Abstract
Endogenous circadian rhythms are entrained to the 24-h light/dark cycle by both light and nonphotic stimuli. During the day, nonphotic stimuli, such as novel-wheel induced exercise, produce large phase advances. Neuropeptide Y (NPY) release from the thalamus onto suprachiasmatic nucleus (SCN) neurons at least partially mediates this nonphotic signal. We examined the hypothesis that NPY-induced phase advances are accompanied by suppression of PER2 and are mediated by long-term depression of neuronal excitability in a phase-specific manner. First, we found that NPY-induced phase advances in PER2::LUC SCN cultures are largest when NPY (2.35 µM) is given in the early part of the day (circadian time [CT] 0–6). In addition, PER2::LUC levels in NPY-treated (compared to vehicle-treated) samples were suppressed beginning 6–7 h after treatment. Similar NPY application to organotypic Per1::GFP SCN cultures resulted in long-term suppression of spike rate of GFP+ cells when slices were treated with NPY during the early or middle of the day (zeitgeber time [ZT] 2 or 6), but not during the late day (ZT 10). Furthermore, 1-h bath application of NPY to acute SCN brain slices decreased general neuronal activity measured through extracellular recordings. Finally, NPY-induced phase advances of PER2::LUC rhythms were blocked by latent depolarization with 34.5 mM [K+] 3 h after NPY application. These results suggest that NPY-induced phase advances may be mediated by long-term depression of neuronal excitability. This model is consistent with findings in other brain regions that NPY-induced persistent hyperpolarization underlies mechanisms of energy homeostasis, anxiety-related behavior, and thalamocortical synchronous firing.
Keywords: Circadian, Suprachiasmatic Nucleus, Entrainment, Nonphotic, NPY, Electrophysiology
INTRODUCTION
The mammalian circadian clock is not only responsible for driving and maintaining 24-h rhythms in physiology, but also for integrating multiple signals into a change in phase that is consistent with the 24-h environmental light/dark (LD) cycle. Different types of stimuli can induce a phase shift, including light and other nonphotic stimuli, such as novel wheel-induced activity, social interaction, restricted feeding, and animal handling accompanied by saline injection (Hastings et al., 1998; Lone & Sharma, 2011; Polidarová et al., 2011; Mead et al., 1992; Mrosovsky, 1988; Reebs & Mrosovsky, 1989). Even in humans, scheduled exposure to nonphotic stimuli, such as exercise, can shift or re-entrain circadian rhythms (Mistlberger & Skene, 2005; Redlin & Mrosovsky, 1997). However, the underlying mechanisms for how a change in phase is effected within the primary central oscillator, the suprachiasmatic nucleus (SCN) of the hypothalamus, during the early phase-shifting period (within the first cycle) need further exploration. We and others have shown that light and light-like stimuli have effects on SCN neurophysiology that persist for hours (Gamble et al., 2007; Kuhlman et al., 2003). Whether there is a persistent effect of nonphotic stimuli on neurophysiology and clock gene regulation is relatively unexplored.
The SCN receives nonphotic input from two main pathways: the geniculohypothalamic tract (GHT) and a pathway from the serotonergic raphe nucleus (Golombek & Rosenstein, 2010). The GHT originates in the thalamic intergeniculate leaflet (IGL) and utilizes neuropeptide Y (NPY), GABA, and endorphins (For review see Yannielli & Harrington, 2004). When presented during the subjective day, novel wheel-induced activity, microinjection of NPY into the SCN region, or application of NPY to SCN slices result in large phase advances of behavior or SCN firing-rate rhythms (Albers & Ferris, 1984; Biello & Mrosovsky, 1996; Biello et al., 1994, 1997; Golombek et al., 1996; Huhman & Albers, 1994; Reebs & Mrosovsky, 1989). In addition to its phase-shifting effects, NPY suppresses rhythmic or light-induced transcription of key molecular clock components, the Period (Per) genes, 1–2 h post-treatment (Fukuhara et al., 2001; Gamble et al., 2006), although it is unknown whether these effects are sustained.
The primary output and a distinguishing characteristic of the SCN network is rhythmic neurophysiological activity (Kuhlman & McMahon, 2006; Schaap et al., 2003). During the day, when action-potential frequency is at its peak, NPY application increases K+ channel conductance and induces a phase shift in day-time peak firing rate (Hall et al., 1999). Interestingly, even brief application of NPY of 8 mins can induce substantial long-term depression of electrical activity for longer than 90 mins in spontaneously active SCN neurons (van den Pol et al., 1996), although acute inhibitory effects of NPY are not correlated with phase-shift size (Gribkoff et al., 1998). However, it may be that sustained effects rather than acute inhibition by NPY is specific to time-of-day.
The purpose of the present study was to test the hypothesis that NPY-induced phase advances are accompanied by persistent suppression of PER2 and are mediated by long-term depression of neuronal excitability in a phase-specific manner. Utilizing SCN slice cultures from PER2::luciferase (PER2::LUC) reporter mice (Yoo et al., 2004), we first examined the phase dependency of NPY-induced phase advances in PER2 rhythms and the time course of Per2 regulation. Second, we used transgenic mice in which a dynamic, short half-life green fluorescent protein (GFP) reports Per1 gene activation (Kuhlman et al., 2000) to examine whether NPY application is sufficient to inhibit neurophysiological activity of Per1::GFP cells in a persistent manner at specific times of the day. Third, we investigated the acute and persistent effects of NPY on general SCN neuronal activity through extracellular recordings in acute brain slices. Finally, we tested the hypothesis that a persistent decrease in spike rate is necessary for NPY-induced phase advances by depolarizing SCN neurons with high [K+] several hours after NPY application using SCN slice cultures from PER2::luciferase (PER2::LUC) reporter mice.
MATERIALS AND METHODS
Animals and Housing
PER2::LUC+/− knock-in mice (16-60-d-old) backcrossed to C57BL/6 for 12 generations (Yoo et al., 2004), were used to measure PER2-driven luminescence. For electrophysiological recordings, Per1::GFP mice (16-30-d-old) hemizygous for the mPer1::d2EGFP transgene on either a C57Bl6J (N8; Figure 3) or a B6C3H hybrid background (Figure 4) and male, wild-type C57BL/6 mice (8–12 wks old; Harlan Laboratories, Indianapolis, IN; Figure 5) were used. All mice were housed in a 12:12 LD cycle for a minimum of 16 d before brains were harvested. All handling of animals was done in accordance with the University of Alabama at Birmingham (UAB) and University of Tennessee at Knoxville (UTK) Institutional Animal Care and Use Committee (IACUC) and National Institutes of Health (NIH) guidelines. The experimental protocol conforms to the international ethical standards as described in Portaluppi et al. (2010).
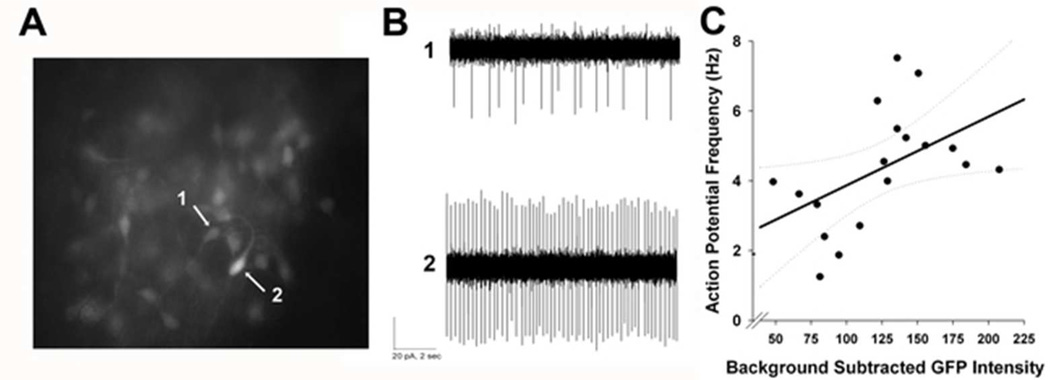
Figure 3. Spike rate is positively correlated with Per1-driven fluorescence.
(A) Representative photomicrograph of an SCN slice (N = 4) from a Per1::GFP mouse. Arrows indicate a dim cell (1) and a bright cell (2) within the same plane of view. (B) Representative traces from loose-patch electrophysiological recordings from cells (1) and (2) shown in (A). (C) Scatter plot depicting a significant correlation between fluorescence intensity and spike rate (Pearson correlation, R = .454, p < .05), as well as the fitted linear regression line and corresponding confidence intervals (95%).
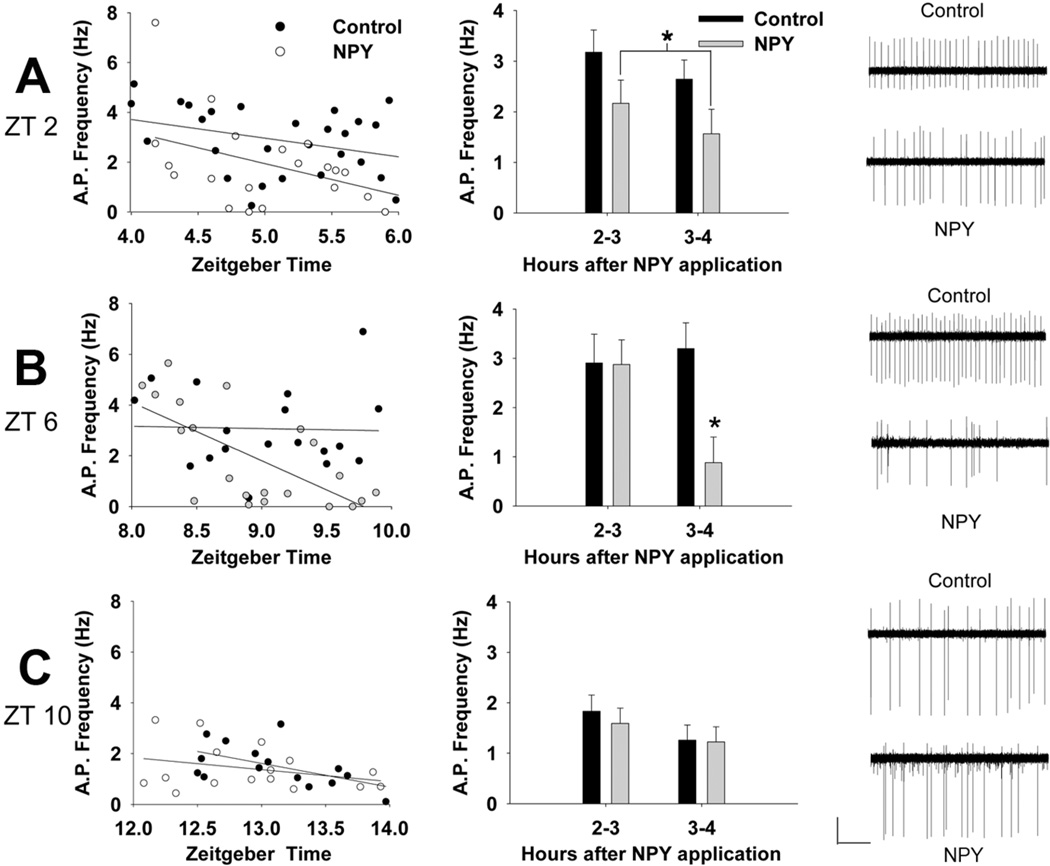
Figure 4. Phase-specific effects of NPY-induced long-term suppression of spike frequency.
(Left) Scatter plots of ZT and action potential frequency of cells treated with NPY at ZT 2 (A), ZT 6 (B), and ZT 10 (C). (Middle) Mean spike rate (± SEM) of NPY- and vehicle-treated groups in the first hour of recording compared to the second hour for NPY application at ZT 2 (A), ZT 6 (B), and ZT 10 (C). (Right) Representative traces for loose patch electrophysiological recordings of spontaneous action potentials from cells treated with NPY or vehicle (Control) at ZT 2 (A), ZT 6 (B), and ZT 10 (C).
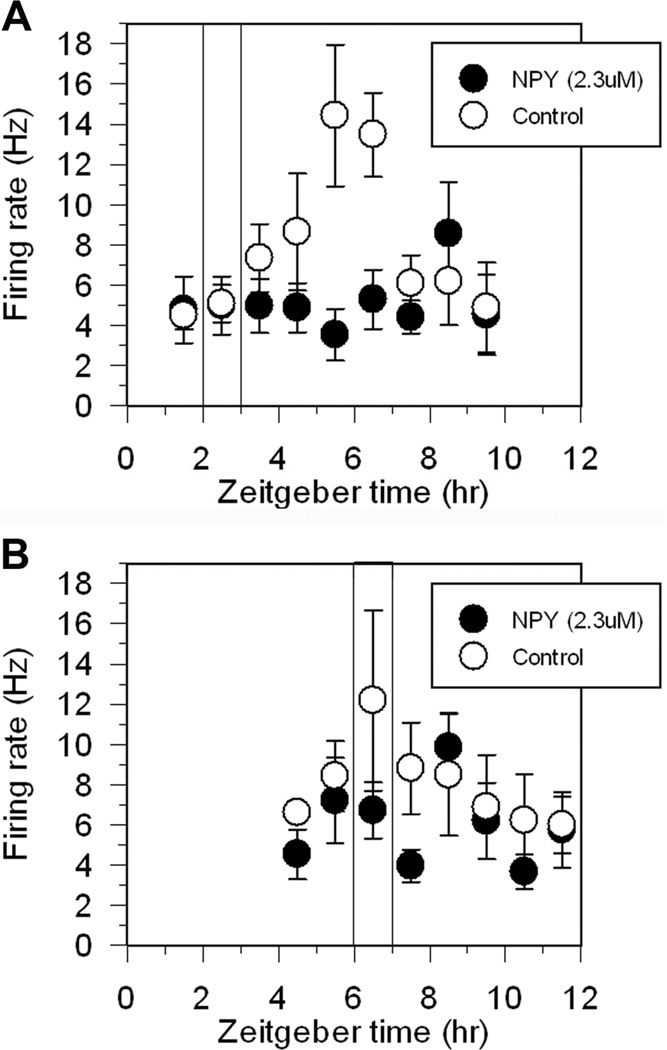
Figure 5. NPY suppresses neuronal activity in acute SCN brain slices.
Shown are the means ± SEM of hourly neuronal activity recorded in individual experiments from acute SCN brain slices. In slices treated with control medium, neuronal activity exhibited a clear peak in activity ~ZT 6, while in slices treated with NPY neuronal activity remained at relatively low levels throughout the recording period. Vertical bar indicates time of treatment: ZT 2–3 (Panel A) and ZT 6–7 (Panel B).
Tissue Preparation for Organotypic Slice Cultures
Using cervical dislocation and rapid decapitation, PER2::LUC mice were killed between 3–8 h after lights-on or zeitgeber time (ZT) 3–8 (where ZT 12 refers to lights-off), and Per1::GFP mice were killed at ZT 1–3 on the day of recording or ZT 10–11 the day before recording. Hypothalamic coronal slices (250 µm) containing the SCN were cut on a vibroslicer (Campden 7000SMZ, World Precision Instruments, Lafayette, IN) at ~4°C in HBSS (14175-103 Gibco, Carlsbad, CA) supplemented with 25 U/ml streptomycin/penicillin, NaHCO3 (7.5%, Sigma, St. Louis, MO), and 1.0 M HEPES. Slices were then trimmed to ~1.5 × 1.5 mm squares and transferred to culture membranes (Millipore, Billerica, MA) in 35-mm culture dishes with 1.0 ml of DMEM (90-013-PB; Cellgro, Manassas, VA) supplemented with 4.5 g/L glucose, 1.0 M HEPES, 25 U/ml penicillin/streptomycin, 2% B27, and 0.1 mM beetle luciferin (Promega, Madison, WI). PER2::LUC slice cultures containing the SCN were maintained at 36°C in a standard incubator. For electrophysiology cultures, hypothalamic slice cultures were prepared in the same conditions, except the slices were cut at 200-µm thickness and trimmed to ~ 4 × 4 mm squares. In addition, these slices were maintained at 34° and 5% CO2 in 1.0 ml of MEM (Invitrogen) containing 30 mM HEPES, 20 mM D-glucose, 5% B27, 5.0 mM L-glutamine, and 25 U/ml streptomycin/penicillin (Gamble et al., 2007; Han et al., 2006).
Pharmacological Treatments
After at least 2 d of stable rhythmicity, PER2::LUC slices were treated with new media by transferring the membrane insert to a new dish of media containing either 10 µl of 0.235 mM NPY (final concentration of 2.35 µM; American Peptide, Sunnyvale, CA) or vehicle (10 µl sterile water). After 1-h incubation, membrane inserts were transferred back into the original dish and media or to the recording chamber for electrophysiology (see below). Treatment times of PER2::LUC slice cultures were between Circadian Time (CT) 0–12, where CT 12 was defined as peak bioluminescence (Field et al., 2000; Gamble et al., 2007; Yoo et al., 2004). Per1::GFP cultures were treated for 1 h at projected ZT 2, 6, or 10. For high [K+] experiments, PER2::LUC cultures were first transferred to a new dish of media containing 10µL of either NPY or vehicle at CT 5.0 to 7.0. After 1-h incubation, membrane inserts were transferred back into the original dish and media. Three hours after the beginning of NPY treatment (CT 8–10, depending on start of NPY treatment), slices were then transferred to a new dish containing 10 µL of either 3.45 M KCl (final concentration of 34.5 mM, Sigma-Aldrich, St. Louis, MO) or vehicle (sterile water) and 1.0 ml of recording media. After 1-h incubation, membrane inserts were transferred back into the original dish and media. Two cultures were treated more than once, and in these cases cultures were rinsed three times and then placed in a new dish of fresh media in between treatments.
PER2::LUC Bioluminescence Analysis
Bioluminescence was measured with a LumiCycle (Actimetrics Wilmette, IL). Data were analyzed by using Lumicycle data analysis software (Actimetrics). For each selection of data, the baseline shift was removed by fitting a polynomial curve with an order of the number of cycles or one less than the number of cycles. After determining goodness of fit, the curve that accounted for at least 80% of the variance in the time series data was used for analysis. To calculate phase shifts, two full cycles before and after treatment were used to determine the period and estimate the peak of the cycle immediately following treatment. For the analysis of only one culture, one cycle was used to determine the peak post-treatment, and in this case the period that was determined pre-treatment was also used for the post-treatment analysis. Two predictions of the peak of the first cycle post-treatment were made: one based on the curve that best fit the cycles preceding treatment, and a second based on the curve that best fit the cycles after treatment. The difference between these two predictions was determined to be the phase shift. For Figures 2 and 6, the data from the two experimental groups (NPY- and vehicle-treated) whose treatment time fell in the range of CT 1.9 – 2.9 (Figure 2A) or CT 5.4 – 6.4 (Figure 2B) were normalized to the peak value for each trace (post treatment) and then averaged by hourly CT time (where CT 12 is peak PER2::LUC). The hourly means per group and standard errors are presented in Figures 2 and 6. The return of the culture dish to the Lumicycle often induced transient increases in bioluminescence that interfered with the above-described normalization procedure for some, but not all, of the cultures. Figure 2 shows the means and SEMs for all of the cultures, including ones in which transient increases in bioluminescence were not observed. However, since the repeated-measures analysis requires a complete dataset for every time point, statistical analysis of the first 12 h (repeated-measures, two-way ANOVA) began 3 h after the onset treatment. Independent t-tests were used to compare NPY and vehicle at each time point for the first 12 h of complete data.
Figure 2. Temporal dynamics of PER2 expression following NPY application at ~CT 2 and 6.
Normalized hourly means (± SEM) of baseline-subtracted PER2::LUC bioluminescence (see Methods) for 1.5 cycles following 1-h treatment with vehicle (CT 2: N = 4; CT 6: N = 5) or NPY (CT 2: N = 5; CT 6: N = 6) at CT 2 (panel A) or CT 6 (panel B). Note: the first two time points in each panel have fewer samples (Panel A, N = 1–4; Panel B, N = 3–6/group) due to transient increases in photon counts after relocation to the incubator/Lumicycle (see Methods).
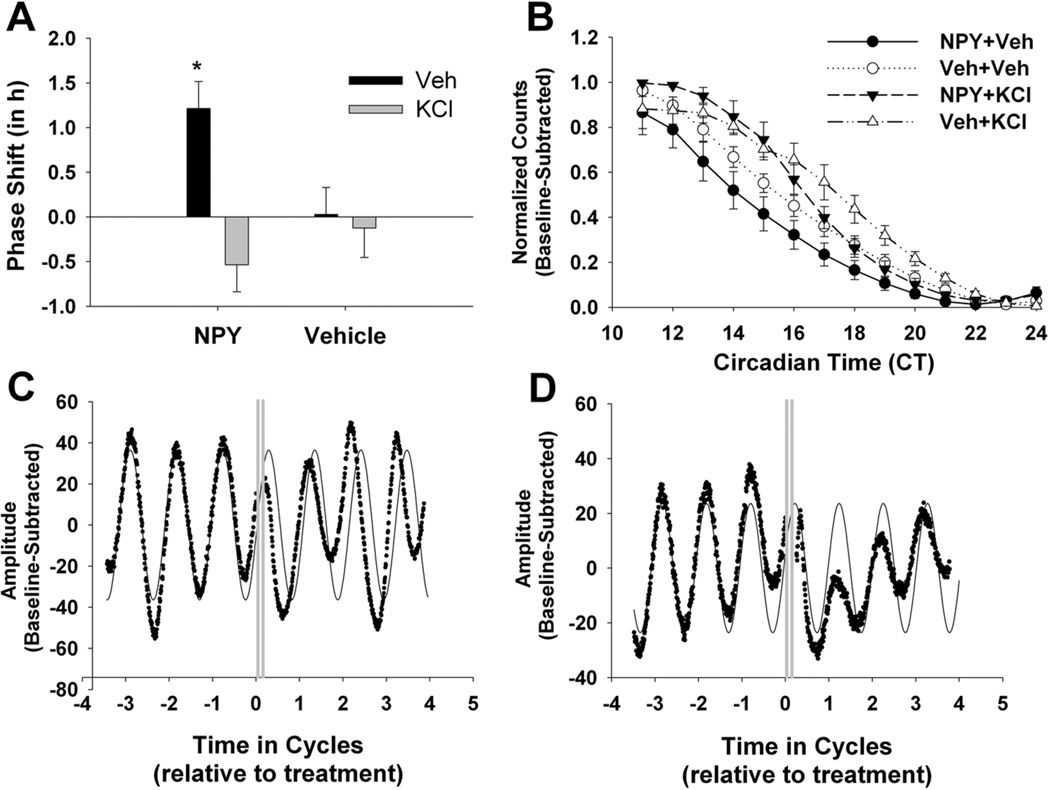
Figure 6. NPY-induced phase shifts and PER2 suppression are blocked by latent depolarization with high [K+].
(A) Means and SEMs of phase shifts resulting from NPY/vehicle (N = 6), NPY/high [K+] (N = 6), vehicle/high [K+] (N = 5), and vehicle/vehicle (N = 6) applied ~CT 6. (B) Group data illustrating the effects of all four treatment groups on PER2::LUC levels the first 12 h after treatment. Normalized and baseline-subtracted photon counts are expressed as mean ± SEM. (C) Representative bioluminescence trace from a PER2::LUC SCN explant after NPY treatment at CT6 and vehicle treatment at CT9. (D) Representative bioluminescence trace from a PER2::LUC SCN explant after NPY at CT6 and high [K+] at CT9. Gray lines indicate treatment. * Significantly different from all other treatment groups, p < .05.
Electrophysiological Recordings
After NPY or vehicle treatment, Per1::GFP slices were transferred to an open recording chamber (Warner Instruments, Hambden, CT) and continuously perfused at a rate of 2 ml/min with extracellular solution (see Nunemaker et al., 2003) bubbled with 5% CO2/95% O2 and heated to 34 ± .5°C. Neurons were visualized with an Axio Examiner microscope (Carl Zeiss Inc., Thornwood, NY) equipped for near-IR-DIC and epi-fluorescence. Loose patch recordings of GFP-positive (GFP+) neurons (confirmed by aligning digital images of the same neuron under near-infrared differential interference contrast and GFP fluorescence illumination) were made between projected ZT 4–6, 8–10, or 12–14 (2–4 h after NPY onset and 1–3 h after washout). Extracellular patch electrodes with a pipette resistance of ~3 MΩ were filled with filtered, normal HEPES solution (Nunemaker et al., 2003). Firing rate was measured as the average of a 120 s recording. Three to six slices were used per treatment type per time point (ZT 2, 6, or 10), and only one slice per animal was used. Electrophysiological signals were processed and controlled by a Multiclamp 700B amplifier, and pClamp 10.2 software (Axon Instruments, Union City, CA) in gap-free mode. Recordings were sampled at 10 kHz and filtered at 1 kHz. Fluorescence intensity was measured for each cell using AxioVision 4.8 software (Carl Zeiss Inc., Thornwood, NY). Images were converted to TIF files, and background intensity was measured from the lateral hypothalamus. Background-subtracted intensity was calculated as a percentage above background.
For acute slice electrophysiology experiments, coronal brain slices (500 µm) containing the SCN were prepared between ZT 0–2, as reported previously (Prosser, 1998; Prosser & Gillette, 1989; Prosser et al., 1993). Slices were maintained at the interface of a Hatton-style brain slice chamber (Hatton et al., 1980), where they were perfused continuously with warm (37°C), oxygenated (5% CO2/95% O2), glucose/bicarbonate-supplemented Earle's Balanced Salt Solution (EBSS; Sigma-Aldrich, St. Louis, MO), pH 7.4–7.5. NPY (2.3 µM) was prepared in warm, oxygenated EBSS. At the onset of the experimental treatment, perfusion of the standard medium was stopped, the medium was completely removed from the chamber, and either the normal EBSS or EBSS-containing NPY was added to the dish. After 1 h, the treatment medium was removed and replaced with normal EBSS, and perfusion was resumed. Single-unit recordings commenced prior to experimental treatment and continued for 6 h following experimental treatment. The recording procedure has been described previously (Prosser, 1998; Prosser et al., 1993). Briefly, the spontaneous activity of single SCN neurons was recorded extracellularly using glass microelectrodes filled with 3M NaCl. Each neuron was recorded for 5 min, and the data stored for later determination of firing rate using a DataWave system (Berthoud, CO). Typically, 4–7 cells were recorded during each hour.
Statistical Analysis
When appropriate, data were analyzed with two-way ANOVA or Pearson’s product-moment correlation. For post-hoc analyses of the phase-response curve data, planned comparisons of NPY versus control at each time bin were made with independent t-tests and a Bonferroni-corrected alpha (p < .0125). For electrophysiology, a two-way ANOVA was used. Except where noted above, a p-value of .05 was used to test for significance.
RESULTS
NPY-induced phase shifts of PER2::LUC rhythms are phase-specific
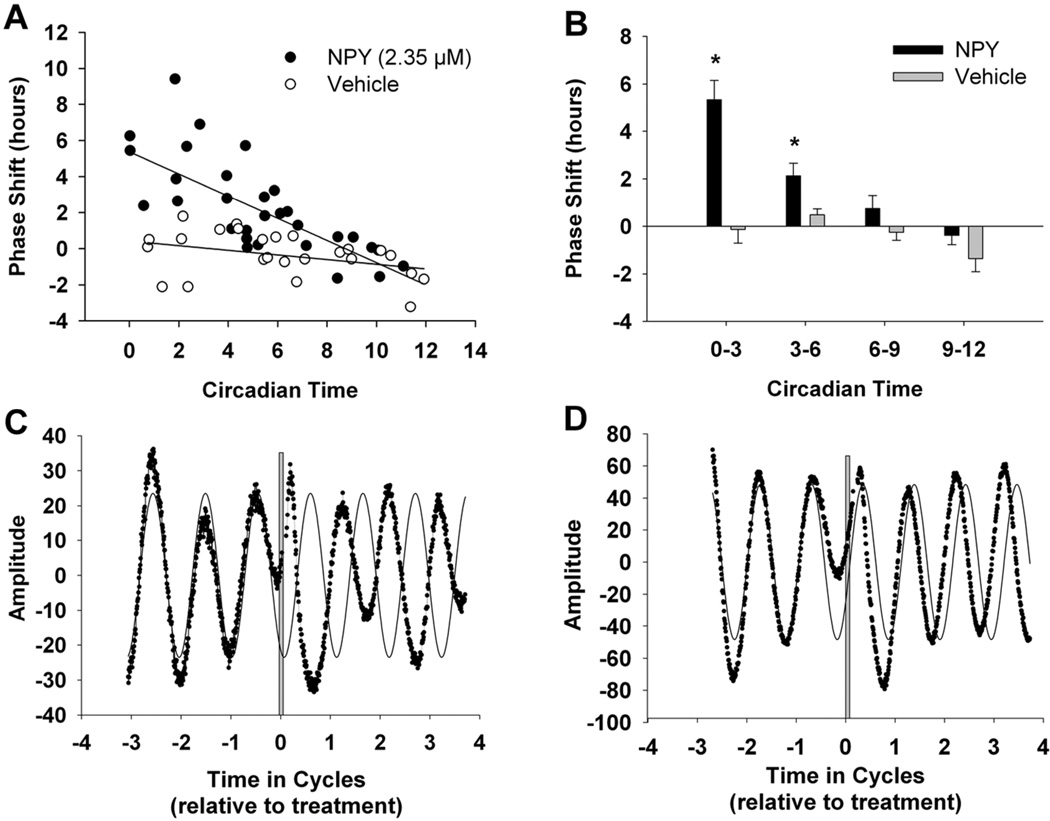
The first step in examining the effects of NPY on PER2 expression during the subjective day was to construct a phase response curve of NPY application at different time points throughout the day. Although NPY application has been shown to produce phase advances in wheel-running behavior (Huhman & Albers, 1994) and firing rate (Harrington & Schak, 2000; Medanic & Gillette, 1993), the phase dependency and time course of NPY-induced changes in PER2 levels have yet to be characterized. Therefore, SCN organotypic cultures from PER2::LUC mice were treated with either 2.35 µM NPY or 10 µL vehicle for 1 h at various times throughout the subjective day. Treatment time was binned into four periods (CT 0–3, 3–6, 6–9, or 9–12), and a two-way ANOVA (Drug X Time) found a significant main effect of Drug (F(1,48) = 29.73; p < .001[Figure 1]), indicating that NPY-treated cultures had significantly larger phase shifts than controls, regardless of CT time of treatment (mean ± SEM; NPY: 1.95 ± .29; control: −.32 ± .30). In addition, there was a significant main effect of Time (F(3,48) = 11.93; p < .001) that was primarily driven by a significant interaction between Drug and Time, such that the phase-shift differences between NPY and control treatments depended on the CT when they were treated (F(3,48) = 7.10; p < .001). Post-hoc analyses indicated significant differences in phase shifts between NPY-treated and control-treated cultures at CT 0–3 and CT 3–6 (Figure 1; p < .05), suggesting the NPY-induced phase shifts of PER2 rhythms are phase-specific.
Figure 1. Phase specific NPY-induced phase advances of PER2 rhythms when applied during subjective day.
(A) Scatterplot of 1-h NPY application to SCN slice cultures from PER2::LUC mice at CT 0–12 and corresponding fitted regression lines. (B) Means and SEMs for phase shifts resulting from NPY- and vehicle-treated samples pooled into 3-h time bins (N = 10–20/NPY or vehicle subgroup). (C–D) Representative bioluminescent traces recorded from SCN explants from PER2::LUC mice after NPY treatment at CT 2 (C) or CT 6 (D). The thin solid black lines represent the fitted sine-wave curves for the pretreatment traces and extended for four cycles. Gray lines indicate treatment. *Significantly different from control treatment at intervals CT 0–3 and CT 3–6, p < .05.
NPY suppresses PER2::LUC levels
Because the PER2::LUC transgenic mouse utilizes a PER2::Luciferase fusion protein that directly reflects PER2 protein levels (Yoo et al., 2004), we measured normalized bioluminescence counts for each circadian hour following treatment with NPY or vehicle at ~CT 2 or 6 to determine the time course of PER2 suppression. We found a significant Drug X Time interaction for the first 12 h (two-way repeated-measures ANOVA: F(11,77) = 6.23, p < .01 and F(11,99) = 7.30, p < .01 for treatment at CT 2 and 6, respectively). Post-hoc analysis revealed no significant differences in the PER2::LUC levels of NPY-treated and vehicle-treated slices until 7 h after the CT 2 treatment (Figure 2A; CT 9, t(7) = 2.8, p < .05) or 6 h after the CT 6 treatment (Figure 2B; CT 12, t(7) = 2.4, p < .05).
During the late day, SCN neuronal excitability is correlated with Per1-driven fluorescence
Light and photic-like stimuli have persistent effects on SCN neuronal excitability (Gamble et al., 2007; 2011; Kuhlman et al., 2003) and light-induced neuronal excitability is correlated with Per1 promoter activity (as reported by Per1::GFP). Therefore, we sought to determine the effect of a phase-shifting pulse of NPY on the excitability of Per1::GFP-expressing neurons. Since NPY induces a phase shift during early and mid-day, we first needed to establish that Per1::GFP neurons are electrically active and spontaneously spiking during the middle to late day at the time of recording. Because a previous study has shown that Per1::GFP neurons are electrically silent during the mid to late day (Belle et al., 2009), we performed loose patch recordings of GFP+ SCN neurons between ZT 6.4 and ZT 9.4. Fluorescence intensity and spike rate were significantly correlated (R = .45, p < .05 [Figure 3]). Out of 18 cells recorded during this time frame (N = 4 slices), 100% were spiking, and action potential frequencies ranged from 1.3 to 7.5 Hz with a mean of 4.0 ± 1.7 (SD).
NPY suppresses action potential frequency of GFP+ cells in a persistent and phase-specific manner
Although NPY-induced acute inhibition of firing rate is not correlated with phase-shift size (Gribkoff et al., 1998), it is possible that the persistent suppression of spike rate by NPY is more important for the ensuing phase change than the acute effects (peak inhibition within minutes). Therefore, we tested the hypothesis that persistent spike-rate suppression induced by NPY is dependent upon the time of day by treating hypothalamic slice cultures from Per1::GFP mice with NPY (2.3 µM) or vehicle at three different phases of the day (ZT 2, 6, or 10). After 1 h, slices were transferred to a recording chamber and 2–3 h after the termination of NPY application (projected ZT 4–6, 8–10, or 12–14), loose patch extracellular recordings were made from Per1::GFP SCN neurons. Spike rates were then grouped into hourly bins for analysis. At ZT 2, a two-way ANOVA (Drug X Time) showed a significant main effect of drug (F(1,45) = 5.58; p < .05 [Figure 4A]), but no main effect of Time nor an interaction, indicating that the firing rates of neurons from vehicle-treated and NPY-treated slices were significantly different, regardless of the amount of time after treatment when the recording was taken.
At ZT 6, a two-way ANOVA (Drug X Time) also revealed a significant main effect of Drug (F(1,35) = 4.84; p < .05) as well as a significant interaction, in that spike rates of GFP+ neurons from NPY-treated slices were significantly reduced in the second time bin only (F(1,35) = 4.60; p < .05 [Figure 4B]). Compared to 5.6% of the vehicle-treated cultures, over half (52.4%) of the NPY-treated cells were nearly silenced (0–1 Hz firing rate). The majority of this silencing was observed during the second hour of recording (27.3% and 70% of cells recorded within the first and second hours, respectively [χ2(1) = 3.83, p = .05, N = 21]). On the contrary, the majority of vehicle-treated cells remained spiking at both 2–3 h after washout (87.5%) and 3–4 h after washout (100%) (χ2(1) = 1.32, p > .05, N = 18).
For NPY treatment at ZT 10, there was no significant effect of Drug (F(1,27) = .20; p > .05) or Time (F(1,27) = 2.34; p > .05), and nor was there a Drug X Time interaction (F(1,27) = .11; p > .05; Figure 4C). These results are consistent with the phase-response data in that there was no significant phase advance of PER2::LUC rhythms generated by NPY treatment at CT 9–12 (Figure 1B).
Fluorescence intensity was measured and background subtracted for each recorded cell. Two-way ANOVA of the GFP intensity for each recording hour following NPY application at ZT 2 revealed no significant effect of Drug (F(1,45) = .58; p > .05), Time (F(1,45) = 1.58; p > .05), or Drug X Time interaction (F(1,45) = .87; p > .05). However, there was a significant main effect of Drug at ZT 6 (F(1,27) = 8.84; p < .05) as well as Time at ZT 10 (F(1,27) = 11.59; p < .05), indicating that fluorescence intensity was significantly decreased following NPY treatment during the mid-day (see Figure S1). It is important to note, however, that the interpretation of these results may be difficult since Per1::GFP degradation may not necessarily reflect PER1 degradation.
NPY suppresses neuronal activity in acute SCN brain slices
A disadvantage of the organotypic slice conditions used in the previous experiment is the lack of electrophysiological recordings from the brain slices prior to and immediately after NPY application. Therefore, we ran an additional set of experiments using acute SCN brain slices prepared from wild-type C57Bl/6J mice. Extracellular recordings of SCN neuronal activity commenced within the first hour after brain slices were prepared. Recordings were continued during the 1-h bath application of either NPY or control media from ZT 2–3 or ZT 6–7, then continued for an additional 6 h. The data from representative experiments are shown in Figure 5. NPY application decreased overall neuronal activity, sometimes apparent as early as during the hour of NPY application, but more consistently seen by the hour after NPY treatment ended. Regardless of the time of treatment, NPY consistently obscured the normal peak in neuronal activity seen during mid-subjective day. However, neuronal activity appeared to recover to normal levels by late-subjective day (ZT 10–12).
Increasing [K+] several hours after NPY blocks phase advances of PER2::LUC rhythms
According to the above experiments, NPY suppression of spike rate was evident between 2–3 h following treatment (Figure 4A & B), whereas NPY suppression of PER2::LUC did not occur until 7 h following NPY onset (Figure 2B). Therefore, we sought to determine whether neuronal inhibition is necessary for NPY-induced, long-term suppression of PER2 and the ensuing phase advance. To test this possibility, we depolarized PER2::LUC organotypic SCN slice cultures with 34.5 mM K+ for 1 h beginning 3 h following NPY treatment at ~CT 6 (CT 5.0–7.0). This [K+] is sufficient to depolarize SCN cells without inducing toxicity (Lundkvist et al., 2005) as well as chronically increase Per1 promoter activity (Maywood et al., 2006). CT 6 was the focal time point of this experiment, because this is the time of NPY treatment that consistently produces phase advances, regardless of animal type or methodology, in vivo or in vitro application (Albers & Ferris, 1984; Biello & Mrosovsky, 1996; Harrington & Schak, 2000; Huhman & Albers, 1994). During both NPY and high [K+] treatments, cultures were transferred to dishes with new media for 1 h and then returned to the original media. A two-way ANOVA (NPY X High Potassium) revealed a significant main effect of High Potassium (F1,19 = 9.42; p < .05) that was primarily driven by a significant interaction between High Potassium and NPY treatments (F1,19 = 6.64: p < .05 [Figure 6A & B]), such that NPY-induced, phase-shift size depended on whether or not cultures were treated with high [K+]. Specifically, post-hoc comparisons revealed that the average phase shift of cultures which received NPY at CT 6 and vehicle at CT 9 was significantly greater than all other groups. These results demonstrate that depolarization even 2 h after NPY washout is sufficient to block NPY-induced phase advances. Furthermore, depolarization also reversed NPY-induced decrease in PER2::LUC levels (Figure 6C), suggesting that long-term depression of neuronal excitability is necessary for NPY-induced phase advances.
DISCUSSION
In addition to light, other nonphotic stimuli, such as exercise, meal timing, and social contact, can entrain the human circadian clock (see Mistlberger & Skene, 2005 for review; Polidarová et al., 2011; Redlin & Mrosovsky, 1997). The purpose of this study was to investigate the underlying molecular and neurophysiological mechanisms that initiate the phase-shifting process following a nonphotic stimulus. NPY may, at least partially, mediate nonphotic entrainment, because NPY is released in the SCN following novel wheel running during the mid-day (Glass et al., 2010), and IGL lesions or injection of NPY antisera block novel wheel-induced phase shifts (Biello et al., 1994; Janik & Mrosovsky, 1994; Wickland & Turek, 1994). One model is that activity-induced serotonin release from the dorsal raphe nucleus suppresses the inhibitory effects of GABA in the IGL, which, in turn, promotes NPY release onto SCN neurons (Glass et al., 2010).
The present study extends this model of nonphotic entrainment by demonstrating three key findings. First, stimulation of SCN neurons with NPY was sufficient to inhibit neuronal excitability of Per1-expressing neurons, and this inhibition persisted for several hours after NPY removal and was phase-specific (limited to the early and middle portions of the day). Using a non-targeted approach, a similar inhibition of neuronal activity lasted for several hours after NPY application to acute SCN brain slices. Based on these results, it is likely that NPY’s inhibition of Per1-expressing neuronal excitability may reflect a more global effect of NPY on SCN neurons. Second, we found that NPY application at either CT 2 or 6 induced phase shifts in PER2 rhythms and suppressed PER2 levels beginning 6–7 h after treatment, suggesting that suppression of PER2 following NPY application may indicate the onset of the ensuing phase shift. A third key finding is that NPY-induced phase advances were completely reversed by depolarization even several hours after NPY was no longer present, suggesting that long-term depression of neuronal excitability is necessary for NPY-induced phase advances. To our knowledge, this is the first evidence that the long-term suppressive effects of NPY are phase-specific and necessary for the ensuing phase change.
In other brain areas, such as the thalamus and other hypothalamic nuclei, NPY has been shown to have primarily inhibitory effects, including hyperpolarization of the resting membrane potential and suppression of action potential frequency (Acuna-Goycolea et al., 2005; Albers et al., 1990; Fu et al., 2004; Roseberry et al., 2004; Sun et al., 2001). Within the SCN, the acute effects (minutes) of NPY during the day primarily consist of inhibition of spike rate (Liou & Albers, 1991) and activation of an inward K+ current (Hall et al., 1999). Interestingly, a brief 8-min application of NPY to SCN neurons induces long-term depression of mini-excitatory postsynaptic current frequency, as well as hyperpolarized membrane potential and decreased intracellular calcium levels for periods longer than 90 min (van den Pol et al., 1996). Although NPY exhibits long-term depression of spike rate, the immediate and maximal inhibitory effect of NPY occurs at all phases of the circadian cycle and is not correlated with the phase-shift size (Gribkoff et al., 1998). Until now, the phase specificity of the long-term effects of NPY has not been empirically tested. The present results are consistent with persistent inhibitory effects of NPY but reveal a dependency on circadian phase, suggesting that these long-term effects of NPY may mediate a change in phase of the oscillator.
One explanation for NPY-induced long-term depression within the SCN may be activation of G protein-coupled inwardly-rectifying potassium (GIRK) channels. G protein-coupled NPY receptors are located throughout the central nervous system (Lindner et al., 2008), including the SCN (Fetissov et al., 2004; Gamble et al., 2005, 2006; Parker & Herzog, 1999; Wolak et al., 2003). In several brain regions, such as the thalamic reticular nucleus, amygdala, hippocampus, and arcuate nucleus, NPY evokes an inwardly-rectifying potassium current that is modulated by barium and non-hydrolyzable GTP analogs (Acuna-Goycolea et al., 2005; Paredes et al., 2003; Sosulina et al., 2008; Sun et al., 2001). This current has yet to be isolated within the SCN, but could provide insight on how membrane physiology and the molecular clock are linked during nonphotic entrainment.
In addition to modulating neuronal activity, phase-shifting stimuli can have an effect on the molecular circadian clock, which is maintained by transcriptional/translational feedback loops in which negative clock regulators (protein products of the Per1, Per2, and Cryptochrome genes) inhibit their own transcription through suppression of promoter activation of the positive clock regulators, Clock and Bmal1 (for review see Takahashi et al., 2008; Welsh et al., 2010). In order to determine whether NPY inhibits the firing rate of Per1-expressing neurons during the subjective day, we first measured the late-day action potential frequency of Per1::GFP SCN neurons (as in Gamble et al. 2007, 2011; Kuhlman et al., 2003), because Per1::GFP SCN neurons have been shown to be highly depolarized but non-spiking in contrast to other SCN cells in which GFP was not visualized (Belle et al., 2009). However, we found that all of the neurons we recorded from were spiking and that the spike rate was significantly and positively correlated with fluorescence intensity (Figure 2), similar to previous reports, following a phase-shifting light pulse (Kuhlman et al., 2003). There were many methodological differences (loose patch versus whole-cell recordings) that may have contributed to these inconsistent results, but they remain beyond the scope of the present study. Following NPY application at ZT 6, we did observe a significant decrease in fluorescence intensity (Figure S1); however, these results are difficult to interpret, since GFP reports promoter activity and transcriptional activation rather than PER1 degradation.
Several lines of evidence suggest that negative clock regulators are suppressed by nonphotic stimuli and NPY. For example, novel wheel confinement and phase-advancing injections of a serotonin receptor agonist during the mid-day significantly reduce Per1 and Per2 mRNA levels (Horikawa et al., 2000; Maywood et al., 1999; Yannielli et al., 2002). Likewise, NPY significantly reduces Per1 and Per2 mRNA levels, but not until 3 h after injection (Brewer et al., 2002; Fukuhara et al., 2001; Gamble et al., 2006; Maywood et al., 2002). In vitro application of NPY results in small, but significant, suppression of Per2 mRNA levels 2 h after stimulation, while Per1 transcripts were reduced only 30 min after stimulation, but not later (Fukuhara et al., 2001). The present data add to these “snapshots” of molecular clock regulation by demonstrating that PER2 suppression does not occur until 6–7 h after NPY application at CT 2 or 6 (Figure 2), which is consistent with previous results (Maywood et al., 2002), given a 3-h lag between transcript and protein expression. Interestingly, firing-rate inhibition was already evident within the first 3 h after NPY application at CT 6 (Figures 4 & 5). Together, these results suggest that a persistent decrease in neuronal excitability may be necessary for NPY-induced changes in PER2. In fact, depolarization of SCN neurons with high [K+] for 1 h simultaneously with NPY application blocks NPY-induced phase advances in firing rate (Biello et al., 1997).
To examine whether persistent electrical inhibition is necessary for NPY-induced phase advances, we depolarized the SCN neurons several hours after the NPY pulse (Figure 6). Not only did latent depolarization block NPY-induced phase advances, but it also completely reversed suppression of PER2 levels. This result may explain why SCN slices prepared from novel wheel-exposed hamsters do not exhibit a phase advance in firing-rate rhythms when the preparation is 3 h after the onset of novel wheel access (Yannielli et al., 2002). It is possible that slice preparation induces depolarization, and this latent depolarization may reverse the phase shift by preventing sustained inhibition.
It is interesting that photic-like phase shifts induced by gastrin-releasing peptide during the night require Per1-gene translation first before a persistent increase in neuronal activity can result (Gamble et al., 2007). The present findings show that NPY-induced PER2 suppression occurred hours later than inhibition of neurophysiological activity and was blocked by latent depolarization. It may be that for nonphotic stimuli, such as NPY, changes at the level of the membrane must occur before molecular clock changes, although the possibility of independent or parallel pathways or the involvement of other clock genes, such as Per1 or Cryptochrome1/2, cannot be ruled out. Alternatively, NPY may shift both of the observed rhythms (firing rate and PER2 expression) rather rapidly; however, the electrical silencing we observed following the CT 6 NPY treatment could not be explained by this alternative hypothesis, because a ~1 h phase advance in a our second hour of recording would have a new phase of ZT 10, a time when most SCN cells are still firing ~3 Hz (Figure 2B).
In summary, the results of this study support the hypothesis that NPY-induced phase advances are accompanied by delayed suppression of PER2 and are mediated by long-term depression of neuronal excitability in a phase-specific manner. Our results demonstrate that the induction of a phase advance by a nonphotic stimulus (NPY) is determined by long-term changes in neuronal excitability. Our findings of long-lasting changes in membrane physiology induced by NPY are consistent with findings in other brain regions that NPY-induced persistent hyperpolarization underlies mechanisms of energy homeostasis in the hypothalamus, anxiety-related behavior in the lateral amygdala, and thalamocortical synchronous firing in the thalamus (Roseberry et al., 2004; Sosulina et al., 2008; Sun et al., 2001).
Supplementary Material
ACKNOWLEDGMENTS
We thank Karla Eubanks for technical assistance and Dr. Martin Young for helpful comments with the writing of this manuscript.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. This work was supported by National Institutes of Health, Grants K99 GM086683 (KLG).
REFERENCES
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Ferris CF. Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci. Lett. 1984;50:163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- Albers HE, Ottenweller JE, Liou SY, Lumpkin MD, Anderson ER. Neuropeptide Y in the hypothalamus: effect on corticosterone and single-unit activity. Am. J. Physiol. 1990;258:R376–R382. doi: 10.1152/ajpregu.1990.258.2.R376. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Biello SM, Mrosovsky N. Phase response curves to neuropeptide Y in wildtype and tau mutant hamsters. J. Biol. Rhythms. 1996;11:27–34. doi: 10.1177/074873049601100103. [DOI] [PubMed] [Google Scholar]
- Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Schak KM, Harrington ME. Circadian phase shifts to neuropeptide Y In vitro: cellular communication and signal transduction. J. Neurosci. 1997;17:8468–8475. doi: 10.1523/JNEUROSCI.17-21-08468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JM, Yannielli PC, Harrington ME. Neuropeptide Y differentially suppresses per1 and per2 mRNA induced by light in the suprachiasmatic nuclei of the golden hamster. J. Biol. Rhythms. 2002;17:28–39. doi: 10.1177/074873002129002311. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J. Comp. Neurol. 2004;470:256–265. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- Field MD, Maywood ES, O'Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J. Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C, Brewer JM, Dirden JC, Bittman EL, Tosini G, Harrington ME. Neuropeptide Y rapidly reduces Period 1 and Period 2 mRNA levels in the hamster suprachiasmatic nucleus. Neurosci. Lett. 2001;314:119–122. doi: 10.1016/s0304-3940(01)02304-7. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Ehlen JC, Albers HE. Circadian control during the day and night: Role of neuropeptide Y Y5 receptors in the suprachiasmatic nucleus. Brain Res. Bull. 2005;65:513–519. doi: 10.1016/j.brainresbull.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Paul KN, Karom MC, Tosini G, Albers HE. Paradoxical effects of NPY in the suprachiasmatic nucleus. Eur. J. Neurosci. 2006;23:2488–2494. doi: 10.1111/j.1460-9568.2006.04784.x. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J. Neurosci. 2007;27:12078–12087. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J. Biol. Rhythms. 2011;26:99–106. doi: 10.1177/0748730410396678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Guinn J, Kaur G, Francl JM. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur. J. Neurosci. 2010;31:1117–1126. doi: 10.1111/j.1460-9568.2010.07139.x. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol. Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. Neuroreport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J. Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Earle-Cruikshanks G, Harrington ME. Role of membrane conductances and protein synthesis in subjective day phase advances of the hamster circadian clock by neuropeptide Y. Eur. J. Neurosc.i. 1999;11:3424–3432. doi: 10.1046/j.1460-9568.1999.00761.x. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J. Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME, Schak KM. Neuropeptide Y phase advances the in vitro hamster circadian clock during the subjective day with no effect on phase during the subjective night. Can. J. Physiol. Pharmacol. 2000;78:87–92. [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chronobiol. Int. 1998;15:425–445. doi: 10.3109/07420529808998700. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Doran AD, Salm AK, Tweedle CD. Brain slice preparation: hypothalamus. Brain Res. Bull. 1980;5:405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H, Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J. Neurosci. 2000;20:5867–5873. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides. 1994;15:1475–1478. doi: 10.1016/0196-9781(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J. Biol. Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport. 2000;11:1479–1482. [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J. Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner D, Stichel J, Beck-Sickinger AG. Molecular recognition of the NPY hormone family by their receptors. Nutrition. 2008;24:907–917. doi: 10.1016/j.nut.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Liou SY, Albers HE. Single unit response of neurons within the hamster suprachiasmatic nucleus to neuropeptide Y. Brain Res. Bull. 1991;27:825–828. doi: 10.1016/0361-9230(91)90216-7. [DOI] [PubMed] [Google Scholar]
- Lone SR, Sharma VK. Timekeeing through social contacts: Social synchronization of circadian locomotor activity rhythm in the carpenter ant Camponotus paria. Chronobiol. Int. 2011;28:862–872. doi: 10.3109/07420528.2011.622676. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J. Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Okamura H, Hastings MH. Opposing actions of neuropeptide Y and light on the expression of circadian clock genes in the mouse suprachiasmatic nuclei. Eur. J. Neurosci. 2002;15:216–220. doi: 10.1046/j.0953-816x.2001.01852.x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Mead S, Ebling FJ, Maywood ES, Humby T, Herbert J, Hastings MH. A nonphotic stimulus causes instantaneous phase advances of the light-entrainable circadian oscillator of the Syrian hamster but does not induce the expression of c-fos in the suprachiasmatic nuclei. J. Neurosci. 1992;12:2516–2522. doi: 10.1523/JNEUROSCI.12-07-02516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Suprachiasmatic circadian pacemaker of rat shows two windows of sensitivity to neuropeptide Y in vitro. Brain Res. 1993;620:281–286. doi: 10.1016/0006-8993(93)90166-k. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J. Biol. Rhythms. 2005;20:339–352. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Phase response curves for social entrainment. J. Comp. Physiol. A. 1988;162:35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol. Proced. Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes MF, Greenwood J, Baraban SC. Neuropeptide Y modulates a G protein-coupled inwardly rectifying potassium current in the mouse hippocampus. Neurosci. Lett. 2003;340:9–12. doi: 10.1016/s0304-3940(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Polidarová L, Sládek M, Soták M, Pácha J, Sumová A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiol. Int. 2011;28:204–215. doi: 10.3109/07420528.2010.548615. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, ouitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Prosser RA. In vitro circadian rhythms of the mammalian suprachiasmatic nuclei: comparison of multi-unit and single-unit neuronal activity recordings. J. Biol. Rhythms. 1998;13:30–38. doi: 10.1177/074873098128999899. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J. Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system: I. In vitro phase shifts by serotonergic agonists and antagonists. J. Biol. Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Exercise and human circadian rhythms: what we know and what we need to know. Chronobiol. Int. 1997;14:221–229. doi: 10.3109/07420529709001157. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J. Biol. Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- Schaap J, Pennartz CM, Meijer JH. Electrophysiology of the circadian pacemaker in mammals. Chronobiol. Int. 2003;20:171–188. doi: 10.1081/cbi-120019311. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Schwesig G, Seifert G, Pape HC. Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Mol. Cell. Neurosci. 2008;39:491–498. doi: 10.1016/j.mcn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J. Physiol. 2001;531:67–79. doi: 10.1111/j.1469-7793.2001.0067j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G, Belousov AB. Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J. Neurosci. 1996;16:5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickland C, Turek FW. Lesions of the thalamic intergeniculate leaflet block activity-induced phase shifts in the circadian activity rhythm of the golden hamster. Brain Res. 1994;660:293–300. doi: 10.1016/0006-8993(94)91302-1. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J. Comp. Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, McKinley Brewer J, Harrington ME. Is novel wheel inhibition of per1 and per2 expression linked to phase shift occurrence? Neuroscience. 2002;112:677–685. doi: 10.1016/s0306-4522(02)00100-8. [DOI] [PubMed] [Google Scholar]
- Yannielli P, Harrington ME. Let there be "more" light: enhancement of light actions on the circadian system through non-photic pathways. Prog. Neurobiol. 2004;74:59–76. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.