Abstract
Background
More than half of persons living with HIV (PLWH) in the US smoke cigarettes, and tobacco use is responsible for considerable morbidity and mortality in this group. Little is known about the efficacy of tobacco treatment strategies in PLWH.
Design
Randomized controlled trial comparing Positively Smoke Free (PSF), an intensive group therapy intervention targeting HIV-infected smokers, to standard care.
Methods
A cohort of 145 PLWH smokers, recruited from an HIV-care center in the Bronx, New York, were randomized 1:1 into the PSF program or standard care. All were offered a 3-month supply of nicotine replacement therapy. PSF is an eight session program tailored to address the needs and concerns of HIV-infected smokers. Sessions were co-facilitated by a graduate student and an HIV-infected peer. The primary outcome was biochemically-confirmed, seven-day point-prevalence abstinence at three months.
Results
In the intention to treat analysis, PSF condition subjects had nearly double the quit rate of controls (19.2% vs 9.7%, P=0.11). In the complete case, as-treated analysis, assignment to PSF was associated with increased odds of quitting (ORadj 3.55, 95% CI: 1.04– 12.0). Latino ethnicity and lower loneliness score were predictive of abstinence. Subjects in the PSF condition exhibited significant decreases in daily cigarette consumption and significant increases in self-efficacy and in motivation to quit. Attendance of ≥7 sessions was associated with higher quit rates.
Conclusions
These findings suggest a positive effect of PSF on cessation rates in PLWH smokers. Loneliness and self-efficacy are influential factors in the smoking behaviors of PLWH.
Keywords: HIV, tobacco use, cigarette, smoking, treatment
Introduction
Over half of persons living with HIV (PLWH) in the US smoke cigarettes.1,2 As longevity has improved secondary to effective antiretroviral therapy, alarming increases in rates of tobacco-related illnesses including cancers3 and coronary events4 have been observed. Cigarette smoking is also associated with increased rates of opportunistic infections,5 poorer quality of life,6 and inferior medication adherence7 in PLWH. One large cohort study estimated that 24% of deaths in PLWH in the modern HAART era are attributable to tobacco use.8
As a group, PLWH smokers have high rates of substance use and comorbid psychiatric illness.9 They are exposed to a range of additional stressors including poverty,10 racism/discrimination,11 stigmatization,12 loneliness,12 and health concerns.13 These factors decrease the likelihood that brief, “simple” cessation interventions will be successful in HIV-infected smokers.14 High smoking rates among PLWH in New York, despite the frequency of advice to quit from providers, ready availability of quitlines and nicotine replacement therapy (NRT), and high motivation to quit attest to the fact that more intensive treatment options are necessary.9,15
There are few published randomized controlled trials of intensive cessation interventions for PLWH smokers.16–19 All of them employed one-on-one counseling strategies combined with an offer of nicotine replacement therapy. Three of the studies demonstrated improved tobacco abstinence rates in recipients of the interventions.16–18 However, cessation programs targeting this group are uncommon, and there are no standard recommendations to guide providers and their patients in achieving and sustaining abstinence.
In 2008, with the input of HIV care specialists, behavioral psychologists, tobacco treatment experts, and HIV-infected smokers, we developed Positively Smoke Free (PSF), an eight-session, group-therapy intervention based on social cognitive theory principles. PSF is an intervention specifically tailored to the HIV-infected population based on the patterns and predictors of tobacco use in PLWH.9 This paper describes a randomized controlled trial of PSF involving a group of HIV-infected smokers in the Bronx, New York. We hypothesized that participants in the PSF intervention would achieve higher abstinence rates than those in a standard care treatment group.
Methods
Research setting and project staff
Montefiore Medical Center is a 1,491-bed teaching hospital located in the Bronx. Its Infectious Diseases Clinic provides care to over 2,700 HIV-infected adults, the majority of whom belong to ethnic minority groups and have incomes below the federal poverty line. The group leaders for PSF consisted of two “professional” and peer-facilitator pairs recruited from the Ferkauf Graduate School of Psychology and the ID Clinic patient roster respectively. All group leaders were ex-smokers, and the two peers were HIV-infected. Before conducting any sessions, all of them completed certified courses in tobacco treatment
Study design
This was a prospective randomized controlled study comparing the PSF program and offer of NRT to standard of care (including offer of NRT). Patients were referred by their clinic providers or recruited from the waiting room. Participants completed self-report questionnaires on days 0, 42, and 132, as well as exhaled carbon monoxide (ECO) measurement. The primary endpoint was 7-day self-reported, point-prevalence abstinence at the final study visit (approximately 3 months post quit date). Subjects reporting abstinence with discrepant ECO levels of ≥ 10 parts per million (ppm) were coded as non-abstinent.
Recruitment
Individuals expressing interest in the study were screened for eligibility by confirming their HIV infection status (with either a positive EIA or a detectable HIV-1 viral load), smoking status (with an affirmative response to “During the past 5 days, did you use any product containing nicotine including cigarettes, pipes, or cigars?”20), and motivation to quit (by scoring ≥6 on the Readiness to Quit Ladder21). Subjects who provided consent were randomized in a 1:1 schedule to the two study conditions.
The standard care condition
Subjects were given a quit smoking brochure, brief advice to quit (i.e. < five minutes), and an offer of a three month supply of NRT.
The Positively Smoke Free (PSF) condition
Subjects were assigned to either a day or evening program group, according to their preference. The study staff aimed to enroll between six and eight subjects to each group. The program emphasized the use of a buddy, a supportive friend or family member, to provide encouragement through the quit attempt. Buddies were welcome to attend sessions. Travel fare was provided to each attendee, including buddies.
PSF is an eight session intervention modeled after the program described in the Tobacco Dependence Treatment Handbook14 and based upon Social Cognitive Theory principles.22 Its content was crafted to address numerous concerns of particular relevance to PLWH smokers (e.g. specific risks of smoking to PLWH, comorbid psychiatric illness and substance use, social isolation, stress reduction, etc.) that were identified in our preliminary work,9 and it was revised and refined through multiple pilot offerings. An overview of the course curriculum, including HIV-referent themes, is presented in Table 1. Each session is led by two cofacilitators (a “professional” and a peer) and encourages the recounting of personal experiences as well as a group interaction dynamic. The same cofacilitators remain with a given group throughout all sessions. Sessions are 90 minutes long and occur weekly, except for the session after Quit Day (Session 5) which is scheduled three days after the quit. At the first session, Quit Day is defined as the day that all tobacco use will cease and is planned for the entire group four weeks thereafter.
Table 1.
An overview of the Positively Smoke Free course curriculum.
| Session # | General Themes | HIV-Referent Themes |
|---|---|---|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
| 7 |
|
|
| 8 |
|
|
Measures
All participants were asked to complete a self-administered, pencil-and-paper questionnaire at the three study visits. Tobacco use and tobacco knowledge was assessed with questions from the CDC QIT Inventory.20 Nicotine addiction was assessed with the Modified Fagerstrom Tolerance Questionnaire.23 Motivation to quit was assessed with the Abrams and Biener Readiness to Quit Ladder.21 Reasons for quitting were assessed with the Reasons for Quitting Scale, which measures intrinsic (concerns about health, desire for self-control) and extrinsic (immediate reinforcement, social influence) motivation for smoking cessation.24 Self-concept was assessed with the Smoker Self-concept and Abstainer Self-concept Questionnaire, which measures the extent to which smokers perceive themselves as more similar to smokers or abstainers.25 Self-efficacy was assessed with the Self-efficacy/Temptations Scale, which measures level of confidence to abstain from smoking in positive affect/social situations, negative affect situations, and habit/craving situations.26 Decisional balance was assessed with the Smoking Decisional Balance Short Form, which measures perceived pros and cons of smoking.27 Social support was assessed with the Partner Interaction Questionnaire, which measures level of predicted support from a significant other for one’s cessation efforts.28 Spirituality was assessed with the Ironson-Woods Spirituality Index, which measures sense of peace, faith in a deity, religious behavior, and compassion for others.29 Loneliness was assessed with the UCLA Loneliness Scale.30 Depression and anxiety were assessed with the Center for Epidemiologic Studies Depression Scale31 and the State-Trait Anxiety Scale.32 Abstinence was assessed using a question from the CDC QIT Inventory,20 “Now, think carefully about the last seven days. Did you smoke cigarettes, even a puff, on any of those days?” Exhaled carbon monoxide (ECO) levels were measured using the Bedfont piCO+ Smokerlyzer (Bedfont Scientific Ltd., Kent, UK) maintained according to the manufacturer’s instructions.
Fidelity monitoring
All sessions were recorded. Fifty-eight percent of the sessions were reviewed by the graduate student who was not leading the group, and topic coverage checklists were completed for each session. Checklists included a mean of 11 items/session, which could be scored as “Yes” or “No” for coverage during the session.
Study contamination survey
Control subjects were asked a series of questions inquiring about discussion of program contents with intervention condition subjects and also inquiring about familiarity with specific cessation strategies (e.g. deep-breathing techniques) taught in PSF.
Program satisfaction survey
PSF subjects rated the various components of the program on a Likert scale ranging from “not helpful at all” to “extremely helpful.”
Sample size considerations
Based upon the two studies of PLWH smokers available at the time,16,17 the investigators estimated that 25% of the PSF group and 8% of the control group would achieve the primary cessation endpoint. A sample size of 148 subjects was predicted to provide 80% power to detect a significant difference in the study outcome between the two conditions with a null hypothesis of no difference in outcome between groups.
Statistical analyses
Dichotomous variables were analyzed using chi-square or Fisher’s exact test. Comparisons of means were accomplished using Student’s t-test or Mann-Whitney U-test. Correlation of two continuous variables was performed using Spearman’s rank correlation. In evaluating factors associated with the abstinence outcome, a backward, stepwise multivariate logistic regression analysis was performed incorporating those variables that were associated (P≤0.10) with abstinence on univariate analysis. Since this was an efficacy trial of PSF, study condition was included in all multivariate models regardless of its level of association on univariate analyses. A mixed-effects linear modeling (MELM) approach was employed to assess the association of study condition with variables repeatedly measured during the three study visits. Changes from assessment 1 to assessment 3 were calculated for those variables found to be significantly associated with study condition in the MELM analyses, and the new variables, reflecting the change in these variables, were individually added into the logistic regression models to assess for independence of association with abstinence and also for mediating effects. Substantial attenuation of the association between study condition and the abstinence outcome after inclusion of one of these covariates was interpreted as a mediating effect of the variable on the study condition-abstinence relationship. Data analyses were performed using SPSS version 18.0 or SAS version 9.13.
Study oversight
The study was reviewed and approved by the Montefiore Medical Center IRB.
Results
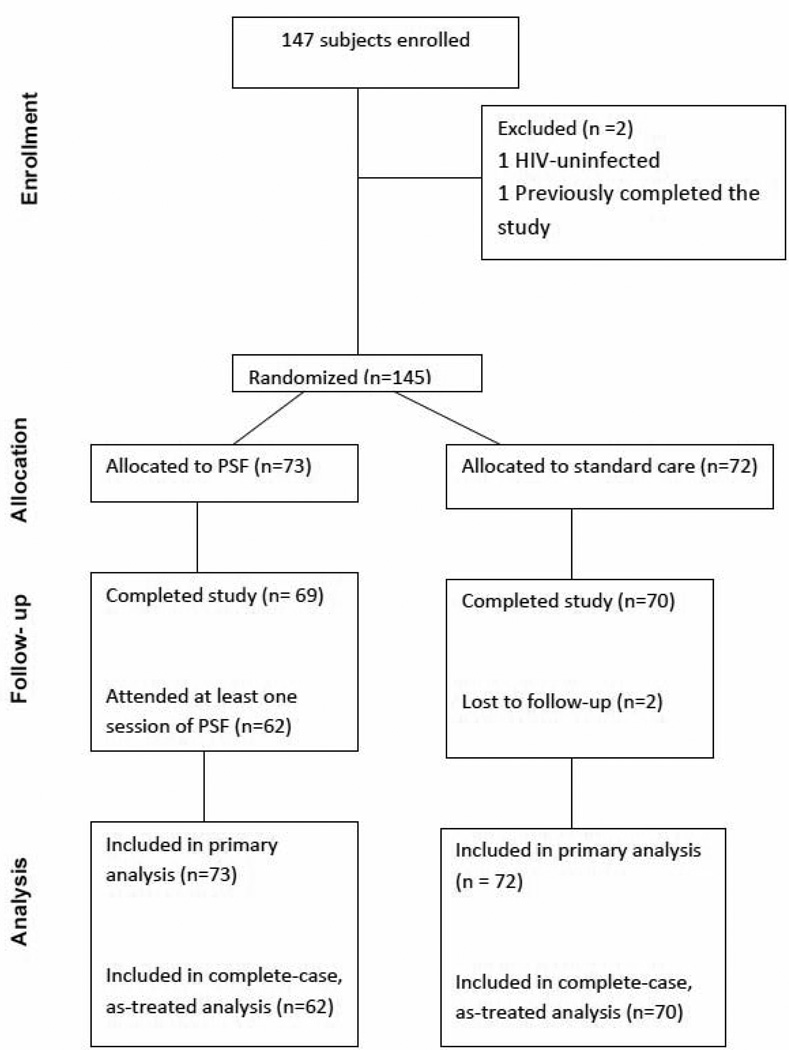
A total of 184 individuals were screened for entry into the study. Thirty-seven were excluded because of: failure to return for the enrollment visit (17), uncertain smoking status with ECO in non-smoker range (7), low motivation to quit score (6), scheduling conflicts (3), decision to try to quit outside of the study setting (2), and successful cessation prior to enrollment (2). Of the 147 subjects who enrolled in the study, two were discovered to be ineligible prior to randomization, yielding a final sample of 145. Seventy-three were randomized to PSF and 72 to the control condition. One hundred thirty-nine subjects (95.9%) completed the study (Figure 1). For analytic purposes, lost-to-follow-up subjects were considered non-abstinent.
Figure 1.
Flow of study subjects
Participants ranged in age from 29 to 70 years with a mean of 48.6±7.0. Forty-nine percent of the cohort were male, 50.3% were female, and 0.7% were transgender, 85.8% were Black/African American, 11.0% were White, and 3.1% were American Indian/Alaskan Native. Across races, the study sample was 22.8% Latino. The HIV risk behavior was heterosexual contact in 57.9%, same-sex contact in 14.5%, injection drug use in 9.0%, transfusion in 2.8%, and unknown in 15.9%. Less than a quarter (23.3%) were married or living with a partner, and 88.0% were unemployed. Recent (within the past 30 days) use of marijuana was reported by 41.7%, cocaine by 28.9%, and heroin by 7.6%. Mean of the cohort’s most recent CD4+ count was 494±289 cells/ul (range: 5 to 1681). Mean duration of cigarette smoking experience for the cohort was 32.7±8.2 years (range: 11.0 to 54.0), and mean daily cigarette intake was 12.0±8.8 (range: 1 to 60). Mean score at enrollment on the Fagerstrom Tolerance Questionnaire was 5.0±2.1 with 24.8% scoring ≥ seven, indicating high nicotine dependence. There were no differences in baseline characteristics between the two conditions (Table 2).
Table 2.
Baseline characteristics of the study cohort.
| Characteristic | Control condition (N=72*) |
Intervention condition (N=73*) |
P |
|---|---|---|---|
| Age | 47.9±6.6 | 49.2±7.4 | 0.24 |
| Gender | |||
| Male | 35 (48.6%) | 36 (49.3%) | |
| Female | 37 (51.4%) | 36 (49.3%) | 0.60 |
| Transgender | 0 (0.0%) | 1 (1.4%) | |
| Ethnicity | |||
| Latino | 18 (25.7%) | 13 (19.7%) | 0.40 |
| Non-Latino | 52 (74.3%) | 53 (80.3%) | |
| Race | |||
| Black/African American | 54 (85.7%) | 55 (85.9%) | |
| White | 7 (11.1%) | 7 (10.9%) | 0.99 |
| American Indian or Alaskan native | 2 (3.2%) | 2 (3.1%) | |
| Marital status | |||
| Married/living with partner | 17 (25.8%) | 13 (20.6%) | 0.49 |
| Single | 49 (74.2%) | 50 (79.4%) | |
| Housing status | |||
| Stable | 63 (88.7%) | 66 (90.4%) | |
| Transitional | 7 (9.9%) | 6 (8.2%) | 0.94 |
| Homeless | 1 (1.4%) | 1 (1.4%) | |
| Employment status | |||
| Unemployed/disabled | 56 (88.9%) | 61 (87.1%) | |
| Part-time employment | 5 (7.9%) | 9 (12.9%) | 0.22 |
| Full-time employment | 2 (3.2%) | 0 (0.0%) | |
| HIV risk behavior | |||
| Heterosexual contact | 46 (63.9%) | 38 (52.8%) | |
| Same sex contact | 8 (11.1%) | 13 (18.1%) | |
| Injection drug use | 5 (6.9%) | 8 (11.1%) | 0.43 |
| Transfusion | 3 (4.2%) | 1 (1.4%) | |
| Other/unknown | 10 (13.9%) | 12 (16.7%) | |
| Most recent CD4+ count (cells/ul) | 483.2±248.9 | 504.4±326.1 | 0.66 |
| Daily cigarette consumption | 11.1±7.3 | 12.8±10.1 | 0.25 |
| Nicotine dependence score** | 5.2±2.0 | 4.8±2.1 | 0.19 |
| Motivation to quit*** | 7.3±0.9 | 7.4±0.9 | 0.44 |
Intention-to-treat analysis
Twenty-one (14.5%) subjects achieved the primary cessation outcome, including 14/73 (19.2%) from the PSF group and 7/72 (9.7%) from the control group. Nine additional subjects (five from PSF) had discrepant ECO results and were classified as non-abstinent. The difference in proportions of subjects achieving the primary study endpoint between the two conditions did not achieve statistical significance (O.R.= 1.97 [0.85—4.60], P=0.11). Factors that were significantly associated with the cessation outcome on univariate analysis included older age (P=0.03), Latino ethnicity (P=0.04), and higher self-efficacy on the Positive Affect/Social Situations subscale (P=0.02), lower score on the decisional balance Pro subscale (P=0.02), and lower loneliness score (P=0.01). On multivariate analysis (Table 3), factors that were independently associated with the cessation outcome were Latino ethnicity and loneliness score. None of the other sociodemographic, clinical, or psychometric measures recorded were significantly associated with the cessation outcome. There was no significant difference in outcome between the two different pairs of group leaders. Fifty-eight subjects (40%) received either NRT, bupropion, or varenicline, during the course of the study, and their quit rate (15.5%) did not differ from those who did not receive pharmacotherapy (13.8%). Subjects with high nicotine dependence scores (≥ seven) were more likely to receive NRT (O.R. = 2.39 [1.06—5.38], P=0.03), but receipt of NRT was not associated with a difference in quit rates according to study condition in this subset of participants. Although the proportion of pharmacotherapy recipients in the PSF condition exceeded that of the control condition (46.6% vs. 33%) the difference was not statistically significant (P=0.10).
Table 3.
Factors associated with three month abstinence on univariate and multivariate intention-to-treat analyses.
| Factor | Abstinent (N=21) |
Non-abstinent (N=124) |
Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | ORadj(95% CI) | P | |||
| Intervention condition | 14/21 (66.7%) | 59/124 (47.6%) | 2.20 (0.83–5.85) | 0.11 | 2.76 (0.82–9.35) | 0.10 |
| Age | 51.6±6.6 | 48.1±6.9 | NA | 0.03 | 1.09 (0.99–1.21) | 0.09 |
| Latino ethnicity | 8/21 (38.1%) | 23/124 (18.5%) | 2.70 (1.00–7.28) | 0.04 | 4.11 (1.06–16.0) | 0.04 |
| Quit attempt in past year | 15/21 (71.4%) | 60/121 (49.6%) | 2.54 (0.92–6.99) | 0.06 | 3.21 (0.96–10.6) | 0.06 |
| Positive affect/Social situations score | 3.0±1.1 | 3.5±0.9 | NA | 0.02 | NS | NS |
| Decisional balance Pros score | 7.3±2.7 | 8.8±2.7 | NA | 0.02 | 0.82 (0.67–1.01) | 0.06 |
| Loneliness score | 20.1±8.3 | 25.4±7.7 | NA | 0.01 | 0.92 (0.85–1.00) | 0.04 |
Complete-case, as-treated analysis
Complete-case, as-treated analyses were performed excluding the six losses to follow-up and 10 subjects in the PSF condition who failed to attend any of the sessions (three subjects met both of these criteria), yielding a cohort of 132 subjects. In these analyses, subjects assigned to PSF were significantly more likely to achieve the cessation endpoint on both univariate (OR 2.63 [0.98–7.01], P=0.05) and multivariate (ORadj 3.55 [1.04–12.0], P=0.04) analyses. Additional factors that were independently associated with abstinence included white race, lower decisional balance Pros score, and lower loneliness score (Table 4).
Table 4.
Factors associated with three month abstinence on univariate and multivariate complete-case, as-treated analyses.
| Factor | Abstinent (N=21) |
Non-abstinent (N=111) |
Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | ORadj(95% CI) | P | |||
| Intervention condition | 14/21 (66.7%) | 48/111 (47.6%) | 2.63 (0.98–7.01) | 0.05 | 3.55 (1.04–12.0) | 0.04 |
| Age | 51.6±6.7 | 48.2±6.9 | NA | 0.04 | NS | NS |
| White race | 4/21 (19.0%) | 8/111 (7.2%) | 3.03 (0.82–11.2) | 0.10 | 4.70 (1.00–22.0) | 0.05 |
| Latino ethnicity | 8/21 (38.1%) | 21/111 (18.9%) | 2.64 (0.97–7.17) | 0.05 | NS | NS |
| Quit attempt in past year | 15/21 (71.4%) | 54/108 (50.0%) | 2.50 (0.90–6.94) | 0.07 | 2.66 (0.82–8.70) | 0.10 |
| Positive affect/Social situations score | 3.0±1.1 | 3.5±0.9 | NA | 0.03 | NS | NS |
| Decisional balance Pros score | 7.3±2.7 | 8.8±2.8 | NA | 0.03 | 0.79 (0.64–0.99) | 0.04 |
| Loneliness score | 20.1±8.3 | 25.3±7.5 | NA | 0.01 | 0.91 (0.83–0.99) | 0.02 |
| Anxiety score | 6.3±6.3 | 8.9±5.9 | NA | 0.07 | NS | NS |
Repeated measures analysis
Repeated measures analyses assessed the effect of PSF over time on the behavioral and psychometric measures. In these analyses, assignment to the PSF condition was associated with increased global self-efficacy (P=0.01), increased Positive Affect/Social Situations self-efficacy (P=0.006), increased Habit/Craving Situations self-efficacy (P=0.04), increased Negative Affect Situations self-efficacy (P=0.04), increased motivation to quit (P=0.003), and a greater reduction in daily cigarette consumption (P=0.002). When only non-abstinent subjects were considered, there was a significantly greater reduction in cigarettes smoked per day in the PSF condition compared to controls (−6.6 vs. −2.6 cigarettes/day, P=0.02).
Mediation analyses
The change in score from visit 1 to visit 3 was calculated for the two behavioral measures that were most significantly associated with the PSF condition on repeated measures analysis (Positive Affect/Social Situations self-efficacy and motivation to quit). These new variables were entered, individually, into the intention-to-treat logistic regression model. In these analyses, change in Positive Affect/Social Situations self-efficacy was independently associated with abstinence (ORadj=3.17 [95% CI: 1.50–6.67], P=0.002) with concurrent attenuation of the association of study condition with the abstinence (ORadj=1.56 [95% CI: 0.44–5.49], P=0.49). Change in motivation to quit was neither associated with the outcome variable nor did it attenuate the association of study condition with the outcome variable. These findings suggest that the effect of PSF on the abstinence outcome was at least partially mediated by a change in Positive Affect/Social Situations self-efficacy.
Intervention-outcome dose-response relationship
PSF condition subjects attended a mean of 4.0 ± 2.6 sessions. Older age was correlated with a higher number of sessions attended (Spearman’s rho=0.24, P=0.04), but gender, ethnicity, race, and baseline motivation to quit were not. Fourteen subjects (19.2%) attended ≥ seven sessions. There was an upward inflection in abstinence associated with attending ≥ seven sessions (42.9% abstinent), compared to those attending < seven sessions (13.6% abstinent), OR=4.78 (1.31–17.4), P=0.01. Whereas the receipt of pharmacotherapy did not appear to affect abstinence rates in the remainder of the cohort, of the eight subjects who attended ≥ seven sessions and received pharmacotherapy, five (62.5%) achieved the abstinence outcome.
Intervention fidelity
Overall fidelity to the program curriculum in the seven group therapy cycles audited was 87.4 ± 5.1%.
Study contamination
Among control condition subjects, 5.7% reported having discussed PSF activities with PSF condition subjects, and 10.0 – 17.1% reported familiarity with cessation strategies presented in the program sessions. There were no significant differences in abstinence rates in control subjects responding affirmatively to the study contamination survey questions as compared to those responding negatively.
Program satisfaction
Program elements that were rated most helpful by the participants were, in order, “Sharing with and hearing from group members,” “Sharing the experience with a group of smokers living with HIV,” “The parts of the sessions that were about living with HIV,” and “Having a group leader who is living with HIV.” The elements that were rated the least helpful were “Role playing during the sessions,” “Weekly homework assignments,” and “Learning how to work with a buddy.”
Discussion
This study reports the results of a tailored group intervention for tobacco use in PLWH. We enrolled 145 subjects into a randomized controlled trial of an intensive group intervention targeting HIV-infected smokers who were motivated to quit. Its emphasis was on building self-efficacy to quit and to remain abstinent. Its curriculum was crafted to address issues of particular importance to PLWH as determined in earlier work published by ourselves and others.9,13 In order to strengthen the personal relevance of the course content, one of the group co-leaders was an HIV-infected ex-smoker.
The outcomes observed in this study show promise. Abstinence rates in those assigned to the PSF condition were almost double those of the control condition. The 19.2% three month abstinence rate in the PSF group compares favorably with the other published trials of intensive interventions in PLWH smokers.17–19 Although the increased abstinence rate that we report did not achieve statistical significance in the primary analysis, several findings suggest that PSF was effective in achieving its aims. A cessation rate of 19.2% in this challenging population compares very favorably with a mean cessation rate of 13.9% (11.6–16.1%) derived from meta-analysis of group counseling interventions conducted in other settings.33 In the complete-case, as-treated analysis, the difference in the abstinence rates between the two conditions was statistically significant. Reduction in daily cigarette consumption was significantly greater in PSF subjects. Finally, we were able to demonstrate a significant advantage for PSF condition subjects over controls in changes on all measures of self-efficacy, the behavioral domain that was most specifically targeted by the program curriculum, and additional post-hoc analyses suggested that change in Positive Affect/Social Situations self-efficacy mediated the effect of PSF on the abstinence outcome.
Retention is a frequent challenge in multisession behavioral interventions. In a study of the program that provided the model for our curriculum, subjects attended a mean of 5.9 out of eight sessions.34 The mean and median number of sessions attended in the PSF condition was four, with only five subjects completing all eight sessions. Quit rates were significantly higher in those who attended ≥ seven sessions, especially if they received pharmacotherapy. This suggests that strategies to improve the efficacy of intensive group therapy in the future should include efforts to maximize attendance and pharmacotherapy acceptance among the participants.
The data that we present on correlates of successful cessation may also be instructive in the development of future interventions. Our findings and those of others19,35 suggest that African American PLWH smokers may face special challenges. The three psychobehavioral domains found to be associated with the abstinence outcome in our study were self-efficacy, decisional balance, and loneliness. Self-efficacy, or confidence in oneself to resist the temptation to smoke, is a critical determinant of successful cessation.26 The Positive Affect/Social Situations subscale (i.e. confidence in oneself to resist smoking in positive and/or social situations) was the most tightly associated with the outcome. The repeated measures and mediation analyses suggest that favorable changes in this measure over time, which was most pronounced in the PSF condition, were predictive of abstinence. These findings are consistent with results reported by three other groups in diverse US PLWH populations.27,36,37 Decisional balance, which is an assessment of positive (e.g. “Smoking cigarettes relieves tension”) and negative (e.g. “My cigarette smoking bothers other people”) aspects of smoking, also influences smoking behaviors.14 Similar to Stanton et al.,27 we found that a lower assessment of the importance of the positive aspects of smoking was associated with abstinence. Program content meant to reduce positive views of smoking and increase negative views should be included and emphasized in cessation programs targeting PLWH. Finally, subjects with lower loneliness scores were more likely to quit smoking. Loneliness is associated with higher rates of tobacco use.38 In our study, this finding did not appear to be a surrogate for social support or depression since our measures of social support and depression were not associated with the outcome. Loneliness is associated with other social realities, such as stigmatization, discrimination, and boredom, that are all related to tobacco use.39,40 It is noteworthy that the most highly rated aspects of the PSF program were those related to the social aspects of the group intervention. This is an observation worthy of additional study.
The PSF program emphasized the potential role of buddies in the quitting process. However, only three subjects brought buddies to the sessions, and the buddy component of PSF was rated as among the least helpful by subjects in their program evaluations. These disappointing findings suggest that buddies may not be the optimal agents of social support in cessation interventions for PLWH smokers.
The study had several limitations worthy of mention. The three month cessation endpoint is appropriate for a pilot trial, but more definitive work will require six or 12 month abstinence data.41 Seven-day point-prevalence abstinence is a standard tobacco treatment outcome that has been closely correlated with six-month continuous abstinence in prior large studies.42 Although the trial was powered to evaluate the efficacy of the PSF intervention, the relatively small sample size may have limited its ability to assess relationships between the range of sociobehavioral data collected and study outcomes. In the PSF condition, over 13% of subjects failed to attend even a single program session, and thus received no dose of the intervention. The group cessation program was associated with significant costs, in terms of labor, time, and space, beyond those of less intensive interventions. As the medical community gains knowledge about the most effective approaches to tobacco treatment in PLWH, cost-benefit analyses will be a necessary component of future public health decisions in this area. Finally, our study was limited to PLWH smokers who were motivated to quit in single center. Although our clinic population is sociodemographically typical of inner city HIV-care clinics throughout the country, our findings may not be generalizable to patients elsewhere nor to HIV-infected smokers who are less motivated to quit.
In conclusion, Positively Smoke Free, a group therapy intervention targeting PLWH smokers, appeared to be effective in promoting smoking cessation. Elements of the program that received the highest praise were the group dynamic, the inclusion of an HIV-infected co-leader, and the HIV-referent material presented in the curriculum. High session attendance rates, although difficult to achieve, were an important factor in program success. African American PLWH smokers were less likely to achieve abstinence, suggesting that this group requires additional or more intensive treatment strategies. Findings relating to loneliness and pro-smoking decisional balance should be considered in the design of future interventions. Multiple studies, including ours, have now shown self-efficacy, especially in positive affect and social situations, to be a critical determinant of program success. Measures to increase self-efficacy should be incorporated into all tobacco treatment efforts targeting PLWH smokers.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms. Kerry Poeggel, Mr. Scott Lloyd, Ms. Daniela Morales, Ms. Rebecca Herskovits, Moonseong Heo, PhD, the Montefiore Medical Center Infectious Diseases Clinic Community Advisory Board, and all of the study participants.
Financial Support: This work was supported by grant R21 DA023362 from the National Institutes of Health/National Institute on Drug Abuse. It was also supported by the Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI-51519).
Conflicts of Interest: Dr. Shuter has received grant support from the American Legacy Foundation and the Abbott Laboratories Investigator Initiated Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reynolds NR. Cigarette smoking and HIV: More evidence for action. AIDS Educ Prev. 2009;21:S106–S121. doi: 10.1521/aeap.2009.21.3_supp.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesoriero JM, Gieryic SM, Carrascal A, et al. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 3.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Canc Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. NEJM. 2003;349:1993–2003. doi: 10.1046/j.1468-1293.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 5.Miguez-Burbano MJ, Burbano Z, Ashkin D, et al. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. Addiction Biology. 2003;8:39–43. doi: 10.1080/1355621031000069864. [DOI] [PubMed] [Google Scholar]
- 6.Crothers K, Griffith T, McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Int Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuter J, Bernstein SL. Cigarette smoking is an independent predictor of non-adherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob Res. 2008;10:731–736. doi: 10.1080/14622200801908190. [DOI] [PubMed] [Google Scholar]
- 8.Lifson AR, Neuhaus J, Arribas JR, et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Publ Health. 2010;100:1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuter J, Bernstein SL, Moadel AB. Cigarette smoking behaviors and beliefs in persons living with HIV/AIDS. Am J Health Behav. 2012;36:75–85. doi: 10.5993/ajhb.36.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley ED, Gandhi M, Hare C, et al. Poverty, unstable housing, and HIV infection among women living in the United States. Curr HIV/AIDS Rep. 2007;4:181–186. doi: 10.1007/s11904-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 11.Boarts JM, Bogart LM, Tabak MA, et al. Relationship of race-, sexual orientation-, and HIV-related discrimination with adherence to HIV treatment: a pilot study. J Behav Med. 2008;31:445–451. doi: 10.1007/s10865-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 12.Bogart LM, Cogwill BO, Kennedy D, et al. HIV-related stigma among people with HIV and their families: a qualitative analysis. AIDS Behav. 2008;12:244–254. doi: 10.1007/s10461-007-9231-x. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV-positive men. JANAC. 2004;15:38–46. doi: 10.1177/1055329003261969. [DOI] [PubMed] [Google Scholar]
- 14.Abrams DB, Niaura R, Brown RA, et al., editors. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. Guilford Press; New York: 2003. [Google Scholar]
- 15.Tesoriero JM, Gieryic SM, Carrascal A, et al. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 16.Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. JANAC. 2000;11:37–44. doi: 10.1016/S1055-3290(06)60353-1. [DOI] [PubMed] [Google Scholar]
- 17.Vidrine DJ, Arduino RC, Lazev AB, et al. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20:253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- 18.Vidrine DJ, Marks RM, Arduino RC, et al. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nic Tob Res. 2012;14:106–110. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd-Richardson EE, Stanton CA, Papandonatos GE, et al. Motivation and patch treatment for HIV+ smokers: A randomized clinical trial. Addiction. 2009;104:1891–1900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Question Inventory on Tobacco (QIT) Retrieved from http://apps.nccd.cdc.gov/qit/index_clt.asp.
- 21.Abrams DB, Biener L. Motivational characteristics of smokers at the workplace: A public health challenge. Prev Med. 1992;21:679–687. doi: 10.1016/0091-7435(92)90075-s. [DOI] [PubMed] [Google Scholar]
- 22.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 23.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 24.Curry S, Wagner EH, Grothaus LC. Intrinsic and extrinsic motivation for smoking cessation. J Cons Clin Psychol. 1990;58:310–316. doi: 10.1037//0022-006x.58.3.310. [DOI] [PubMed] [Google Scholar]
- 25.Mermelstein R, Shadel WG, Shadel Individual differences in self-concept among smokers attempting to quit: Validation and predictive utility of measures of the smoker self-concept and abstainer self-concept. Annals of Behavioral Medicine. 1996;18:151–156. doi: 10.1007/BF02883391. [DOI] [PubMed] [Google Scholar]
- 26.Velicer WF, DiClemente C, Rossi JS, et al. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 27.Stanton CA, Lloyd-Richardson EE, Papandonatos GD, et al. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Educ Prev. 2009;21(Supp A):63–78. doi: 10.1521/aeap.2009.21.3_supp.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mermelstein R, Cohen S, Lichtenstein E, et al. Social support and smoking cessation and maintenance. Journal of Consulting and Clinical Psychology. 1986;54:447–453. doi: 10.1037//0022-006x.54.4.447. [DOI] [PubMed] [Google Scholar]
- 29.Ironson G, Solomon GF, Balbin EG, et al. Ironson-Woods Spirituality/Religiousness index is associated with long survival, health behaviors, less distress, and low cortisol in people with HIV/AIDS. Ann Behav Med. 2002;24:34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- 30.Russell D. The UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 32.Savard J, Laberge B, Gauthier JG, et al. Evaluating anxiety and depression in HIV-infected patients. J Pers Assess. 1998;71:349–367. doi: 10.1207/s15327752jpa7103_5. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services. Treating Tobacco Use and Dependence. Public Health Service. 2000 Jun [Google Scholar]
- 34.Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69:471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinidad DR, Perez-Stable EJ, White MM, et al. A nationwide analysis of of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Amer J Publ Health. 2011;101:699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nic Tob Res. 2006;8:S103–S108. doi: 10.1080/14622200601039451. [DOI] [PubMed] [Google Scholar]
- 37.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions in HIV positive smokers. AIDS Behav. 2009;13:545–554. doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauder W, Mummery K, Jones M, et al. A comparison of health behaviors in lonely and non-lonely populations. Psychol Health & Med. 2006;11:233–245. doi: 10.1080/13548500500266607. [DOI] [PubMed] [Google Scholar]
- 39.Herskovits R, Knackmuhs E, Stanton C, Shuter J. The Relationship between Perceived Racism/Discrimination and Smoking Habits in Persons Living with HIV and AIDS. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 40.Peretti-Watel P, Constance J. “It’s all we got left" Why poor smokers are less sensitive to cigarette price increases. Int J Environ Res Publ Health. 2009;6:608–621. doi: 10.3390/ijerph6020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes JR, Keely JP, Niaura RS, et al. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine and Tobacco Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 42.Velicer WF, Prochaska JO. A comparison of four self-report smoking cessation outcome measures. Addict Behav. 2004;29:51–60. doi: 10.1016/s0306-4603(03)00084-4. [DOI] [PubMed] [Google Scholar]