Abstract
This study explored the role of the melatonin receptors in methamphetamine (METH)-induced locomotor sensitization during the light and dark phases in C3H/HeN mice with genetic deletion of theMT1 and/or MT2 melatonin receptors. Six daily treatments with METH (1.2 mg/kg, i.p.) in a novel environment during the light phase led to the development of locomotor sensitization in wild-type (WT), MT1KO and MT2KO mice. Following four full days of abstinence, METH challenge (1.2 mg/kg, i.p.) triggered the expression of locomotor sensitization in METH-pretreated but not in vehicle (VEH)-pretreated mice. In MT1/MT2KO mice, the development of sensitization during the light phase was significantly reduced and the expression of sensitization was completely abrogated upon METH challenge. During the dark phase the development of locomotor sensitization in METH-pretreated WT, MT1KO and MT2KO mice was statistically different from VEH-treated controls. However, WT and MT2KO, but not MT1KO mice receiving repeated VEH pretreatments during the dark phase expressed a sensitized response to METH challenge that is of an identical magnitude to that observed upon 6 days of METH pretreatment. We conclude that exposure to a novel environment during the dark phase, but not during the light phase, facilitated the expression of sensitization to a METH challenge in a manner dependent on MT1 melatonin receptor activation by endogenous melatonin. We suggest that MT1 and MT2 melatonin receptors are potential targets for pharmacotherapeutic intervention in METH abusers.
Keywords: melatonin, methamphetamine, mouse, MT1 melatonin receptor, MT2 melatonin receptor, sensitization
Introduction
Repeated administrations of psychostimulants such as cocaine or methamphetamine (METH) induce a state of heightened sensitivity to subsequent drug exposures. This process, termed sensitization, is thought to reflect neuroadaptations that confer enhanced ‘incentive salience’ onto a drug and its associated contextual cues, which has implications for the motivational value of drug-associated stimuli and their ability to stimulate craving or drug-seeking behavior [1]. Overt manifestations of psychostimulant-induced sensitization in humans have been demonstrated sparsely, but the process has been proposed to reflect neurological alterations that underlie the transition from casual drug use to the compulsive use that signifies drug addiction [1, 2]. In contrast, sensitization is readily observed in mice as a progressive increase in locomotor activity after repeated intermittent METH pretreatments, culminating in an enhanced locomotor response to a challenge dose of the psychostimulant relative to vehicle-pretreated controls [3–5]. Over the past two decades, a wide variety of receptor-mediated mechanisms were reported as targets for the modulation of locomotor sensitization induced by drugs of abuse such as cocaine, amphetamine, morphine and methamphetamine. In mice, METH-induced locomotor sensitization is reduced by aripiprazole acting as a partial agonist at the 5HT1A and D2 receptors [6], by the μ/κ opioid receptor antagonist naltrexone [3], and by genetic deletion of the μ opioid receptors [7], but is enhanced in mice lacking the corticotrophin-releasing factor gene [8]. Together, these reports identified endogenous targets that regulate METH-induced sensitization and may have potential for treating maladies related to METH abuse.
Melatonin, a hormone secreted into the circulation by the pineal gland, is a candidate for the modulation of psychostimulant responses [9]. The synthesis and release of melatonin from the pineal gland occurs according to a rhythm controlled by the suprachiasmatic nucleus of the hypothalamus, with highest levels measured at night [10, 11]. Melatonin signals the duration of nighttime to virtually all central and peripheral tissues through its interactions with two high-affinity G-protein-coupled receptors termed MT1 and MT2 [12, 13]. These receptors are expressed throughout the brain [13], with mRNA for the MT1 receptor present in the reward- and motivation-associated sites targeted by METH and other psychostimulants modulating dopaminergic neurotransmission, and include the ventral tegmental area (VTA), nucleus accumbens, and caudate-putamen [14].
By virtue of its importance in circadian physiology, melatonin is a viable candidate for modulating behavioral responses to drugs of abuse, which are well known to vary according to time of day [15]. Kuribara and Takadoro [16] demonstrated that acute METH treatment at 4 hr intervals elicits a diurnal rhythm of locomotor responses in mice with higher levels at night compared to day. Locomotor sensitization in cocaine-treated mice and rats is also subject to time-of-day regulation, with the strongest responses observed during the day in both mice [17–19] and rats [20]. These studies suggested that removal of the pineal gland and hence pineal products abolish the diurnal rhythm of cocaine sensitization in mice [18], leading to the overall conclusion that endogenous melatonin may be counteracting the sensitizing effect of cocaine. Results with exogenous melatonin on sensitization induced by psychostimulants are inconclusive, with no effect of melatonin observed on the expression of sensitization induced by this drug after 2-wk abstinence [21], or the expression of sensitization to METH in mice [22].
Here we demonstrated that both the MT1 and MT2 melatonin receptors are needed for METH pretreatments to induce the development, and the expression upon METH challenge of locomotor sensitization in the C3H/HeN mice. Melatonin receptors activated by endogenous melatonin through the 24 hr day/night period may participate in the signaling and molecular processes leading to the development and expression of METH-induced locomotor sensitization.
Materials and methods
Animals
Male C3H/HeN mice were bred in colonies maintained in the Laboratory Animal Facility at the University at Buffalo. C3H/HeN mice homozygous for the MT1 melatonin (MT1KO) receptor gene deletion and their WT controls were generated by back-crossing C57BL/6J MT1 knockout mice (donated by Dr. Steven Reppert, University of Massachusetts Medical School, Worcester, MA, USA) with C3H/HeN mice (Harlan, Indianapolis, IN, USA) [23]. Mice were back-crossed for seven generations and were congenic with the C3H/HeN strain expressing the wild-type allele at the arylalkylamine N-acetyltransferase (AA-NAT) gene as well as the rd (retina degeneration) mutation on the rod photoreceptor cGMP phosphodiesterase gene [24]. C3H/HeN mice homozygous for the MT2 melatonin receptor gene deletion (MT2KO) were also donated by Dr. Steve Reppert. MT1/MT2 double knockout C3H/HeN mice (MT1/MT2KO) were generated by back-crossing C3H/HeN MT1KO with C3H/HeN MT2KO. Male mice were bred under a 14:10 hr light/dark cycle, in a temperature– (22 ± 1°C) and humidity-controlled environment with food and water provided ad libitum. Genotype was confirmed by PCR analysis of DNA samples prepared from tail tips periodically during the tenure of the colony and repeated when animals were sacrificed. All animal procedures were performed in accordance with the guidelines set forth by the National Institutes of Health and the Institutional Animal Care and Use Committee of the University at Buffalo.
At weaning, male mice were group-housed (4 per cage) and maintained in a 14:10 hr light/dark cycle, with 150–200 lux light illumination at the level of the cage. Mice (2–3 months old) were individually housed (cage 30 × 19 cm) and maintained in a 12/12 light/dark cycle inside ventilated and light-tight cabinets for 14 days prior to initiation of the locomotor sensitization experiment. Mice were weighed on the day before the first METH pretreatment (Day −1), after every locomotor activity test (Days 1–6), and 1 day prior to the sensitization test. Occasionally, mice losing more than 10% of their body weight during the course of the experiment were nutritionally supplemented with NutriCal. Only two mice (one WT, one MT1/MT2KO) were excluded from the experimental procedures after exceeding the weight loss threshold of 15%.
Drugs
WT, MT1KO, MT2KO, MT1/MT2KO mice were randomly assigned to groups receiving either vehicle (saline) or METH (1.2, 2.4 or 4.8 mg/kg in saline). Vehicle or appropriate METH doses were administered via intraperitoneal (i.p.) injections to deliver 0.01 ml solution per gram of mouse weight.
Chambers and data acquisition
The 16 novel environment chambers consisted of cylindrical (20 cm diameter × 20 cm height) Plexiglas arenas [25]. The walls of the cylindrical arena were lined with black aluminum sheets to reduce shadows and reflections. The tops were open to allow top-view imaging and the floor was transparent to allow illumination from below. Locomotor activity was recorded by 4 overhead-mounted video cameras. Each camera recorded locomotor activity from four arenas simultaneously under white (light phase) or infrared (dark phase) light using the LocoScan behavior analysis system (CleverSys, Inc., Reston, VA, USA). Analog-to-digital conversion of the video data was accomplished by a Dell Precision workstation. Locomotor activity was quantified from the digital video by the TopScan software package running on the same computer.
Development and expression of METH-induced sensitization
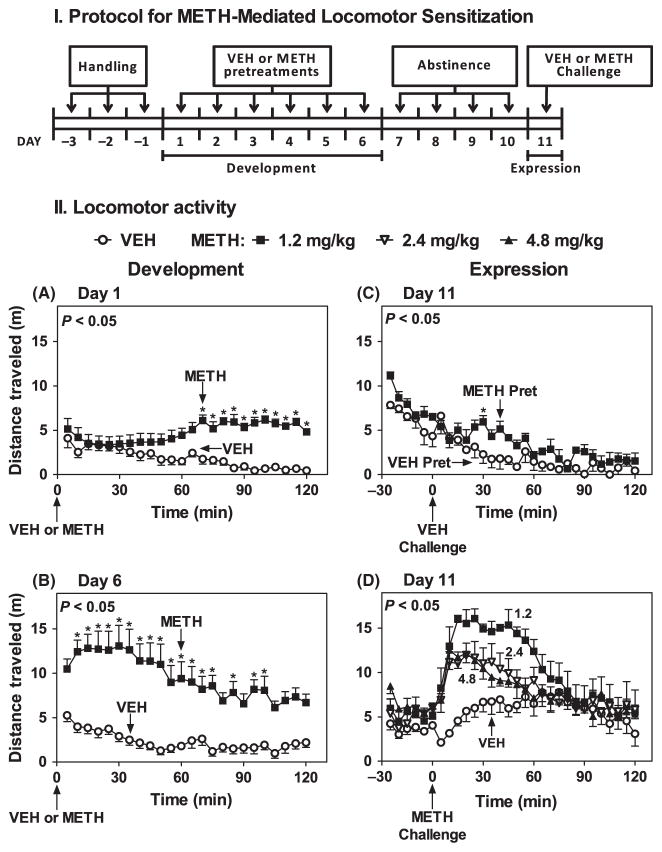
Figure 1(I) shows the experimental protocol used to induce locomotor sensitization by METH during the development and expression phases. Experiments were conducted either under ambient light of approximately 50 lux at the level of the arena floor between Zeitgeber time (ZT) 5 and 7, (ZT 0: lights on), or in complete darkness between ZT 19 and 21, with the experimenter visualizing the work through night-vision goggles. Mice were moved to the testing room at least 1 hr before the start of the experiment, i.e., at ZT 4 or at ZT 12 for experiments conducted during the light and dark phases, respectively.
Fig. 1.
Optimization of the METH-induced locomotor sensitization protocol. Part I: protocol for METH induction of locomotor sensitization used during the light (ZT 5–7) or dark (ZT 19–21) phases. Days −3 to −1: mice handling and saline injections. Days 1–6: VEH or METH pretreatments (1.2 mg/kg, i.p.) (2.4 and 4.8 mg/kg, i.p., not shown). Locomotor activity was collected at 5 min intervals for 2 hr after treatment on Days 1, 2, 3 and 6. Day 7–10: abstinence period: no treatments. Day 11: VEH or METH challenge (1.2 mg/kg, i.p.). Locomotor activity was recorded 30 min prior to and 2 hr following treatment. Part II: ordinates represents distance traveled in 5 min intervals as a function of time (abscissae) during development (A, B) and expression (C, D) of sensitization in VEH (○) and METH (■) pretreated mice. Pretreatments were administered at times indicated by arrows under the abscissae. The main effects of drug treatment were F(1,14) = 27.58, P < 0.05 on Day 1 (A) and F(1,14) = 32.98, P < 0.05 on Day 6 (B). The main effects of drug pretreatment was F(1,6) = 6.36, P < 0.05 in mice challenged with VEH and F(1,6) = 6.36, P < 0.05 in mice challenged with METH at times indicated by arrows under the abscissae. Significance values for the main effect of drug pretreatment are noted in the upper left corners of the graphs. *P < 0.05 when compared with the corresponding VEH 5 min bin (Bonferroni test).
Mice were handled and injected with sterile saline (0.25 ml, i.p.) during three daily sessions (Days −3 to −1) to habituate them to the experimental procedures and return to the home cage. Next, during the development phase (Days 1–6) mice were treated with either VEH (n = 8) or METH (1.2 mg/kg, i.p.) (n = 8), unless otherwise indicated, and immediately placed in the novel environment arenas (cylindrical chambers). Locomotor activity was recorded continuously for 2 hr from the time mice were placed in the novel environment and then returned to their home cage. Expression of locomotor sensitization was assessed on Day 11, after four full days of abstinence (Days 7–10). Mice pretreated with VEH (n = 8) or METH (1.2 mg/kg, i.p.) (n = 8) were then challenged with either VEH (VEH-VEH, n = 4; METH-VEH, n = 4) or METH (1.2 mg/kg, i.p.) (METH-VEH, n = 4; METH-METH, n = 4). Mice were moved to the arenas for 30 min of baseline recording at either ZT 4.5 or ZT 18.5 for light or dark phase experiments, respectively. Mice were given either VEH or METH as appropriate, and placed back into the arenas starting at ZT 5 or ZT 18, respectively for 2 hr of locomotor activity recording. These procedures were conducted during both the light (ZT 5–7) and dark (ZT 19–21) phases in WT, MT1KO and MT2KO mice. Based on the results observed with the MT1/MT2KO mice during the light phase and the reduced availability of this genotype after a prolonged interruption in successful breeding, sensitization was conducted only during the light phase.
Statistical analysis
Locomotor activity was indexed by distance traveled during the development and expression (challenge) phases of sensitization. Sixteen mice were tested in each experiment with VEH or METH administered to 8 mice per group during repeated pretreatments, that is, development of sensitization stage (i.e., VEH [n = 8] and METH [n = 8]). During the expression of sensitization stage, each group was challenged with either VEH (n = 4) or METH (n = 4). Experiments were repeated 2–3 times. Time course data were analyzed and then grouped in 5- or 30-min bins and time point averages for each experimental condition expressed as mean ± S.E.M. Time course studies were analyzed by two-way mixed design analysis of variance (ANOVA) having the between-groups factor of pretreatment drug (VEH or METH) and time. The development of sensitization across test day was analyzed by two-way mixed design ANOVA having the between-groups factor of pretreatment drug and the within-groups factor of test day. Treatment X Genotype between-subjects analysis of variance (ANOVA) was conducted on behavioral domains at each time (ZT 5–7 or ZT 19–21) in mice treated with VEH (saline, i.p.) or METH (1.2 mg/kg, i.p., unless otherwise indicated) in the four genotype groups (WT, MT1KO, MT2KO, MT1/MT2KO). The Bonferroni correction was used for post hoc comparisons. Analyses were computed using GraphPad Prism v. 5 (GraphPad Software, Inc., LaJolla, CA, USA). For all analysis P values <0.05 were considered statistically significant.
Results
Figure 1(I) shows the protocol used for inducing the development and expression of locomotor sensitization with METH in male C3H/HeN (WT, MT1KO, MT2KO, MT1/MT2KO) mice during the light (ZT 5–7) and dark phases (ZT 19–21). VEH or METH pretreatments and challenge after four full days of abstinence were administered in a novel environment that is, cylindrical arenas 20 cm in diameter [25]. Locomotor activity was recorded continuously for 2 hr after treatment to assess the temporal development of sensitization by METH.
Each experiment included 16 mice, half treated with VEH and the other half treated with METH at the appropriate dose during the 6-day development (pretreatment) stage. Mice treated with METH (1.2 mg/kg, i.p.) during the light phase on Day 1 expressed a gradual increase in locomotor activity compared to VEH controls over the 2 hr recording period (Fig. 1-IIA). Treatment with METH on Day 2–6 gradually increased locomotor activity beginning immediately after treatment (Fig. 1-IIB; Days 2–5 not shown). Pretreatment with VEH during 6 days did not affect total locomotor activity: 44.6 ± 7.5 (n = 8) (Day1) versus 56.0 ± 6.9 (n = 8) (Day 6) (P > 0.05) (Fig. 1-IIA versus B). Total locomotor activity per 2 hr during repeated pretreatments with METH (1.2 mg/kg, i.p.), on Day 6 was significantly increased over Day 1: 113.7 ± 10.8 m (n = 8) versus 229.4 ± 29.4 m (n = 8), P < 0.05, Fig. 1-IIA versus B. Similar patterns were observed for total locomotor sensitization elicited by the higher doses of METH (2.4 and 4.8 mg/kg, i.p.) (data not shown). Maximal development of sensitization was observed with the lowest dose of METH (1.2 mg/kg, i.p.), with the higher doses producing significant stereotypic behaviors (e.g., rearing, grooming) (data not shown).
The expression of sensitization after the abstinence period (Days 7–10) was assessed by a challenge dose of either VEH or METH (1.2 mg/kg, i.p.) on Day 11 (see Fig. 1-IIC,D). VEH challenge induced a small conditioned-locomotor response in 1.2 mg/kg METH-pretreated mice compared to VEH-pretreated controls (Fig. 1-IIC). By contrast, METH (1.2 mg/kg, i.p.) challenge produced large increases in locomotor activity in mice pretreated with any of the three doses of METH (1.2, 2.4, 4.8 mg/kg), with the greatest expression observed in mice pretreated with 1.2 mg/kg METH (Fig. 1-IID). Thus, a sequence of six doses of METH (1.2 mg/kg, i.p.) induced the development of a state of sensitization expressed by METH challenge and distinct from a conditioned response triggered by the injection (Fig. 1-IIC,D). Thus we selected the 1.2 mg/kg METH dose for both the development and expression of locomotor sensitization in all subsequent experiments conducted during the light (ZT 5–7) or dark (ZT 19–21) periods.
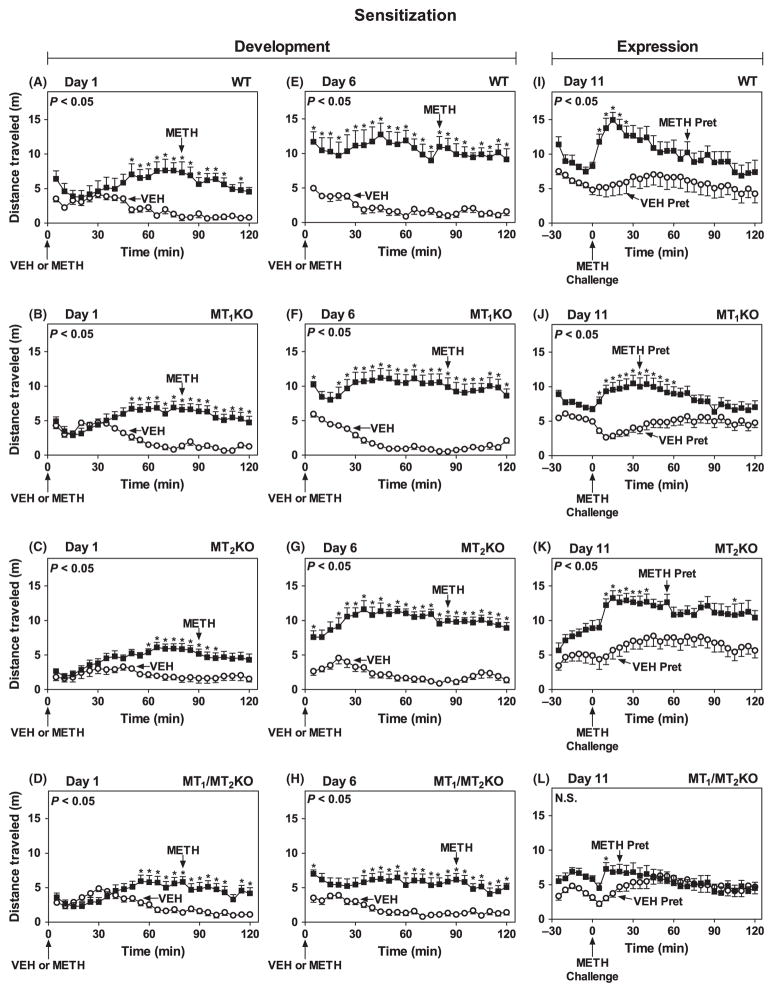
During the light phase (ZT 5–7), METH (1.2 mg/kg, i.p.) increased locomotor activity on Day 1 to a similar extent in WT, MT1KO, MT2KO and MT1/MT2KO when compared with VEH-treated controls (Fig. 2A–D). Mice exhibited little change in total locomotor activity in a 2 hr period across pretreatment days in response to VEH injections (Fig. 3A), with the MT1/MT2KO exhibiting a small but significant decrease in distance traveled on Days 2, 3 and 6 compared to Day 1. In contrast, METH-treated WT, MT1KO and MT2KO mice expressed larger total locomotor activity responses after successive daily treatments (Day 1 to Day 6) with a maximal effect at Day 6 when compared with VEH pretreatment (Figs 2E–G and 3B). However, the magnitude of the METH pretreatment-induced development of sensitization of MT1/MT2KO mice during Days 2–6 was significantly attenuated (Figs 2H and 3B). On Day 11, METH challenge (1.2 mg/kg, i.p.) induced a robust expression of locomotor sensitization in METH-pretreated WT, MT1KO and MT2KO (Fig. 2I–K), but not in MT1/MT2KO mice compared to their VEH-pretreated counterparts (Fig. 2L).
Fig. 2.
Development and expression of locomotor sensitization during the light phase (ZT 5–7) in mice lacking MT1 and/or MT2 melatonin receptors. Ordinates represent distance traveled in 5 min intervals as a function of time (abscissae) during the development (Day 1: A, B, C, D; Day 6: E, F, G, H) and expression (Day 11: I, J, K, L) of sensitization, in mice pretreated with VEH (○) or METH (1.2 mg/kg, i.p.) (■) (A–H) and challenged with METH (1.2 mg/kg, i.p.) (I–L) at times indicated by arrows under the abscissae. The main effects of drug treatment for each genotype were as follows: Day 1: WT: F(1,14) = 16.73, P < 0.05 (A); MT1KO: F(1,29) = 24.88, P < 0.05 (B); MT2KO: F(1,19) = 22.33, P < 0.05 (C); and MT1/MT2KO: F(1,23) = 15.40, P < 0.05 (D). Day 6: WT: F(1,14) = 37.69, P < 0.05 (E); MT1KO: F(1,29) = 52.71, P < 0.05 (F); MT2KO: F(1,19) = 112.40, P < 0.05 (G); and MT1/MT2KO: F(1,23) = 25.08, P < 0.05 (H). Day 11: WT: F(1,14) = 7.98, P < 0.05 (I); MT1KO: F(1,29) = 17.18, P < 0.05 (J); MT2KO : F(1,19) = 14.38, P < 0.05 (K); MT1/MT2KO: F(1,23) = 0.79, P > 0.05 (L). Significance of the main effect of drug (A–H) or drug pretreatment (I–L) is noted in the upper left of each graph. NS, not significant. *P < 0.05 when compared with the corresponding 5 min VEH interval (Bonferroni test).
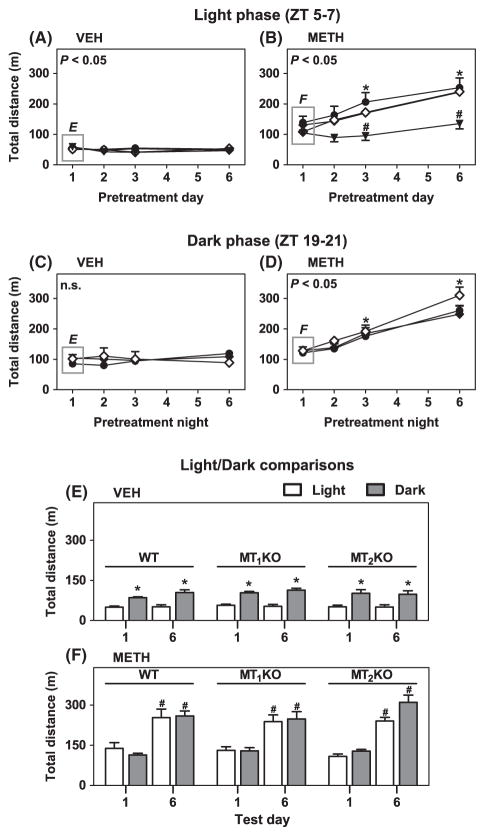
Fig. 3.
Development of locomotor sensitization during the light (ZT 5–7) and dark (ZT 19–21) phases across pretreatment days. Panels A–D: ordinates represent total distance traveled in 2 hr as a function of pretreatment in Day 1 to Day 6 (abscissae) during development of sensitization in the light phase (ZT 5–7) (A, B) and the dark phase (ZT 19–21) (C–D) in WT (●), MT1KO (◆), MT2KO (◇) and MT1/MT2KO (▼) C3H mice. Significance of the main effect of test day (A: F(3,123) = 3.15, P < 0.05; B: F(3,132) = 47.24, P < 0.05) and test night (C: F(3,72) = 0.74, P > 0.05; D: F(3,75) = 77.85, P < 0.05) is noted in the upper left of the graphs. Panel B: *P < 0.05 when compared to Day 1 in each genotype; #P < 0.05 when compared to corresponding values in WT, MT1KO and MT2KO mice. Panel D: *P < 0.05 when compared to Day 1 in each genotype. Panels E and F: ordinates represent total locomotor distance traveled in 2 hr during the light (ZT 5–7) (open columns) and dark (ZT 19–21) (gray columns) phases at Day/Night 1 and Day/Night 6 of pretreatment with VEH or METH (1.2 mg/kg, i.p.). Panel E: *P < 0.05 when compared with corresponding light phase values. Panel F: #P < 0.05 when compared with corresponding Day 1 values.
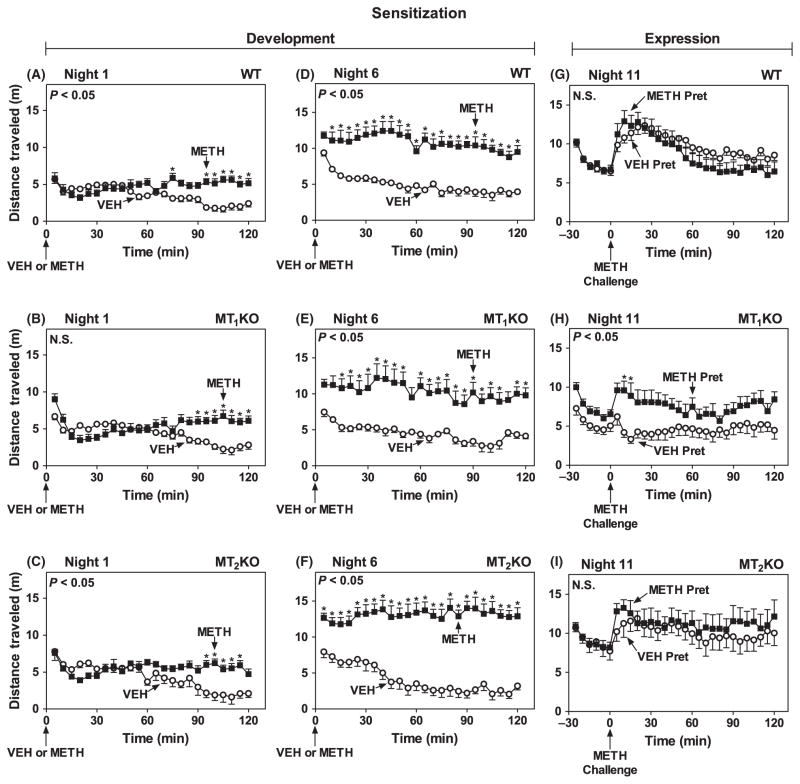
During the dark phase, when circulating melatonin peaks in C3H mice [22, 25], METH pretreatment on Night 1 significantly increased locomotor activity in WT (Fig. 4A), MT1KO (Fig. 4B) and MT2KO (Fig. 4C) mice relative to VEH controls. No change in total locomotor responses was observed in VEH-pretreated mice across six consecutive test nights (Fig. 3C). In METH-pretreated WT, MT1KO and MT2KO mice the increases in locomotor responses were statistically different from VEH-pretreated and became progressively higher with the successive nightly treatments, with a maximal development of sensitization on Night 6 (Figs 3D and 4D–F). The magnitude of locomotor responses during the development phase in each test night was not significantly different among either VEH- or METH-pretreated WT, MT1KO and MT2KO mice, suggesting that deletion of either MT1 or MT2 receptors has no effect on the development of sensitization at night. On Night 11, however, METH challenge triggered statistically significant expression of behavioral sensitization when compared with vehicle only in the MT1KO (Fig. 4H versus G and I). Expression of locomotor sensitization in METH-pretreated WT (Fig. 4G) and MT2KO mice (Fig. 4I) was of similar magnitude as in VEH-pretreated controls.
Fig. 4.
Development and expression of locomotor sensitization during the dark phase (ZT 19–21) in mice lacking MT1 or MT2 melatonin receptors. Ordinates represent distance traveled in 5 min intervals as a function of time (abscissae) during the development (Night 1: A, B, C; Night 6: D, E, F) and expression (Night 11: G, H, I) of sensitization, in mice pretreated VEH (○) or METH (1.2 mg/kg, i.p.) (■) (A–F) and challenged with METH (1.2 mg/kg, i.p.) (G–I) at times indicated by arrows under the abscissae. The main effects of drug treatment for each genotype were as follows: Night 1: WT: F(1,19) = 17.63, P < 0.05 (A); MT1KO: F(1,15) = 3.17, P > 0.05 (B); and MT2KO: F(1,17) = 4.75, P < 0.05 (C). Night 6: WT: F(1,19) = 54.45, P < 0.05 (D); MT1KO: F(1,15) = 21.55, P < 0.05 (E); and MT2KO: F(1,17) = 63.73, P < 0.05 (F). Night 11: WT: F(1,19) = 0.72, P > 0.05 (G); MT1KO: F(1,15) = 5.98, P < 0.05 (H); MT2KO: F(1,17) = 0.30, P > 0.05 (I). Significance of the main effect of drug (A–F) or drug pretreatment (G–I) is noted in the upper left of each graph. NS, not significant. *P < 0.05 when compared with the corresponding VEH 5 min interval (Bonferroni test).
The total distance traveled by WT, MT1KO and MT2KO mice pretreated with VEH were significantly higher at night compared to day, with no significant differences observed between Day 1 and Day 6 at either time point (Fig. 3E). The converse was true for METH-treated mice, which exhibited no significant difference between night and day but did express larger locomotor responses on Day 6 relative to Day 1 at both time points (Fig. 3F).
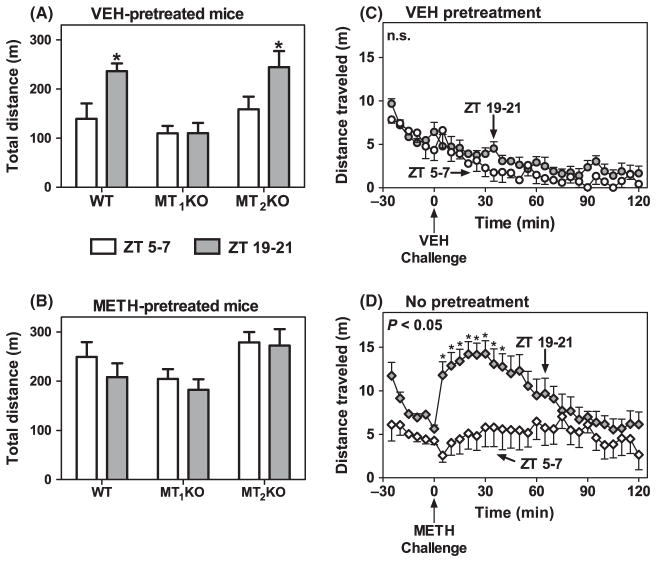
Locomotor responses triggered by METH challenge during the light and dark phases on Day 11 are summarized in Fig. 5A,B. In VEH-pretreated WT and MT2KO mice (Fig. 5A), the expression of locomotor sensitization was significantly higher after METH challenge during the dark phase (ZT 19–21) than the light phase (ZT 5–7). By contrast, mice lacking the MT1 receptors and pretreated with VEH show the same magnitude of locomotor sensitization upon a METH challenge on Day 11 during the light and dark phase (Fig. 5A). The magnitude of locomotor sensitization induced by METH challenge in METH-pretreated WT, MT1KO, or MT2KO mice was identical during the light and dark periods (Fig. 5B). VEH-pretreated mice challenged with VEH showed no difference between the light and dark phases (Fig. 5C).
Fig. 5.
Time-of-day differences in the expression of locomotor sensitization in VEH-pretreated mice. Panels A and B: ordinates represent total distance traveled for 2 hr after METH challenge on Day/Night 11 during the light (ZT 5–7) (open columns) and dark (ZT 19–21) (gray columns) phases following pretreatments with VEH (A) or METH (1.2 mg/kg, i.p.) (B). *P < 0.05 when compared to corresponding ZT 5–7 values in VEH-pretreated WT and MT2 KO mice (Bonferroni test). Panels C and D: ordinate represents distance traveled in 5 min intervals as a function of time before and after VEH (C) or METH (1.2 mg/kg, i.p.) (D) challenge at the times indicated by arrows under the abscissae. The main effect of time of day was in C: F(1,17) = 2.45, P > 0.05 and in D: F(1,6) = 7.21, P < 0.05. *P < 0.05 when compared with the corresponding 5 min VEH bin (Bonferroni test).
VEH-pretreated WT and MT2KO mice became sensitized to the effects of METH on Day 11 uniquely at night. Interestingly, the dramatic locomotor sensitization to METH challenge was also observed during the dark period in mice not pretreated with VEH during the development of sensitization stage (Fig. 5D). These mice were subjected to an identical locomotor testing protocol, i.e., for example, placement in novel arenas for six consecutive days. Moreover, this response to the novel arenas was comparable to the sensitized response observed in METH-pretreated WT mice during the light (Fig. 2I) and dark (Fig. 4G) phases.
Discussion
This study reports the involvement of the MT1 and MT2 melatonin receptors in modulating the development and expression of METH-induced locomotor sensitization in melatonin proficient C3H/HeN mice. In C3H/HeN WT mice, treatment with consecutive daily doses of METH during the light phase (ZT 5–7) when endogenous melatonin levels are low induced a gradual and statistically significant increase in the development of locomotor sensitization from Day 1 to Day 6 when compared with vehicle-treated controls. A METH challenge dose after abstinence during the light phase induced a robust expression of sensitization in mice pretreated with METH as compared with those pretreated with vehicle. Mice with genetic deletion of the MT1 (MT1KO) or the MT2 (MT2KO) melatonin receptors exhibited similar development and expression sensitization after METH pretreatments during the light phase, but these responses were significantly attenuated in MT1/MT2KO mice. During the dark phase (ZT 19–21), when endogenous melatonin levels are elevated [26], the locomotor responses to METH challenge were identical in VEH-and METH-pretreated mice in both WT and MT2KO groups. This sensitization-like response to METH challenge was observed in mice exposed to novel environments only during the dark phase, with or without VEH pretreatments. This nocturnal enhancement of the response to METH challenge after repeated VEH pretreatments was not seen in MT1KO mice. The data suggest that repeated exposure to a novel environment during the dark phase triggers sensitization to METH via endogenous melatonin activation of MT1 receptor-mediated signaling and/or other circadian factors.
Melatonin or opioid receptor activation modulates METH-induced locomotor sensitization as this response was significantly attenuated by deletion of both the MT1 and MT2 melatonin receptors or μ opioid receptors [7]. The μ/κ opioid receptor antagonist naltrexone, as well as the 5HT1A/D2 receptor partial agonist aripiprazole, blocked METH-induced development and expression of sensitization in mice [3, 6]. In the present studies, genetic deletion of both the MT1 and MT2 melatonin receptors was necessary to abrogate locomotor sensitization, which is consistent with other reports aiming to block G-protein couple receptor functional responses. Genetic deletion of both the presynaptic α2a and α2c adrenergic inhibitory autoreceptors on noradrenergic neurons was necessary to enhance norepinephrine release from cultured mouse atrium and cortical slices [27]. Further, deletion of both alpha adrenergic receptors was necessary to increase the circulating levels of norepinephrine. With the caveats associated with the use of global knockout mice [28], our findings in the MT1/MT2KO mice could reflect redundancy or compensation in the function of the MT1 and MT2 melatonin receptors.
The progressive increase in locomotor activity induced by daily doses of psychostimulants correlates with increases in dopamine release from presynaptic terminals in the nucleus accumbens [29, 30]. Presynaptic melatonin heteroreceptors modulate calcium-dependent release of dopamine in the retina and brain, playing an important role in modulating dopaminergic transmission [31–34]. Melatonin attenuates the excitation of striatal neurons induced by glutamatergic cortical inputs, which is counteracted by the D2 dopamine receptor antagonist sulpiride [35]. Activation of somadendritic MT1 receptors inhibiting neuronal firing, and/or presynaptic MT1 or MT2 heteroreceptors inhibiting dopamine release on dopaminergic neurons originating in the VTA and/or substantia nigra pars compacta provides inhibitory and modulatory inputs [14, 30, 36, 37]. It follows that the circadian release of endogenous melatonin through activation of receptors on somadendritic and/or axon terminals may exert a net diurnal inhibitory effect on basal dopaminergic activity, thereby establishing a relatively low, primed, basal level of dopaminergic activity in areas where METH exerts its effects. The sensitizing properties of METH may require this primed state which could be precluded by removal of the MT1 and MT2 receptors leading to an abnormally elevated basal level of dopamine signaling and the obscuring of sensitized activity after repeated METH.
Removal of presynaptic melatonin heteroreceptors on dopaminergic neurons may lead to persistent increases in dopamine release. In MT1/MT2KO mice persistent increases in extracellular dopamine could promote oxidative stress leading to programmed cell death [38]. The neurotoxic effects of METH are partially mediated by programmed cell death leading to increases in dopamine release. METH-mediated increases in dopamine release are known to cause apoptosis through activation of D1 receptor-mediated mitochondrial dysfunction [39] and autophagy through inhibition of the mammalian target of rapamycin [40]. Neuroprotective effects of melatonin on multiple pathways coupling METH to apoptotic and autophagic cell death have been reported [40–44]. Removal of presynaptic inhibitory MT1 and MT2 melatonin hetero-receptors on dopaminergic neuron receptors could potentially increase the apoptotic effects of METH via increases in dopamine release [31, 33]. Further, genetic deletion of MT1 mitochondrial receptors may potentiate METH-induced programmed cell death as activation of this receptor reduces cytochrome c and caspase activation [45]. Activation of the MT1 receptors blocks mitochondrial dysfunction and apoptosis in a mouse model of Huntington’s disease [45], and melatonin reverses the effects of METH on mTOR-controlled autophagy and activation of pro-apoptotic proteins including Bax, calpain and caspases [40–42]. It is then conceivable that in dopaminergic neurons expressing melatonin receptors, activation of the MT1 and perhaps the MT2 melatonin receptors may attenuate the neurotoxic effect of METH, by reducing dopamine release [31, 33] and/or mitochondrial mediated apoptosis [45]; hence preserving the health and proper function of dopaminergic pathways throughout the repeated treatments with METH. Deletion of the MT1 and MT2 melatonin receptors in dopaminergic neurons since birth may lead to neuronal dysfunction by depriving the neuron of the circadian modulation of dopaminergic activity via inhibition of dopamine release and apoptosis.
The mechanism of novelty-induced expression of sensitization to METH challenge during the dark period is not known. Exposure to a novel environment stimulates the transient release of dopamine in the nucleus accumbens as measured by voltammetry [46], suggesting a mechanism by which METH could facilitate sensitization under conditions in which dopaminergic transmission via MT1 receptors is inhibited by endogenous melatonin. Notably, the magnitude of this putative novelty-induced sensitized response to single METH challenge is identical to that induced by repeated METH pretreatments, in mice endowed with MT1 receptors, indicating that novelty may have a greater role in determining the magnitude of sensitization at night than does METH. This phenomenon may have its roots in an association between novelty and stress, as environmental novelty up-regulates stress-related hormones (e.g., corticosterone) [47] and repeated mild stressors (e.g., tail pressure) induce locomotor sensitization to amphetamine [48]. Activation of signaling pathways involves circadian molecules such as Per1, which is linked to MT1 melatonin receptor activation [18, 49] and necessary for METH-mediated sensitization [50].
The nocturnal nature of the effect of novelty in our studies is likely explained by intracellular signaling through the MT1 receptor. Signaling targets for this receptor in C3H/HeN mice includes Period 1 (Per1) [51], a clock gene whose protein oscillates in the caudate-putamen and nucleus accumbens with high levels during the day (ZT 5) and low levels at night (ZT 17–24) in a manner contingent on the presence of the pineal gland [18]. In the pars tuberalis, a similar rhythmic pattern of Per1 expression requires the MT1 receptor [49]. METH treatment leads to the acute induction of Per1 expression in the striatum through activation of D1-like dopamine and N-methyl-D-aspartate (NMDA) receptors. Further, METH challenge triggers a sensitized response in Per1 induction [50]. Interactions between METH (acting through dopamine and its receptors) and the MT1 receptor at the level of oscillating Per1 protein may account for the appearance of a sensitization-like state during the dark phase in mice exposed to novel environments. Specifically, MT1 receptor activation by endogenous melatonin in a setting of low Per1 protein abundance may render cells in the dopaminergic pathway to be more responsive to the dopamine release events triggered by exposure to the locomotor arenas. Consistent with this hypothesis, deletion of CLOCK (circadian locomotor output cycles kaput) protein, a transcriptional initiator of Per1 expression, has been shown to induce hyperactivity in the dopaminergic pathways and increased responsiveness to cocaine in behavioral assays of reward in mice [51, 52].
Our results demonstrate that deletion of the MT1 and MT2 melatonin receptors decreases the development and expression of METH-induced locomotor sensitization during the light phase and that the MT1 receptor uniquely confers enhanced susceptibility to the effect of novelty on METH-induced locomotor responses during the dark phase. The effect of novelty on the enhancement of METH-induced locomotor activity exclusively at night has important implications for understanding the factors that regulate sensitivity to METH in humans, that is, the time of day and the novelty of the context surrounding drug use. Circadian changes in MT1 melatonin receptor signaling together with changes in circadian molecules might play an important role in mediating the expression of sensitization by a single dose of METH to mice exposed in consecutive days to a novel environment at night. Sensitization is widely considered a viable model for the neuroadaptations that underlie the advancement of drug craving; therefore, the melatonin receptors may be a target worthy of additional investigations for their potential in treating METH abusers.
Acknowledgments
The authors would like to thank Iwona Stepien, Sarada Vissapragada, Kathleen A. McGowan, Jason Ma and Peter Crombe for their kind and capable technical assistance with animal management and equipment maintenance, and to Shannon J. Clough and Ekue B. Adamah-Biassi for discussions and comments on the manuscript. We are indebted to Drs. Jerrold C. Winter and Alexis C. Thompson for their expert guidance on experimental design, perspectives on data interpretation, and critical feedback on the manuscript.
Footnotes
Author contributions
All authors contributed to the conception and design, acquisition of data, or analysis and interpretation of data (AJH, MLD, RLH). AJH drafted the manuscript, which was substantially edited by MLD. All authors approved the manuscript before submission.
Conflict of interest
The authors declare that this work was funded by R01DA021870 to MLD. The authors declare that over the last 3 yr MLD was a consultant for and received compensation from Adolor, and from Takeda Pharmaceutical North America Inc.
References
- 1.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 2.Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67:100–109. doi: 10.1016/j.brainresbull.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirabayashi M, Alam MR. Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol Biochem Behav. 1981;15:925–932. doi: 10.1016/0091-3057(81)90056-3. [DOI] [PubMed] [Google Scholar]
- 5.Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- 6.Futamura T, Akiyama S, Sugino H, et al. Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1115–1119. doi: 10.1016/j.pnpbp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Purser C, Tien LT, et al. mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010;88:2294–2302. doi: 10.1002/jnr.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giardino WJ, Pastor R, Anacker AM, et al. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manev H, Uz T. Dosing time-dependent actions of psychostimulants. Int Rev Neurobiol. 2009;88:25–41. doi: 10.1016/S0074-7742(09)88002-1. [DOI] [PubMed] [Google Scholar]
- 10.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinology. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 11.Stehle JH, Saade A, Rawashdeh O. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51:17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 12.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 14.Uz T, Arslan AD, Kurtuncu M, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Falcon E, Mcclung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuribara H, Tadokoro S. Circadian variation in methamphetamine- and apomorphine-induced increase in ambulatory activity in mice. Pharmacol Biochem Behav. 1982;17:1251–1256. doi: 10.1016/0091-3057(82)90129-0. [DOI] [PubMed] [Google Scholar]
- 17.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uz T, Akhisaroglu M, Ahmed R, et al. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 19.Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- 20.Sleipness EP, Sorg BA, Jansen HT. Time of day alters longterm sensitization to cocaine in rats. Brain Res. 2005;1065:132–137. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Sircar R. Effect of melatonin on cocaine-induced behavioral sensitization. Brain Res. 2000;857:295–299. doi: 10.1016/s0006-8993(99)02460-9. [DOI] [PubMed] [Google Scholar]
- 22.Itzhak Y, Martin JL, Black MD, et al. Effect of melatonin on methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- induced dopaminergic neurotoxicity and methamphetamine- induced behavioral sensitization. Neuropharmacology. 1998;37:781–791. doi: 10.1016/s0028-3908(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 23.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant- like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39:170–177. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 24.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirabayasi M, Saito T, Tadokoro S. Differential sensitization to ambulation-increasing effect of methamphetamine after repeated administration to mice in activity cages of different sizes. Jpn J Pharmacol. 1991;57:91–97. doi: 10.1254/jjp.57.91. [DOI] [PubMed] [Google Scholar]
- 26.Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28:185–192. doi: 10.1034/j.1600-079x.2001.280309.x. [DOI] [PubMed] [Google Scholar]
- 27.Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 28.Crusio WE, Goldowitz D, Holmes A, et al. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;31:14496–14507. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa T, Suzuki Y, Nagayasu K, et al. Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures. PLoS One. 2011;6:e24865. doi: 10.1371/journal.pone.0024865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- 32.Mcclung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. The Scientific World Journal. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cellular Mol Neurobiol. 2001;21:605–616. doi: 10.1023/A:1015187601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zisapel N, Egozi Y, Laudon M. Inhibition of dopamine release by melatonin: regional distribution in the rat brain. Brain Res. 1982;246:161–163. doi: 10.1016/0006-8993(82)90157-3. [DOI] [PubMed] [Google Scholar]
- 35.Escames G, Acuna Castroviejo D, Vives F. Melatonin-dopamine interaction in the striatal projection area of sensorimotor cortex in the rat. NeuroReport. 1996;7:597–600. doi: 10.1097/00001756-199601310-00053. [DOI] [PubMed] [Google Scholar]
- 36.Dubocovich ML, Masana MI, Iacob S, et al. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedebergs Arch Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 38.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauvais G, Atwell K, Jayanthi S, et al. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One. 2011;6:e28946. doi: 10.1371/journal.pone.0028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kongsuphol P, Mukda S, Nopparat C, et al. Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J Pineal Res. 2009;46:199–206. doi: 10.1111/j.1600-079X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 41.Wisessmith W, Phansuwan-Pujito P, Govitrapong P, et al. Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2009;46:433–440. doi: 10.1111/j.1600-079X.2009.00680.x. [DOI] [PubMed] [Google Scholar]
- 42.Suwanjang W, Phansuwan-Pujito P, Govitrapong P, et al. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cells. J Pineal Res. 2010;48:94–101. doi: 10.1111/j.1600-079X.2009.00731.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaewsuk S, Sae-Ung K, Phansuan-Pujito P, et al. Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin, and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int. 2009;55:397–405. doi: 10.1016/j.neuint.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Nopparat C, Porter JE, Ebadi M, et al. The mechanism for the neuroprotective effect of melatonin against methamphetamine- induced autophagy. J Pineal Res. 2010;49:382–389. doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Sirianni A, Pei Z, et al. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J Neurosci. 2011;31:14496–14507. doi: 10.1523/JNEUROSCI.3059-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebec GV, Grabner CP, Johnson M, et al. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 47.Hennessy MB. Sensitization of the plasma corticosterone response to novel environments. Physiol Behav. 1991;50:1175–1179. doi: 10.1016/0031-9384(91)90579-d. [DOI] [PubMed] [Google Scholar]
- 48.Antelman SM, Eichler AJ, Black CA, et al. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 49.Von GALL C, Weaver DR, Moek J, et al. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci. 2005;1040:508–511. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- 50.Nikaido T, Akiyama M, Moriya T, et al. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- 51.Imbesi M, Arslan AD, Yildiz S, et al. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal ‘clock’ gene expression in striatal neurons in vitro. J Pineal Res. 2009;46:87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 52.Mcclung CA, Sidiropoulou K, Vitaterna M, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]