Abstract
The control of infectious bovine rhinotracheitis induced by bovine herpesvirus 1 (BoHV-1) requires sensitive and specific diagnostic assays. As BoHV-1 is antigenically and genetically related to four other alphaherpesviruses of ruminants—namely, BoHV-5, caprine herpesvirus 1 (CpHV-1), cervine herpesvirus 1 (CvHV-1) and CvHV-2—diagnostic tests able to discriminate BoHV-1 from these related viruses are needed to avoid misdiagnosis, especially because some of these viruses are able to cross the species barrier. In this study, murine monoclonal antibodies (MAbs) specific for BoHV-1, BoHV-5, CpHV-1, CvHV-1, and CvHV-2 were produced with the aim of setting up an immunofluorescence assay able to discriminate between these related herpesviruses. Produced MAbs were selected for their viral specificity by enzyme-linked immunosorbent assay and indirect immunofluorescence staining of virus-infected cells. Radioimmunoprecipitation characterization of the selected MAbs revealed that four of them are directed against glycoprotein C (gC) and one of them is directed against gD of these related viruses. The obtained results demonstrate that the antibodies produced allow an unambiguous discrimination of each of the four alphaherpesviruses related to BoHV-1.
Bovine herpesvirus 1 (BoHV-1) is a worldwide pathogen of cattle associated with a variety of clinical diseases, including infectious bovine rhinotracheitis (IBR), infectious pustular vulvovaginitis, and infectious pustular balanoposthitis (17, 26, 69). Due to the significant losses in the cattle industry, there is an increased interest in the control and eradication of IBR. In this context, it is of primary importance to take into consideration the potential risk of infection of cattle with heterologous ruminant alphaherpesviruses closely related to BoHV-1. Four ruminant alphaherpesviruses are related to BoHV-1 and have a potential for cross-infection of cattle in Europe: BoHV-5, caprine herpesvirus 1 (CpHV-1), cervine herpesvirus 1 (CvHV-1), and CvHV-2. Although not present in Europe, buffalo herpesvirus 1 and elk herpesvirus are also closely related to BoHV-1 (5, 11). BoHV-5 is responsible for fatal meningoencephalitis reported in calves in several countries, including Argentina, Hungary, Italy, Australia, and the United States (1, 7, 19, 20). CpHV-1 causes enteritis and generalized infection in neonatal kids. Although most infections in adults are subclinical, CpHV-1 can induce vulvovaginitis (23, 25), balanoposthitis (61), or abortion (4, 29). This virus was first isolated in the 1970s from young kids with a severe generalized infection in California (57) and Switzerland (42). CpHV-1 infections occur worldwide but with high prevalence in Mediterranean countries (24, 28, 30, 32, 62). CvHV-1 was first isolated in 1982 from an outbreak of ocular disease on a red deer farm in Scotland (27, 45). The virus is widespread in free-living and farmed red deer (45). CvHV-2 was isolated from reindeer in Finland (14, 15), and serological evidence of infection with a virus related to BoHV-1 has been reported in reindeer in the United States (12) but also in caribou from Canada (16). Although these viruses differ considerably in their virulence, they are closely related both genetically (18, 51, 53, 55, 66) and antigenically (28, 36, 39, 47). Moreover, all these viruses establish latent infection in a manner similar to that of BoHV-1 (3, 6, 15, 52).
Some experiments have shown that the related herpesviruses described above are able to cross the species barrier and establish infection in heterologous animal species. For example, CpHV-1 can infect cattle, but reactivation of latent CpHV-1 was not detected in cattle although viral CpHV-1 DNA was detected in trigeminal ganglia of cattle (60). Experimental infection of goats with BoHV-1 clearly showed that this virus is able to infect the heterologous host and establish latent infection (60). Moreover, BoHV-1 has been isolated from naturally infected goats (64). Natural BoHV-1 infection and experimental BoHV-5 infection in sheep have also been reported (2, 56, 59, 65). Cattle were refractory to CvHV-1 by intranasal challenge but successfully infected with CvHV-2 (46, 63). Red deer could become infected following a profound BoHV-1 challenge, whereas reindeer did not seem susceptible to BoHV-1 (46).
The capacity of these related herpesviruses to circulate in the ruminant population is a major threat to a BoHV-1 eradication scheme. Indeed infection by related viruses could lead to false-positive diagnosis of BoHV-1. Moreover, the heterologous ruminant species could serve as a BoHV-1 reservoir. Consequently, diagnostic tools able to distinguish all these related viruses are needed. Successful control of IBR relies on sensitive and specific diagnostic methods. Since BoHV-1 and related viruses (BoHV-5, CpHV-1, CvHV-1, and CvHV-2) cross-react in enzyme-linked immunosorbent assay (ELISA) and seroneutralization tests (36, 39), the available serological tests are unable to discriminate between the related viruses. Only one method was described to distinguish BoHV-1- from BoHV-5-positive sera (67). Tests based on virus isolation or viral DNA detection could avoid misinterpretation due to these cross-reactions. Restriction endonuclease analysis (REA) performed after viral isolation allowed the identification and distinguishing between the related viruses (17, 30, 49, 52, 68). However, this procedure is too burdensome for general use in diagnostic laboratories. Specific methods using viral DNA amplification were also described (37, 53). In these PCR assays a pair of consensus primers was selected for the detection of bovine, caprine, and cervine herpesviruses, and discrimination was achieved by subsequent REA of PCR products. A specific PCR system using four primer pairs has also been developed to specifically amplify a part of the glycoprotein C (gC) gene (54). However, a specific diagnostic method based on antigen detection is not available so far to differentiate between these alphaherpesvirus infections.
Since viral isolation and subsequent characterization using PCR and/or REA are often laborious, expensive, and time-consuming, we undertook this study to develop an immunofluorescence assay able to differentiate in one step between the five related herpesviruses.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
Herpesviruses used in this study are listed in Table 1. They were propagated in Madin-Darby bovine kidney (MDBK) (ATCC CCL-22) cells grown in Earle's minimal essential medium (MEM) (Invitrogen S.A., Merelbeke, Belgium) supplemented with 2% penicillin (5,000 U/ml)-streptomycin (5,000 μg/ml) (PS; Invitrogen S.A.) and 5% heat-inactivated fetal bovine serum (BioWhittaker, Verviers, Belgium).
TABLE 1.
Ruminant alphaherpesvirus strains used in this study
Viral stocks were produced by infection of confluent MDBK cells at a multiplicity of infection (MOI) of 0.1 in MEM supplemented with 2% PS and 5% heat-inactivated horse serum (BioWhittaker). When the cytopathic effect reached 90%, the culture medium was removed and clarified by centrifugation at 1,500 × g for 20 min. The supernatants were aliquoted, frozen at −80°C, and titrated by plaque assay on MDBK cells as previously described (33).
One BoHV-5 monoclonal antibody (MAb) (2915) (21) was kindly provided by M. Engels (Faculty of Veterinary Medicine, University of Zurich, Zurich, Switzerland). The following reference BoHV-1 MAbs were kindly provided by G. J. Letchworth: MAb 3402 is directed against BoHV-1 gD, MAb 4807 is directed against BoHV-1 gB, and MAb 1507 is directed against BoHV-1 gC (38). A serum sample from a rabbit immunized with a previously described recombinant human adenovirus type 5 expressing the BoHV-1 gD (22) and that was found to cross-react with the five related herpesviruses (data not shown) was also used.
Virus purification for immunization.
BoHV-1 LA, BoHV-5, CpHV-1 E/CH, CvHV-1, and CvHV-2 were propagated on MDBK cells. When the cytopathic effect reached 90%, the culture medium was clarified by centrifugation at 1,500 × g for 20 min. Viruses were pelleted at 100,000 × g for 45 min at 4°C in a Beckman Optima LE 80K centrifuge. The viral pellets were resuspended in phosphate-buffered saline (PBS) and centrifuged at 100,000 × g through a 10 to 25% Ficoll (Amersham, Biosciences Europe GmbH, Roosendal, The Netherlands) step gradient for 60 min at 4°C. The virus band at the 10 to 25% Ficoll interface was gently removed by pipetting, resuspended in PBS, and stored at −80°C until used. The protein concentration was determined with the BCA protein assay kit (Pierce Chemical Company, Rockford, Ill.).
MAb production.
Five groups of two BALB/c mice were immunized by three intraperitoneal injections of purified BoHV-1, BoHV-5, CpHV-1, CvHV-1, or CvHV-2 (50 μg/mouse for each injection) emulsified in Freund's complete adjuvant for the two first injections and incomplete Freund's adjuvant for the last one. Injections were spaced at 3 weeks. After a further 21 days mice were boosted by intraperitoneal injection of antigen (30 μg of viral proteins without adjuvant). Fusions were performed 4 days after the boost according to the method of Köhler and Milstein (31). Splenocytes were fused with cell line SP2/0-Ag 14 (ATCC CRL-8287) using polyethylene glycol 1500 (Roche Diagnostics Belgium, Vilvoorde, Belgium).
Ascitic fluids were produced by intraperitoneal injection of hybridoma cells in BALB/c mice treated with pristane 7 to 15 days before. MAbs from immunoglobulin G (IgG) isotype were purified on protein G-Sepharose (Amersham, Biosciences Europe GmbH) according to the manufacturer's instructions. All these procedures were performed in accordance with Belgian law on animal care and were approved when the experiments were performed.
ELISA for antibody screening.
Hybridomas secreting specific antibodies raised against viral antigens were identified by indirect ELISA. Preparation of viral antigens was performed as described above for virus purification with the exception of the Ficoll gradient step. Ninety-six-well microplates (MaxiSorb; Nunc) were coated overnight with either infected or noninfected MDBK cell lysate, used as viral and negative control antigen, respectively, and diluted in 50 mM carbonate buffer-3 mM sodium azide, pH 9.6, to obtain the final concentration of 500 ng of antigen per well. After a wash step with 0.15 M NaCl containing 0.01% Tween 80 (washing solution), the wells were saturated with PBS containing 3% bovine albumin and 0.1% Tween 80 at 37°C for 3 h. After each incubation, wells were extensively washed with washing solution. Fifty microliters of hybridoma supernatants was added, and plates were incubated for 1 h at 37°C. Binding antibodies were detected by an incubation of 60 min at room temperature with horseradish peroxidase-conjugated rabbit anti-mouse IgG (Dako Diagnostics N.V., Heverlee, Belgium) 2,000-fold diluted in PBS, 0.5 mM EDTA, and 0.1% Tween 80 containing 4% casein hydrolysate. After addition of 100 μl of 3,3′,5,5′-tetramethyl-benzidine (Biosource Europe S.A., Nivelles, Belgium) as substrate-chromogen, the microtiter plate was incubated 20 min at room temperature. Fifty microliters of H2SO4 (2 N) was subsequently added to stop the reaction. The optical densities were measured at 450 nm in a microplate reader (MIOS; Merck).
Determination of the immunoglobulin subclasses.
The immunoglobulin isotypes were determined using a commercial kit (Isostrip; Roche) following the manufacturer's recommendations.
Immunofluorescence assay.
In order to set up an immunofluorescence assay, MAbs were first selected by flow cytometric analysis. MDBK cells were infected with the five related viruses at an MOI of 2 in MEM containing 4% horse serum. Fifteen hours after infection cells were incubated 30 min at 37°C in PBS containing EDTA (0.5 mM), resuspended, extensively washed with PBS containing 10% fetal calf serum (BioWhittaker) (PBSF), and incubated 45 min on ice with PBSF containing the tested antibody (undiluted for hybridoma supernatant or 1,000-fold diluted for ascitic fluid). After two additional washes in PBSF, cells were incubated 30 min on ice with PBSF containing fluorescein isothiocyanate (FITC)-conjugated rabbit immunoglobulin anti-mouse IgG (2 μg/ml; Dako) and analyzed with a FACStar Plus cytometer (Becton & Dickinson) after a final PBS wash.
An immunofluorescence assay was performed as described by Schynts et al. (58) with minor modifications. Briefly, MDBK cells grown on glass coverslips (Assistent) were infected with the different viruses and incubated 48 h in MEM containing 4% horse serum and 0.6% carboxymethylcellulose. The coverslips with individual plaques were fixed in PBS containing 2% (wt/vol) paraformaldehyde and incubated with undiluted hybridoma supernatant or 1,000-fold-diluted ascitic fluid in PBSF. Bound antibodies were revealed by FITC-conjugated rabbit anti-mouse IgG (2 μg/ml; Dako). Coverslips were mounted with a Prolong Antifade kit (Molecular Probes Europe BV, Leiden, The Netherlands). Pictures were collected with a charge-coupled device Leica DC 300F camera (with Leica IM 50 V1.20 software) installed on an epifluorescence microscope.
RIPA.
Confluent MDBK cells were infected with BoHV-1 Jura, BoHV-5, CpHV-1 E/CH, CvHV-1, or CvHV-2 at an MOI of 1 in MEM. After 90 min of adsorption, the monolayers were overlaid with either methionine-free medium (ICN Biomedicals S.A., Asse-Relegem, Belgium) or glucose-reduced (10%) medium (Gibco). Five hours after infection, cells that were previously overlaid with methionine-free MEM were labeled with a 25-μCi/ml concentration of Tran35S-label methionine (ICN), whereas cells overlaid with glucose-reduced MEM were labeled with [3H]glucosamine (50 μCi/ml; Amersham). For glycosylation inhibition experiments, tunicamycin (1 μg/ml; Sigma-Aldrich N.V., Bornem, Belgium) was added from the time of inoculation with the virus until infected cells were collected. Twenty-four hours after infection cells were washed in PBS, disrupted with radioimmunoprecipitation assay (RIPA) buffer [0.15 M NaCl, 0.05 M Tris-HCl (pH 7.2), 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1 mM EDTA, 0.1% NaN3, 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride] (RIPA I) and centrifuged at 20,000 × g for 60 min at 4°C. Cells lysates were stored at −80°C. Immunoprecipitation was performed as described by Dubuisson et al. (13) with minor modifications. Briefly, protein A-Sepharose CL-4B beads (Amersham) coated with rabbit IgG anti-mouse immunoglobulin (Dako) were incubated for 60 min with 500 μl of hybridoma supernatant or 30 μl of ascitic fluid. Unbound material was removed by washing beads twice with phosphate buffer (0.1 M, pH 8.1). Beads were then saturated 90 min with unlabeled uninfected cell lysates and incubated for 90 min with radiolabeled cell lysates. Beads were successively washed with RIPA I buffer, water, and RIPA II buffer (0.05 M Tris-HCl [pH 7.2], 0.005 M NaCl), and resuspended in NuPage sample buffer containing reducing agent before electrophoresis on 4 to 12% NuPage Bis-Tris acrylamide gel under reducing conditions according to the instructions of the manufacturer (Invitrogen). 14C-labeled molecular weight standard (Amersham) was used as the molecular weight marker. Polyacrylamide gels were dried under vacuum prior to autoradiography on Hyperfilm MP (Amersham).
RESULTS
MAb reactivity against the five related alphaherpesviruses.
With the aim to identify specific MAbs for each of the five related alphaherpesviruses, a first screening of the produced MAbs was performed by either ELISA (for MAbs obtained after BoHV-5, CpHV-1, CvHV-1, and CvHV-2 immunizations) or indirect immunofluorescence staining revealed by fluorescence-activated cell sorting (FACS) (for MAbs obtained after BoHV-1 immunization). Results (shown in Table 2) indicated that most of the MAbs cross-reacted with one or more related alphaherpesviruses. However, among 14 MAbs obtained after BoHV-1 immunization, 4 (G13G1D3B10, G14G11F5, G6B7G2, and G6D7G2) reacted only with BoHV-1 (Table 2). Among six MAbs resulting from BoHV-5 immunization, two (2D6 and 2H7) distinguished BoHV-5 from the other related viruses (Table 2). Among eight MAbs generated after CpHV-1 immunization, seven reacted only against CpHV-1. Two of these MAbs (2E5G5G1 and 5F11G7D8) were selected for the following experiments because they generate the highest optical densities by ELISA (data not shown). From five MAbs obtained after CvHV-1 immunization, two (6C2 and 6C3) were CvHV-1 specific. From the CvHV-2 immunization, eight MAbs were obtained. Results indicated that two of these MAbs were CvHV-2 specific (2A6 and 5D9).
TABLE 2.
Reactivities of the produced MAbs against related herpesvirusesd
| Immunization virus | MAb designation | Isotypeb | Specificityc
|
||||
|---|---|---|---|---|---|---|---|
| BoHV-1 | BoHV-5 | CpHV-1 | CvHV-1 | CvHV-2 | |||
| BoHV-1 | G12H3D10 | IgG1 | + | + | − | − | − |
| G13G1D3B10a | IgG2a | + | − | − | − | − | |
| G14G11F5 | IgG2b | + | − | − | − | − | |
| G16B6G10 | IgG2a | + | + | + | + | + | |
| G16F3A11 | IgG2a | + | + | − | + | − | |
| G17D8E9 | IgG1 | + | + | − | − | − | |
| G1G7G8 | IgG2a | + | + | − | + | + | |
| G3D7E8D12 | IgG1 | + | + | − | − | − | |
| G5C12F2 | IgG1 | + | + | − | + | + | |
| G6B7G2 | IgG1 | + | − | − | +/− | − | |
| G6B9E7 | IgG1 | + | + | − | − | − | |
| G6D7G2 | IgG1 | + | − | − | +/− | − | |
| G6G5G2 | IgG2a | + | + | + | + | − | |
| G6H4D6 | IgG2a | + | + | + | + | + | |
| BoHV-5 | 2D2 | ND | + | + | − | − | − |
| 2D6 | IgM | − | + | − | − | − | |
| 2H7 | IgG1 | − | + | − | − | − | |
| 3D10 | IgG1 | + | + | + | + | + | |
| 4E10 | NT | + | + | − | − | − | |
| 5D6 | NT | + | + | − | − | + | |
| CpHV-1 | 2E5E1 | IgG2a | − | − | + | − | − |
| 2E5G5G1 | IgG2a | − | − | + | − | − | |
| 3A4D9 | IgG2a | − | − | + | − | − | |
| 3A4C8G8 | IgG2a | − | − | + | − | + | |
| 4G2H2 | IgG2a | − | − | + | − | − | |
| 4G2G5G1 | IgG2a | − | − | + | − | − | |
| 5F11G10 | IgG2a | − | − | + | − | − | |
| 5F11G7D8 | IgG2a | − | − | + | − | − | |
| CvHV-1 | 1B7 | IgG1 | + | + | + | + | + |
| 5D3 | ND | + | + | + | + | + | |
| 6B9 | ND | − | + | − | + | − | |
| 6C2 | IgG3 | − | − | − | + | − | |
| 6C3 | ND | − | − | − | + | − | |
| CvHV-2 | 2A6 | IgG1 | − | − | − | − | + |
| 3A8 | NT | + | − | +/− | +/− | + | |
| 3F3 | NT | +/− | − | − | − | + | |
| 4B11 | ND | − | − | +/− | − | + | |
| 4G5 | NT | +/− | − | − | − | + | |
| 5B12 | NT | − | − | − | +/− | + | |
| 5D9 | IgG1 | − | − | − | − | + | |
| 5G10 | IgG1 | − | − | − | + | + | |
Boldface type indicates MAbs used in the next experiments.
Abbreviations: ND, not determined; NT, not tested.
Symbols: +, positive signal; −, negative signal; +/−, weak signal.
BoHV-1 MAb reactivity was tested by FACS analysis, and other MAb reactivities were tested by ELISA.
Selection of discriminative MAbs by indirect immunofluorescence labeling.
In order to set up a simple discriminative immunofluorescence test that could be performed on plaques resulting from infection with one of the five related viruses, MAbs selected for their specificity (shown in Table 2) were further analyzed by indirect immunofluorescence labeling revealed by FACS (Table 3). Since no permeabilization step was performed on infected cells prior to immunofluorescence staining and FACS analysis, this methodology allowed the detection of cell surface antigens. From the four BoHV-1 selected MAbs, two (G13G1D3B10 and G14G11F5) clearly and specifically stained BoHV-1-infected cells. However, we selected MAb G13G1D3B10 because the immunofluorescence generated by this MAb, as determined by FACS analysis, was greater than that generated by MAb G14G11F5. In contrast to results depicted in Table 2, obtained after ELISA analysis, the two selected BoHV-5 MAbs (2D6 and 2H7) were not BoHV-5 specific since they cross-reacted with one or more other related alphaherpesviruses when analyzed by FACS. For this reason, a previously described MAb (MAb 2915) (21) was tested and was demonstrated to be BoHV-5 specific. From the two CpHV-1 selected MAbs (2E5G5G1 and 5F11G7D8), only one (2E5G5G1) specifically stained CpHV-1-infected cells. Both CvHV-1 selected MAbs (6C2 and 6C3) were CvHV-1 specific, but only 6C2 MAb clearly stained CvHV-1-infected cells. Finally, none of the CvHV-2 selected MAbs was demonstrated to be CvHV-2 specific when tested by FACS analysis. To encounter this apparent problem, we selected CvHV-2 5G10 MAb even if it cross-reacted with CvHV-1. According to these results, five MAbs (shown in Table 3) were selected to test their ability to discriminate between the five related alphaherpesviruses in an immunofluorescence test performed on virus induced plaques.
TABLE 3.
Reactivities of selected MAbs against related herpesviruses as determined by FACS analysis
| Immunization virus | MAb designation | Reactivity with virus used for cell infectionsb
|
||||
|---|---|---|---|---|---|---|
| BoHV-1 | BoHV-5 | CpHV-1 | CvHV-1 | CvHV-2 | ||
| BoHV-1 | G13G1D3B1a | + | − | − | − | − |
| G14G11F5 | + | − | − | − | − | |
| G6B7G2 | + | − | − | +/− | − | |
| G6D7G2 | + | − | − | +/− | − | |
| BoHV-5 | 2D6 | + | + | + | + | + |
| 2H7 | − | + | − | + | − | |
| 2915c | − | + | − | − | − | |
| CpHV-1 | 2E5G5G1 | − | − | + | − | − |
| 5F11G7D8 | − | − | − | − | − | |
| CvHV-1 | 6C2 | − | − | − | + | − |
| 6C3 | − | − | − | +/− | − | |
| CvHV-2 | 2A6 | + | + | + | + | + |
| 5D9 | + | + | + | + | + | |
| 5G10 | − | − | − | + | + | |
Boldface type indicates MAbs selected to be assayed by immunofluorescence labeling on virus-induced plaques.
Symbols: +, positive signal; −, negative signal; +/−, weak signal.
Kindly provided by M. Engels (Faculty of Veterinary Medicine, University of Zürich, Zürich, Switzerland).
Differential diagnosis of bovine, caprine, and cervine alphaherpesviruses.
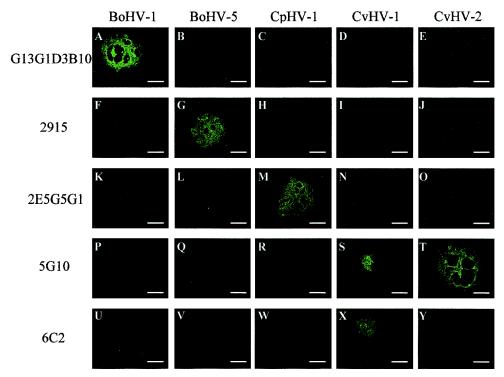
The five selected MAbs were tested by immunofluorescence assay on cells infected separately with the five related alphaherpesviruses (Fig. 1). The immunofluorescence assay was performed on infected cell monolayers where well-separated plaques were observed by transmission light microscopy. Each MAb specifically detected one virus species, except MAb 5G10, which detected both CvHV-2 and CvHV-1 (Fig. 1S and T) as predicted by FACS analysis (Table 3). Finally, in order to determine whether the selected MAbs are specific for viral strains other than those we used for mice immunizations, they were also assayed on cells infected with different BoHV-1 and CpHV-1 strains. Unfortunately, we were unable to perform the same experiments on different strains of BoHV-5, CvHV-1, and CvHV-2 because only a few strains of these viral species are available. Results demonstrated that the specificity of the selected MAbs was similar to those depicted in Fig. 1 when tested on cells infected with the different BoHV-1 and CpHV-1 strains. Taken together these results demonstrate that the five selected MAbs are powerful tools to discriminate unambiguously between bovine, cervine, and caprine alphaherpesviruses.
FIG. 1.
Indirect immunofluorescence staining of MDBK cells infected with BoHV-1 (A, F, K, P, and U), BoHV-5 (B, G, L, Q, and V), CpHV-1 (C, H, M, R, and W), CvHV-1 (D, I, N, S, and X), or CvHV-2 (E, J, O, T, and Y). Cells were incubated until viral plaques were visible and were then treated as described in Materials and Methods. G13G1D3B10 (A to E), 2915 (F to J), 2E5G5G1 (K to O), 5G10 (P to T), and 6C2 (U to Y) were the primary antibodies and were detected by FITC-conjugated rabbit immunoglobulin anti-mouse IgG. Presence of viral plaque was verified by transmission microscopy before epifluorescence microscopy. All immunofluorescence stainings were performed three times. Bars, 200 μm.
Identification of viral antigens targeted by the discriminative MAbs.
To identify viral antigens targeted by the five discriminative MAbs depicted in Fig. 1, [35S]methionine-labeled proteins from cells infected separately with the five related herpesviruses were analyzed by RIPA. Because it was previously shown that BoHV-1 immunization of mice leads mainly to the production of MAbs directed against BoHV-1 major glycoproteins, the reactivities of the five MAbs in this RIPA experiment were compared with those of previously described MAbs directed against BoHV-1 gB (MAb 4807), gC (MAb 1507), and gD (MAb 3402) major glycoproteins (38).
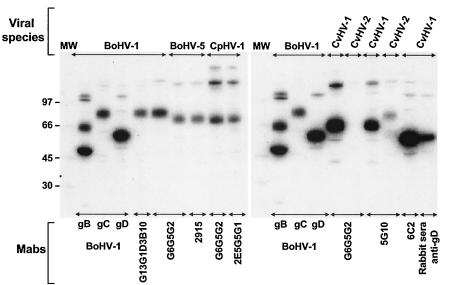
As demonstrated by results presented in Fig. 2, MAb G13G1D3B10 (BoHV-1 specific) is directed against BoHV-1 gC since this MAb and the anti-BoHV-1 gC reference MAb precipitated a single peptide with a similar molecular weight. Moreover, MAbs 2915 (BoHV-5 specific), 2E5G5G1 (CpHV-1 specific), and 5G10 (CvHV-2 specific) precipitated a single peptide that exhibited a molecular weight slightly different from but approximately equivalent to that of BoHV-1 gC, suggesting that these three MAbs are directed against gC of BoHV-5, CpHV-1, and CvHV-2, respectively. However, due to the fact that the BoHV-1 anti-gC reference MAb does not cross-react with these three related viral species (data not shown), we were unable to use it to demonstrate that the peptides precipitated by MAb 2915, 2E5G5G1, and 5G10 are the gC homologues of BoHV-1. To solve this problem, we screened by RIPA some of the previously nonselected MAbs (shown in Table 2) to find an anti-gC MAb which cross-reacts with BoHV-5, CpHV-1, and CvHV-2. As shown in Fig. 2, we found that MAb G6G5G2 is directed against BoHV-1 gC since this MAb and the anti-BoHV-1 gC reference MAb precipitated a single peptide with a similar molecular weight. MAb G6G5G2 was then used in RIPA to precipitate gC from BoHV-5-, CpHV-1-, and CvHV-2-infected cells to demonstrate that the single peptide precipitated by MAb 2915, 2E5G5G1, and 5G10 are the gC homologues of BoHV-1. Results demonstrated that MAbs 2915 and 2E5G5G1 precipitated a single peptide with a molecular weight similar to that precipitated by MAb G6G5G2 on cells infected with BoHV-5 and CpHV-1, respectively, while no CvHV-2 peptide was precipitated by MAb G6G5G2, as predicted by results presented in Table 2. However, we reasoned that the CvHV-2 peptide precipitated by 5G10 is most probably the CvHV-2 homologue of BoHV-1 gC, because 5G10 MAb (i) reacts with both CvHV-1 and CvHV-2 and (ii) is directed against CvHV-1 gC homologue since MAb 5G10 and MAb G6G5G2 precipitated an equivalent peptide.
FIG. 2.
[35S]methionine-labeled peptides immunoprecipitated from BoHV-1-, BoHV-5-, CpHV-1-, CvHV-1-, and CvHV-2-infected cells and analyzed by electrophoresis on 4 to 12% NuPage Bis-Tris acrylamide gel under reducing conditions. Peptides were precipitated by selected MAbs G13G1D3B10, 2915, 5G10, 2E5G5G1, 6C2, gC MAb (G6G5G2); by previously described MAbs gB 4807, gC 1507, gD 3402; and by serum from a rabbit immunized with recombinant human adenovirus type 5 expressing BoHV-1 gD, which cross-reacts with all the related viruses. MW, 14C-labeled molecular weight marker (Amersham), expressed in thousands.
Finally, MAb 6C2, which was demonstrated to be CvHV-1 specific, precipitated a peptide with a molecular weight approximately similar to that of BoHV-1 gD. To demonstrate that MAb 6C2 precipitated the CvHV-1 homologue of BoHV-1 gD, we used a cross-reactive polyclonal rabbit anti-gD sera (24). Results indicated that MAb 6C2 is directed against a CvHV-1 gD homologue since this MAb and the rabbit anti-gD sera precipitated a peptide with a similar molecular weight. In summary, results obtained in these RIPA experiments demonstrate that G13G1D3B10, 2915, 2E5G5G1, 5G10, and 6C2 are directed against BoHV-1 gC, BoHV-5 gC, CpHV-1 gC, CvHV-2 gC, and CvHV-1 gD, respectively.
It is noteworthy that no tested MAbs precipitated peptides when they were analyzed by RIPA after labeling of mock-infected cells. Moreover, RIPA experiments performed after viral protein labeling using [3H]glucosamine or after the use of a glycosylation inhibitor (tunicamycin) during infection confirmed that all tested MAbs were directed against glycoproteins (data not shown).
DISCUSSION
Herpesviruses have generally coevolved with their hosts (10, 40, 41). Consequently, phylogenetically related host species can be infected by phylogenetically and antigenically related herpesviruses. Illustrating this concept, at least five alphaherpesviruses of ruminants have been shown to be genetically and antigenically related, namely, BoHV-1, BoHV-5, CvHV-1, CvHV-2, and CpHV-1. The existence of antigenic cross-reactions between these viruses added to their ability to establish, at least in some cases, cross-species infections needed to be considered in the development of a BoHV-1 eradication scheme. In addition, diagnostic tools able to discriminate all these related viruses need to be developed. With the latter goal in mind, the present study was devoted to the production of MAbs able to identify specifically each of the five related alphaherpesviruses. Among these MAbs, five were selected for their viral specificity by ELISA and FACS analysis before being assayed by immunolabeling of cells infected separately with the five related herpesviruses. Our results demonstrate that the five selected MAbs unambiguously discriminate between bovine, caprine, and cervine alphaherpesviruses. RIPA characterization of the selected MAbs revealed that four of them are directed against the gC and one of them is directed against the gD homologues of these related viruses.
Approximately half of the produced MAbs cross-reacted with one or more related viruses (Table 2). This observation confirms previous studies that reported a very high antigenic relationship between the five related viruses (47, 39, 36). Identification of the antigens targeted by the selected MAbs demonstrated that four MAbs (G13G1D3B10, 2915, 2E5G5G1, and 5G10) are directed against BoHV-1, BoHV-5, CpHV-1, and CvHV-2 gC homologues, respectively. BoHV-5, CpHV-1, and CvHV-2 gC exhibited a lower apparent molecular weight than BoHV-1 gC, confirming previous observations that demonstrated a lower molecular weight for BoHV-5 gC (9). Using MAbs that react against BoHV-1, BoHV-5, and CpHV-1 (gB, gC, and gD), Friedli and Metzler (21) also found differences in the sizes of the corresponding viral proteins, including variation in the apparent molecular weight of gC, similar to results reported here. These variations could probably result from differences in the open reading frame length but especially from differences in the type of glycosylation and number of glycosylation sites as previously described for BoHV-5 gC (8). The comparison of the peptides precipitated by 6C2 MAb and serum from a rabbit immunized with recombinant human adenovirus type 5 expressing BoHV-1 gD, which cross-reacts with all the related viruses, revealed that 6C2 MAb is directed against CvHV-1 gD. Because gCs of the related viruses were shown to be genetically and antigenically less conserved than gBs or gDs of the same viruses (9, 53, 54, 55), it is not surprising that the majority of the discriminative MAbs are directed against gC, although MAb G6G5G1 cross-reacts with gC of all the related viruses except CvHV-2. The comparison of the related herpesvirus gC coding sequences has revealed a conserved central part of the gC gene, while the N-terminal part was highly variable, with large deletions and insertions (54). Consequently, the specific MAbs produced during this study were probably directed against epitopes present in the variable region, while the cross-reactive MAb is probably directed against a common epitope from the gC conserved region. Moreover, glycoprotein gC plays an important but not essential role in attachment of BoHV-1 to the targeted cells (48, 35). It could then be hypothesized that these differences in the gC amino acid sequence may correlate with in vivo tropism differences. Additional experiments are required to identify the epitopes recognized by the discriminative MAbs produced during this study.
Until now, PCR and/or REA (17, 29, 30, 37, 49, 52, 53, 55, 68) were the only available methods able to discriminate between these closely related herpesviruses. The antigen detection method developed here is a complementary tool to detect and identify ruminant herpesviruses. This new method is easy to perform, inexpensive, and faster than an REA performed after viral isolation. MAbs are directly useful for detection and identification of the related viruses from antemortem samples (after viral isolation during both the excretion and the reexcretion period) and from postmortem samples (in tissue sections or in triturated organs after virus isolation). Contrary to PCR, immunofluorescence assay is unfortunately unable to detect viruses at latency sites in postmortem samples. However, the immunofluorescence assay can detect and discriminate between viruses after experimental reactivation and viral isolation. Moreover, MAbs developed here could be useful to perform immunolabeling on organ sections. In summary, PCR and immunofluorescence assay are complementary tools able to discriminate the related viruses in latently infected animals at excretion or reexcretion time or from postmortem samples but not in the no-(re-)excretion period.
Until now, no diagnostic tests have been available to discriminate between animals latently infected with one of the five related herpesviruses. Therefore, the availability of the produced MAbs in this study is also an opportunity to develop discriminative ELISAs which could serologically differentiate BoHV-1 infection from other related viruses in latent carriers. Most of the discriminative MAbs are directed against gC, and this glycoprotein shows an extensive variation between members of the ruminant herpesvirus group. Therefore, gC-blocking ELISAs using recombinant glycoproteins containing the variable regions of gC as antigens are potential strategies to serologically detect latently infected animals.
The newly developed immunofluorescence assay is useful in epidemiological situations near areas of IBR eradication or where IBR is eradicated from a region or a country. In these cases, each BoHV-1-positive diagnosis should be confirmed by a test able to differentiate between the five alphaherpesvirus infections. Special concerns are CvHV-1 in the United Kingdom and continental Europe; CvHV-2 infection in Scandinavia and North America; and CpHV-1 in North America, Switzerland, and Mediterranean countries. Further developments should focus on the design of a discriminative ELISA for serological testing of these five alphaherpesvirus infections.
Acknowledgments
A. Vanderplasschen is a Senior Research Associate of the “Fonds National Belge de la Recherche Scientifique” (FNRS).
We are thankful to G. Meyer (Toulouse, France); M. Schwyzer, M. Engels, A. Six, and M. Ackermann (Zürich, Switzerland); H. Reid and I. Campbell (Edinburgh, United Kingdom); C. Ek-Kommonen (Helsinki, Finland); C. Ros and S. Belàk (Uppsala, Sweden); and M. Banks (Addlestone, United Kingdom) for providing helpful comments, advice, or strains. Thanks are due to M. Tempesta for providing Ba-1 reference strain and to G. J. Letchworth for BoHV-1 reference MAbs. We thank M. Loncar for technical assistance.
This work was financially supported by Bayer S.A. and concerted actions 98/03-220 of the French Community of Belgium.
REFERENCES
- 1.Bartha, A., G. Hajdu, P. Aldasy, and G. Paczolay. 1969. Occurrence of encephalitis caused by infectious bovine rhinotracheitis virus in calves in Hungary. Acta Vet. Acad. Sci. Hung. 19:145-151. [PubMed] [Google Scholar]
- 2.Belak, K., L. Kucsera, C. Ros, G. Kulcsar, L. Makranszki, T. Soos, and S. Belak. 1999. Studies on the pathogenicity of bovine herpesvirus type 5 in sheep. Comp. Immunol. Microbiol. Infect. Dis. 22:207-220. [DOI] [PubMed] [Google Scholar]
- 3.Belknap, E. B., J. K. Collins, V. K. Ayers, and P. C. Schultheiss. 1994. Experimental infection of neonatal calves with neurovirulent bovine herpesvirus type 1.3. Vet. Pathol. 31:358-365. [DOI] [PubMed] [Google Scholar]
- 4.Berrios, P. E., D. G. McKercher, and H. D. Knight. 1975. Pathogenicity of a caprine herpesvirus. Am. J. Vet. Res. 36:1763-1769. [PubMed] [Google Scholar]
- 5.Bulach, D. M., and M. J. Studdert. 1990. Comparative genome mapping of bovine encephalitis herpesvirus, bovine herpesvirus 1, and buffalo herpesvirus. Arch. Virol. 113:17-34. [DOI] [PubMed] [Google Scholar]
- 6.Buonavoglia, C., M. Tempesta, A. Cavalli, V. Voigt, D. Buonavoglia, A. Conserva, and M. Corrente. 1996. Reactivation of caprine herpesvirus 1 in latently infected goats. Comp. Immunol. Microbiol. Infect. Dis. 19:275-281. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo, B. J., A. Ambrogi, A. A. Schudel, M. Vazquez, E. Dahme, and A. Pospischil. 1983. Meningoencephalitis caused by IBR virus in calves in Argentina. Zentbl. Veterinarmed. B 30:327-332. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury, S. I. 1995. Molecular basis of antigenic variation between the glycoproteins C of respiratory bovine herpesvirus 1 (BHV-1) and neurovirulent BHV-5. Virology 213:558-568. [DOI] [PubMed] [Google Scholar]
- 9.Collins, J. K., V. K. Ayers, C. A. Whetstone, and S. van Drunen Littel-van den Hurk. 1993. Antigenic differences between the major glycoproteins of bovine herpesvirus type 1.1 and bovine encephalitis herpesvirus type 1.3. J. Gen. Virol. 74:1509-1517. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J. 2002. Evolution of the herpesviruses. Vet. Microbiol. 86:69-88. [DOI] [PubMed] [Google Scholar]
- 11.Deregt, D., L. T. Jordan, S. van Drunen Littel-van den Hurk, S. A. Masri, S. V. Tessaro, and S. A. Gilbert. 2000. Antigenic and molecular characterization of a herpesvirus isolated from a North American elk. Am. J. Vet. Res. 61:1614-1618. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich, R. A. 1981. Respiratory viruses, p 28-29. In R. A. Dietrich (ed.), Alaskan wild life diseases. University of Alaska, Fairbanks.
- 13.Dubuisson, J., D. Boulanger, M. Bublot, E. Thiry, and P. P. Pastoret. 1989. Proteins specified by bovine herpesvirus type 4: structural proteins of the virion and identification of two major glycoproteins by using monoclonal antibodies. J. Gen. Virol. 70:1743-1753. [DOI] [PubMed] [Google Scholar]
- 14.Ek-Kommonen, C., P. Veijalainen, M. Rantala, and E. Neuvonen. 1982. Neutralizing antibodies to bovine herpesvirus 1 in reindeer. Acta Vet. Scand. 23:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ek-Kommonen, C., S. Pelkonen, and P. F. Nettleton. 1986. Isolation of a herpesvirus serologically related to bovine herpesvirus 1 from a reindeer (Rangifer tarandus). Acta Vet. Scand. 27:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Azhary, M. A., R. S. Roy, and J. L. Frechette. 1979. Serological evidence of IBR and BVD infection in caribou (Rangifer tarandus). Vet. Rec. 105:336. [DOI] [PubMed] [Google Scholar]
- 17.Engels, M., F. Steck, and R. Wyler. 1981. Comparison of the genomes of infectious bovine rhinotracheitis and infectious pustular vulvovaginitis virus strains by restriction endonuclease analysis. Arch. Virol. 67:169-174. [DOI] [PubMed] [Google Scholar]
- 18.Engels, M., C. Giuliani, P. Wild, T. M. Beck, E. Loepfe, and R. Wyler. 1986. The genome of bovine herpesvirus 1 (BHV-1) strains exhibiting a neuropathogenic potential compared to known BHV-1 strains by restriction site mapping and cross-hybridization. Virus Res. 6:57-73. [DOI] [PubMed] [Google Scholar]
- 19.Eugster, A. K., Angulo, A. B., and L. P. Jones. 1975. Herpesvirus encephalitis in range calves. Proc. Annu. Meet. Am. Assoc. Vet. Lab. 17:267-281. [Google Scholar]
- 20.French, E. L. 1962. A specific virus encephalitis in calves: isolation and characterization of the causal agent. Aust. Vet. J. 38:216-221. [Google Scholar]
- 21.Friedli, K., and A. E. Metzler. 1987. Reactivity of monoclonal antibodies to proteins of a neurotropic bovine herpesvirus 1 (BHV-1) strain and to proteins of representative BHV-1 strains. Arch. Virol. 94:109-122. [DOI] [PubMed] [Google Scholar]
- 22.Gogev, S., N. Vanderheijden, M. Lemaire, F. Schynts, J. D'Offay, I. Deprez, M. Adam, M. Eloit, and E. Thiry. 2002. Induction of protective immunity to bovine herpesvirus type 1 in cattle by intranasal administration of replication-defective human adenovirus type 5 expressing glycoprotein gC or gD. Vaccine 20:1451-1465. [DOI] [PubMed] [Google Scholar]
- 23.Grewal, A. S., and R. Wells. 1986. Vulvovaginitis of goats due to a herpesvirus. Aust. Vet. J. 63:79-82. [DOI] [PubMed] [Google Scholar]
- 24.Guercio, A., A. Greco, G. Ianizzoto, V. Di Marco, and M. Todaro. 1998. Valutazione della diffusione di anticorpi anti Herpes virus della capra in allevamenti caprini della Sicilia. Atti SIPAOC 12:138-142. [Google Scholar]
- 25.Horner, G. W., R. Hunter, and A. M. Day. 1982. An outbreak of vulvovaginitis in goats caused by a caprine herpesvirus. N. Z. Vet. J. 30:152. [DOI] [PubMed] [Google Scholar]
- 26.House, J. A. 1972. Bovine herpesvirus IBR-IPV. Strain differences. Cornell Vet. 62:431-453. [PubMed] [Google Scholar]
- 27.Inglis, D. M., J. M. Bowie, M. J. Allan, and P. F. Nettleton. 1983. Ocular disease in red deer calves associated with a herpesvirus infection. Vet. Rec. 113:182-183. [DOI] [PubMed] [Google Scholar]
- 28.Kao, M., T. Leiskau, G. Koptopoulos, O. Papadopoulos, G. W. Horner, B. Hyllseth, M. Fadel, A. H. Gedi, O. C. Straub, and H. Ludwig. 1985. Goat herpesvirus infections: a survey on specific antibodies in different countries, p. 93-97. In P.-P. Pastoret, E. Thiry, and Sapliki (ed.), Immunity to herpesvirus infections of domestic animals. Report EUR 9737 EN. Commission of the European Communities, Luxembourg, Luxembourg.
- 29.Keuser, V., S. Gogev, F. Schynts, and E. Thiry. 2002. Demonstration of generalized infection with caprine herpesvirus 1 diagnosed in an aborted caprine fetus by PCR. Vet. Res. Commun. 26:221-226. [DOI] [PubMed] [Google Scholar]
- 30.Keuser V., J. Espejo-Serrano, F. Schynts, J.-P. Georgin, and E. Thiry. Isolation of caprine herpesvirus 1 in Spain. Vet. Rec., in press. [DOI] [PubMed]
- 31.Kölher, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody to predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 32.Koptopoulos, G., M. Papanastasopoulou, O. Papadopoulos, and H. Ludwig. 1988. The epizootiology of caprine herpesvirus (BHV-6) infections in goat populations in Greece. Comp. Immunol. Microbiol. Infect. Dis. 11:199-205. [DOI] [PubMed] [Google Scholar]
- 33.Lemaire, M., F. Schynts, G. Meyer, and E. Thiry. 1999. Antibody response to glycoprotein E after bovine herpesvirus type 1 infection in passively immunised, glycoprotein E-negative calves. Vet. Rec. 144:172-176. [DOI] [PubMed] [Google Scholar]
- 34.Leung-Tack, P. 1993. L'herpèsvirus bovin de type 1. Caractérisation de la région Us du génome viral (souche ST) et étude de mutants délétés. Ph.D. thesis. University of Strasbourg, Strasbourg, France.
- 35.Liang, X. P., L. A. Babiuk, S. van Drunen Littel-van den Hurk, D. R. Fitzpatrick, and T. J. Zamb. 1991. Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J. Virol. 65:1124-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyaku, J. R., P. F. Nettleton, and H. Marsden. 1992. A comparison of serological relationships among five ruminant alphaherpesviruses by ELISA. Arch. Virol. 124:333-341. [DOI] [PubMed] [Google Scholar]
- 37.Lyaku, J. R., S. Vilcek, P. F. Nettleton, and H. S. Marsden. 1996. The distinction of serologically related ruminant alphaherpesviruses by the polymerase chain reaction (PCR) and restriction endonuclease analysis. Vet. Microbiol. 48:135-142. [DOI] [PubMed] [Google Scholar]
- 38.Marshall, R. L., L. L. Rodriguez, and G. J. Letchworth. 1986. Characterization of envelope proteins of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) by biochemical and immunological methods. J. Virol. 57:745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, W. B., G. Castrucci, F. Frigeri, and M. Ferrari. 1990. A serological comparison of some animal herpesviruses. Comp. Immunol. Microbiol. Infect. Dis. 13:75-84. [DOI] [PubMed] [Google Scholar]
- 40.McGeoch, D. J., S. Cook, A. Dolan, F. E. Jamieson, and E. A. Telford. 1995. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 247:443-458. [DOI] [PubMed] [Google Scholar]
- 41.McGeoch, D. J., A. Dolan, and A. C. Ralph. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 74:10401-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mettler, F., Engels, M., Wild, P., and A. Bivetti. 1979. Herpesvirus-Infektion bei Zieklein in der Schweiz. Schweiz. Arch. Tierheilkd. 121:655-662. [PubMed] [Google Scholar]
- 43.Metzler, A. E., H. Matile, U. Gassmann, M. Engels, and R. Wyler. 1985. European isolates of bovine herpesvirus 1: a comparison of restriction endonuclease sites, polypeptides, and reactivity with monoclonal antibodies. Arch. Virol. 85:57-69. [DOI] [PubMed] [Google Scholar]
- 44.Miller, J. M., and M. J. Van der Maaten. 1984. Reproductive tract lesions in heifers after intrauterine inoculation with infectious bovine rhinotracheitis virus. Am. J. Vet. Res. 45:790-794. [PubMed] [Google Scholar]
- 45.Nettleton, P. F., J. A. Sinclair, J. A. Herring, D. M. Inglis, T. J. Fletcher, H. M. Ross, and M. A. Bonniwell. 1986. Prevalence of herpesvirus infection in British red deer and investigations of further disease outbreaks. Vet. Rec. 118:267-270. [DOI] [PubMed] [Google Scholar]
- 46.Nettleton, P. F., C. Ek-Kommonen, R. Tanskanen, H. W. Reid, J. A. Sinclair, and J. A. Herring. 1988. Studies in the epidemiology and pathogenesis of alphaherpesviruses from red deer (Cervus elaphus) and reindeer (Rangifer tarandus), p. 143-147. In H. W. Reid (ed.), The management and health of farmed deer. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 47.Nixon, P., S. Edwards, and H. White. 1988. Serological comparisons of antigenically related herpesviruses in cattle, red deer and goats. Vet. Res. Commun. 12:355-362. [DOI] [PubMed] [Google Scholar]
- 48.Okazaki, K., M. Watanabe, E. Honda, and T. Kumagai. 1991. Immune recognition pattern of bovid herpesvirus 1-infected rabbits against major glycoproteins. J. Vet. Med. Sci. 53:137-139. [DOI] [PubMed] [Google Scholar]
- 49.Pratelli, A., G. Greco, P. Dall'ara, M. Engels, M. Tempesta, and C. Buonavoglia. 2000. Restriction endonuclease analysis of the genome of two Italian caprine herpesvirus 1 strains. Arch. Virol. 145:845-851. [DOI] [PubMed] [Google Scholar]
- 50.Probst, U., R. Wyler, U. Kihm, M. Ackermann, L. Bruckner, H. K. Muller, and F. Ehrensperger. 1985. Excretion of IBR virus, especially in milk, in experimentally infected cows. Schweiz. Arch. Tierheilkd. 127:723-733. [PubMed] [Google Scholar]
- 51.Rimstad, E., R. Krona, and B. Hyllseth. 1992. Comparison of herpesviruses isolated from reindeer, goats, and cattle by restriction endonuclease analysis. Arch. Virol. 123:389-397. [DOI] [PubMed] [Google Scholar]
- 52.Ronsholt, L., L. S. Christensen, and V. Bitsch. 1987. Latent herpesvirus infection in red deer: characterization of a specific deer herpesvirus including comparison of genomic restriction fragment patterns. Acta Vet. Scand. 28:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ros, C., and S. Belak. 1999. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J. Clin. Microbiol. 37:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ros, C., M. E. Riquelme, K. Öhman-Forslund, and S. Belak. 1999. Improved detection of five closely related ruminant alphaherpesviruses by specific amplification of viral genomic sequences. J. Virol. Methods 83:55-65. [DOI] [PubMed] [Google Scholar]
- 55.Ros, C., and S. Belak. 2002. Characterization of the glycoprotein B gene from ruminant alphaherpesviruses. Virus Genes 24:99-105. [DOI] [PubMed] [Google Scholar]
- 56.Rosadio, R. H., J. F. Evermann, and J. C. DeMartini. 1984. A preliminary serological survey of viral antibodies in Peruvian sheep. Vet. Microbiol. 10:91-96. [DOI] [PubMed] [Google Scholar]
- 57.Saito, J. K., D. H. Gribble, P. E. Berrios, H. D. Knight, and D. G. McKercher. 1974. A new herpesvirus isolate from goats: preliminary report. Am. J. Vet. Res. 35:847-848. [Google Scholar]
- 58.Schynts, F., A. Vanderplasschen, E. Hanon, F. A. Rijsewijk, J. T. van Oirshot, and E. Thiry. 2001. Use of PCR and immunofluorescence to detect bovine herpesvirus 1 recombinants. J. Virol. Methods 92:99-104. [DOI] [PubMed] [Google Scholar]
- 59.Silva, A. M., R. Weiblen, L. F. Irigoyen, P. M. Roehe, H. J. Sur, F. A. Osorio, and E. F. Flores. 1999. Experimental infection of sheep with bovine herpesvirus type-5 (BHV-5): acute and latent infection. Vet. Microbiol. 66:89-99. [DOI] [PubMed] [Google Scholar]
- 60.Six, A., M. Banks, M. Engels, C. R. Bascunana, and M. Ackermann. 2001. Latency and reactivation of bovine herpesvirus 1 (BHV-1) in goats and of caprine herpesvirus 1 (CapHV-1) in calves. Arch. Virol. 146:1325-1335. [DOI] [PubMed] [Google Scholar]
- 61.Tarigan, S., R. F. Webb, and D. Kirkland. 1987. Caprine herpesvirus from balanoposthitis. Aust. Vet. J. 64:321. [DOI] [PubMed] [Google Scholar]
- 62.Tempesta, M., A. Cavalli, V. Voigt, and D. Buonavoglia. 1994. Presenza di anticorpi per caprine herpesvirus 1 (CapHV. 1) in allevamenti caprini dell'Italia meridionale. Atti SIPAOC 11:121-122. [Google Scholar]
- 63.Thiry, E., and M. Lemaire. 2001. Infection de ruminants par des herpèsvirus hétérologues. Point Vet. 207:20-25. [Google Scholar]
- 64.Tolari, F., H. White, and P. Nixon. 1990. Isolation and reactivation of bovid herpesvirus 1 in goats. Microbiologica 13:67-71. [PubMed] [Google Scholar]
- 65.Trueblood, M. S., B. L. Swift, and L. McHolland-Raymond. 1978. A bovine herpesvirus isolated from sheep. Can. J. Comp. Med. 42:97-99. [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderplasschen, A., M. Bublot, P. P. Pastoret, and E. Thiry. 1993. Restriction maps of the DNA of cervid herpesvirus 1 and cervid herpesvirus 2, two viruses related to bovine herpesvirus 1. Arch. Virol. 128:379-388. [DOI] [PubMed] [Google Scholar]
- 67.Wellenberg, G. J., M. H. Mars, and J. T. van Oirshot. 2001. Antibodies against bovine herpesvirus (BHV) 5 may be differentiated from antibodies against BHV1 in a BHV1 glycoprotein E blocking ELISA. Vet. Microbiol. 78:79-84. [DOI] [PubMed] [Google Scholar]
- 68.Whetstone, C. A., and J. F. Evermann. 1988. Characterization of bovine herpesviruses isolated from six sheep and four goats by restriction endonuclease analysis and radioimmunoprecipitation. Am. J. Vet. Res. 49:781-785. [PubMed] [Google Scholar]
- 69.Wyler, R., M. Engels, and M. Schwyzer. 1989. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1), p. 1-72. In G. Wittmann (ed.), Herpesvirus diseases of cattle, horses and pigs. Kluwer Academic Publishers, Boston, Mass.