Abstract
The effects of varying the electron energy and cationizing agents on electron activated dissociation (ExD) of metal-adducted oligosaccharides were explored, using permethylated maltoheptaose as the model system. Across the examined range of electron energy, the metal-adducted oligosaccharide exhibited several fragmentation processes, including electron capture dissociation (ECD) at low energies, hot-ECD at intermediate energies, and electronic excitation dissociation (EED) at high energies. The dissociation threshold depended on the metal charge carrier(s), whereas the types and sequence spans of product ions were influenced by the metal-oligosaccharide binding pattern. Theoretical modeling contributed insight into the metal-dependent behavior of carbohydrates during low-energy ECD. When ExD was applied to a permethylated high mannose N-linked glycan, EED provided more structural information than either collision-induced dissociation (CID) or low-energy ECD, thus demonstrating its potential for oligosaccharide linkage analysis.
INTRODUCTION
Oligosaccharides participate in a variety of cellular processes, such as protein folding, cell signaling, and cell-cell recognition.1 Better understanding of their roles in these biological processes often requires detailed structural characterization of the relevant oligosaccharides. Among the methodologies currently employed for structural analysis of oligosaccharides, tandem mass spectrometry (MS/MS or MSn) has been proven to be one of the most powerful, as it offers high speed and high sensitivity, and has minimum sample requirements yet offers the capability for determination of the components in complex mixtures.2
Dissociation of oligosaccharides can generate both glycosidic and cross-ring fragments.3 For detailed structural analysis, cross-ring fragments are more informative, as they can be used to determine linkage types between adjacent monosaccharide residues.4 For production of sufficient cross-ring fragments to fully define the oligosaccharide structure, a combination of different MS/MS methods is often necessary. Collisionally activated dissociation (CAD), or collision-induced dissociation (CID), can be easily implemented on most types of mass spectrometers and is the most commonly used method.4–5 Low-energy CID of oligosaccharides produces mostly glycosidic fragments, which do not furnish any information on linkage types. In addition, internal monosaccharide residue loss,6 fucose migration,7 and elimination of residues that are linked through labile bonds, such as sialic acid,8 may further complicate the interpretation of CID mass spectra. Other slow-heating fragmentation methods such as infrared multiphoton dissociation (IRMPD)9 also suffer from similar problems. High-energy CID10 and vacuum ultraviolet (157 nm) photodissociation (VUVPD)11 result in more cross-ring cleavages, but these two fragmentation methods are not widely available. Recently, several electron activated dissociation (ExD) techniques, including electron capture dissociation (ECD)9b, 12a,12b and electron transfer dissociation (ETD)13, have been applied to oligosaccharide structural characterization, and have shown great promise. In addition, a number of dissociation methods have been developed for oligosaccharide analysis in the negative ion mode, including negative CID,14 electron detachment dissociation (EDD),15 and negative electron transfer dissociation (nETD).16 These methods are well suited for structural analysis of acidic oligosaccharides, such as those containing sialic acid residues or sulfate substituents.
The fragmentation behavior of oligosaccharides depends on the nature of the charge carriers.5a, 9b, c, 17 The relationship between the cationizing agents and the fragmentation pattern has been well characterized for positive-ion CID. Protonated oligosaccharides produce only glycosidic cleavages; larger alkali metal cations increase the dissociation threshold and are themselves often eliminated during fragmentation. Lithium and sodium have therefore been suggested as the two best candidates for MS/MS analysis.17b, 17d, e, 18 In CID, the preferred positions for glycosidic bond cleavages were found to be dependent on the size of the alkali metal ion, whereas the types of cross-ring cleavages were not.17b CID of oligosaccharides coordinated with divalent cations produced more abundant ions than had been observed from adduction of alkali metals.5a A similar trend was also observed in ECD of native oligosaccharides.9b While Na+- and K+-adducted maltoheptaose fragmented poorly under ECD, the alkaline-earth or divalent transition metal-adducted species underwent efficient fragmentations, producing abundant cross-ring fragments.
The amount of energy available also influences the fragmentation behavior of oligosaccharides. ECD is a softer fragmentation technique than CID. It is initiated by the dissociative recombination of electrons with polycationic molecules and generates charge-reduced radical species and product ions.19 Although the electron capture efficiency usually reaches its maximum at low electron energies,20 higher-energy electrons may also induce fragmentation, as observed in hot-ECD.21 For peptides, in addition to the c- and z-type ions commonly observed in low-energy ECD, secondary side-chain fragments can be produced in hot-ECD, and this strategy has been utilized for isomeric residue differentiation.21 When compared to CID, hot-ECD of sodium-adducted permethylated oligosaccharides was found to generate more cross-ring fragments, including types which were not present in the low-energy ECD spectra of native oligosaccharides. However, it was unclear whether the fragmentation pattern change was the result of permethylation or could be attributed to the electron energy difference between ECD and hot-ECD.12 Although hot-ECD appears to be a valuable tool for the structural analysis of oligosaccharides, its potential has remained underexplored.
In order to better define the ExD behavior of metal-adducted oligosaccharides, a model oligosaccharide maltoheptaose was reduced and permethylated and subjected to ExD analysis under different experimental conditions. Judicious selection of the electron energy and metal charge carriers resulted in different fragmentation patterns and new types of informative fragmentation. ExD was further applied to a high mannose N-linked glycan to investigate its potential in the oligosaccharide linkage analysis.
METHODS
Sample Preparation
Materials and sample preparation are detailed in the Supporting Information. Briefly, high-mannose N-linked glycans were released from ribonuclease B by PNGase F. Although some mass spectra were acquired for native glycans, most native and released glycans were reduced and permethylated before MS analysis.
Tandem MS Analysis
The maltoheptaose study was carried out on a custom-built 7-T qQq-FTICR mass spectrometer.22 The study of high mannose N-linked glycans was performed on a 12-T solariX™ hybrid Qq-FTICR mass spectrometer (Bruker Daltonics, Billerica, MA). Samples were directly infused into the mass spectrometer by nanoESI at a concentration of 1–5 µM. Target ions were externally isolated and accumulated before being transferred to the ICR cell. Fragmentation conditions and data analysis are detailed in the Figure captions and Supporting Information.
Theoretical Modeling
The singly Li+- and Na+-adducted methyl-β-glucosides (GlcOMe) were used as model systems to investigate the ECD behavior of alkali-metal coordinated oligosaccharides. The potential energy surfaces of the metal loss and methyl loss channels of the model systems were calculated using density functional theory (DFT) based quantum chemical approaches, with the hybrid of Becke’s exchange and Lee-Yang-Parr’s correlation functionals (B3LYP) and 6-31G(d) Gaussian type basis set. The electronic energies were corrected by zero point vibrational energy scaled by a factor of 0.9806, and used as zero Kelvin enthalpies to estimate the reaction exothermicities and energy barriers. All reactant and product ions were verified to be local minima, and all tight transition states were verified as desired first-order saddle points via normal mode analysis. All calculations were performed using Gaussian 03 program suite23 at the supercomputing facility at Boston University.
RESULTS AND DISCUSSION
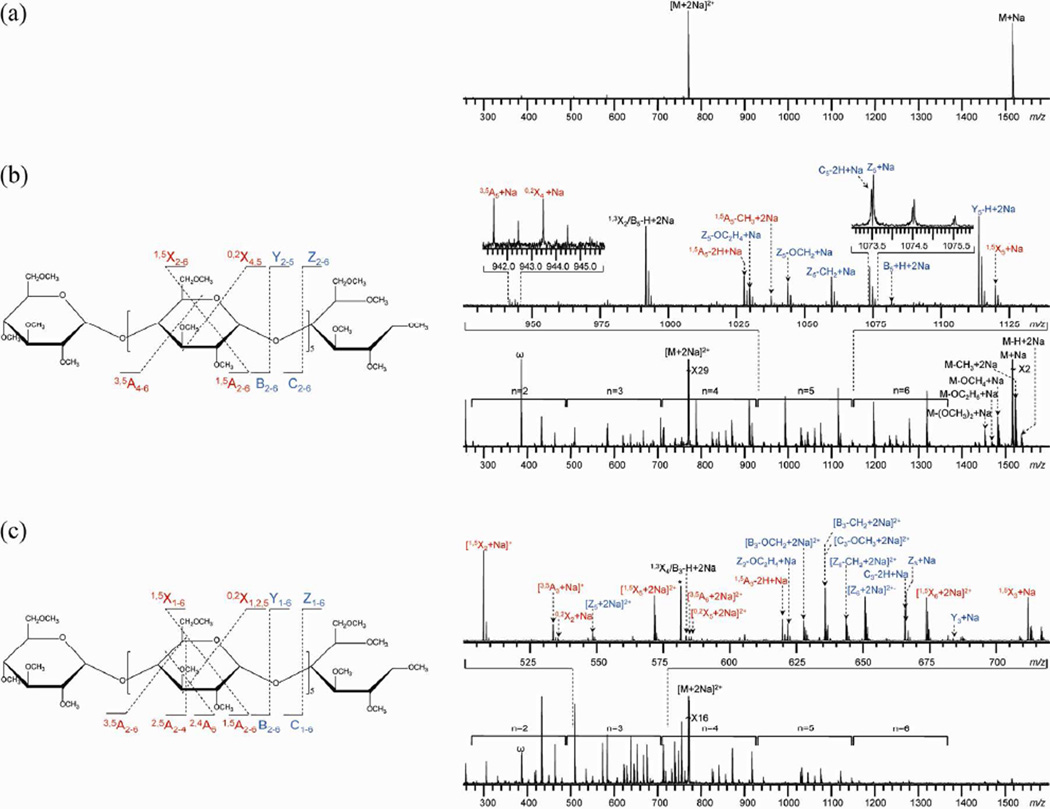
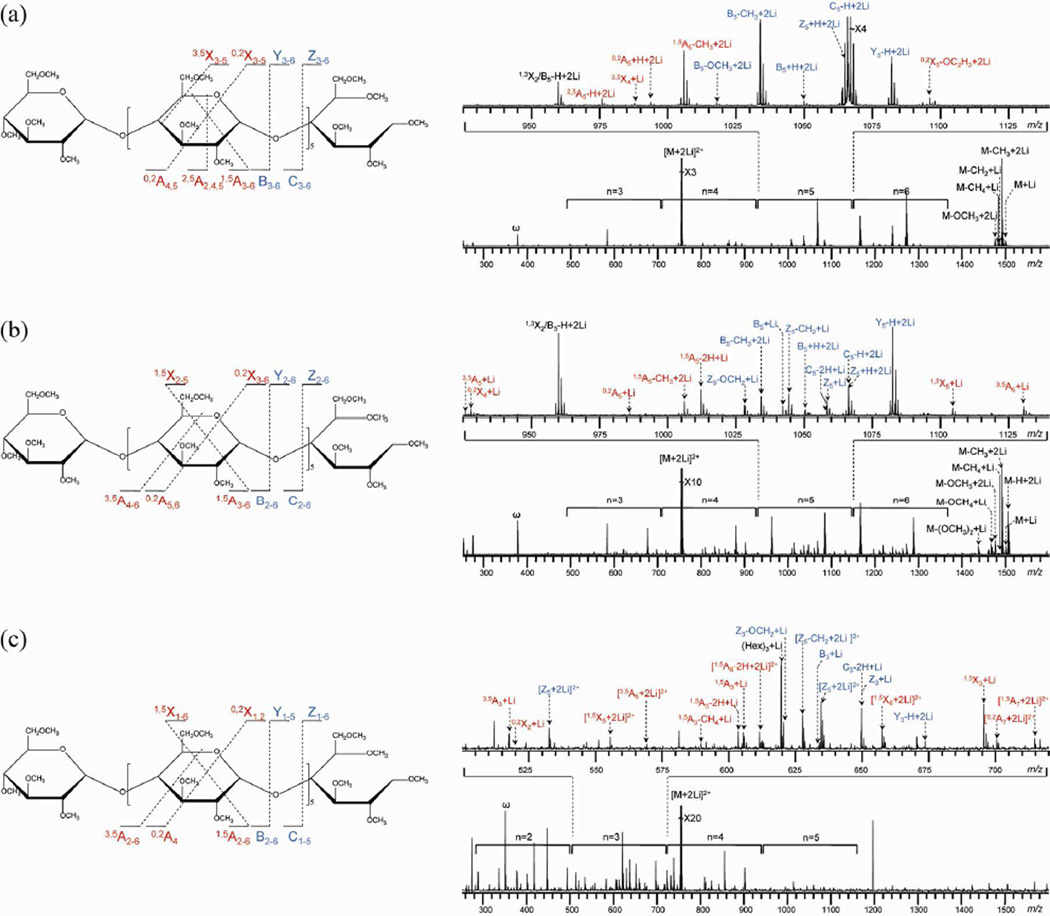
Figures 1, 2 and S-1 show the ExD spectra of permethylated maltoheptaose coordinated with Na+, Li+, and Mg2+, respectively, acquired at three different electron energies. For each experimental condition, the bottom panel shows the spectrum in its full range, with the number above each bracket indicating, roughly, the number of monosaccharide units contained in that set of fragment ions observed within the bracketed range. The expansion of a selected m/z range is shown above each full spectrum to demonstrate the various types of fragment ions observed. Peaks resulting from glycosidic bond cleavages are labeled in blue, whereas peaks resulting from cross-ring cleavages are labeled in red. A schematic representation of all glycosidic and cross-ring fragment ions observed is shown to the left of each ECD spectrum. Detailed peak assignments can be found in supporting tables (Tables S1–8).
Figure 1.
ExD spectra and cleavage maps of permethylated maltoheptaose [M + 2Na]2+ m/z 768.3750 at different electron energy levels: (a) 1.5 eV, (b) 9 eV, and (c) 14 eV.
Figure 2.
ExD spectra and cleavage maps of permethylated maltoheptaose [M + 2Li]2+ m/z 752.4012 at different electron energy levels: (a) 1.5 eV, (b) 9 eV, and (c) 14 eV.
ExD of the Sodium-Adducted Permethylated Maltoheptaose
In the 1.5 eV ECD spectrum of doubly sodiated permethylated maltoheptaose, sodium loss was the dominant fragmentation channel (Figure 1a). However, at 9 eV, i.e., under the hot-ECD condition, the same precursor ion underwent extensive fragmentation, generating both glycosidic and cross-ring product ions (Figure 1b and Table S-1). Due to its symmetric structure, native or permethylated maltoheptaose can produce multiple pairs of isobaric product ions, such as Bn and Zn, Cn and Yn, and 0,2Xn and 2,4An+1. In order to facilitate unambiguous peak assignment, maltoheptaose was reduced before permethylation, since this step introduces a 16-Da mass shift for all reducing end-containing fragment ions. Peak assignments were also independently verified by 18O-labeling at the reducing end of native maltoheptaose. The superior mass resolving power and mass measurement accuracy afforded by the FTICR instrument are essential for correct peak assignments, as illustrated by the peaks at m/z 1073.5 (inset), which correspond to a doublet consisting of both C5 ([C5 − 2H + Na]+) and Z5 ([Z5 + Na]+) ions. This pair of ions are separated from one another by 36 mDa, a common splitting seen in oligosaccharide fragment ions due to the mass difference between a CH4 group and an O atom, requiring a mass resolving power of ~60,000 for baseline separation at this m/z value.
The majority of the fragment ions were glycosidic fragments, including C-, Y- and Z-ions, whereas 1,5A- and 1,5X-type product ions were the most abundant cross-ring fragments. These fragment ions are useful for sequence determination, but they do not provide linkage information. A few informative cross-ring fragment ions were also present in the hot-ECD spectrum, including 3,5A- and 0,2X-type ions. In comparison, Håkansson and coworkers reported that ECD of the doubly sodiated native maltoheptaose primarily resulted in the formation of 0,2- and/or 2,4-designated A- and X-type cross ring fragments,9b although the assignments had ambiguity due to the symmetry of the non-reduced maltoheptaose used in their study. To remove this uncertainty in peak assignment, we have now performed ECD on 18O-labeled native maltoheptaose, and have determined that the most abundant fragments are 2,4A-type ions (Figure S-2). It is interesting to note that definition of the 1→4 linkage in maltoheptaose requires both 3,5A- (from hot-ECD of permethylated maltoheptaose) and 2,4A-type ions (from ECD of native maltoheptaose). In the hot-ECD spectrum of permethylated maltoheptaose, a series of high-abundance fragment ions was also observed at masses ~106 Da lighter than corresponding Cn ions, and we have tentatively assigned this series as the 1,3Xm/B7-m-H ions (e.g. the 1,3X2/B5-H ion at m/z ~991.4). It appears that generation of these product ions is specific to the 1,4-linkage, as they were also observed in the hot-ECD spectra (data not shown) of permethylated cellohexaose (β-1,4-linkage) and mannohexaose (β-1,4-linkage), but not in those of permethylated isomaltohexaose (α-1,6-linkage) and laminarihexaose (β-1,3-linkage).
Many of the fragment ions observed in the hot-ECD spectrum of permethylated maltoheptaose, including many of the glycosidic and cross-ring fragments, were also present in its CID spectrum.12 The 2,4An product ion series are still the most abundant fragments in the hot-ECD spectrum of the same precursor ion (data not shown). However, hot-ECD also produced abundant neutral losses from the charge-reduced species and from B- and Z-type ions, which were absent in the CID spectrum. No neutral losses from C- or Y-type ions were observed in the hot-ECD spectrum. This is expected, as the homolytic cleavage of the glycosidic bond after electron capture at a glycosidic oxygen-binding Na+ will produce an odd-electron B- (or Z-) ion, and even-electron C- (or Y-) ion, depending on which glycosidic bond is cleaved and which fragment retains the charge (Scheme S-1). Only the odd-electron B- and Z-ions can undergo further radical-induced dissociations. Possible mechanisms for several neutral losses from the charge-reduced molecular ion and from Z-ions in hot-ECD are illustrated in Schemes S-2 and S-3, respectively. Thus, hot-ECD is likely a radical-driven process rather than a slow-heating method, capable of producing new types of fragment ions (e.g., the 1,3Xm/B7-m-H ions mentioned above) that can be used for linkage determination.
At 14 eV, the distribution of fragment ions shifted to the lower m/z region (Figure 1c and Table S-2). This shift in fragmentation pattern was partly due to the ultimate formation of smaller fragments upon irradiation at the higher energy, as reflected in the pattern of relative abundances of product ions (Tables S-1 and S-2), and partly due to the presence of doubly-charged fragments, which could not have resulted from ECD of the doubly-charged precursor ion. At the high mass end of the 14-eV spectrum, charge-reduced species and neutral losses are barely observable, further suggesting that electron capture may not play an important role here.
One possibility is that the impact of high-energy electrons resulted in vibrational excitation (VE) of the precursor ion, as in the electron impact excitation of ions from organics (EIEIO).24 Since no charge reduction takes place in EIEIO, subsequent fragmentation of the vibrationally excited precursor ion can generate doubly-charged product ions. However, as a VE method, EIEIO usually produces CID-type fragments, and cannot explain the observation of several ECD-like, doubly-charged fragments, such as those arising via neutral losses from B- and Z-ions.
A second possibility is that the precursor ion could undergo a second ionization step upon encounter with high-energy electrons, generating a radical species in a 3+ charge state that can produce 2+ fragment ions, via a process known as electron ionization dissociation (EID).25 The most likely electron detachment site here would be an ether or acetal oxygen, which has an ionization potential of ~10 eV, well below the electron energy used. The resulting radical cation could undergo further fragmentation, similar to what occurs in electron impact ionization (EI) mass spectrometry. Scheme S-4 illustrates a possible pathway for B- and Y-ion production via this EID mechanism. However, ionization alone does not necessarily lead to dissociation, and additional energy is often needed to produce fragmentation; this could be imparted through collisions with high-energy electrons. An alternative EID mechanism has been proposed by Zubarev and coworkers to explain the ECD-like fragmentation behavior of peptide and protein ions when irradiated with >20 eV electrons.25 These investigators proposed that EID involves double ionization of the precursor ion which subsequently captures one of the ejected electrons and fragments. This hypothesis was supported by their observation that abundant backbone fragments were observed only at electron energies exceeding ~40 eV, the level at which doubly-ionized species started to appear. Neither EID mechanism is likely to play a major role here, as the ~14 eV electrons had much lower energy, which was neither energetic enough to induce extensive fragmentations after single ionization nor sufficient for double ionization. Further, no triply-charged molecular or fragment ions were observed in support of either EID mechanism.
A third possibility is electronic excitation dissociation (EED), which has been invoked to explain the formation of ECD-like fragment ions upon irradiation of singly-charged peptide ions with >10 eV electrons.26 In this case, the Zubarev group proposed that, following ionization, the charge (hole) could migrate to a remote site, such as the N terminus. Subsequent H-atom transfer from a carboxylic acid group would generate a peptide dication that could capture a low-energy electron and undergo fragmentation. In oligosaccharides, electron detachment from the lone pair of an oxygen atom produces an oxygen radical cation, which can abstract a hydrogen atom from a spatially adjacent carbon to form protonated oxygen. Because of its larger recombination energy, the newly protonated oxygen becomes favored, over sodiated sites, as the location for electron capture. Subsequent electron capture at the protonated oxygen can produce doubly-charged glycosidic and cross-ring fragments (Scheme S-5). Since this charge neutralization occurs at a protonated site, all doubly-charged product ions are expected to contain both sodium cations, consistent with the experimental observation (Table S-2). The EID mechanism (Scheme S-4) would predict that doubly-charged fragment ions can carry either one or two sodium atoms, but no singly-sodiated product ions were observed experimentally.
ExD of Lithium-Adducted Permethylated Maltoheptaose
ExD of permethylated maltoheptaose adducted with larger alkali metal cations (K+, Rb+ or Cs+) exhibited fragmentation behavior similar to that of the sodium adducts, producing only metal loss at low electron energy, while undergoing extensive fragmentations at higher electron energies (Figures S-3 and S-4). However, the Li+ adduct readily fragmented at 1.5 eV (Figure 2a and Table S-3), indicating that the ECD fragmentation threshold has a dependence on the charge carrier. At higher energies, the hot-ECD (Figures 2b and Table S-4) and EED (Figures 2c and Table S-5) spectra of lithiated and sodiated (Figure 1b, c) permethylated maltoheptaose had many parallel features. The types of fragments present were very similar, except for a few low-abundance cross-ring fragments and neutral losses. Thus, although the identity of the metal charge carrier still demonstrated some influence on the fragmentation behavior of oligosaccharides at higher energies, the effect was much more subtle than for irradiation with low energy electrons, and likely arose from the difference in metal-oligosaccharide binding pattern, rather than from a change in fragmentation mechanisms.
As in the case of the Na+ adduct, the electron energy was observed to have a very strong effect on the fragmentation behavior of the Li+-adducted permethylated maltoheptaose. At 1.5 eV, the vast majority of product ions contained two lithium atoms, with C-type ions ([Cn − H + 2Li]+) being the most abundant. At 9 eV, product ions containing both one and two lithium atoms were observed, and Y-type ions ([Yn − H + 2Li]+) became the most abundant glycosidic fragments. At 14 eV, doubly-charged fragment ions were produced, the abundance of Y-type ions diminished, and the C-type ions ([Cn − 2H + Li]+) became one of the most abundant sets of glycosidic fragments. It is important to note that many seemingly similar types of fragment ions observed at different energies were actually different species, likely produced by different processes. For example, at 1.5 eV, the only C-type ions produced were [Cn − H + 2Li]+ ions, with a possible mechanism for their formation illustrated in Scheme S-6a. At 14 eV, only [Cn − 2H + Li]+ ions were observed; these could be produced via the mechanism shown in Scheme S-6b.
As was observed for the sodiated species, an increase in the electron energy shifted the distribution of the Li+ adducts to favor formation of smaller ions. For example, the range of C- and Y-type ions produced changed from C3–6 and Y3–6 at 1.5 eV to C2–6, and Y2–6 at 9 eV, and to C1–5 and Y1–5 at 14 eV. One possible explanation is that the production of small fragments requires the charge neutralization site to be near an end (reducing or non-reducing) to initiate cleavage, and its successful detection requires the remaining charge-carrying Li+ to be coordinated at the same end. The proximity of the two charge carriers would cause significant Coulombic repulsion, and such a conformer would only be accessible at higher energies. EED may also contribute to this shift by allowing smaller fragment ions to be generated and detected from thermodynamically more favored conformers where the two charge carriers are farther apart, because the EED cleavage is initiated by charge neutralization at a protonated site without the involvement of either metal charge carrier. Finally, it is also possible that, under the EED conditions, the more energetic initial products could undergo further fragmentation to generate abundant low m/z fragments, as is observed in EI mass spectra.
ExD of Alkaline Earth Metal-Adducted Permethylated Maltoheptaose
Previous reports suggested that divalent cation-adducted oligosaccharides can produce more abundant and informative fragment ions than are produced by alkali metal-coordinated species under low-energy ECD and ETD conditions.9b, 13 Thus, the electron-energy dependence of the fragmentation behavior of alkaline earth metal coordinated oligosaccharides was further investigated here. The ExD spectra of Mg2+-adducted permethylated maltoheptaose are shown in Figure S-1. For the most part, the ExD behavior of the Mg2+ adduct resembled that of the Li+ adduct, which underwent extensive fragmentation at 1.5 eV, and produced abundant EED fragments (as exemplified by the presence of doubly-charged fragment ions) at 14 eV. However, ECD of the Mg2+ adduct produced a slightly broader range of fragments, possibly because it does not need to overcome Coulombic repulsion to adopt conformations producing small fragments. Further, unlike those of the alkali metal adducts, the ExD spectra of the Mg2+ adduct underwent minimal change when the electron energy was raised from 9 to 14 eV: the range of observed fragments did not shift towards smaller fragments, and no new members of the 3,5An ion series were produced. One explanation is that EED of the alkali metal adducts was initiated by electron capture at the protonated site, and this could produce cleavages distant from the original metal binding sites, whereas Mg2+ remained as the preferred electron capture site owing to its much higher recombination energy. Maltoheptaose adducted with other alkaline metals (Ca2+, Sr2+ and Ba2+) showed similar energy-dependent ExD fragmentation behavior (Figures S-4 and S-5), and will not be discussed further.
Table S-9 summarizes the ExD fragments observed, at three different electron energies, from permethylated maltoheptaose with Li+, Na+, Cs+ and Mg2+ adductions. Although the Mg2+ adduct provided the most information under low-energy ECD conditions, its favorable performance was surpassed by results from analyses that used the alkali metal adducts at higher energies. At higher electron energies, the fragments of the Cs+ adducts are (almost) the same as those of the Li+ adducts. The largest alkali metal (Cs+) adduct in this set generated a wider range of fragment ions than the Li+ and Na+ adducts, possibly because Cs+ can simultaneously coordinate with oxygen atoms that may be located in several monosaccharide units than can the smaller metals Li+ or Na+, and this property allows it to initiate fragmentation at more positions. Considering that 0,2-, 2,4- and 3,5-types of cross-ring cleavages are the most informative, EED of the Cs+-adduct seemed to offer an advantage for linkage analysis.
Theoretical Modeling
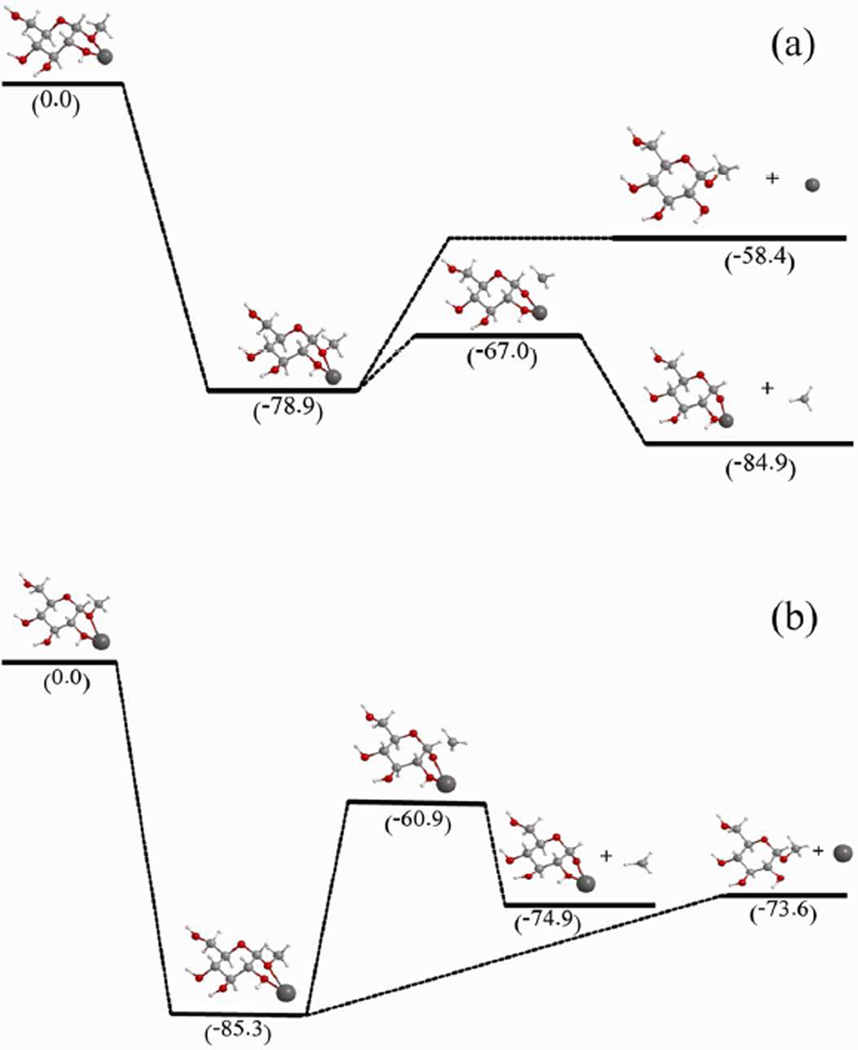
While all metal-adducted maltoheptaoses underwent extensive fragmentation when irradiated with high-energy electrons, their fragmentation behaviors differed drastically at lower energies. For the Na+, K+, Rb+ and Cs+ adducts, metal loss was the dominant channel (Figures 1a and S-3), whereas for the Li+ and alkaline earth metal adducts (Figures 2a, S-1 and S-5), the most abundant fragment ions were the C-type ions and the products resulting from methyl loss, the latter of which would correspond to the C7 ion, if the methyl radical is eliminated from the reducing end. The Mg2+ adduct fragmented more extensively under low-energy ECD conditions, probably because it has a much higher recombination energy (second IP of Mg is ~15 eV) than alkali metals (e.g., the first IP of Cs is ~3.89 eV). However, the drastic difference between the low-energy ECD behaviors of the Li+ and Na+ adducts was somewhat surprising, considering that their first ionization potentials are fairly close (Li: 5.39 eV, Na: 5.14 eV). It was also interesting to note that, when the doubly charged precursor ion contained both Li+ and Na+, Na loss was still the predominant fragmentation channel at 1.5 eV (Figure S-7a). At 9 eV, the [M + Li + Na]2+ species fragmented extensively, producing a mixture of product ions that contained either Na+ or Li+ or both, which indicated that either Li+ or Na+ could serve as the neutralization site initiating fragmentations (Figure S-7b and Table S-10).
These phenomena can be rationalized using theoretical modeling carried out on alkali-metal coordinated β-methyl-glucosides (GlcOMe) as the corresponding model systems. The Na+-GlcOMe complex has a larger recombination energy (85 kcal/mol) than the Li+-GlcOMe complex (79 kcal/mol). Figure 3 illustrates the potential energy surfaces (PESs) for the metal loss and methyl loss channels of the Li+- or Na+-adducted GlcOMe, as calculated at the B3LYP/6-31g(d) level of theory. For the Li+ adduct, Li loss is 20 kcal/mol endothermic relative to the charge-reduced species, whereas the methyl loss channel is 6 kcal/mol exothermic with an energy barrier of 12 kcal/mol (Figure 3a). Therefore, methyl loss is both thermodynamically and kinetically favored over Li loss. For the Na+ adduct, although methyl loss and Na loss have similar exothermicities, the methyl loss channel is associated with a 24 kcal/mol energy barrier, while Na loss is barrier-less, making Na loss the preferred fragmentation pathway (Figure 3b), By analogy, the initial electron capture for the [M + Li + Na]2+ of permethylated maltoheptaose is likely to occur at the Na+ site, leading to predominant Na loss, consistent with the experimental observation (Figure S-7).
Figure 3.
The unscaled potential energy surfaces of the methyl and metal loss channels of (a) [Glc-OMe + Li]+ and (b) [Glc-OMe + Na]+ complexes upon electron capture, as calculated at the B3LYP/6-31G(d) level of theory. Numbers in brackets are in units of kcal/mol.
Therefore, the ECD threshold depends not only on the recombination energy of the charge carrier, but also on the detailed PESs for the metal loss and bond dissociation channels. However, at higher electron energies, the relatively small differences in recombination energies and activation barriers became less important, as was reflected by the similarity in the hot-ECD spectra of the Li+ and Na+ adducts (Figures 2b and 1b), as well as by the presence of both Li+- and Na+-adducted fragment ions in the hybrid adduct spectrum (Figure S-7b and Table S-10). The details in fragmentation patterns, including both the types and ranges of fragments generated, were still metal-dependent at higher energies, e.g., in hot-ECD and EED spectra of the alkali metal adducts, as shown in Table S-9. This was also true at lower energies, where the Li+ and Mg2+ adducts displayed substantial differences in the types and ranges of fragment ions produced. Theoretical calculations by Leary and coworkers showed that Li+ and Mg2+ have different binding patterns with monosaccharides.27 Thus, a hypothesis may be made that, once the energy requirement is met for fragmentation to occur, it is the coordination pattern between the metal and the oligosaccharide that determines which fragments are actually produced. As we have shown herein, complementary information can be obtained by conducting ExD experiments using different metal charge carriers at different electron energies.
Linkage Determination of the High Mannose N-linked Glycan by ExD
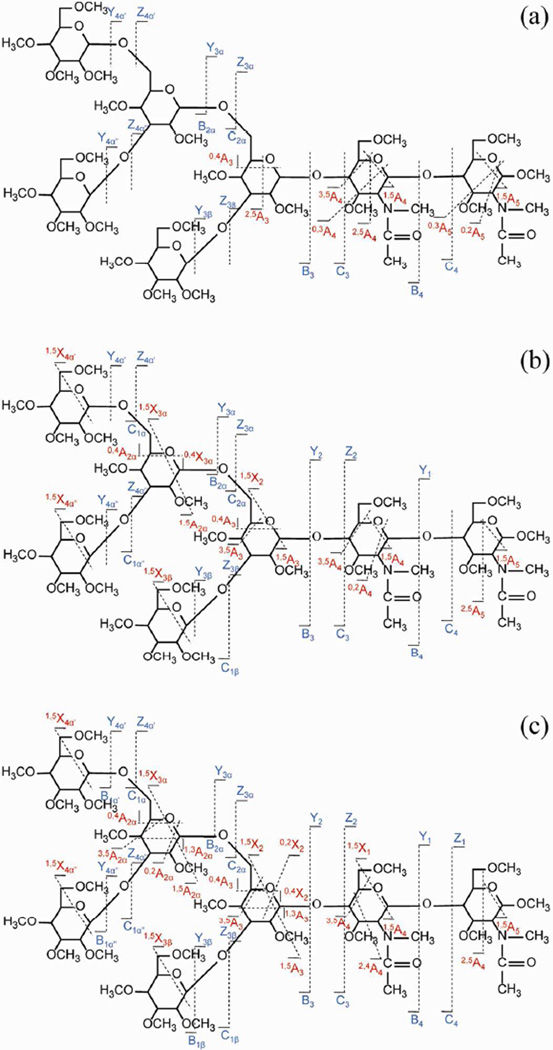
The ExD techniques characterized above were applied to the structural analysis of an N-linked glycan released from Ribonuclease B, Man5GlcNAc2 (Man: mannose, GlcNAc: N-acetyl glucosamine). Li+ was selected as the charge carrier, because the earlier results indicated that it is able to initiate fragmentations at all electron energies, and Li-induced cleavages reached both the reducing and non-reducing ends in the maltoheptaose study. Doubly lithiated Man5GlcNAc2 was isolated and fragmented by ExD at three electron energies, as investigated above, and was also subjected to CID for comparison. The tandem mass spectra are shown in Figure S-8, and the cleavage patterns are summarized in Figures 4 and S-9. Note that, this branched N-linked glycan produced quite a few fragment ions for which more than one assignment is possible. For example, the Y4α', Y4α" and Y3β ions all have the same calculated mass, as do the 1,5X4α', 1,5X4α" and 1,5X3β ions. Figures 4 and S-9 includes all possibilities, although some of the labeled cleavages may not have actually occurred. The assignments could be fully clarified with higher stages of MSn but, since these monosaccharide losses do not inform about branching and thus do not contribute significantly to the structural determination, such analyses were not relevant to this study.
Figure 4.
ExD cleavage maps of the Man5GlcNAc2 [M + 2Li]2+ m/z 785.4121 at different electron energy levels: (a) 1.5 eV, (b) 9 eV, and (c) 14 eV.
As expected, CID (28 eV) generated mostly glycosidic bond cleavages, producing complete B- and Y-ion series, as well as abundant cross-ring products in the 1,5A and 1,5X series (Figure S-9). A few informative cross-ring fragments were also observed, which identified the two 1→6 linkages based on the presence of the 0,4A2α and 0,4A3 fragments. The linkage positions for the other four glycosidic bonds could not be determined unambiguously by CID.
The low-energy ECD (1.5 eV) spectrum is shown in Figure S-8b and its cleavage map is shown in Figure 4a. The four dominant fragments resulted from glycosidic cleavages around the chitobiose core, indicating that the preferred electron capture site was the GlcNAc-bound Li+ near the reducing end. The presence of B3 and C3 ions suggested that the other Li+ was probably distant, coordinated by mannose residues on the 6-branch to minimize the Coulombic repulsion between the two charge carriers. Several Y- and Z-type ions resulting from cleavages near the non-reducing end were also observed, indicating that the non-reducing end Li+ could also initiate ECD pathways, albeit with lower probabilities. Although more informative cross-ring cleavages were produced by low-energy ECD than by CID, they mostly occurred around the GlcNAc residues, and no evidence indicated that cross-ring products formed via cleavages in the antennae regions. Among the six glycosidic linkages, only the 1→6 linkage at the first branch point could be defined. The absence of cross-ring cleavages near the termini of the antennae was likely the consequence of competitive electron capture near the chitobiose core, together with competition from glycosidic cleavages, even when the Li+ located on an antenna did capture an electron.
The results reported above indicated that, in such a case, increasing the electron energy should help to access the thermodynamically and/or kinetically unfavored fragmentation channels of the released and permethylated high mannose N-linked glycan.
Figures S-8c and S-8d show the hot-ECD (9 eV) and EED (14 eV) spectra of the doubly lithiated Man5GlcNAc2, and their cleavage maps are shown in Figures 4b and 4c, respectively. Hot-ECD was able to produce cross-ring cleavages reaching the second branching point, identifying the second 1→6 linkage via the presence of the 0,4A2α and 0,4X3α ions. At 14 eV, additional, new cross-ring fragments were generated, including the 1,3A2α and 0,2A2α, as well as the 1,3A3 and 0,2X2 ions, which were essential for the delineation of the 1→3 linkages at both branch points. Altogether, five out of the six glycosidic linkage positions could be identified by EED, including all linkages in the antennae regions, and thus EED provided much more complete information than either CID or low-energy ECD.
CONCLUSIONS
Using maltoheptaose as the model system, the ExD fragmentation behaviors of metal-adducted permethylated oligosaccharides were investigated. At low electron energies, adducts formed with divalent alkaline metals or lithium produced abundant glycosidic and cross-ring cleavages, whereas adducts containing larger alkali metals generated predominantly metal losses. This metal-dependent ECD fragmentation behavior was rationalized on the basis of theoretical modeling and could be attributed to the difference in the metal cation recombination energies and the detailed PESs that governed various dissociation channels. At higher energies, hot-ECD and EED processes took over, generating types of fragments different from those that had been observed in low-energy ECD, and often yielding a broader range of fragments as well. The types and ranges of fragments observed depended on the specific metal-oligosaccharide binding patterns, which were influenced by both the electron energy and the metal charge carriers. When ExD was applied to the structural analysis of a released and permethylated high mannose N-linked glycan, EED provided cross-ring cleavages more informative than those obtained with CID and low-energy ECD, and enabled definition of the ring positions five out of the six glycosidic linkages, including those in the antennae.
Although further experimental and theoretical investigations are needed to fully understand the electron activated dissociation of metal coordinated oligosaccharides, the present study has clearly demonstrated the substantial potential of ExD techniques for detailed structural characterization of oligosaccharides.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from NIH Grants P41 RR010888/GM104603 and S10 RR025082. The authors also acknowledge the technical support and computing resources provided by the Scientific Computing and Visualization Group at Boston University.
Footnotes
ASSOCIATED CONTENT
Supporting Material includes details of experimental methods, additional MS/MS spectra of native and permethylated glycans, proposed fragmentation schemes and tables containing lists of accurate masses and assignments for fragment ions corresponding to spectra shown in the Figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dwek RA. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ashline D, Singh S, Hanneman A, Reinhold V. Anal. Chem. 2005;77:6250–6262. doi: 10.1021/ac050724z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zaia J. Mass Spectrom. Rev. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- 3.Domon B, Costello CE. Glycoconjugate J. 1988;5:397–409. [Google Scholar]
- 4.Reinhold VN, Reinhold BB, Costello CE. Anal. Chem. 1995;67:1772–1784. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 5.(a) Harvey DJ. J. Am. Soc. Mass. Spectrom. 2001;12:926–937. doi: 10.1016/S1044-0305(01)00268-9. [DOI] [PubMed] [Google Scholar]; (b) Lemoine J, Fournet B, Despeyroux D, Jennings KR, Rosenberg R, Dehoffmann E. J. Am. Soc. Mass. Spectrom. 1993;4:197–203. doi: 10.1016/1044-0305(93)85081-8. [DOI] [PubMed] [Google Scholar]
- 6.(a) Brull LP, Heerma W, ThomasOates J, Haverkamp J, Kovacik V, Kovac P. J. Am. Soc. Mass. Spectrom. 1997;8:43–49. [Google Scholar]; (b) Kovacik V, Hirsch J, Kovac P, Heerma W, Thomasoates J, Haverkamp J. J. Mass Spectrom. 1995;30:949–958. [Google Scholar]
- 7.Ernst B, Muller DR, Richter WJ. Int. J. Mass Spectrom. Ion Processes. 1997;160:283–290. [Google Scholar]
- 8.(a) Harvey DJ. Proteomics. 2005;5:1774–1786. doi: 10.1002/pmic.200401248. [DOI] [PubMed] [Google Scholar]; (b) Zhang JH, LaMotte LT, Dodds ED, Lebrilla CB. Anal. Chem. 2005;77:4429–4438. doi: 10.1021/ac050010o. [DOI] [PubMed] [Google Scholar]
- 9.(a) Adamson JT, Hakansson K. J. Proteome Res. 2006;5:493–501. doi: 10.1021/pr0504081. [DOI] [PubMed] [Google Scholar]; (b) Adamson JT, Hakansson K. Anal. Chem. 2007;79:2901–2910. doi: 10.1021/ac0621423. [DOI] [PubMed] [Google Scholar]; (c) Xie YM, Lebrilla CB. Anal. Chem. 2003;75:1590–1598. doi: 10.1021/ac026009w. [DOI] [PubMed] [Google Scholar]
- 10.Harvey DJ, Bateman RH, Green MR. J. Mass Spectrom. 1997;32:167–187. doi: 10.1002/(SICI)1096-9888(199702)32:2<167::AID-JMS472>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MS, Cui WD, Reilly JP. Angew. Chem. Int. Ed. 2004;43:4791–4794. doi: 10.1002/anie.200460788. [DOI] [PubMed] [Google Scholar]
- 12.(a) Budnik BA, Haselman KF, Elkin YN, Gorbach VI, Zubarev R. Anal. Chem. 2003;75:5994–6001. doi: 10.1021/ac034477f. [DOI] [PubMed] [Google Scholar]; (b) Zhao C, Xie B, Chan SY, Costello CE, O'Connor PB. J. Am. Soc. Mass. Spectrom. 2008;19:138–150. doi: 10.1016/j.jasms.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Han L, Costello C. J. Am. Soc. Mass. Spectrom. 2011;22:997–1013. doi: 10.1007/s13361-011-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaia J, Seymour JL, Costello CE. Glycobiology. 2005;15:1209–1209. [Google Scholar]
- 15.Wolff JJ, Amster IJ, Chi LL, Linhardt RJ. J. Am. Soc. Mass. Spectrom. 2007;18:234–244. doi: 10.1016/j.jasms.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff JJ, Leach FE, Laremore TN, Kaplan DA, Easterling ML, Linhardt RJ, Amster IJ. Anal. Chem. 2010;82:3460–3466. doi: 10.1021/ac100554a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Cancilla MT, Penn SG, Carroll JA, Lebrilla CB. J. Am. Chem. Soc. 1996;118:6736–6745. [Google Scholar]; (b) Cancilla MT, Wang AW, Voss LR, Lebrilla CB. Anal. Chem. 1999;71:3206–3218. doi: 10.1021/ac9813484. [DOI] [PubMed] [Google Scholar]; (c) Fura A, Leary JA. Anal. Chem. 1993;65:2805–2811. doi: 10.1021/ac00068a017. [DOI] [PubMed] [Google Scholar]; (d) Harvey DJ. J. Mass Spectrom. 2000;35:1178–1190. doi: 10.1002/1096-9888(200010)35:10<1178::AID-JMS46>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]; (e) Ngoka LC, Gal JF, Lebrilla CB. Anal. Chem. 1994;66:692–698. doi: 10.1021/ac00077a018. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeister GE, Zhou Z, Leary JA. J. Am. Chem. Soc. 1991;113:5964–5970. [Google Scholar]
- 19.Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]
- 20.Zubarev RA. Mass Spectrom. Rev. 2003;22:57–77. doi: 10.1002/mas.10042. [DOI] [PubMed] [Google Scholar]
- 21.Kjeldsen F, Haselmann KF, Budnik BA, Jensen F, Zubarev RA. Chem. Phys. Lett. 2002;356:201–206. [Google Scholar]
- 22.O'Connor PB, Pittman JL, Thomson BA, Budnik BA, Cournoyer JC, Jebanathirajah J, Lin C, Moyer S, Zhao C. Rapid Commun. Mass Spectrom. 2006;20:259–266. doi: 10.1002/rcm.2307. [DOI] [PubMed] [Google Scholar]
- 23.Frisch MJ. C.02 edn. Wallingford CT: Gaussian, Inc.; 2004. [Google Scholar]
- 24.Cody RB, Freiser BS. Anal. Chem. 1979;51:547–551. [Google Scholar]
- 25.Fung YME, Adams CM, Zubarev RA. J. Am. Chem. Soc. 2009;131:9977–9985. doi: 10.1021/ja8087407. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen ML, Budnik BA, Haselmann KF, Olsen JV, Zubarev RA. Chem. Phys. Lett. 2000;330:558–562. [Google Scholar]
- 27.Zheng YJ, Ornstein RL, Leary JA. J. Mol. Struc.-Theochem. 1997;389:233–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.