Abstract
β-ketoesters are robust probes for labeling sulfenic acid (−SOH) proteins allowing quantitative cleavage of the tag for improved analysis of the labeled peptides by MS.
Cysteine oxidative transformation to sulfenic acid (−SOH) is a transient protein posttranslational modification of importance to physiological and pathological processes.1 The metastable −SOH moiety in proteins can be converted into more stable species such as: (a) disulfides formed from its reaction with free thiols (−SH) like glutathione (GSH) and cysteine in proteins; (b) sulfenamides produced after condensation with amine (−NH2) from lysine residues or amide (−NHCO−) in the protein backbone;2 and, (c) sulfinic or sulfonic acid in the presence of higher concentrations of reactive oxygen/nitrogen species (RO/NS). Sulfenic acid and its secondary products disulfide and sulfenamide can be reversibly reduced to −SH by thiol-containing reductants and phosphines (TCEP).3 This reversibility is akin to phosphorylation, a critical regulatory protein posttranslational modification. Like phosphorylation, the reversible cysteine oxidation can regulate protein function4 and impact the relay of signaling and metabolic events through H2O2-mediated processes.5
The scarcity of −SOH in proteins makes its detection challenging. Most commonly available methods for selective labeling of −SOH rely on the use of dimedone (5,5-dimethyl-1,3-cyclohexanedione) or dimedone-like derivatives that have complex synthesis schemes.6 We have recently described a two-step synthesis and kinetic characterization of new 1,3-cyclopentanedione-based chemical probes for selective labeling of −SOH in proteins.7 Similar to dimedone-based probes, these compounds exhibit mild reactivity with −SOH at physiological pH. They are also not suitable for mass spectrometry (MS) analysis in positive ion mode due to their acid–base properties, which generates negatively charged species and lowers the charge states of modified peptides.
In this study, we demonstrate that linear β-ketoesters can be utilized as robust chemical probes for labeling and analysis of −SOH modified proteins (Scheme 1). The advantages over the previous reagents are: (a) facile derivatization can be achieved through boric acid catalyzed transesterification; and, (b) pH dependence of −SOH labeling with β-ketoesters is uniquely distinct from dimedone or 1,3-cyclopentanedione probes with improved reactivity at physiological pH. Moreover, similar to dimedone and 1,3-cyclopentanedione derivatives, the β-ketoester probe discussed here is cell membrane permeable; however, it does not induce accumulation of ROS in the cells and does not cause cell death, important parameters when protein oxidation is studied in cell culture or in vivo.
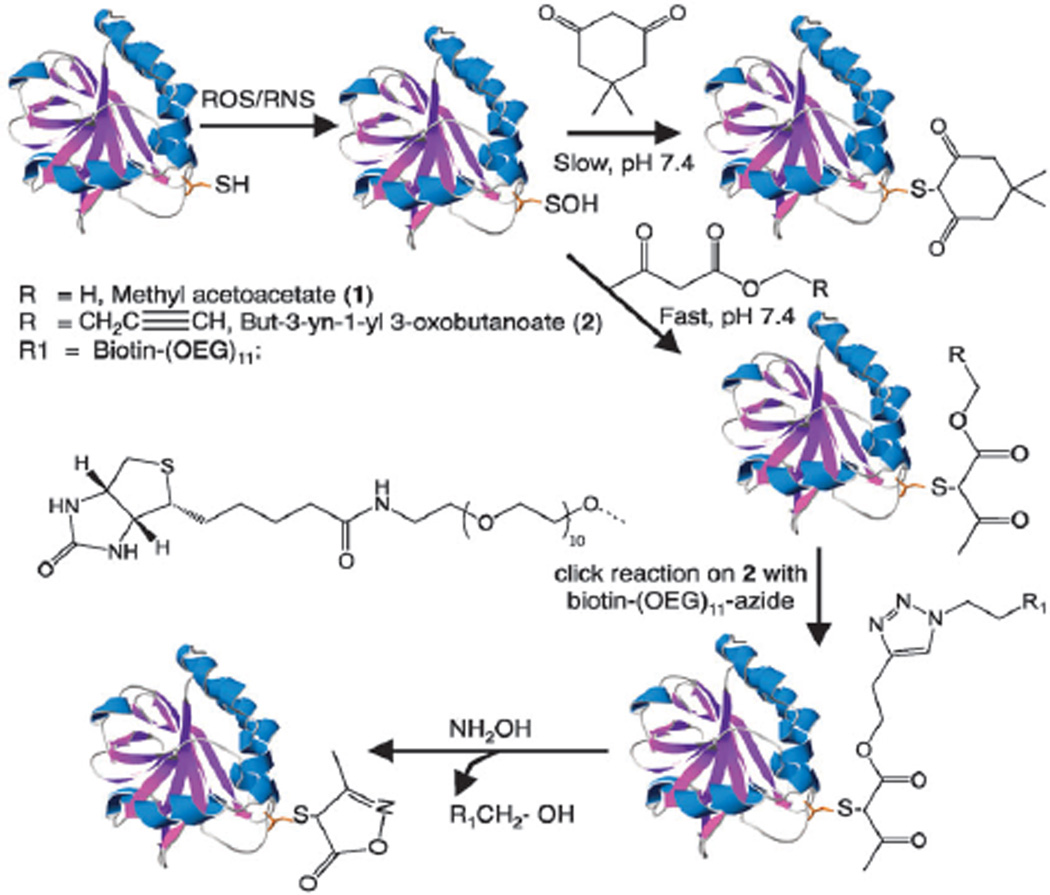
Scheme 1.
Reaction of −SOH in proteins with dimedone or β-ketoesters 1 and 2. Proteins labeled by 2 can be conjugated with reporter tags via click-reaction. The reporter tag can then be removed using NH2OH. The protein structure shown is C165S AhpC and was generated using Swiss PDB Viewer 4.0.1 based on the PDB entry 3EMP.
Initial experiments were performed using methyl acetoacetate (1, in Scheme 1) and AhpC protein. AhpC is a cysteine-based peroxidase from bacteria known to form a stable intersubunit disulfide bond by condensing the sulfenic acid at reactive C46 with C165 from neighboring AhpC monomers upon oxidation. Mutation of C165 to serine stabilizes the −SOH at C46 and enables reactivity evaluation of chemical probes against this otherwise transient species. The reactivity of 1 with C165S AhpC–SOH was monitored by electrospray ionization time-of-flight MS (ESI-TOF MS).7 A product peak was observed at 20 714 amu (Fig. S1A, ESI†) indicating the labeling of C165S AhpC–SOH by 1. Control experiments showed that 1 did not react with −SH, −S–S−, −SO2/3H, or other amino acid residues (Fig. S2A–C, ESI†). The envisioned advantage of the ester linkage in C165S AhpC–1 was the potential reaction with hydroxylamine (NH2OH) to generate 3-methyl-5-isoxazolone.8 As anticipated, the yield was nearly quantitative after treatment with 50 mM NH2OH for 1 h at 37 °C (product peak at 20 697 amu in Fig. S1B, ESI†). Further MS analysis confirmed modification of C165S AhpC at C46 with an Xcorr of 6.0 (Fig. S3 (top), ESI†) (Xcorr is indicative of the quality of experimental MS/MS fragmentation spectrum of a peptide—a higher number represents a better match with the predicted MS/MS spectrum); by comparison, the Xcorr for the dimedone labeled peptides is much lower typically between 2 and 3 (Fig. S3 (bottom), ESI†). This could be due to better ionization of the 3-methyl-5-isoxazolone labeled peptides and/or as a result of lower interference from metal ion chelation, a common problem for diketone-containing molecules.9
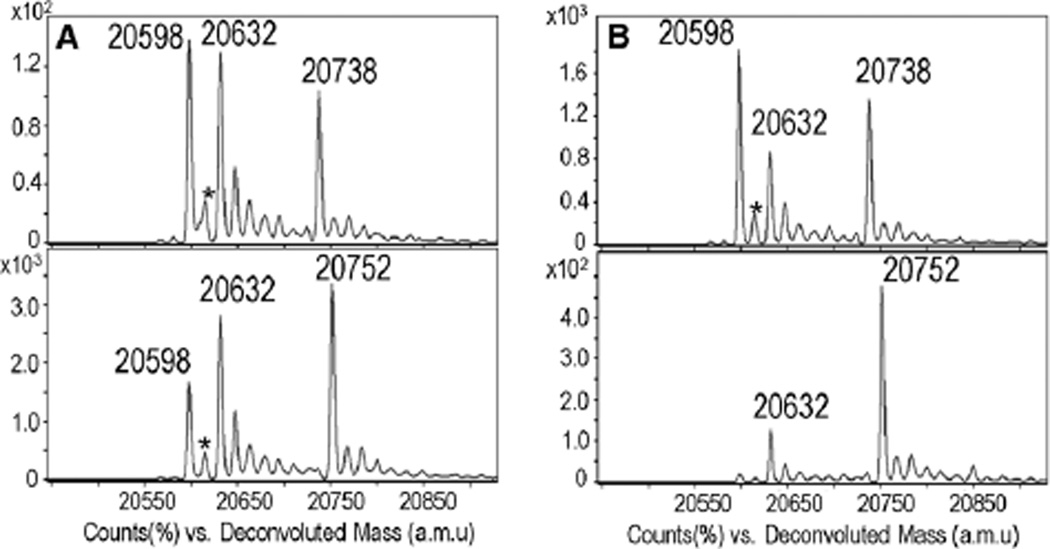
The alkyne analogue of 1 (compound 2 in Scheme 1) was then synthesized via boric acid catalyzed transesterification10 (Fig. S4A and B, ESI†). Labeling of C165S AhpC–SOH by 2 was compared with dimedone at pH 7.4 and 8.5. Product peaks at amu 20 738 and 20 752 correspond to dimedone and 2 adducts with C165S AhpC, respectively (Fig. 1). Increased labeling of −SOH with 2 compared with dimedone was observed at both pH 7.4 and 8.5.
Fig. 1.
ESI-TOF MS spectra of C165S AhpC–SOH reaction with dimedone (top) and 2 (bottom) at pH 7.4 (A) and pH 8.5 (B) AhpC adduct peak with dimedone and 2 was observed at 20 738 and 20 752 amu, respectively. Peaks at 20 598, 20 616 (*), 20 632 amu correspond to sulfenamide (−SN), −SOH, and sulfinic acid (−SO2H) formation in C165S AhpC, respectively. Note: Sulfenamide is likely formed in gas phase from sulfenic acid after loss of one molecule of H2O (Scheme S1, ESI†).
Our previous studies using 1,3-cyclopentanedione compounds showed enhanced labeling of AhpC–SOH at lower pH.7 In contrast, the pH dependence studies of −SOH labeling by 2 showed an increase in the reaction rate at higher pH (Fig. S5A, ESI†). Data were fit as described in ESI† to obtain the pKa 7.8 ± 0.2. This value most likely represents the pKa of the −SOH in C165S AhpC, as the pKa of the ethylene carbon in β-ketoesters is typically much higher (10.6 for 1).11 This is consistent with the reported values of the −SOH pKa in the range of 6–10.12 Control experiments using the reduced form of C165S AhpC showed no adduct formation at pH 9.5 (Fig. S2D, ESI†). The reported pKa for the carbon between keto groups for dimedone and 1 is 4.3 and 10.6, respectively.11,13 With regard to the keto–enol tautomerism in these compounds, while dimedone was reported to exist exclusively in the enol state,14 1 is primarily found in the ketone form (7.4% enol content in aqueous solution).11 Dimedone showed a slight increase in reactivity at mild acidic or basic conditions compared to pH 7.4 (Fig. S6, ESI†), which can be partly explained by the acid/base catalyzed enol-formation process.7 In contrast, 2 reacts very slowly with AhpC–SOH at pH 5.5 and 6.5 but becomes highly reactive as pH increases. These studies suggest that important aspects to consider for the reaction mechanism of this class of probes with −SOH are: (a) tautomerism of 1,3-dicarbonyls, (b) equilibrium between the −SOH and HS=O tautomers and (c) the pKa at the reactive ethylene carbon in relation to the pH of reaction mixture.
To determine the dependence of labeling reaction on the concentration of 2, the reaction with C165S AhpC–SOH was monitored for 2 h at pH 7.4 using increasing concentrations of 2. There was an increase in the labeling efficiency when the concentration of 2 was increased from 0.2 mM to 5 mM (Fig. S5B, ESI†). Data were fit to a hyperbolic equation and the K0.5 was determined as 3.0 ± 0.3 mM.
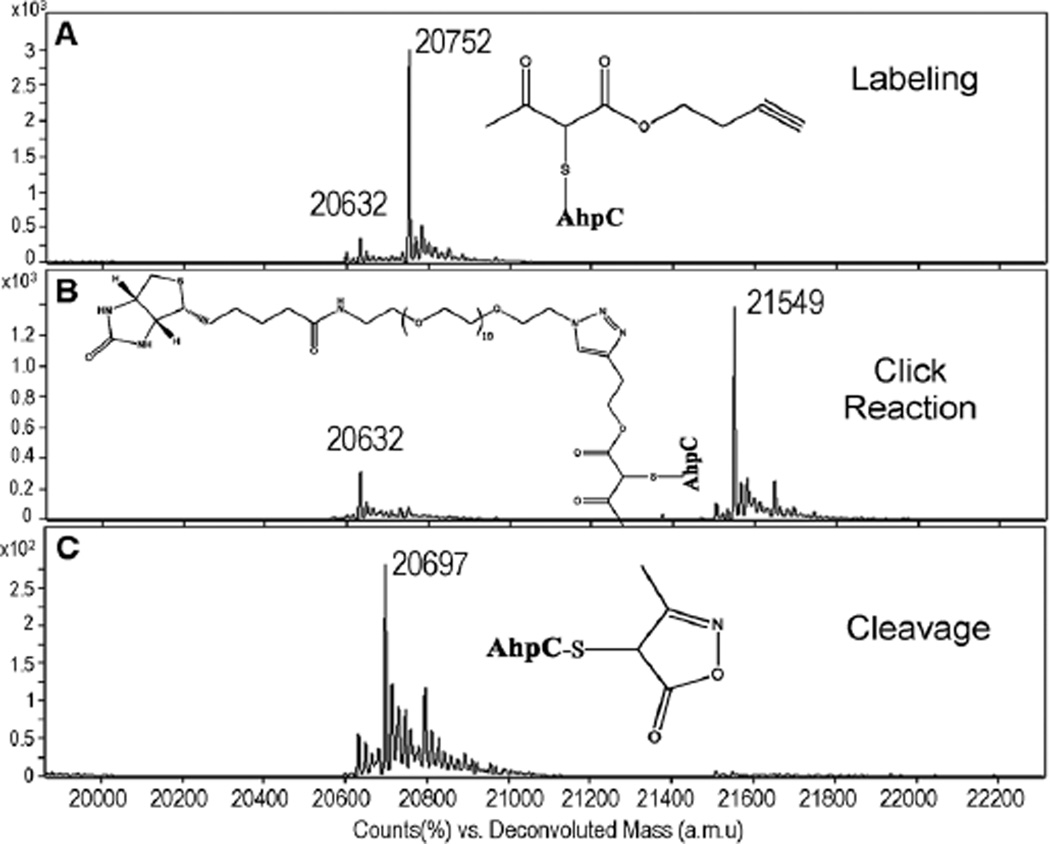
To establish the feasibility of our workflow in Scheme 1, the C165SAhpC–2 was then coupled to biotin using the Cu+-catalyzed click reaction. Near 100% conversion was achieved after 1 h reaction at rt, resulting in a mass increase of 797 amu (Fig. 2A and B). The removal of the biotin tag was then performed in the presence of NH2OH. The biotin moiety was easily removed to yield the cleaved adduct of C165S AhpC with 100% completion (Fig. 2C). The complete elution of biotin-labeled biomolecules could then be achieved through the NH2OH cleavage of the biotin tag, a task otherwise difficult in traditional biotin–avidin enrichment methods. Moreover, potential complications from the biotin tag in MS analysis would be avoided.
Fig. 2.
ESI-TOF MS spectra showing the labeling of C165S AhpC–SOH by 2 (adduct peak: 20 752 amu) (A); biotinylation of AhpC–2 adduct via click reaction (adduct peak: 21 549 amu) (B); and removal of the biotin moiety after NH2OH treatment (adduct peak: 20 697 amu) (C).
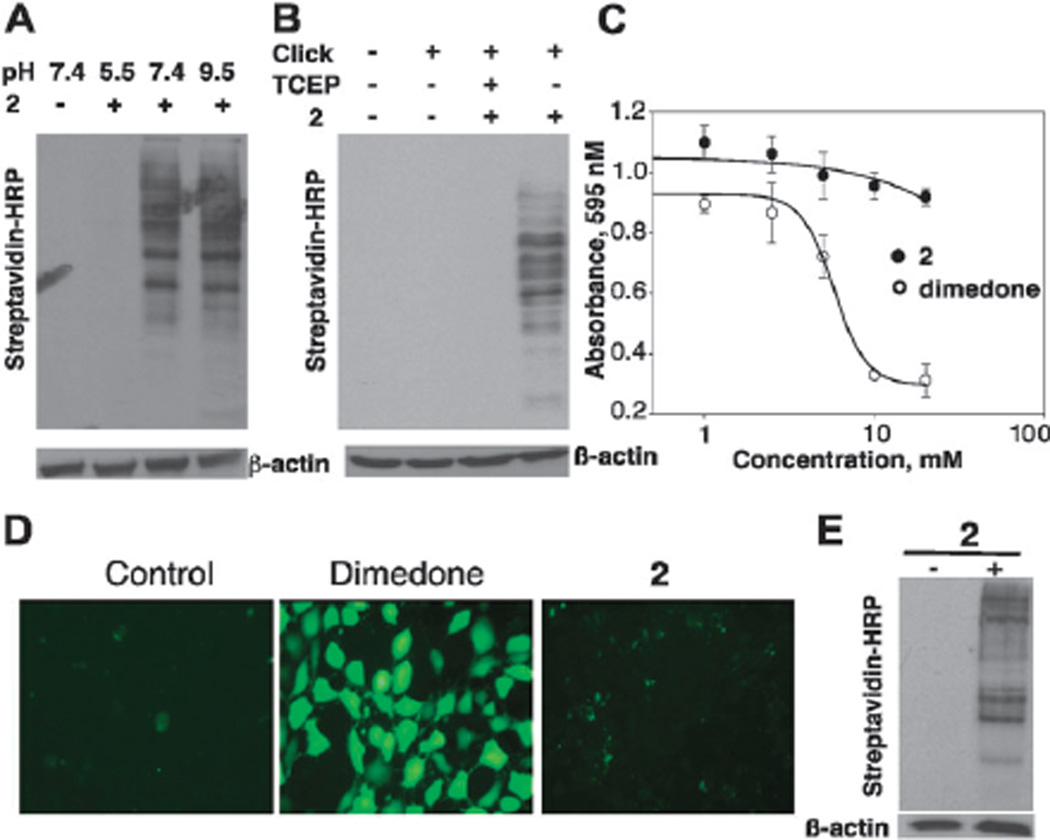
The pH dependence of the labeling reaction was further examined by incubating cell lysates from NIH 3T3 fibroblasts with 2 (5 mM) at pH 5.5, 7.4 and 9.5. Biotin-labeled proteins after click reaction with biotin-(OEG)11-azide were visualized using streptavidin–HRP. Nearly equal labeling was observed at pH 7.4 and 9.5, while no visible signal was detected at pH 5.5 (Fig. 3A). The results confirmed the higher reactivity of 2 at pH 7.4 than pH 5.5. Unlike the results with C165S AhpC–SOH, the labeling of lysate proteins was not improved at pH 9.5. This could be explained by considering the instability of −SOH at higher pH in complex cell lysate. The −SOH species may decay and form a disulfide bond with free thiols like glutathione (GSH) or cysteines in proteins since the nucleophilicity of these thiols is also increased at pH 9.5. Also, some of the −SOH proteins may become further oxidized to −SO2H or −SO3H under aerobic conditions. The net effect of the enhanced reactivity of 2 at pH 9.5 and the rapid decay of −SOH into other more stable species may result in equal labeling of cellular −SOH proteins at pH 7.4 and pH 9.5. To avoid adventitious oxidation, the follow-up studies were performed at pH 7.4. The selectivity of 2 in labeling −SOH proteins was then examined in cell lysate at pH 7.4. TCEP is known to reduce −SOH to −SH.3 The results in Fig. 3B demonstrate that 2 does not react with other amino acid residues or functional groups (e.g., glycosyl, phosphoryl, thiols, disulfides, etc.) and is selective towards −SOH in cell lysates.
Fig. 3.
(A) Labeling of oxidized proteins with 2 at pH 5.5, 7.4 and 9.5 was monitored by Western blot. (B) Selectivity of −SOH labeling with 2 in lysates: cell lysates pre-reduced with TCEP were incubated with 2 at rt for 1 h (lane 3). (C) MTT assay to determine cell toxicity. (D) Intracellular ROS level was determined using DCF labeling in NIH 3T3 cells treated with 10 mM dimedone and 2. (E) Cell permeability assay. Cells treated with 2 (10 mM) for 2 h were washed extensively and then lysed in the absence of 2. Click reaction was performed to attach the biotin tag followed by Western blot analysis.
To test the potential cytotoxicity of 2, cell viability in these experiments was measured using the MTT assay at increasing concentrations of dimedone and 2 (Fig. 3C). Dimedone caused cell death with an IC50 of 5.3 ± 0.2 mM, while 2 did not significantly affect cell viability. Imaging and labeling of ROS with DCF were applied to further determine whether the difference in cytotoxicity could be due to variations in ROS accumulation inside the cells. The DCF assay is a measurement of intracellular ROS level based on the fluorescent intensity observed from cells treated with DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate). A significant increase in ROS was observed when cells were incubated with 10 mM dimedone (Fig. 3D); pre-incubation of cells with 2 did not alter the basal level of ROS (Fig. 3D). Caution should therefore be taken when performing whole-cell labeling experiments or when interpreting results obtained from cells pre-treated with dimedone-based probes. Working at dimedone concentrations of 1 mM or lower would be recommended to avoid these artifacts. To assess whether 2 is cell permeable, SCC-61 cells were pretreated with 2 for 2 h, washed repeatedly to remove excess 2, and lysed at pH 7.4. The labeled lysates after the click reaction were analyzed by Western blot. A strong biotin signal was observed when cells were treated with 2, indicating that 2 is cell permeable, similar to dimedone-based probes (Fig. 3E).15
In summary, we present here the synthesis and primary evaluation of alkyne β-ketoester probes for labeling of −SOH proteins. These probes display improved kinetics compared to dimedone at pH 7.4; biotin or other tags can be added through the robust click reaction; the tags can be then removed using NH2OH to yield a derivative amenable for MS analysis. Moreover, we demonstrate that the alkyne β-ketoester 2 is cell permeable with minimum effect on the cellular redox status and cell viability. Thus, these compounds have improved properties over 1,3-cyclodione probes and well suited to detect and identify −SOH proteins in vivo and in vitro.
Supplementary Material
Acknowledgments
This research was supported by the NIH R01 CA136810 (CMF), and R33 CA126659/CA126659Z (LBP).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cc17868k
Notes and references
- 1.(a) Roos G, Messens J. Free Radical Biol. Med. 2011;51:314–326. doi: 10.1016/j.freeradbiomed.2011.04.031. [DOI] [PubMed] [Google Scholar]; (b) Jacob C, Burkholz T, Du P, Battaglia E, Bagrel D, Montenarh M. Chem. Res. Toxicol. 2011 doi: 10.1021/tx200342b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 3.Mansuy D, Dansette PM. Arch. Biochem. Biophys. 2011;507:174–185. doi: 10.1016/j.abb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Wani R, Qian J, Yin L, Bechtold E, King SB, Poole LB, Paek E, Tsang AW, Furdui CM. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10550–10555. doi: 10.1073/pnas.1011665108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole LB, Nelson KJ. Curr. Opin. Chem. Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Charles RL, Schroder E, May G, Free P, Gaffney PRJ, Wait R, Begum S, Heads RJ, Eaton P. Mol. Cell. Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]; (b) Poole LB, Klomsiri C, Knaggs SA, Furdui CM, Nelson KJ, Thomas MJ, Fetrow JS, Daniel LW, King SB. Bioconjugate Chem. 2007;18:2004–2017. doi: 10.1021/bc700257a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Leonard SE, Garcia FJ, Goodsell DS, Carroll KS. Angew. Chem., Int. Ed. 2011;50:4423–4427. doi: 10.1002/anie.201007871. [DOI] [PubMed] [Google Scholar]; (d) Truong TH, Garcia FJ, Seo YH, Carroll KS. Bioorg. Med. Chem. Lett. 2011;21:5015–5020. doi: 10.1016/j.bmcl.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 7.Qian J, Klomsiri C, Wright MW, King SB, Tsang AW, Poole LB, Furdui CM. Chem. Commun. 2011;47:9203–9205. doi: 10.1039/c1cc12127h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen N, Kolindandersen H, Christensen J. Can. J. Chem. 1984;62:1940–1944. [Google Scholar]
- 9.Hall BJ, Brodbelt JS. J. Am. Soc. Mass Spectrom. 1999;10:402–413. doi: 10.1016/S1044-0305(98)00141-X. [DOI] [PubMed] [Google Scholar]
- 10.Kondaiah GCM, Reddy LA, Babu KS, Gurav VM, Huge KG, Bandichhor R, Reddy PP, Bhattacharya A, Anand RV. Tetrahedron Lett. 2008;49:106–109. [Google Scholar]
- 11.Bunting JW, Kanter JP. J. Am. Chem. Soc. 1993;115:11705–11715. [Google Scholar]
- 12.Nagy P, Ashby MT. J. Am. Chem. Soc. 2007;129:14082–14091. doi: 10.1021/ja0737218. [DOI] [PubMed] [Google Scholar]
- 13.Wong FM, Keeffe JR, Wu WM. Tetrahedron Lett. 2002;43:3561–3564. [Google Scholar]
- 14.Cremlyn RJ, Osborne AG, Warmsley JF. Spectrochim. Acta. 1996;52:1423–1432. [Google Scholar]
- 15.Seo YH, Carroll KS. Bioorg. Med. Chem. Lett. 2009;19:356–359. doi: 10.1016/j.bmcl.2008.11.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.