Summary
Glypican-3 (GPC3) is an oncofetal protein that has been demonstrated to be a useful diagnostic immunomarker for hepatocellular carcinoma and hepatoblastoma. Its expression in mesenchymal tumors of the liver, particularly undifferentiated embryonal sarcoma (UES) and mesenchymal hamartoma (MH), has not been investigated. In this study, a total of 24 UESs and 18 MHs were immunohistochemically stained for GPC3 expression. The results showed cytoplasmic staining for GPC3 in 14 (58%) UESs, of which 6 exhibited diffuse immunoreactivity and the remaining 8 showed focal positivity. The patients with GPC3-positive UES tended to be younger (mean 18 years; median 11 years) than those with GPC3-negative tumors (mean 39.4 years; median 27 years), although the difference did not reach statistical significance (P = .06). Eight MHs also exhibited GPC3 immunoreactivity (44%; 4 diffuse and 4 focal). Positive staining in all 8 cases was primarily seen in entrapped nonlesional hepatocytes with a canalicular and cytoplasmic staining pattern. In only 4 cases (22%) was GPC3 immunoreactivity also observed in the mesenchymal component. The patients with positive staining also tended to be younger (mean 2.6 years; median 1.1 years) compared with those with negative staining (mean 16.3 years; median 4.5 years), but the difference was not statistically significant (P = .15). Our data demonstrate that GPC3 is expressed in a subset of UES and MH of the liver. Caution should thus be exercised when evaluating a GPC3-expressing hepatic neoplasm, particularly on a needle biopsy when the differential diagnosis includes poorly differentiated hepatocellular carcinoma or hepatoblastoma.
Keywords: Glypican-3, Liver, Undifferentiated embryonal sarcoma, Mesenchymal hamartoma, Immunohistochemistry
1. Introduction
Mesenchymal hamartoma (MH) is an uncommon benign hepatic tumor primarily affecting the pediatric population within the first 2 years of life. Uncommonly, this tumor is seen in older children and adults [1-4]. It encompasses approximately 8% of all primary pediatric hepatic tumors and is the second most common primary hepatic tumor after hepatoblastoma. These tumors can be solid or cystic and quite large. The common histological features include a biphasic growth pattern composed of bland myxomatous stroma intertwined with branching duct structures, reminiscent of ductal plate malformation. Residual benign hepatocytes may be seen intermixed within the tumor but are more commonly noted at the periphery of the lesion. Malignant transformation to undifferentiated embryonal sarcoma (UES) has been reported but is rare [1,5,6].
On the other hand, UES is a malignant hepatic tumor with aggressive behavior. It primarily affects children between 2 and 5 years old and young adults. Rare cases have been reported in middle-aged and older patients [7-10]. These tumors are typically large, hemorrhagic, necrotic, and can be solid or cystic. Histologically, these tumors usually show proliferation of pleomorphic spindle cells in a myxoid stroma, which may surround or entrap benign bile ducts at the periphery. Intracellular and extracellular periodic acid–Schiff-positive hyaline globules may be seen [7,8,11]. Occasionally, UES may exhibit clinical, radiographic, or even histological features overlapping with hepatocellular carcinoma, hepatoblastoma, or MH, which makes the diagnosis difficult in a small biopsy specimen. In this regard, ancillary immunohistochemical studies may serve an important role in helping establish the diagnosis.
Glypican-3 (GPC3) is a member of the heparan sulfate proteoglycan family, which is linked to the cell surface through a glycosylphosphatidylinositol anchor [12]. This oncofetal protein is widely expressed in fetal tissues and is involved in organogenesis and growth control during development. In adults, GPC3 is exclusively expressed in neoplastic processes, notably hepatocellular carcinoma, hepatoblastoma, germ cell tumors, and Wilms tumor [13-18]. Studies have shown that serum GPC3 can be detected in 40% to 53% of patients with hepatocellular carcinoma, including a subset of patients that are seronegative for α-fetoprotein [19]. Immunohistochemical studies further demonstrate GPC3 to be a useful diagnostic immunomarker for hepatocellular carcinoma and hepatoblastoma, as it shows at least focal immunoreactivity in the majority of cases evaluated whereas benign liver lesions are typically negative for GPC3 expression [18,20-25]. However, the expression of GPC3 in nonvascular mesenchymal tumors of the liver, particularly UES and MH, has not been investigated.
2. Materials and methods
A total of 42 liver resection specimens were included in this study. These included 24 cases of UES and 18 cases of MH retrieved from surgical pathology archives of authors’ institutions. The clinical history, pathology reports, hematoxylin and eosin–stained slides, and various diagnostic immunohistochemical stains were reviewed by the submitting pathologists to confirm the diagnosis. The study was approved by the Institutional Review Board at Cedars-Sinai Medical Center and coauthors’ institutions.
Immunohistochemical staining for GPC3, α-fetoprotein, and hepatocyte antigen (Hep Par 1) was performed on all 42 cases. Formalin-fixed, paraffin-embedded tissue sections (4 μm) were deparaffinized, rehydrated, and treated with 3% hydrogen peroxide for 15 minutes to quench endogenous peroxidase. For GPC3 and Hep Par 1 immunostains, heat-induced epitope retrieval in 0.1 M citrate buffer at pH 6.0 for 20 minutes was performed. Antigen retrieval was not required for anti–α-fetoprotein antibody. The slides were incubated with a mouse monoclonal antibody (clone 1G12) specific for GPC3 obtained from BioMosaics (Burlington, VT), a rabbit polyclonal antibody specific for α-fetoprotein obtained from Dako (Carpinteria, CA), and a mouse monoclonal antibody (clone OCH1E5) specific for hepatocyte antigen obtained from Dako at room temperature at dilutions of 1:200 for 1 hour, 1:500 for 30 minutes, and 1:200 for 30 minutes, respectively. After incubation with an anti-mouse or anti-rabbit secondary antibody, a reaction was performed using the EnVision+ detection system that contained biotin-free horseradish peroxidase–labeled polymers obtained from Dako. The staining was visualized using 3,3′-diaminobenzidine substrate-chromogen solution and counterstained with hematoxylin.
Immunohistochemically stained slides were evaluated and a case was considered negative if less than 1% of the cells of interest exhibited immunoreactivity. Those cases with positive staining were graded as weak, intermediate, or strong for staining intensity. The percentage of positively stained cells in each case was also recorded. In general, the staining was considered focal if 1% to 50% of the cells of interest were stained and diffuse if more than 50% of the cells stained. Staining pattern (canalicular or cytoplasmic) was also recorded.
Statistical analysis was performed using the 2-tailed unpaired t test or the 2-tailed χ2 test with Yates continuity correction, when applicable. A P value of <.05 was considered statistically significant.
3. Results
3.1. Clinicopathological findings
The demographic data of the patients and the size of the tumors are summarized in Table 1 for UES and Table 2 for MH. The ages of all patients included in this study ranged from 2 months to 87 years (mean 19.8 years; median 11.0 years). There were 23 males and 18 females. The demographic data were not available for 1 UES case. The ages of the patients with UES ranged from 2 to 87 years (mean 27.3 years; median 16.0 years). There were 13 males and 10 females. The patients with MH ranged in age from 2 months to 72 years (mean 10.2 years; median 1.2 years). These patients were younger in comparison to those with UES (P = .03). All patients had liver masses, 5 of which were described to be partially cystic on image studies. Some patients also presented with obstructive jaundice, abdominal pain, and abdominal distension. In 2 cases, the liver masses were detected incidentally during workup for unrelated pancreatic and brain lesions.
Table 1.
Patient demographic data, tumor size, and GPC3 expression in cases of UES (n = 24)
| Case | Age (y) | Sex | Tumor size (cm) | GPC3 expression |
|---|---|---|---|---|
| 1 | 2 | M | n/a | 80% |
| 2 | 2 | M | n/a | 70% |
| 3 | 6 | M | 9 | neg |
| 4 | 7 | M | 4.5 | 80% |
| 5 | 9 | F | n/a | 80% |
| 6 | 10 | F | 13 | neg |
| 7 | 10 | F | 16 | 90% |
| 8 | 11 | F | 13 | 40% |
| 9 | 11 | M | 5.5 | 70% |
| 10 | 12 | M | 12.5 | 5% |
| 11 | 13 | F | 12 | 20% |
| 12 | 16 | M | n/a | 10% |
| 13 | 18 | M | 2.4 | neg |
| 14 | 19 | M | 6.4 | 10% |
| 15 | 19 | F | 33 | neg |
| 16 | 19 | M | 29 | neg |
| 17 | 35 | F | 17 | neg |
| 18 | 40 | M | 2.6 | 10% |
| 19 | 61 | M | 13.3 | neg |
| 20 | 65 | M | 16.5 | neg |
| 21 | 74 | F | 20 | neg |
| 22 | 82 | F | 5 | 10% |
| 23 | 87 | F | n/a | neg |
| 24 | n/a | n/a | n/a | 30% |
Abbreviations: M, male; F, female; n/a, data not available; neg, negative.
Table 2.
Patient demographic data, tumor size, and GPC3 expression in cases of MH (n = 18)
| Case | Age | Sex | Tumor size (cm) | GPC3 expression |
|---|---|---|---|---|
| 1 | 2 mo | F | 4 | pos |
| 2 | 6 mo | M | 9 | pos |
| 3 | 7 mo | F | 15 | neg |
| 4 | 7 mo | F | 15 | neg |
| 5 | 10 mo | M | 15 | pos |
| 6 | 12 mo | F | 16.5 | neg |
| 7 | 12 mo | M | 9 | pos |
| 8 | 12 mo | F | 13.5 | neg |
| 9 | 14 mo | M | 14 | pos |
| 10 | 17 mo | M | 15 | pos |
| 11 | 2 y | F | 9.5 | neg |
| 12 | 3 y | M | 15 | pos |
| 13 | 7 y | M | 3 | neg |
| 14 | 12 y | M | n/a | neg |
| 15 | 13 y | M | 2.2 | pos |
| 16 | 16 y | F | 34 | neg |
| 17 | 51 y | M | 2.4 | neg |
| 18 | 72 y | F | 1.7 | neg |
Abbreviations: M, male; F, female; n/a, data not available; pos, positive; neg, negative.
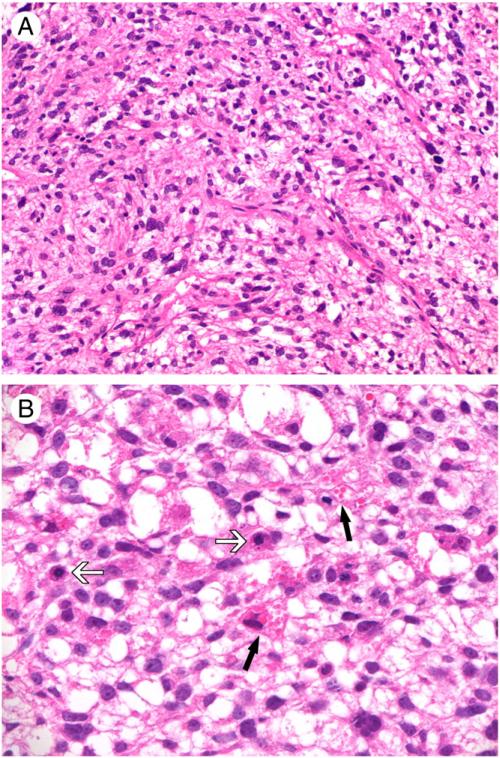
The tumor size for UES ranged from 2.4 to 33 cm (mean 12.8 cm). There did not appear to be a correlation between patient age and tumor size. Histologically, UES was characterized by proliferation of spindle, stellate, ovoid, and pleomorphic tumor cells with variable cellularity, either loosely or compactly distributed in a myxoid or fibrous matrix (Fig. 1A). Multinucleated or bizarre nuclei were occasionally seen. Mitotic figures, including atypical mitoses, were readily identified. Variably sized intracytoplasmic eosinophilic globules were present in some of the tumor cells (Fig. 1B). Focal, multifocal, or diffuse tumor necrosis was seen in the majority of the cases, and the extent of necrosis did not correlate with tumor size. Intratumoral hemorrhage was noted in some cases. Entrapped thin cords of benign hepatocytes and bile ducts were noted at the periphery of 3 tumors.
Fig. 1.
Histological features of UES. A, Short-spindled and ovoid tumor cells distributed in a loose stroma with delicate vasculature (original magnification, ×200). B, Intracytoplasmic hyaline globules noted in some tumors (black arrows). Note the presence of frequent mitotic figures (white arrows; original magnification, ×400).
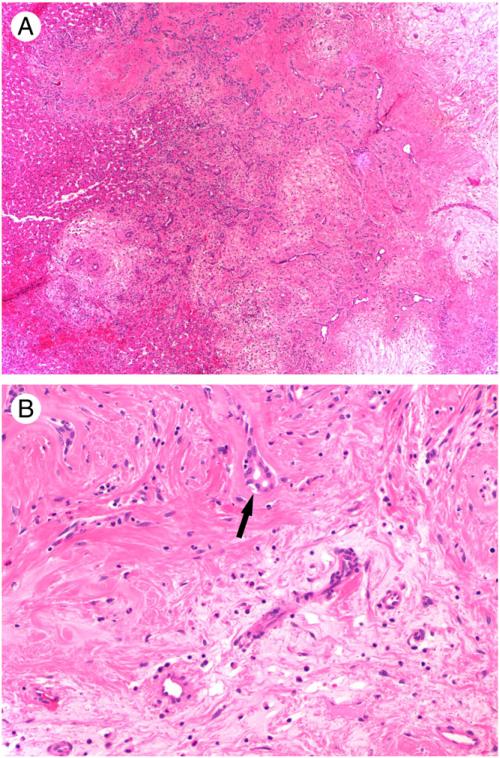
The tumor size for MH ranged from 1.7 to 34 cm (mean 11.4 cm). There did not appear to be a correlation between patient age and tumor size. Cystic degeneration was noted in one third of the cases. MH was histologically characterized by nodular collections of bland spindle cells and proliferating bile ducts, which were usually surrounded by islands of normal-appearing hepatocytes (Fig. 2A). The proportion of these different elements in each tumor varied but did not correlate with the age or sex of the patient or the size of the tumor. The stroma around the spindle cells was loose, edematous, or myxoid in some cases but hyalinized in others (Fig. 2B). The spindle cell areas were hypocellular or, at most, mildly hypercellular and mitotic activity was absent.
Fig. 2.
Histological features of MH. A, Nodular collection of hypocellular myxoid and hyalinized stroma with proliferating bile ducts (original magnification, ×40). Note the presence of normal-appearing hepatocytes at the periphery. B, Higher power view of myxoid (bottom) and hyalinized (upper) stroma with compressed bile ducts (black arrow), sparse bland spindle cells, and admixed inflammatory cells (original magnification, ×200).
3.2. Immunohistochemical findings
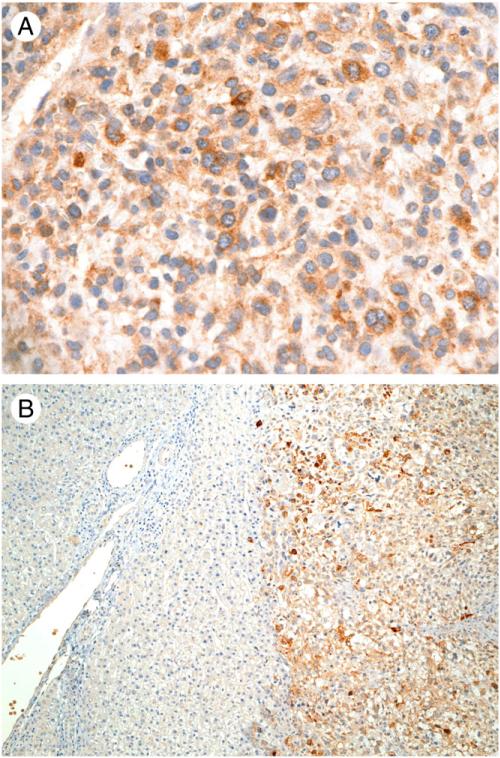
A variable degree of cytoplasmic staining for GPC3 was observed in 14 (58%) of 24 UESs (Table 1). Among these 14 positively stained tumors (Table 3), 13 (93%) showed strong or intermediate immunoreactivity in tumor cells (Fig. 3A). The staining was diffuse in 6 cases (43%) and focal in 8 cases (57%). In the other 10 cases, GPC3 staining was negative in tumor cells. Background nonneoplastic liver tissue was present in 13 cases, which was also negative for GPC3 expression (Fig. 3B). The patients with GPC3-positive tumors tended to be younger (mean 18 years; median 11 years) than those with GPC3-negative tumors (mean 39.4 years; median 27 years), but the difference did not reach statistical significance (P = .06). The tumor cells in all UES cases showed negative immunostaining for α-fetoprotein and Hep Par 1.
Table 3.
Summary of GPC3 expression in UES (n = 24)
| Staining characteristics in tumor cells |
||||
|---|---|---|---|---|
| Strong | Intermediate | Weak | Total | |
| Diffuse | 4 | 2 | 0 | 6 |
| Focal | 5 | 2 | 1 | 8 |
| Total | 9 | 4 | 1 | 14 |
Fig. 3.
Immunohistochemical expression of glypican-3 in UES. A, Positive cytoplasmic staining in tumor cells (original magnification, ×400). B, No immunoreactivity detected in nonneoplastic hepatic tissue (left) whereas the adjacent tumor cells (right) were positively stained (original magnification, ×100).
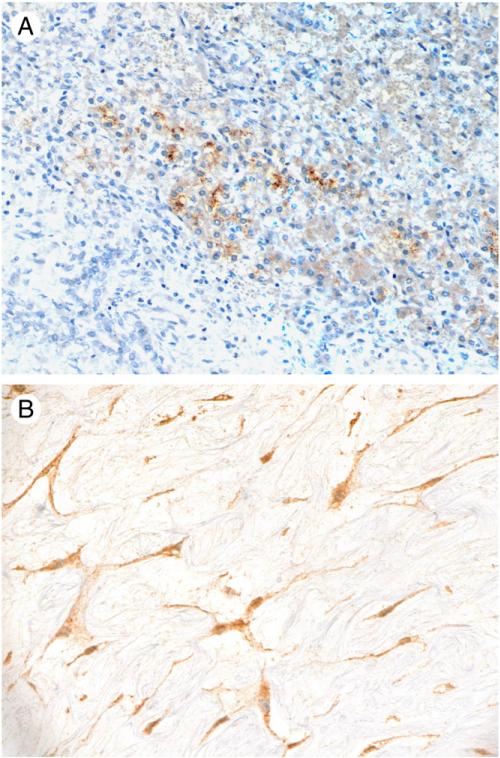
Positive GPC3 immunostaining was observed in 8 (44%) of 18 MHs (Table 2), a frequency comparable to that seen in UES cases (P = .56). The staining intensity was strong in 5 (63%) and intermediate in 3 (37%) cases. GPC3 immunoreactivity was detected in several components of the tumors (Table 4), including tumoral hepatocytes in all 8 positive cases with a canalicular and/or cytoplasmic staining pattern (Fig. 4A), in spindle cells in 5 cases (63%; Fig. 4B), and in intratumoral bile ducts in 2 cases (25%). Although the overall frequency of GPC3 expression in spindle cells in MH was lower (28%) than that in UES (58%), the difference was not statistically significant (P = .098). Nonneoplastic liver parenchyma was present in 10 cases, 2 of which showed reactivity against GPC3 in hepatocytes adjacent to tumor (1 diffuse and 1 focal). The patients with GPC3-positive MH tended to be younger (mean 2.6 years; median 1.1 years) than those with GPC3-negative tumors (mean 16.3 years; median 4.5 years), but the difference did not reach statistical significance (P = .15).
Table 4.
Summary of GPC3 expression in MH (n = 18)
| Tumor components | Strong (n = 5) |
Intermediate (n = 3) |
||
|---|---|---|---|---|
| Diffuse (n = 3) | Focal (n = 2) | Diffuse (n = 1) | Focal (n = 2) | |
| Hepatocytes | 3 | 2 | 1 | 2 |
| Bile ducts | 2 | 0 | 0 | 0 |
| Spindle cells | 2 | 0 | 1 | 2 |
Fig. 4.
Immunohistochemical expression of glypican-3 in MH. A, Intratumoral hepatocytes showing canalicular and cytoplasmic staining but no immunoreactivity was detected in bile ducts and stromal cells (original magnification, ×200). B, Positive cytoplasmic staining in stromal cells in a loose myxoid background (original magnification, ×400).
Four MH cases (22%) showed strong cytoplasmic α-fetoprotein immunoreactivity in hepatocytes within the tumors (3 diffuse, 1 focal). No staining was observed in spindle cells or bile duct epithelium. Three of these 4 cases also showed strong and diffuse GPC3 immunoreactivity. Interestingly, all 3 tumors that coexpressed GPC3 and α-fetoprotein were from patients younger than 10 months, whereas the single case that was positive for α-fetoprotein but negative for GPC3 was from a 16-year-old patient. The nontumoral hepatocytes outside the tumor present in 2 of these 4 cases were negative for α-fetoprotein expression.
Thirteen cases (72%) of MH showed strong and diffuse cytoplasmic staining for Hep Par 1 in intratumoral hepatocytes. No immunoreactivity was detected in spindle cells or bile ducts. Ten of the 18 cases that contained nonneoplastic liver parenchyma also showed strong and diffuse cytoplasmic staining for Hep Par 1 in hepatocytes outside the tumors.
4. Discussion
In this study, we demonstrate that GPC3 is expressed in a subset of UES and MH of the liver. GPC3 immunoreactivity is detected in the cytoplasm of tumor cells in UES and in both the epithelial and mesenchymal components of MH. These observations extend our knowledge regarding the immunophenotypic profile of UES and MH and expand the differential diagnosis list for GPC3-expressing hepatic tumors. The detection of GPC3 immunoreactivity in the spindle cell component in 28% of MH cases examined in this study is intriguing and differs from previous observations in hepatocellular lesions where GPC3 expression is essentially exclusively seen in malignant tumors and only rarely detectable in benign hepatocellular lesions [20,22-25]. These data thus indicate that GPC3 is not a useful immunomarker in differentiating malignant UES from benign MH or in facilitating the recognition of malignant transformation from MH to UES.
Only a few studies have investigated the immunohistochemical phenotype of UES. One series of 6 cases reported positive immunostaining for vimentin in all cases and Bcl2 immunoreactivity in 5 cases. Focal expression of CD10, p53, pancytokeratin (AE1/AE3), calponin, and desmin was observed in some of the cases. The tumors were negative for Hep Par 1, myogenin, CD34, SMMS, h-caldesmon, PE10, ALK-1, CD117 (c-kit), and S100. The Ki-67 proliferation index ranged from 30% to 95% in tumor cells [7]. Another study examined 14 primary and 2 recurrent UESs and similarly showed strong immunostaining for vimentin in most of the tumor cells. Focal immunoreactivity was observed for desmin, smooth muscle actin, and cytokeratins 18 and 19. Immunostains for Hep Par 1, myoglobin, CD117, CD34, S100, HMB45, and α-fetoprotein were negative in all primary tumors, but focal CD34, S100, and myoglobin expression was detected in 2 recurrent tumors [11].
In our series, immunostains for vimentin and smooth muscle actin performed at the submitting institutions at the time of tumor resection revealed similar findings. Our study also corroborated previous studies with negative immunore-activity for Hep Par 1 and α-fetoprotein in all UES cases, which can be helpful in the distinction of this tumor from other GPC3-expressing hepatic tumors, such as poorly differentiated or sarcomatoid hepatocellular carcinoma and hepatoblastoma with prominent mesenchymal or small cell component, particularly when the diagnostic material is limited. It should be noted, however, that the mesenchymal component in hepatoblastoma is nearly always negative for GPC3 immunostaining [18]. It should also be mentioned that GPC3 immunostaining is not useful in distinguishing UES from hepatic rhabdomyosarcoma. In 3 hepatic rhabdomyosarcomas we encountered, 2 showed strong cytoplasmic GPC3 immunoreactivity in tumor cells (1 diffuse and 1 focal), results that were similar to those observed in UES (data not shown).
Immunohistochemical studies on MH are limited. Two studies examined a total of 5 cases and showed positive cytokeratins 7 and 19 expression and negative cytokeratin 20 staining in the ductal structures within the tumors. Smooth muscle actin, desmin, and vimentin immunoreactivity was detected in the mesenchymal component [3,4]. Markedly elevated serum α-fetoprotein levels, mimicking hepatoblastoma clinically, has also been documented in case reports [26-28]. By immunohistochemistry, the hepatocytes within the tumors expressed α-fetoprotein, but no nuclear accumulation of β-catenin was detected [27]. Our study expands on these data and further demonstrates that GPC3, Hep Par 1, and α-fetoprotein can be expressed in the various components of MH.
In summary, we have demonstrated that in addition to hepatocellular carcinoma and hepatoblastoma, benign and malignant hepatic nonvascular mesenchymal tumors can express GPC3. The expression does not appear to correlate directly with the young age of the patients, despite the oncofetal nature of the protein. Our findings broaden the differential diagnosis of primary hepatic neoplasms that express GPC3, which can be a potential diagnostic pitfall, especially when interpreting small biopsy specimens.
Footnotes
This work is partly supported by grants R01DK080736 and R01DK081417 (to R.A.A.).
Presented in abstract form at the 97th annual meeting of the United States and Canadian Academy of Pathology, Boston, March 7-13, 2009.
References
- 1.Siddiqui MA, McKenna BJ. Hepatic mesenchymal hamartoma: a short review. Arch Pathol Lab Med. 2006;130:1567–9. doi: 10.5858/2006-130-1567-HMHASR. [DOI] [PubMed] [Google Scholar]
- 2.Klaassen Z, Paragi PR, Chamberlain RS. Adult mesenchymal hamartoma of the liver: case report and literature review. Case Rep Gastroenterol. 2010;4:84–92. doi: 10.1159/000260183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook JR, Pfeifer JD, Dehner LP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum Pathol. 2002;33:893–8. doi: 10.1053/hupa.2002.127442. [DOI] [PubMed] [Google Scholar]
- 4.Yesim G, Gupse T, Zafer U, et al. Mesenchymal hamartoma of the liver in adulthood: immunohistochemical profiles, clinical and histopathological features in two patients. J Hepatobiliary Pancreat Surg. 2005;12:502–7. doi: 10.1007/s00534-005-1025-9. [DOI] [PubMed] [Google Scholar]
- 5.Lauwers GY, Grant LD, Donnelly WH, et al. Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol. 1997;21:1248–54. doi: 10.1097/00000478-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Shehata B, Gupta NA, Katzenstein H, et al. Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: a review of eleven cases. Pediatr Dev Pathol. 2011;14:111–6. doi: 10.2350/09-07-0681-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Kiani B, Ferrell LD, Qualman S, et al. Immunohistochemical analysis of embryonal sarcoma of the liver. Appl Immunohistochem Mol Morphol. 2006;14:193–7. doi: 10.1097/01.pai.0000173052.37673.95. [DOI] [PubMed] [Google Scholar]
- 8.Wei ZG, Tang LF, Chen ZM, et al. Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J Pediatr Surg. 2008;43:1912–9. doi: 10.1016/j.jpedsurg.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Lenze F, Birkfellner T, Lenz P, et al. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008;112:2274–82. doi: 10.1002/cncr.23431. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga Y, Ryo J, Hoppou T, et al. Hepatic undifferentiated (embryonal) sarcoma in an adult: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2000;12:1247–51. doi: 10.1097/00042737-200012110-00014. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JM, Tao X, Xu AM. Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology. 2007;51:195–203. doi: 10.1111/j.1365-2559.2007.02746.x. [DOI] [PubMed] [Google Scholar]
- 12.Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82:184–9. doi: 10.1016/j.yexmp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832–8. doi: 10.1158/0008-5472.CAN-08-1973. [DOI] [PubMed] [Google Scholar]
- 14.Nakano K, Orita T, Nezu J, et al. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279–84. doi: 10.1016/j.bbrc.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Toretsky JA, Zitomersky NL, Eskenazi AE, et al. Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol. 2001;23:496–9. doi: 10.1097/00043426-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Zynger DL, Dimov ND, Luan C, et al. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570–5. doi: 10.1097/01.pas.0000213322.89670.48. [DOI] [PubMed] [Google Scholar]
- 17.Capurro MI, Xiang YY, Lobe C, et al. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–54. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 18.Zynger DL, Gupta A, Luan C, et al. Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum Pathol. 2008;39:224–30. doi: 10.1016/j.humpath.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–81. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HL, Anatelli F, Zhai QJ, et al. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723–8. doi: 10.5858/132.11.1723. [DOI] [PubMed] [Google Scholar]
- 21.Anatelli F, Chuang ST, Yang XJ, et al. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol. 2008;130:219–23. doi: 10.1309/WMB5PX57Y4P8QCTY. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican-3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–8. doi: 10.1038/modpathol.3800436. [DOI] [PubMed] [Google Scholar]
- 23.Coston WMP, Loera HT, Lau SK, et al. Distinction of hepatocellular carcinoma from benign mimickers using glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol. 2008;32:433–44. doi: 10.1097/PAS.0b013e318158142f. [DOI] [PubMed] [Google Scholar]
- 24.Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405–11. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 25.Wang XY, Degos F, Dubois S, et al. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435–41. doi: 10.1016/j.humpath.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Unal E, Koksal Y, Akcoren Z. Mesenchymal hamartoma of the liver mimicking hepatoblastoma. J Pediatr Hematol Oncol. 2008;30:458–60. doi: 10.1097/MPH.0b013e318169171b. [DOI] [PubMed] [Google Scholar]
- 27.Cajaiba MM, Sarita-Reyes C, Zambrano E, et al. Mesenchymal hamartoma of the liver associated with features of Beckwith-Wiedemann syndrome and high serum alpha-fetoprotein levels. Pediatr Dev Pathol. 2007;10:233–8. doi: 10.2350/06-07-0128.1. [DOI] [PubMed] [Google Scholar]
- 28.Boman F, Bossard C, Fabre M, et al. Mesenchymal hamartomas of the liver may be associated with increased serum alpha fetoprotein concentrations and mimic hepatoblastomas. Eur J Pediatr Surg. 2004;14:63–6. doi: 10.1055/s-2004-815784. [DOI] [PubMed] [Google Scholar]