Abstract
Objective
Mitral annular calcification (MAC) is a degenerative process of the mitral fibrous annulus associated with cardiac disease and stroke. Although thought to be more prevalent in type 2 diabetes (T2DM), MAC remains poorly characterized in this population, due to confounding by renal and cardiac disease. Our goal was to study the risk factors for MAC in asample of T2DM subjects without renal and cardiac disease.
Methods
The Penn Diabetes Heart Study (PDHS) is a cross-sectional study of diabetic individuals without clinical cardiovascular or renal disease. We quantified and analyzed MAC Agatston scores in baseline cardiac CTs from 1753 individuals. Logistic and tobit regression were used to assess MAC’s relationship with risk factors and coronary artery calcification (CAC).
Results
MAC was present in 12.0% of -subjects, with a median Agatston score of 72.3 [Interquartile range (22.2 256.9)]. Older age, diabetes female gender, Caucasian race, and longer duration were independently associated with both the presence and extent MAC even after controlling for the CAC; hypertension, hyperlipidemia, comorbidities however, tobacco use, CRP levels, and other were not associated. CAC was strongly associated with MAC [OR of 4.0, (95% CI 2.4-6.6)] in multivariable models.
Conclusions
Age, AC female gender, Caucasian race, and diabetes duration were associated with the presence and extent of MAC in T2DM subjects, independent of CAC, which was also strongly associated with MAC. These data suggest that additional mechanisms for MAC formation in diabetics may exist which are distinct from those related to generalized atherosclerosis and deserve further investigation.
Keywords: Diabetes, Mitral Annular Calcification, Coronary Heart Disease, Risk Factors
Introduction
Mitral annular calcification (MAC) represents a degeneration of the fibrous ring of the mitral annulus 1. It is one of the most common sites of calcification after the coronary arteries 2and has been increasingly recognized in cardiovascular imaging studies, especially cardiac CT. Once thought to be a mostly benign process, more recent evidence suggests that MAC is independently associated with cardiovascular disease (CVD) events 3, heart failure 4, stroke 1, 5, 6, carotid disease 7, atrial fibrillation, 8 as well as cardiovascular mortality 9 and overall mortality10. Hence, the burden of MAC in at risk populations and mechanisms of MAC’s association with cardiovascular risk factors and CVD deserve further investigation.
To date, the etiology of MAC remains unclear. Histological findings in early disease reveal calcium deposition with apoptotic or necrotic interstitial cell material11 but the pathogenesis still remains largely unknown. Abnormalities of calcium and phosphate homeostasis as in chronic kidney disease (CKD) and end stage renal disease (ESRD) may contribute12, 13. Hemodynamic stress on the mitral annulus as occurs with hypertension and diastolic dysfunction may play a role2, 14, 15. Chronic inflammation may also lead to mitral annular degeneration, or MAC may be simply an extension or manifestation of generalized atherosclerosis2, 16. A combination of several of these processes is also plausible.
Prior studies have found consistent associations between diabetes and MAC17, 18. Yet, additional research is required to determine whether MAC may be a clinically useful marker and additive to established risk stratification in CVD or stroke risk assessment in diabetes. Because MAC has also been associated with kidney disease—both end-stage kidney disease (ESKD) and milder forms of chronic kidney disease (CKD) 19—diabetic nephropathy in prior studies might have complicated the study of MAC in diabetics. In addition, many prior studies employed M-mode or 2D echocardiography for a semi-quantitative assessment of MAC, whereas contemporary cardiac CT18, 20 approaches provide a more sensitive and quantitative modality for assessment of MAC. We therefore examined the risk factors for MAC as CT in patients with T2DM, but without clinical cardiovascular or kidney disease.
Methods
Study Participants
Details regarding the Penn Diabetes Heart Study (PDHS) have been published previously 21. In brief, PDHS recruited a cohort of 2032 subjects with T2DM between 2001and 2011 from primary care and endocrinology outpatient clinics affiliated with the Hospital of the University of Pennsylvania and the Philadelphia VA Medical Center. The inclusion criteria were a clinical diagnosis of T2DM (defined as fasting blood glucose ≥126 mg/dl, 2-h postprandial glucose ≥200 mg/dl, or use of oral hypoglycemic agents/insulin in a subject greater than age 40 yr), age of 35 to 75 years, and negative pregnancy test (if female). University of Pennsylvania IRB approval was obtained for this study and informed consent was obtained from all study subjects.
We excluded those with clinical coronary artery disease (defined as myocardial infarction, coronary revascularization, angiographic coronary disease or positive stress test), insulin use prior to age 35, a serum creatinine > 2.5 mg/dl and a weight > 300 lb (136.4 kg). Of 2032 subjects originally recruited, several individuals were excluded from this analysis: 143 individuals did not have a cardiac CT either because of withdrawal from study or technical issues related to the scanner; of those with CT scans, 12 individuals had uninterpretable studies for MAC and 124 had missing covariate data. Thus, a sample of 1753 with complete data remained for analysis. Individuals with missing covariate data and no CT scans were similar to those with all available data based on age, gender, and race.
Clinical Parameters
Participants were evaluated at Penn’s Clinical and Translational Research Center (CTRC) after a 12-hour overnight fast. All individuals underwent a detailed questionnaire for medical information and anthropomorphic measurements. Standard lipid panels were measured in real-time in Penn’s Centers for Disease Control-certified lipid laboratory using enzymatic assays (Hitachi 912, Roche Diagnostic Systems Inc., NJ, USA) in lipoprotein after ultracentrifugation (β-quantification technique). C-Reactive Protein fractions (CRP) levels were measured using two different assays over time: the initial 1000 participants had CRP measured using a high-sensitivity latex turbidimetric immunoassay (Wako Ltd., Osaka Japan), whereas the later 1032 were measured by a BNII nephelometer (Dade-Behring, Newark DE). A subset of PDHS CRP samples (n = 35) were measured using both assays with a Spearman rho correlation of 0.94 between samples and small systematic difference in levels (with mean ± SDs of 1.02 ± 0.50 mg/L vs. 1.03 ± 0.50 mg/L). Linear regression was used to adjust original assay results to reflect the more recent assay. Laboratory test results were generated by personnel blinded to the clinical characteristics and CT data of study participants. The MDRD estimated GFR evaluation was not applied because the vast majority of PDHS participants (>95%) had eGFR values of >60 mL/min/1.73 m2 and this estimation lacks sensitivity and discrimination in mild kidney dysfunction 22 .
Cardiac CT measurements
CT studies and global Agatston MAC scores were performed using an Imatron C-150 CT scanner (GE-Imatron, South San Francisco, CA). Scans were obtained using a single breath hold and with section thickness of 3 mm, field of view of 35 cm, and matrix of 512 × 512 to reconstruct raw image data. Images were calibrated to a standard phantom attenuation. MAC was defined as the presence of calcium visually seen along the mitral annulus in 3 orthogonal views; a specific Hounsfield cutpoint was not used. MAC was quantified using the Agatston scoring method provided by AquariusNET viewer software (TeraRecon, INC. Foster City, CA). Two trained readers interpreted all of the studies and were blinded to all other subject data. Five percent of the studies were randomly re-assigned to be re-read by each reader and 5% to the other reader for quality control. The intra reader kappa statistics were 0.96 (95%CI 0.87-1.00) and 0.90 (95% 0.76-1.00). The inter-reader kappa statistic for the presence of MAC was 0.87 (95% CI 0.73-1.00). Quantification of MAC Agatston scores was performed by 3 readers, who had an interclass correlation coefficient of 0.98 on a subset of 25 randomly selected studies. Coronary artery calcium (CAC) was quantified by the Agatston method as previously described.23
Statistical Analysis
Data are reported as median (interquartile range = IQR) or mean ± standard deviation for continuous variables and as proportions for categorical variables. For univariate comparisons of cardiovascular risk factors between individuals with and without MAC, the chi-square test, the Student t-test and the Wilcoxon rank sum test were used. We used logistic regression and tobit regression for multi-variable analysis of MAC data. Logistic regression was used to test the association of CVD risk factors with the presence of MAC; tobit conditional regression of ln(MAC+1) was used to assess the association of risk factors with the both presence and quantity of MAC scores. Tobit regression is useful in situations with zero scores but also a marked skew and has been used for other similar distributions such as with coronary artery calcium 23. Tobit combines logistic regression of the presence of MAC (any MAC present vs. no MAC zero score) with a linear regression (of log-transformed MAC) when MAC is present to produce a single estimate for the relationship of risk factors with MAC data.
Initial covariates were obtained from univariate analysis and included, age, gender, race and variables that were different between those with and without MAC with a p value cut point of <0.2. Plasma triglyceride and CRP levels were log transformed. Backward stepwise elimination was used to obtain variables in the final model which were significantly associated with MAC (with a p value above 0.05 warranting elimination). We also examined the association of the presence of CAC and CAC Agatston scores with MAC using logistic and tobit regression models, respectively. All analysis was done using STATA version 12.0 software (Statacorp, College Station, Texas).
Results
Characteristic of the Study Sample
The mean age of the full study sample was 60 years (IQR 52-66 years); 36% were female, 61% were Caucasian, and 33% were African American. Most individuals were hypertensive with a history of hyperlipidemia, and median LDL levels treated to below 100 mg/dl. Roughly two-thirds were on one or more types of lipid lowering agent and one-fifth were using insulin.
MAC Prevalence and Association with CVD Risk Factors
Table 1 shows the characteristics of the study sample comparing those with and without MAC. Overall, 12% of individuals had MAC with a median Agatston score of 72.3 (IQR 22.2-256.9).The range of MAC scores (1.3 to 6448.9) had a rightward skew with more people having lower Agatston scores and few individuals having higher scores (Figure 1A). After (natural logarithm of log transformation MAC score + 1) a more normal distribution was achieved for analysis(Figure 1B).
Table 1. Characteristics of the Study Population.
Median and interquartile ranges are presented unless otherwise specified
| No MAC (n=1541) | MAC (n=212) | p value | |
|---|---|---|---|
| Demographics | |||
| Age | 58 (53-65) | 66 (61-70) | <0.001 |
| Men (%) | 63.9 | 61.8 | 0.53 |
| Race (%) | <0.001 | ||
| Caucasian | 59.6 | 72.6 | |
| African American | 34.5 | 23.6 | |
| Other | 5.9 | 3.8 | |
| History | |||
| Hypertension (%) | 85.5 | 92 | 0.01 |
| Tobacco use (past or present, %) | 58.2 | 62.3 | 0.26 |
| Pack years | 13(4-30) | 14 (5-28) | 0.90 |
| Hyperlipidemia (%) | 80 | 86 | 0.04 |
| Diabetes duration, yrs (mean, SD) | 7.6 (6.6) | 9.8 (9.3) | <0.001 |
| Anthropomorphic | |||
| Waist circumference (in) | 41.5 (38.0-45.5) | 42 (38.5-46.0) | 0.13 |
| BMI | 32.1 (28.4-36.2) | 31.9 (28.6-36.4) | 0.99 |
| Labs | |||
| LDL-C (mg/dL) | 94 (77-116) | 94 (79-118) | 0.46 |
| Total Cholesterol (mg/dL) | 171 (148-196) | 170 (152-197) | 0.28 |
| HDL-C (mg/dL) | 45 (37-55) | 47 (41-56) | 0.01 |
| Triglycerides (mg/dL) | 115 (82-174) | 113 (84-161) | 0.46 |
| Serum Creatinine (mg/dl, mean, SD) | 0.9 (0.7-1.0) | 0.9 (0.7-1.0) | 0.46 |
| Hemoglobin A1c | 6.7 (6.1-7.7) | 6.7 (6.1-7.5) | 0.16 |
| C Reactive Protein (mg/L, mean SD) | 3.6 (5.3) | 3.7 (6.6) | 0.94 |
| Medications (%) | |||
| Statin | 57.2 | 62.8 | 0.12 |
| Fish oil | 14.1 | 16.5 | 0.35 |
| Fibrate | 6.8 | 5.7 | 0.55 |
| Metformin | 64.2 | 64.6 | 0.91 |
| Sulfonylurea | 34.5 | 40.1 | 0.14 |
| Thiazolidinedione | 22.9 | 25.5 | 0.41 |
| Insulin | 21.7 | 19.3 | 0.43 |
| Aspirin | 43.2 | 51.2 | 0.03 |
| Coronary Artery Calcium (%) | 61.4 | 90.4 | <0.001 |
| Coronary Artery Calcium Score | 22 (0-242) | 278 (42-746) | <0.001 |
Figure 1.
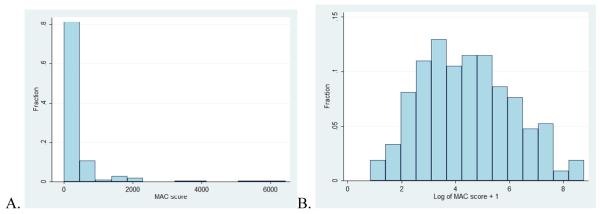
Distribution of MAC scores are shown for those with scores of greater than zero. Panel A shows untransformed Agatston scores. Panel B shows the distribution after log transformation of the MAC scores
On average, participants with MAC were almost a decade older than those without (66 vs. 58 years) and had had diabetes on average for 2 years long than those without MAC. Those with MAC were more predominantly Caucasian (72.6%) compared to those without MAC (59.6%) and had AC a significantly higher prevalence and median score of CAC. In univariate analysis, several CVD related risk factors were more predominant among those with MAC including: history of high blood pressure, systolic blood pressure, and hyperlipidemia. However, many other variables including BMI, tobacco use, and serum creatinine did not differ between groups. Markers of diabetes control and severity including hemoglobin A1c, fasting blood glucose, and insulin use, were not different between groups with and without MAC. There was also no difference in baseline CRP levels between groups.
Multivariable Regression
After backward stepwise elimination, in multivariable logistic regression, only older age, female gender, Caucasian race, the duration of diabetes and CAC scores were independently associated with the presence of MAC (Table 2). Adding back additional risk factors to the final model failed to reveal significant associations for serum creatinine, waist circumference, systolic blood pressure, hemoglobin A1c, fasting glucose, lipid parameters, use of statin medications or CRP levels. Additional analysis, excluding these individuals with low GFR (<60 ml/kg/min) which accounted for 3.6% of the study population, yielded similar results (data not shown). There was no interaction between race and gender in the association with MAC in the final model (interaction p = 0.7).
Table 2. Multivariable Association of Cardiac Risk Factors with the presence of MAC.
Statistical models include all of the variables listed above: age, gender, race, diabetics duration and the presence of CAC. The tobit regression ratio (TRR) represents the risk of one unit increase in the log of MAC score for the pre-specified variable. Values with confidence intervals that do no cross zero are considered significant.
| Logistic Regression | Tobit Regression | |||
|---|---|---|---|---|
|
| ||||
| Final Model Variables | OR [95% CI] | P Value | TRR [95% CI] | P Value |
| Age (per 10 years) | 2.4 [1.9 to 3.0] | <0.001 | 3.1 [2.3 to 3.9] | <0.001 |
| Gender (Women compared to Men) | 1.9 [1.3 to 2.6] | <0.001 | 2.3 [1.1 to 3.6] | <0.001 |
| White vs. Black Race | 1.5 [1.0 to 2.1] | 0.046 | 1.6 [0.2 to 2.9] | 0.02 |
| Duration of Diabetes (per 10 years) | 1.2 [1.0 to 1.5] | 0.045 | 0.8 [0.1 to 1.5] | 0.03 |
| Coronary Artery Calcium | 4.0 [2.4 to 6.6] | <0.001 | 4.7 [3.0 to 6.4] | <0.001 |
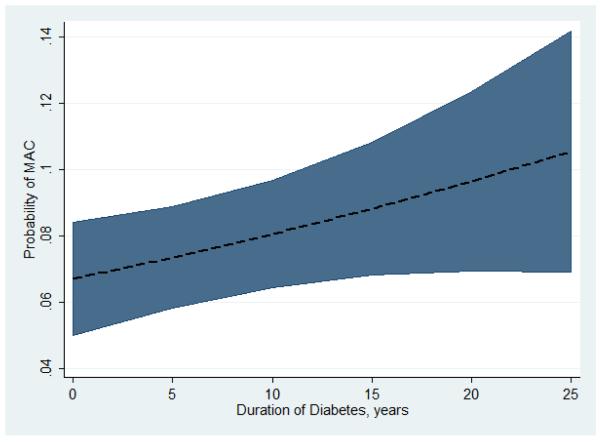
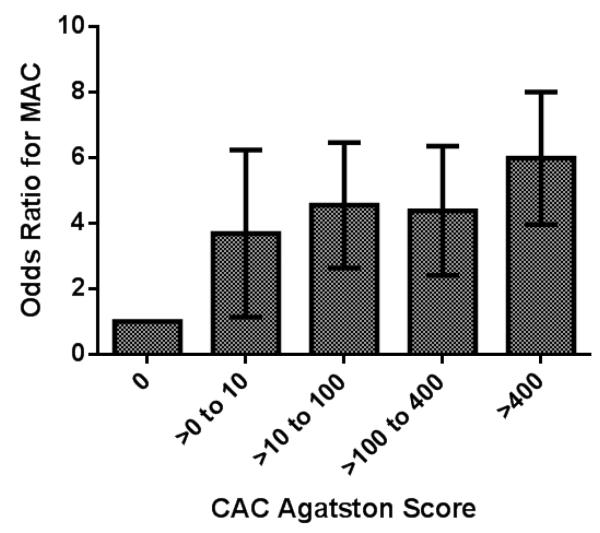
After adjusting for age, gender race and CAC score, the duration of diabetes was directly associated with an increasing probability of MAC (Figure 2). In adjusted models, the presence of any CAC was also strongly associated with a higher odds of MAC [odds ratio of 4.0, (95% CI 2.4-6.6)]. In addition, higher CAC Agatston scores were directly associated with a higher odds ratio forMAC (Figure 3) in adjusted models. In multivariable tobit regression, the same variables age, female gender, Caucasian race, the duration of diabetes, and CAC were also significantly associated with the burden of MAC as measured by the MAC Agatston score (Table 2). All tobit regression ratios were significantly greater than zero, again without gender and race interaction.
Figure 2.
The graph above shown the probability of having MAC (dashed line) with 95% CI (shaded area)based on the number of years subjects had diabetes, after adjustment for age, gender, race and CAC in a logistic regression model.
Figure 3.
The odds ratio for the presence of MAC based on CAC Agatston score category is shown. This is after adjustment for age, race, gender and diabetes duration. P value for trend is <0.001
Discussion
We demonstrated in this study that age, female gender, Caucasian race and duration of diabetes, but not other cardiovascular risk factors, were significantly associated with both the presence and extent of MAC in this T2DM sample free of clinical CVD or CKD. CAC was also strongly associated with the presence and extent of MAC.
The prevalence of MAC in our T2DM sample was 12% (14.3% in Caucasians and 8.3% in African Americans). Prior data show marked differences in prevalence estimates based on the population studied and the imaging modality used. Older studies using M-mode echocardiography suggested MAC was present in anywhere from 2.8% to 6.3% of individuals in general population based samples3, 15, 24. This increased beyond 15% in subjects of advanced age 8, and in another study more than one-third patients with clinical CAD were shown to have MAC 25. More recent CT based imaging studies such as Multiethnic Study of Atherosclerosis the found that 9% of healthy middle aged individuals had MAC (12% for Caucasians and 7.4% for African Americans), although only 14% of the MESA sample was diabetic at time of measurement of MAC. 26
Our higher prevalence of MAC in T2DM is worth noting relative to MESA given age similarities and our recruitment of a relatively healthy sample, and may suggest that MAC is increased in T2DM before the onset of overt kidney or vascular disease. A consistent feature across all studies, including ours, is that MAC appears to have an increased prevalence with age independent of racial and metabolic influences. This is not surprising given that MAC is proposed to have a chronic degenerative component that is expected to accelerate with increasing biological age 18.
Why female gender is associated MAC is not so clear. Several other studies have noted that MAC is more common in women than men7, 15, 27 in contrast to the observed association of male gender with clinical CVD. One proposed mechanism involves increased bone demineralization in women, particularly in postmenopausal women. Davutoglu and colleagues showed that MAC was more common in postmenopausal women with severe osteoporosis, and hypothesized that bone loss led to ectopic calcium deposition on the mitral annulus 28. Others have shown that calcium from bones may deposit on the mitral valve 29 and perhaps therefore also the mitral annulus. We did not have bone mineral density data on the women in this population to test this hypothesis. The lack of relation of MAC with many traditional atherogenic CVD risk factors, however, may suggest that MAC is more influenced by renal and diabetes metabolism (including calcium and phosphate homeostasis) than CAC which may be more driven by atherogenic factors.
Caucasians had a higher prevalence of MAC compared to blacks and other race/ethnicities, similar to findings in other studies. In MESA, the prevalence of MAC among Caucasians was 12% and lower in other races; CAC and valvular calcification were also more prevalent in Caucasians in MESA as well as other studies 30-32 after adjustment for risk factors. Atherosclerotic plaque is more common in Caucasians than African Americans at autopsy studies 33.It is known also that Caucasians have lower bone density and more osteoporosis than African Americans34 and that bone density is inversely related to vascular calcification 35. Therefore racial differences in bone metabolism and calcium deposition may explain these findings. Despite these findings, however, African Americans have more clinical CVD events than Caucasians suggesting that this may be related to differences in CVD risk factors that have less influence on vascular calcification e.g., hypertension.
To date several studies have demonstrated an association of MAC with diabetes16-18, 36 regardless of the imaging modality. Noteworthy among these is a large case-control study by Boon (657 with and 562 without MAC) demonstrating an odds ratio of 2.49 for the presence of MAC in those who were diabetic 16. Kanjanauthai found in the MESA cohort that diabetes was associated with an increased prevalence of MAC (adjusted odds ratio 1.58 [95% CI (1.25-1.99)], (N=971 with T2DM). The prevalence MAC of in our large T2DM sample is given notable our recruitment of a relatively healthy sample without CVD orCKD and is consistent with observations in MESA. We also found that the duration of diabetes was associated with MAC independent of age. However, measures of glycemic control and disease complications including HbA1c, fasting glucose and use of specific diabetic medications were not related to MAC. This might be because these cross-sectional data lack sensitivity to the influence of T2DM and glycemic control over time. Overall, data in PDHS and MESA support the concept that MAC is increased in T2DM before the onset of overt clinical kidney or vascular disease.
Several explanations for MAC’s association with T2DM are possible. Diabetes is frequently associated with kidney dysfunction, a strong risk factor for MAC. Prior studies such as MESA did not fully exclude individuals with early kidney disease and part of the association might be explained on the basis of sub-clinical diabetic nephropathy as renal disease itself was not a major exclusion criteria. PDHS used a creatinine cut-point for exclusion and not GFR. In our study only 3.6% of the participants had MDRD eGFRs values <60 mL/min/1.73 m2, and results were similar when these individuals were excluded from analysis. However, data on more accurate biomarkers of early kidney disease, including microalbumin and cystatin-C, are required to better answer this question; currently we do not this data available in our study population. In MESA, Ix and colleagues did find an association with MAC and eGFR (when less than 60 mL/min/1.73 m2) as well as greater Cystatin C levels, but this was only among individuals with diabetes. Diabetes may be associated with certain forms of sub-clinical structural heart disease and diastolic dysfunction which may predispose to MAC; this is not directly assessed in many studies. Diabetes is also associated with increased vascular calcification and atherosclerosis and MAC may be an extension of this generalized increase pre-clinical vascular disease in T2DM. Chronic low grade inflammation, also present in T2DM, in seems to be a less plausible mechanism for MAC formation in these individuals. We did not find any relationship between CRP and MAC. Furthermore, several studies mostly of non-diabetic samples, that measured CRP and other inflammatory markers, have also been negative 18, 37.
Traditional CVD risk factors such as hypertension and hyperlipidemia have had variable but predominantly negative associations with MAC 18; smoking has more consistently demonstrated no association3, 25, 38. Differences in findings across studies for CVD risk factors and MAC may have resulted from small study sizes, variation in MAC assessment by different imaging modalities, and the variable use of lipid lowering and antihypertensive medications. In our analyses, we did not find an association of hypertension, blood pressure medications, hyperlipidemia, lipid lowering medications or smoking with MAC in this T2DM sample. It is possible that these CVD risk factors have less influence on MAC than on atherosclerosis, clinical CVD and CAC, and that such heterogeneity may partly contribute to opposite association of gender with MAC relative to CVD and CAC.
The only other large study to look at the association between CAC and MAC was published by Hamirani and colleagues who found a strong positive association between the two phenotypes in the MESA population 26. Along with our parallel findings in diabetics, this suggests that MAC and CAC may be the result of partially overlapping calcification pathophysiology. At the same time, our findings of unique risk factors for MAC, independent of CAC, suggest that both are distinct processes which may have unique value with respect to risk stratification for cardiovascular disease. Several large studies have shown an association of MAC with clinical CHD 3, heart failure, 4, and stroke 1, 5, 6 but studies of incident CVD in large samples with CT-defined MAC are required to understand the nature mechanisms association with incident clinical CVD.
Strengths of our study include the large T2DM sample size and use of CT for assessment of both presence and quantity of MAC. Limitations include the cross sectional design making causation difficult to prove. While we adjusted for lipid and diabetic treatments that changed over the course of recruitment, it is possible that other changes in disease management during this time influenced MAC but were not controlled for in our analyses. We cannot rule out subtle renal disease in our cohort that may partially explain these findings, since we did not have more sensitive measures of renal dysfunction. We also could not assess the role calcium and phosphate homeostasis in our analysis because we did not have comprehensive measures of these factors.Finally our study may not be generalizable to all diabetics with clinically apparent CVD and CKD, since they were excluded from our study.
In conclusion, we found that MAC had strong associations with CAC but that MAC was associated with age, Caucasian race, female gender and diabetes duration independent of CAC while having no relation to many traditional CVD risk factors in this diabetic sample. It remains to be determined whether MAC is largely a manifestation of generalized atherosclerosis or if it is predominantly regulated by metabolic factors in diabetes, kidney disease and bone metabolism. Additional investigation is needed to determine how diabetes contributes to accelerated MAC formation and whether and how MAC is an independent risk factor for CHD and stroke.
Highlights/Condensed Abstract.
Mitral annular calcification (MAC), a degenerative process of the mitral fibrous annulus, is associated with coronary disease, heart failure and stroke and appears to be increased in those with type 2 diabetes (T2DM). In this study we, investigated whether MAC, measured accurately by CT, was associated with specific cardiovascular disease (CVD) risk factors. Using a cohort of 1753 subjects with T2DM who did not have kidney disease or clinical cardiac disease, we quantified MAC by Cardiac CT. MAC was not associated with traditional CVD risk factors: hypertension, hyperlipidemia, tobacco use, or inflammation as measured by CRP. The presence and extent of MAC was associated with age, female gender, Caucasian race, and a longer duration of diabetes even after controlling for coronary artery calcium. This study suggests that MAC may form in diabetics via mechanisms distinct from those related to generalized atherosclerosis or renal dysfunction, and therefore its relationship to cardiovascular disease deserves further study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fulkerson PK, Beaver BM, Auseon JC, et al. Calcification of the mitral annulus: etiology, clinical associations, complications and therapy. Am J Med. 1979;66:967–977. doi: 10.1016/0002-9343(79)90452-2. [DOI] [PubMed] [Google Scholar]
- [2].Roberts WC. The senile cardiac calcification syndrome. Am J Cardiol. 1986;58:572–574. doi: 10.1016/0002-9149(86)90045-7. [DOI] [PubMed] [Google Scholar]
- [3].Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- [4].Barasch E, Gottdiener JS, Marino Larsen EK, et al. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study) Am J Cardiol. 2006;97:1281–1286. doi: 10.1016/j.amjcard.2005.11.065. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin EJ, Plehn JF, D’Agostino RB, et al. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- [6].Kizer JR, Wiebers DO, Whisnant JP, et al. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36:2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- [7].Adler Y, Koren A, Fink N, et al. Association between mitral annulus calcification and carotid atherosclerotic disease. Stroke. 1998;29:1833–1837. doi: 10.1161/01.str.29.9.1833. [DOI] [PubMed] [Google Scholar]
- [8].Fox CS, Parise H, Vasan RS, et al. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173:291–294. doi: 10.1016/j.atherosclerosis.2003.12.018. [DOI] [PubMed] [Google Scholar]
- [9].Sharma R, Pellerin D, Gaze DC, et al. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. 2007;191:348–354. doi: 10.1016/j.atherosclerosis.2006.03.033. [DOI] [PubMed] [Google Scholar]
- [10].Potpara TS, Vasiljevic ZM, Vujisic-Tesic BD, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality in middle-aged patients with atrial fibrillation: the Belgrade Atrial Fibrillation Study. Chest. 2011;140:902–910. doi: 10.1378/chest.10-2963. [DOI] [PubMed] [Google Scholar]
- [11].Arounlangsy P, Sawabe M, Izumiyama N, et al. Histopathogenesis of early-stage mitral annular calcification. J Med Dent Sci. 2004;51:35–44. [PubMed] [Google Scholar]
- [12].Maher ER, Young G, Smyth-Walsh B, et al. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;2:875–877. doi: 10.1016/s0140-6736(87)91370-5. [DOI] [PubMed] [Google Scholar]
- [13].Linefsky JP, O’Brien KD, Katz R, et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- [15].Savage DD, Garrison RJ, Castelli WP, et al. Prevalence of submitral (anular) calcium and its correlates in a general population-based sample (the Framingham Study) Am J Cardiol. 1983;51:1375–1378. doi: 10.1016/0002-9149(83)90315-6. [DOI] [PubMed] [Google Scholar]
- [16].Boon A, Cheriex E, Lodder J, et al. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chu H, Chen J, Guo R. The association between cardiac calcification and coronary artery disease. Acta Cardiol. 2009;64:531–535. doi: 10.2143/AC.64.4.2041619. [DOI] [PubMed] [Google Scholar]
- [18].Kanjanauthai S, Nasir K, Katz R, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;213:558–562. doi: 10.1016/j.atherosclerosis.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- [20].Budoff MJ, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- [21].Qasim AN, Martin SS, Mehta NN, et al. Lipoprotein(a) is strongly associated with coronary artery calcification in type-2 diabetic women. Int J Cardiol. 2011;150:17–21. doi: 10.1016/j.ijcard.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fesler P, Mimran A. Estimation of glomerular filtration rate: what are the pitfalls? Curr Hypertens Rep. 2011;13:116–121. doi: 10.1007/s11906-010-0176-5. [DOI] [PubMed] [Google Scholar]
- [23].Reilly MP, Wolfe ML, Localio AR, et al. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [24].Lewandowski BJ, Winsberg F. Incidence of aortic cusp and mitral annulus calcification as determined by echocardiography: significance and interrelationship. AJR Am J Roentgenol. 1982;138:829–832. doi: 10.2214/ajr.138.5.829. [DOI] [PubMed] [Google Scholar]
- [25].Atar S, Jeon DS, Luo H, et al. Mitral annular calcification: a marker of severe coronary artery disease in patients under 65 years old. Heart. 2003;89:161–164. doi: 10.1136/heart.89.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hamirani YS, Nasir K, Blumenthal RS, et al. Relation of mitral annular calcium and calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2011;107:1291–1294. doi: 10.1016/j.amjcard.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [27].Tenenbaum A, Fisman EZ, Pines A, et al. Gender paradox in cardiac calcium deposits middle-aged and elderly patients: mitral annular and coronary calcifications interrelationship. Maturitas. 2000;36:35–42. doi: 10.1016/s0378-5122(00)00120-1. [DOI] [PubMed] [Google Scholar]
- [28].Davutoglu V, Yilmaz M, Soydinc S, et al. Mitral annular calcification is associated with osteoporosis in women. Am Heart J. 2004;147:1113–1116. doi: 10.1016/j.ahj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- [29].Tohno S, Tohno Y, Minami T, et al. Accumulation of calcium and phosphorus in the mitral valve in comparison with the abdominal aorta and the scaphoid bone. Biol Trace Elem Res. 2000;77:33–42. doi: 10.1385/BTER:77:1:33. [DOI] [PubMed] [Google Scholar]
- [30].Orakzai SH, Orakzai RH, Nasir K, et al. Subclinical coronary atherosclerosis: racial profiling is necessary! Am Heart J. 2006;152:819–827. doi: 10.1016/j.ahj.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [31].Budoff MJ, Yang TP, Shavelle RM, et al. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408–412. doi: 10.1016/s0735-1097(01)01748-x. [DOI] [PubMed] [Google Scholar]
- [32].Nasir K, Katz R, Takasu J, et al. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;198:104–114. doi: 10.1016/j.atherosclerosis.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Strong JP, McGill HC., Jr. The Natural History of Aortic Atherosclerosis Relationship to Race, Sex, and Coronary Lesions in New Orleans. Exp Mol Pathol. 1963;52(SUPPL1):15–27. [PubMed] [Google Scholar]
- [34].Henry YM, Eastell R. Ethnic and gender differences in bone mineral density and bone turnover in young adults: effect of bone size. Osteoporos Int. 2000;11:512–517. doi: 10.1007/s001980070094. [DOI] [PubMed] [Google Scholar]
- [35].Kiel DP, Kauppila LI, Cupples LA, et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- [36].Nair CK, Sudhakaran C, Aronow WS, et al. Clinical characteristics of patients younger than 60 years with mitral anular calcium: comparison with age- and sex-matched control subjects. Am J Cardiol. 1984;54:1286–1287. doi: 10.1016/s0002-9149(84)80082-x. [DOI] [PubMed] [Google Scholar]
- [37].Fox CS, Guo CY, Larson MG, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97:1502–1505. doi: 10.1016/j.amjcard.2005.11.086. [DOI] [PubMed] [Google Scholar]
- [38].Fox E, Harkins D, Taylor H, et al. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J. 2004;148:979–984. doi: 10.1016/j.ahj.2004.05.048. [DOI] [PubMed] [Google Scholar]