Abstract
The nucleotide sequences of the groEL genes, the flagellin genes, and the 16S rRNA genes from 22 reference strains of Borrelia were compared. groEL sequence analysis is useful not only in interspecies differentiation but also in intraspecies differentiation of Borrelia afzelii and Borrelia garinii isolates.
Borrelia burgdorferi sensu lato, the causative spirochete of Lyme disease, is transmitted to humans and animals through Ixodes ticks (1, 3). Lyme disease is one of the most prevalent tick-borne infectious diseases in Europe and North America and now occurs all over the world (1, 12). Various DNA-based techniques have recently been developed for the species identification of B. burgdorferi because characterization and identification by conventional methods are time-consuming and expensive (2, 5, 6, 11). A previous study proposed that groEL gene analysis is useful for the differentiation of B. burgdorferi sensu lato (9). In the present study, groEL gene analysis was compared with the sequence analyses of the 16S rRNA and the flagellin gene to determine the role of the groEL gene in defining evolutionary relationships among strains of B. burgdorferi sensu lato.
Twelve Borrelia strains were recently isolated from Ixodes granulatus, Ixodes nipponensis (tick vectors for Lyme spirochetes in rare cases), and Ixodes persulcatus, and 11 strains were isolated from Apodemus agrarius (8, 10). In previous studies, they were characterized as Borrelia afzelii, Borrelia garinii, and unclassified Haenam strains (8, 10). In the present study, a comparative sequence analysis of the groEL gene from Korean isolates was performed to determine their relationships with the known species of the genus Borrelia.
Twenty-two reference strains (Table 1) and 23 Korean isolates of the genus Borrelia were used in this study. The strains were cultivated at 32°C in Barbour-Stoenner-Kelly II (BSKII) medium. DNA was extracted by a modified version of a previously described method (4). The groEL genes of 22 reference strains and 23 Korean isolates and the flagellin genes and the 16S rRNA genes of 22 reference strains were amplified as presented in Table 2. The nucleotide sequences of the recombinant DNA were determined using the CEQ L DNA Analysis System and the CEQ 2000 Dye Terminator Cycle Sequencing kit (Beckman Coulter Inc., Fullerton, Calif.) with forward and reverse sequencing primers (M13) and sequencing primers (Table 2). The multiple-alignment algorithm in the MegAlign software package (Windows version 3.12e; DNASTAR, Madison, Wis.) was used to align the sequences. All positions with alignment gaps were excluded from the pairwise sequence comparison. Phylogenetic trees were constructed by the unweighted pair group method with arithmetic averages using the MEGA program (7). A bootstrap analysis (100 replicates) was performed to evaluate the topology of the phylogenetic tree.
TABLE 1.
Borrelia reference strains used in this study
| Borrelia species | Strain | Source | Geographic location | GenBank accession no.
|
||
|---|---|---|---|---|---|---|
| groEL | Flagellin gene | 16S rRNA | ||||
| B. burgdorferi | B31T | Ixodes scapularis | USb | AE001166 | X15661 | U03396 |
| Sh-2-82 | Ixodes dammini | US | AF517948 | AY342019 | M60969 | |
| 20004 | Ixodes ricinus | France | AF517951 | AY342018 | M64310 | |
| IP2 | Human (CSFa) | France | AF517952 | AB057452 | AY342028 | |
| B. afzelii | Iper3 | Ixodes ricinus | Russia | AF517953 | AY342020 | M84815 |
| VS461T | Ixodes ricinus | Switzerland | AF517954 | D63365 | AY342034 | |
| ACA1 | Human (skin) | Sweden | X54059 | AB035613 | AB035404 | |
| Pko-85 | Skin | Germany | AF517956 | AY342021 | AY342030 | |
| B. garinii | PBi | Human (CSF) | Germany | AF517957 | AB035595 | X85199 |
| PD89 | Human (blood) | China | AF517958 | AY342022 | AY342031 | |
| IP90 | Ixodes persulcatus | Russia | AF517959 | L42885 | M89937 | |
| G1 | Human (CSF) | Germany | AF517960 | AY342023 | M64311 | |
| G2 | Human (CSF) | Germany | AF517961 | AY342024 | M60967 | |
| Sika1 | Ixodes ovatus | Japan | AF517963 | AY342025 | AY342029 | |
| K48 | Ixodes ricinus | Slovakia | AF517968 | AY342026 | AY342032 | |
| IP89 | Ixodes persulcatus | Russia | AF517969 | AY342027 | AY342033 | |
| B. japonica | HO14T | Ixodes ovatus | Japan | AF517970 | D82852 | L40597 |
| B. valaisiana | VS116T | Ixodes ricinus | Switzerland | AF517976 | D82854 | X98232 |
| B. lusitaniae | PotiB2T | Ixodes ricinus | Portugal | AF517971 | D82856 | X98228 |
| B. bissettii | DN127T | Ixodes pacificus | US | AF517974 | D82857 | L40596 |
| B. andersonii | 21123 | Ixodes dentatus | US | AF517975 | D83764 | NDc |
| B. andersonii | 21038 | Ixodes dentatus | US | ND | ND | L46701 |
| B. hermsii | HS1T | Ornithodoros coriaceus | US | AF518000 | M86838 | U42292 |
CSF, cerebrospinal fluid.
US, United States.
ND, not done.
TABLE 2.
Sequences of primers and PCR conditions
| Gene (DNA size) | Primer | Sequence | Positionsc | PCR conditions
|
|
|---|---|---|---|---|---|
| No. of cycles | Cycle stepsd | ||||
| groEL (310 bp) | GFa | 5′-TACGATTTCTTATGTTGAGGG-3′ | 552-572 | 30 | 94°C for 30 s, 59°C for 45 s, 72°C for 45 s |
| GRa | 5′-CATTGCTTTTCGTCTATCACC-3′ | 861-841 | |||
| Flagellin gene (584 bp) | F1a | 5′-GCAGTTCAATCAGGTAACGG-3′ | 280-299 | 30 | 94°C for 30 s, 56°C for 45 s, 72°C for 45 s |
| F2a | 5′-AGGTTTTCAATAGCATACTC-3′ | 863-844 | |||
| 16S RNA (1,427 bp) | B1a,b | 5′-CAGTGCGTCTTAAGCATGC-3′ | 40-58 | 30 | 94°C for 30 s, 59°C for 45 s, 72°C for 45 s |
| B2b | 5′-CGACCTTCTTCATTCACGC-3′ | 416-398 | |||
| B3b | 5′-GCAGCTAAGAATCTTCCGCAATGG-3′ | 340-373 | |||
| B4b | 5′-AAGTTCGCCTTCGCCTCCGGTA-3′ | 735-714 | |||
| B5b | 5′-TGTAAGGGTGGAATCTGTTG-3′ | 681-700 | |||
| B6b | 5′-CAACCATGCAGCACCTGTATAT-3′ | 1053-1032 | |||
| B7b | 5′-TATACAGGTGCTGCATGG-3′ | 1033-1040 | |||
| B8a,b | 5′-CCTTAAATACCTTCCTCCC-3′ | 1466-1448 | |||
Oligonucleotide primers used for PCR amplication.
Oligonucleotide primers used for sequencing.
Position numbers were determined from B. burgdorferi B31T.
Steps in one cycle of PCR.
In this study, interspecies differences in the groEL genes (positions 552 to 861 in B. burgdorferi B31T numbering; 310 bp) of B. burgdorferi strains sensu lato were compared with those in the flagellin genes (positions 280 to 789 in the B. burgdorferi B31T numbering; 510 bp) and 16S rRNA genes (positions 44 to 849 in B. burgdorferi B31T numbering; 806 bp). Moreover, intraspecies differences in the groEL genes from B. burgdorferi, B. afzelii, and B. garinii were compared with those in the flagellin and 16S rRNA genes. However, intraspecies differences in the groEL genes of other Borrelia species could not be compared with those in the flagellin and 16S rRNA genes, because the groEL gene sequence of just one strain per species was available (9).
groEL gene analysis has several characteristics different from those of analyses of other genes. Compared with the 16S rRNA genes, groEL sequences have higher divergence for strains of B. burgdorferi sensu lato. More than 91.6% similarity of the groEL gene sequences was observed among strains of B. burgdorferi sensu lato. On the other hand, more than 95.4% similarity of the 16S rRNA gene sequences was observed among strains of B. burgdorferi sensu lato (data not shown). The groEL gene sequence similarities in B. burgdorferi, B. afzelii, and B. garinii strains were 99.7 to 100%, 99.0 to 99.4%, and 96.8 to 100%, respectively (Table 3). On the other hand, the 16S rRNA gene sequence similarities in B. burgdorferi, B. afzelii, and B. garinii strains were 99.6 to 100%, 99.6 to 100%, and 99.1 to 100%, respectively (Table 3). These results showed that the groEL gene is more heterogeneous than the 16S rRNA gene and is useful in intraspecies differentiation. Compared with the flagellin gene analysis, more than 92.0% similarity of the flagellin gene sequences was observed in strains of B. burgdorferi sensu lato (data not shown). The groEL gene sequence similarities in B. burgdorferi, B. afzelii, and B. garinii strains were 99.7 to 100%, 99.0 to 99.4%, and 96.8 to 100%, respectively, whereas the flagellin gene sequence similarities in B. burgdorferi, B. afzelii, and B. garinii strains were 99.0 to 99.8%, 99.6 to 100%, and 98.0 to 100%, respectively (Table 3). These results showed that the groEL gene is more heterogeneous than the flagellin gene in B. afzelii and B. garinii, whereas the flagellin gene is more heterogeneous than the groEL gene in B. burgdorferi.
TABLE 3.
Intraspecies variation in the groEL, flagellin, and 16S rRNA gene sequences of Borrelia strains
| Species | No. of strains | % Identity
|
||
|---|---|---|---|---|
| groEL gene | Flagellin gene | 16S rRNA gene | ||
| B. burgdorferi | 4 | 99.7-100.0 | 99.0-99.8 | 99.6-100.0 |
| B. afzelii | 4 | 99.0-99.4 | 99.6-100.0 | 99.6-100.0 |
| B. garinii | 8 | 96.8-100.0 | 98.0-100.0 | 99.1-100.0 |
The B. garinii IP89 strain showed the lowest similarity (96.8 to 98.4%) to other B. garinii strains (data not shown). This strain was previously classified as a different group with B. garinii using multilocus enzyme electrophoresis (2). This strain also showed a different restriction fragment length polymorphism (RFLP) pattern of 5S-23S intergenic spacer amplicons from one of the B. garinii strains (11). These results showed that groEL sequence analysis is useful not only in interspecies differentiation but also in intraspecies differentiation of B. garinii strains. groEL gene sequence analysis may be useful for intraspecies differentiation of B. afzelii and B. garinii strains, whereas flagellin gene sequence analysis may be useful for intraspecies differentiation of B. burgdorferi strains.
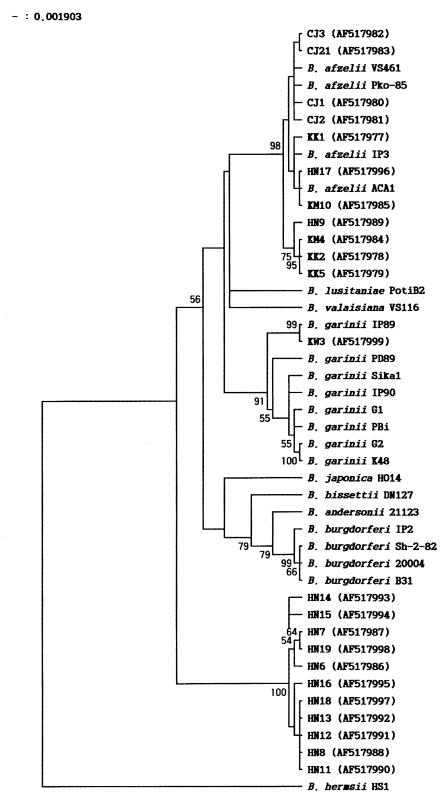
Twenty-three Korean isolates were characterized by phylogenetic analysis based on groEL gene sequences. Eleven strains (KK1, KK2, KK5, KM4, KM10, CJ1, CJ2, CJ3, CJ21, HN9, and HN17), identified as B. afzelii through PCR-RFLP analyses of the ospC gene and the rrf-rrl intergenic spacer in a previous study (10), were also identified as B. afzelii by groEL gene analysis (Fig. 1). The nucleotide sequence of strain KW3 was identical to that of B. garinii IP89 (Fig. 1), and KW3 also showed the same restriction pattern as B. garinii IP89 in RFLP analysis of the 5S-23S intergenic spacer amplicons (data not shown). Eleven Haenam strains formed a distinctive cluster, separated from other strains of B. burgdorferi sensu lato in the phylogenetic tree (Fig. 1). The sequence similarities among 11 Haenam strains (HN6, HN7, HN8, HN11, HN12, HN13, HN14, HN15, HN16, HN18, and HN19) were 98.7 to 100%. In general, they showed 89.7 to 94.8% similarity with other strains of B. burgdorferi sensu lato. The MseI and DraI restriction patterns of the 5S-23S intergenic spacer amplicons of Haenam strains differed from those of other strains of B. burgdorferi sensu lato. Furthermore, in the phylogenetic tree based on 16S ribosomal DNA sequences, Haenam strains also formed a distinctive cluster (8).
FIG. 1.
Phylogenetic tree based on groEL gene sequences of Borrelia strains. The phylogenetic tree was constructed by the unweighted pair group method with arithmetic averages using MEGA software. Bootstrap analysis was performed with 100 replicates. The GenBank accession numbers are shown in parentheses.
In conclusion, the groEL gene is useful for the identification and characterization of B. burgdorferi sensu lato despite the fact that it has a shorter nucleotide sequence (310 bp) than the flagellin gene (510 bp) and the 16S rRNA gene (806 bp).
Acknowledgments
This work was supported in part by the Ministry of Science and Technology through the Bio-Food and Drug Research Center at Konkuk University, Chungju, Korea.
REFERENCES
- 1.Anderson, J. F. 1989. Epizootiology of Borrelia in Ixodes tick vectors and reservoir hosts. Rev. Infect. Dis. 11:S1451-S1459. [DOI] [PubMed] [Google Scholar]
- 2.Balmelli, T., and J. C. Piffaretti. 1996. Analysis of the genetic polymorphism of Borrelia burgdorferi sensu lato by multilocus enzyme electrophoresis. Int. J. Syst. Bacteriol. 46:167-172. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., and C. F. Garon. 1987. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science 237:409-411. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G., R. A. Heiland, and T. R. Howe. 1985. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J. Infect. Dis. 152:478-484. [DOI] [PubMed] [Google Scholar]
- 6.Canica, M. M., F. Nato, L. du Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25:441-448. [DOI] [PubMed] [Google Scholar]
- 7.Kumar, S., K. Tamura, and N. Masatoshi. 1993. MEGA: molecular evolutionary genetics analysis, version 1.01. The Pennsylvania State University, University Park.
- 8.Lee, S.-H., B.-J. Kim, J.-H. Kim, K.-H. Park, S.-J. Yeo, S. J. Kim, and Y. H. Kook. 2000. Characterization of Borrelia burgdorferi strains isolated from Korea by 16S rDNA sequence analysis and PCR-RFLP analysis of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Evol. Microbiol. 50:857-863. [DOI] [PubMed] [Google Scholar]
- 9.Lee, S.-H., J.-H. Lee, H.-S. Park, W.-J. Jang, S.-E. Koh, Y.-M. Yang, B.-J. Kim, Y.-H. Kook, and K.-H. Park. 2003. Differentiation of Borrelia burgdorferi sensu lato through groEL gene analysis. FEMS Microbiol. Lett. 222:51-57. [DOI] [PubMed] [Google Scholar]
- 10.Lee, S.-H., K.-D. Jung, J.-H. Lee, S.-C. Kim, J.-H. Kim, W.-J. Jang, and K.-H. Park. 2002. Characterization of Borrelia afzelii isolated from Ixodes nipponensis and Apodemus agrarius in Chungju, Korea, by PCR-RFLP analyses of ospC gene and rrf (5S)-rrl (23S) intergenic spacer. Microbiol. Immunol. 46:677-683. [DOI] [PubMed] [Google Scholar]
- 11.Postic, D., M. V. Assous, P. A. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 44:743-752. [DOI] [PubMed] [Google Scholar]
- 12.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]