related to their hypoxic tolerant phenotype 36 given prior evidence for downregulation of mTOR pathway through HIF1α activation in hypoxic states 37-39 and given the association of HIF1α overexpression with poor outcome in UrCa 40-44. We are in the process of evaluating HIF1α expression in the current cohort in relation to mTOR pathway expression hoping to test such hypothesis.
In addition to promoting translation, phos S6 is recognized to repress PIK3-AKT pathway through the inhibition of insulin receptor substrates 1 and 2 (IRS1/IRS2) 7. Accordingly, we found phos S6 expression, but not 4E-BP1 to correlate with other AKT-regulated members: p27 and c-Myc. In fact, the strongest correlation among pathway members was between c-Myc and phos S6. Furthermore, high c-Myc expression was an independent predictor of disease progression in our cohort. These results are also in keeping with a recently suggested MYC-dependent mechanism for phos S6 translation 45.
Lower p27 expression in our UrCa tumors and its inverse correlation with pT stage and aggressive divergent histology is in agreement with prior reports 46-48. The significantly positive correlation between p27 and Pten expression levels in our UrCa cohort could be interpreted as additional evidence of a potential oncogenic role for downregulation of p27 in PTEN deficient bladders as it has been recently pointed by Yoo et al 31. Unlike some of the prior studies on p27 in UrCa 46, 48, we did not find loss of p27 expression to be an unfavorable predictor of disease progression or DSS. One possible reason for the difference could be that these studies had a larger proportion of superficial tumors. Interestingly, recent studies have pointed to a favorable effect on outcome for loss of p27 in UrCa especially in combination with other cell cycle markers 21, 49. Additional large cohort studies are needed to better resolve the prognostic role if any of p27 in UrCa patients.
In summary, our study represent the first attempt at simultaneous assessment of key members of PI3K- mTOR pathway and its downstream affected biomarkers, in relation to clinicopathologic parameters and outcome. We found phos S6 expression to be an independent predictor of DSS (p=0.03) and c-Myc expression to be an independent predictor of disease progression (p=0.02) in addition to TNM pathologic stage grouping in our cohort. Our intriguing novel findings of a statistically significant prognostic role for phos S6 and c-Myc in a multivariate model that included established clincopathologic prognostic parameters are promising and certainly warrant further confirmation in an independent cystectomy cohort, preferably in a prospective setting and in combination with cell cycle markers evaluation. Additional studies to address potential value of mTOR pathway markers expression in predicting therapeutic response to mTOR inhibitors are also warrented.
Table 5.
Two multivariate cox-regression analysis models correlating clinicopathological parameters and expression levels of mTOR related biomarkers with disease specific survival, overall survival and disease progression. Model 1 includes TNM stage grouping while Model 2 includes organ confined disease status and lymph node status as a substitute. Both models include aggressive histology concomitant CIS; Phos S6 and c-myc. DSS: disease specific survival, OS: overall survival; phos Akt: phosphorylated Akt; phos S6: phosphorylated S6 protein. HR: Hazard ratio. 95%CI: 95% confidence interval. NS: not significant.
| Multivariate analysis – MODEL 1 | |||
|---|---|---|---|
| Outcome | |||
| DSS | OS | Progression | |
| phos S6 | p=0.03 | p=NS | p=NS |
| HR: -.19 | |||
| 95%CI: -.37 to -.01 | |||
| c-Myc | p=NS | p=NS | p=0.02 |
| HR: -.38 | |||
| 95%CI: -.70 to -.06 | |||
| TNM stage grouping | p=0.004 | p=NS | p=0.01 |
| HR: .11 | HR: .10 | ||
| 95%CI: .03 to .18 | 95%CI: .02 to .18 | ||
| Divergent aggressive histology | p=NS | p=NS | p=NS |
| Concomitant CIS | p=NS | p=NS | p=NS |
| Multivariate analysis – MODEL 2 | |||
| Outcome | |||
| DSS | OS | Progression | |
| phos S6 | p=0.03 | p=NS | p=NS |
| HR: -.21 | |||
| 95%CI: -.40 to -.02 | |||
| c-Myc | p=NS | p=NS | p=0.03 |
| HR: -.34 | |||
| 95%CI: -.67 to -.02 | |||
| Organ confined disease status | p=NS | p=NS | p=0.03 |
| HR: .22 | |||
| 95%CI: .02 to .42 | |||
| Lymph node status | p=NS | p=NS | p=NS |
| Divergent aggressive histology | p=NS | p=NS | p=NS |
| Concomitant CIS | p=NS | p=NS | p=NS |
Acknowledgments
Helen Fedor and Marcella Southerland, Johns Hopkins Tissue Microarray Core Facility.
Supported in part by PO1# CA077664 NCI/NIH grant
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand J Urol Nephrol Suppl. 2008;(218):154–165. doi: 10.1080/03008880802291915. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Manning BD. A complex interplay between akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Viglietto G, Motti ML, Fusco A. Understanding p27(kip1) deregulation in cancer: Down-regulation or mislocalization. Cell Cycle. 2002;1:394–400. doi: 10.4161/cc.1.6.263. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, et al. Prolactin induces c-myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene. 2004;23:7378–7390. doi: 10.1038/sj.onc.1208002. [DOI] [PubMed] [Google Scholar]
- 7.Sabatini DM. mTOR and cancer: Insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Montironi R, Davidson DD, Lopez-Beltran A. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22(2):S70–95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- 9.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 10.Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and akt in papillary thyroid cancer tissues. Endocr J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- 11.Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res. 1996;56:3895–3897. [PubMed] [Google Scholar]
- 12.Tampellini M, Longo M, Cappia S, et al. Co-expression of EGF receptor, TGFalpha and S6 kinase is significantly associated with colorectal carcinomas with distant metastases at diagnosis. Virchows Arch. 2007;450:321–328. doi: 10.1007/s00428-007-0370-2. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Brown RE, Trung CD, et al. Morphoproteomic profile of mTOR, Ras/Raf kinase/ERK, and NF-kappaB pathways in human gastric adenocarcinoma. Ann Clin Lab Sci. 2008;38:195–209. [PubMed] [Google Scholar]
- 14.Hartman TR, Nicolas E, Klein-Szanto A, et al. The role of the birt-hogg-dube protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellvi J, Garcia A, Rojo F, et al. Phosphorylated 4E binding protein 1: A hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–1811. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- 16.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 17.Okada T, Sawada T, Kubota K. Rapamycin inhibits growth of cholangiocarcinoma cells. Hepatogastroenterology. 2009;56:6–10. [PubMed] [Google Scholar]
- 18.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2009 doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 19.Baba HA, Wohlschlaeger J, Cicinnati VR, et al. Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int. 2009;29:399–405. doi: 10.1111/j.1478-3231.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Hage JA, van den Broek LJ, Legrand C, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004;90:1543–1550. doi: 10.1038/sj.bjc.6601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shariat SF, Chade DC, Karakiewicz PI, et al. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol. 2009 doi: 10.1016/j.juro.2009.08.115. [DOI] [PubMed] [Google Scholar]
- 22.Shariat SF, Karam JA, Lerner SP. Molecular markers in bladder cancer. Curr Opin Urol. 2008;18:1–8. doi: 10.1097/MOU.0b013e3282f1c5c1. [DOI] [PubMed] [Google Scholar]
- 23.Wu XR. Urothelial tumorigenesis: A tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 24.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Obata T, Khan Q, Highshaw RA, De Vere White R, Sweeney C. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU Int. 2004;93:143–150. doi: 10.1111/j.1464-410x.2004.04574.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris LD, De La Cerda J, Tuziak T, et al. Analysis of the expression of biomarkers in urinary bladder cancer using a tissue microarray. Mol Carcinog. 2008;47:678–685. doi: 10.1002/mc.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: Implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 30.Qian CN, Furge KA, Knol J, et al. Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: Identification in human tumors and confirmation in animal models. Cancer Res. 2009;69:8256–8264. doi: 10.1158/0008-5472.CAN-09-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo LI, Liu DW, Le Vu S, Bronson RT, Wu H, Yuan J. Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res. 2006;66:1929–1939. doi: 10.1158/0008-5472.CAN-05-1986. [DOI] [PubMed] [Google Scholar]
- 32.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koksal IT, Yasar D, Dirice E, et al. Differential PTEN protein expression profiles in superficial versus invasive bladder cancers. Urol Int. 2005;75:102–106. doi: 10.1159/000085933. [DOI] [PubMed] [Google Scholar]
- 34.Bose S, Chandran S, Mirocha JM, Bose N. The akt pathway in human breast cancer: A tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 36.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 37.Connolly E, Braunstein S, Formenti S, Schneider RJ. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol. 2006;26:3955–3965. doi: 10.1128/MCB.26.10.3955-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tinton SA, Buc-Calderon PM. Hypoxia increases the association of 4E-binding protein 1 with the initiation factor 4E in isolated rat hepatocytes. FEBS Lett. 1999;446:55–59. doi: 10.1016/s0014-5793(99)00185-4. [DOI] [PubMed] [Google Scholar]
- 39.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 40.Chai CY, Chen WT, Hung WC, et al. Hypoxia-inducible factor-1alpha expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J Clin Pathol. 2008;61:658–664. doi: 10.1136/jcp.2007.050666. [DOI] [PubMed] [Google Scholar]
- 41.Zhou JT, Cai ZM, Li NC, Na YQ. Expression of hypoxia inducible factor-1alpha and glucose transporter protein 1 in renal and bladder cancers and the clinical significance thereof. Zhonghua Yi Xue Za Zhi. 2006;86:1970–1974. [PubMed] [Google Scholar]
- 42.Ioachim E, Michael M, Salmas M, Michael MM, Stavropoulos NE, Malamou-Mitsi V. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha expression in bladder cancer and their associations with other angiogenesis-related proteins. Urol Int. 2006;77:255–263. doi: 10.1159/000094819. [DOI] [PubMed] [Google Scholar]
- 43.Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1alpha and glut-1 in human bladder cancer. Oncol Rep. 2005;14:909–913. doi: 10.3892/or.14.4.909. [DOI] [PubMed] [Google Scholar]
- 44.Theodoropoulos VE, Lazaris AC, Sofras F, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46:200–208. doi: 10.1016/j.eururo.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt EV, Ravitz MJ, Chen L, Lynch M. Growth controls connect: Interactions between c-myc and the tuberous sclerosis complex-mTOR pathway. Cell Cycle. 2009;8:1344–1351. doi: 10.4161/cc.8.9.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbani F, Koppie TM, Charytonowicz E, Drobnjak M, Bochner BH, Cordon-Cardo C. Prognostic significance of p27(Kip1) expression in bladder cancer. BJU Int. 2007;100:259–263. doi: 10.1111/j.1464-410X.2007.06927.x. [DOI] [PubMed] [Google Scholar]
- 47.Sgambato A, Migaldi M, Faraglia B, et al. Loss of P27Kip1 expression correlates with tumor grade and with reduced disease-free survival in primary superficial bladder cancers. Cancer Res. 1999;59:3245–3250. [PubMed] [Google Scholar]
- 48.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481–7. doi: 10.1016/j.juro.2006.09.038. discussion 487. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblatt R, Jonmarker S, Lewensohn R, et al. Current status of prognostic immunohistochemical markers for urothelial bladder cancer. Tumour Biol. 2008;29:311–322. doi: 10.1159/000170878. [DOI] [PubMed] [Google Scholar]