Abstract
Although many human cancers such as melanoma express tumor antigens recognized by T cells, host immune responses often fail to control tumor growth for as yet unexplained reasons. Here, we found a strong association between melanocyte expression of B7-H1 (PD-L1), an immune-inhibitory molecule, and the presence of tumor-infiltrating lymphocytes (TILs) in human melanocytic lesions: 98% of B7-H1+ tumors were associated with TILs compared with only 28% of B7-H1− tumors. Indeed, B7-H1+ melanocytes were almost always localized immediately adjacent to TILs. B7-H1/TIL colocalization was identified not only in melanomas but also in inflamed benign nevi, indicating that B7-H1 expression may represent a host response to tissue inflammation. Interferon-γ, a primary inducer of B7-H1 expression, was detected at the interface of B7-H1+ tumors and TILs, whereas none was found in B7-H1− tumors. Therefore, TILs may actually trigger their own inhibition by secreting cytokines that drive tumor B7-H1 expression. Consistent with this hypothesis, overall survival of patients with B7-H1+ metastatic melanoma was significantly prolonged compared with that of patients with B7-H1− metastatic melanoma. Therefore, induction of the B7-H1/PD-1 pathway may represent an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous antitumor activity and may explain how melanomas escape immune destruction despite endogenous antitumor immune responses. These observations suggest that therapies that block this pathway may benefit patients with B7-H1+ tumors.
INTRODUCTION
Melanoma is considered an immunogenic neoplasm, and tumor-specific T cells can often be isolated from both peripheral blood and melanoma lesions. These T cells recognize tumor-associated or tumor-specific antigens (1). However, most melanomas will progress if left untreated, and spontaneous regression is rare (2), which suggests that endogenous immunity fails to control tumor growth. Local factors within the tumor microenvironment, such as secreted inhibitory cytokines (for example, transforming growth factor–β), regulatory T cells and myeloid-derived suppressor cells, and cell membrane–bound immunomodulatory proteins, have been proposed to contribute to creating a suppressive milieu that protects tumor cells from immune destruction. Indeed, the co-inhibitory molecule B7-H1 (also called PD-L1), which is often up-regulated on tumor cells, impairs T cell responses—leading to anergy, exhaustion, or apoptosis upon engagement with its cognate co-inhibitory receptor PD-1, which is often highly expressed on tumor-infiltrating lymphocytes (TILs) (3–8).
Current clinical efforts in immunotherapy are directed toward overcoming systemic and local immunoregulation, which is thought to have thwarted cancer vaccines, adoptive cellular therapies, and other anti-tumor immunotherapies in the past (9). Ipilimumab, a blocking monoclonal antibody (mAb) directed against the co-inhibitory T cell receptor CTLA-4 (cytotoxic T lymphocyte–associated antigen 4), systemically activates T cells, which led to enhancement of antitumor T cell responses and demonstrated a survival benefit in patients with advanced metastatic melanoma in randomized trials (10, 11). However, serious immune-related adverse events have been experienced by 15 to 30% of patients receiving this therapy (12–14).
The modest therapeutic window for anti–CTLA-4 therapy is due in part to the complete lack of tumor-specific expression of its cognate ligands, B7.1 and B7.2. In contrast, the ligand-receptor interactions of the B7-H1/PD-1 axis occur more selectively within the tumor microenvironment. The B7 Homolog One (B7-H1, also called PD-L1) protein is not detectable in most normal tissues (5, 15–17); however, its expression can be induced to a high level in various cell types by pro-inflammatory cytokines, particularly interferon-γ (IFN-γ) (5, 18), and B7-H1 is highly expressed on many human tumors, including melanoma (3). In addition to the immunosuppressive effects of B7-H1 binding to PD-1 on activated T and B cells, it also mediates suppressive functions via interactions with CD80 on activated T cells (19). Whereas CTLA-4−/− mice die at a young age from uncontrolled lympho-proliferation, B7-H1−/− mice demonstrate only a mild lymphocyte accumulation in vital organs with no signs of autoimmune disease (20, 21). PD-1−/− mice develop late-onset organ-specific autoimmunity, which is strain-dependent (22). Therefore, blockade of the B7-H1/PD-1 axis may be a more tumor microenvironment–specific and less toxic approach to reversing tumor-induced immune tolerance in the clinic than CTLA-4 blockade.
B7-H1 expression has been observed in various solid malignancies, including melanoma, squamous cell carcinoma of the head and neck, and carcinomas of the esophagus, ovary, gastrointestinal tract, breast, lung, and kidney (3, 23). In some tumor types, such as renal cell, esophageal, gastric, and pancreatic carcinomas, cell surface expression (also referred to as “membranous” expression) by more than 5 to 10% of primary tumor cells has been reported to be an independent predictor of adverse patient outcomes (24–26). Additionally, expression of B7-H1 by TILs has been reported to correlate with decreased patient survival (27, 28), suggesting that B7-H1 expression in the tumor microenvironment may exert an immunosuppressive function. Moreover, early clinical trials of a fully human mAb against PD-1 have demonstrated clinical activity in patients with advanced melanoma and other cancers, associated with generally manageable side effects (29).
Here, we used immunohistochemistry (IHC) with a specific mAb to human B7-H1 to examine patterns of membranous B7-H1 expression by both melanocytes/melanoma cells and TILs in 150 melanocytic lesions of various malignant potentials, including nevi, melanomas in situ, primary melanomas of various histologic subtypes, and regional and distant metastases. A significant correlation was observed between the presence of TILs and B7-H1 expression in the tumor microenvironment, which was associated with local production of the inflammatory cytokine IFN-γ. These data support an adaptive mechanism rather than constitutive oncogene-driven expression of B7-H1.
RESULTS
Patient demographics and follow-up
Patient demographic information is provided in Table 1. Tissue samples were obtained from 150 patients (74 women and 76 men) treated at Johns Hopkins Hospital over 20 years. Patients had a median age of 53 years (range, 7 to 94 years). Neither patient gender nor the pathologic subtype of the melanocytic lesion was significantly associated with the degree of melanocyte B7-H1 expression. At the time of last follow-up, 54 of 110 patients with melanoma in situ, invasive primary melanoma, or metastatic melanoma were alive. Median follow-up for the patients who were alive was 100 months (range, 24 to 319 months). Of the 56 melanoma patients who expired, the median time to death was 18 months (range, 1 to 220 months).
Table 1.
Correlation of B7-H1 expression by melanocytes with clinicopathologic features in 150 patients.
| Number of patients | P* | |||
|---|---|---|---|---|
| Total | B7-H1+ (≥5%) |
B7-H1− (<5%) |
||
| All patients | 150 | 57 | 93 | |
| Gender | ||||
| Male | 76 | 28 | 48 | |
| Female | 74 | 29 | 45 | 0.867 |
| Lesion type | ||||
| Nevus | 40 | 14 | 26 | |
| Melanoma in situ | 11 | 6 | 5 | |
| Invasive primary melanoma | 43 | 13 | 30 | |
| Metastasis | 56 | 24 | 32 | 0.378 |
χ2 Analysis.
Patterns of B7-H1 expression in melanocytic lesions
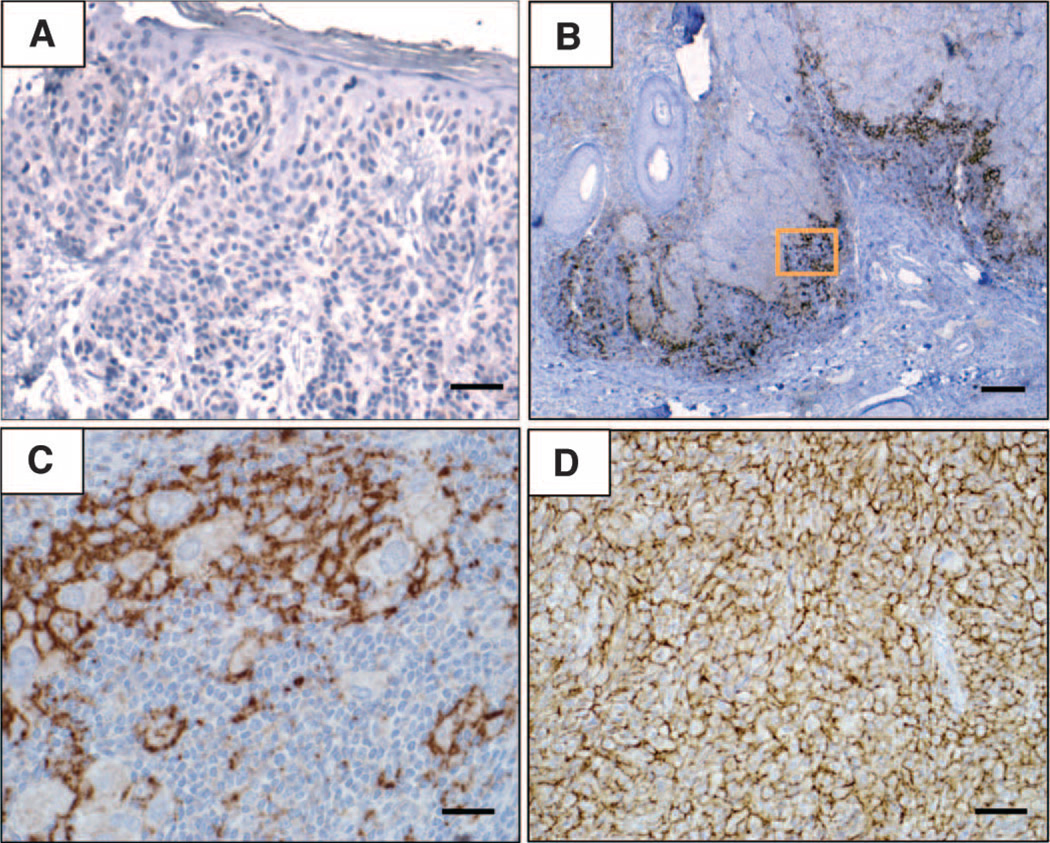
Membranous B7-H1 expression by melanocytes within the tumors (including benign melanocytes and malignant melanoma cells) demonstrated three major patterns on IHC, as shown in Fig. 1: the absence of B7-H1 (Fig. 1A); regional expression of B7-H1 on melanocytes, highly colocalized with TILs (Fig. 1, B and C, and fig. S1, B and C); and B7-H1 expression in the presence of rare or no TILs (Fig. 1D and fig. S1D). B7-H1 expression at the interface with TILs was by far the predominant pattern and often occurred in discrete geographic foci (Fig. 2, A and B). Among 150 melanocytic lesions spanning the spectrum of benign nevi to metastatic melanoma, 57 (38%) were B7-H1+. Of these, only a single case of metastatic melanoma demonstrated broad B7-H1 expression unassociated with TILs. Additionally, only one case of primary melanoma and two metastases demonstrated mixed patterns, including distinct regions of B7-H1+ tumor cells either associated or not with TILs.
Fig. 1.
Patterns of B7-H1 expression observed in melanocytic lesions stained with the anti–B7-H1 mAb 5H1. (A) No B7-H1 expression (brown chromogen) by melanocytes in a benign nevus. Original magnification, ×200 (scale bar, 50 µm). Note the paucity of TILs in this case. (B) B7-H1 expression by both melanocytes and TILs at the advancing edge of an invasive primary melanoma, nodular histologic subtype. Original magnification, ×40 (scale bar, 200 µm). (C) Original magnification of the boxed area shown in (B), ×400 (scale bar, 20 µm). Overall, 10% of total melanoma cells in this specimen expressed B7-H1 (scoring was performed as described in Materials and Methods). The inflammatory host response to tumor was graded as “moderate” in this case and included B7-H1+ TILs. (D) Diffuse B7-H1 expression by melanocytes in a subcutaneous melanoma metastasis to the scalp, associated with singular TILs. Original magnification, ×200 (scale bar, 50 µm). Representative photomicrographs of CD3 staining for TILs corresponding to each of these examples are shown in fig. S1. The interface pattern with B7-H1 expression by both melanocytes and TILs at the advancing edge pre-dominated in this study.
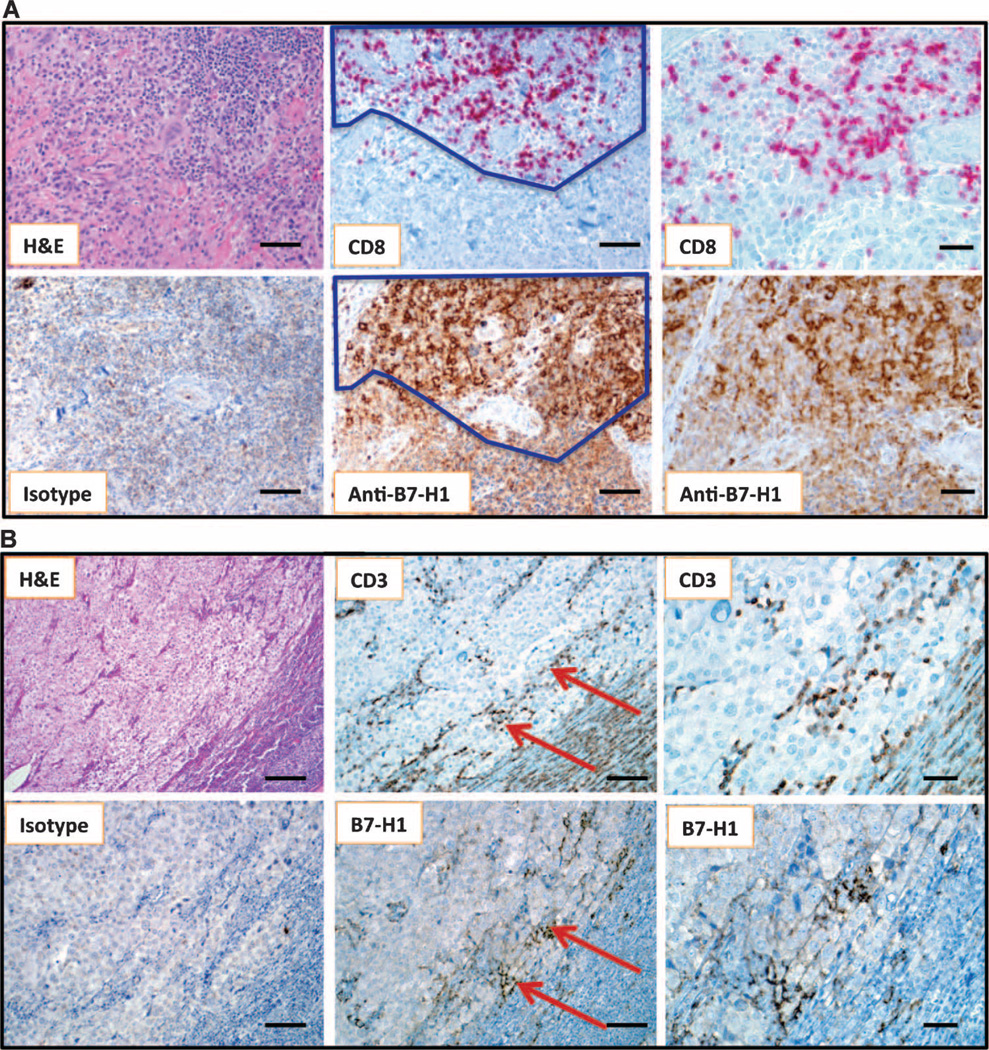
Fig. 2.
Geographic association of B7-H1 expression and TILs. (A) Primary nodular melanoma with associated “severe” grade of lymphocytic infiltration, highlighted by CD8 immunostaining. The dark line demarcates the area of colocalizing T cells and B7-H1+ tumor cells. Overall, 30% of the tumor cells demonstrated cell surface B7-H1 expression. Note that the melanoma cell cytoplasm is heavily pigmented due to abundant melanin (see isotype control). Original magnifications, ×200 for left and middle column panels (scale bars, 50 µm) and ×400 for right column panels (scale bars, 25 µm). (B) Metastatic deposit of melanoma in a lymph node, associated with “mild” grade of lymphocyte infiltration shown by CD3 immunostaining. Original magnifications, ×100 for left column panels (scale bars, 100 µm), ×200 for middle column panels (scale bars, 50 µm), and ×400 for right column panels (scale bars, 25 µm). Arrows highlight the geographic area of concordance of CD3+ TILs with B7-H1 expression by melanoma, and also point to the area shown at higher magnification in the right-hand panels. Five percent of tumor cells demonstrated B7-H1 expression in this case.
Membranous B7-H1 expression was also observed in the TILs and associated macrophages/histiocytes in the host response to tumor (Fig. 1C and fig. S2). In some cases, lymphohistiocytic aggregates unassociated with melanocytes also expressed B7-H1. For example, 3 of the 10 common melanocytic nevi examined showed lymphohistiocytic aggregates within the biopsy specimen (most often located perivascularly and not infiltrating among melanocytes), with some degree of B7-H1 positivity in the macrophages and in occasional lymphocytes.
Quantification of B7-H1 expression by melanocytes and the associated host inflammatory response
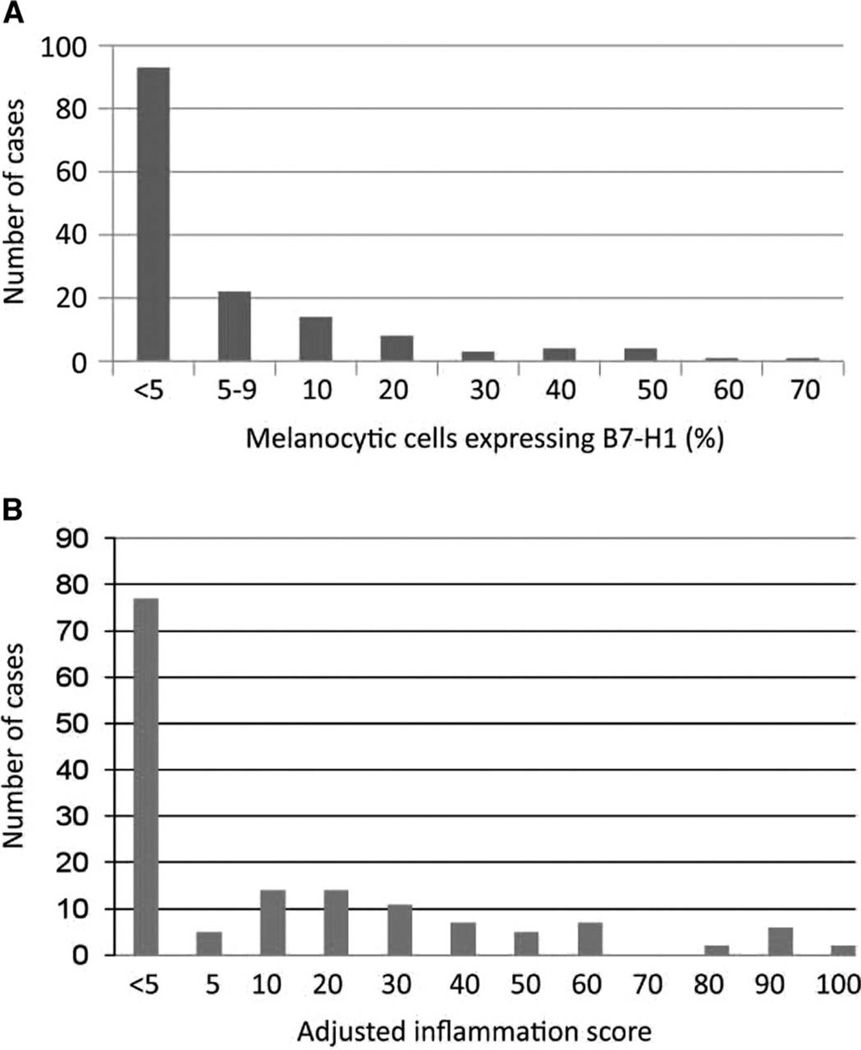
Formalin-fixed, paraffin-embedded (FFPE) tissue sections from each case studied were evaluated for B7-H1 expression. Among 150 melanocytic lesions, 93 were negative for membranous B7-H1 expression (defined as <5% B7-H1+ cells) (Fig. 3A). A 5% expression threshold was chosen to define B7-H1 positivity, in keeping with previous studies (26, 30). Thirty-eight percent (57 of 150) of the study specimens were above this threshold, including 35% (14 of 40) of nevi and 39% (43 of 110) of malignant lesions (Table 1). Thus, B7-H1 expression on melanocytic cells did not correlate with benign compared with malignant lesions.
Fig. 3.
Distribution of intensity of B7-H1 expression by melanocytic cells and immune infiltrates in 150 lesions. (A) Percent B7-H1 expression by melanocytes ≥5% was considered positive. (B) AIS, defined as the intensity of intratumoral inflammation including TILs and histiocytes (graded 0 to 3, see text) multiplied by the percent B7-H1+ inflammatory cells.
A similar analysis was conducted for expression of B7-H1 in infiltrating host inflammatory cells (TILs and associated macrophages/histiocytes), as expressed by the adjusted inflammation score (AIS; density of inflammation multiplied by the percent B7-H1 expression by infiltrating host inflammatory cells) (Fig. 3B). Only true intratumoral immune infiltrates, and not perivascular inflammation, were recorded in the AIS. A threshold AIS of 5 was chosen to parallel the threshold designated for melanocytes. Seventy-seven of 150 specimens (51%) exhibited an AIS of <5, reflecting either absent/scant infiltrating immune cells or absent/scant B7-H1 expression on infiltrating cells. Seventy-three of 150 (49%) samples, including nevi, demonstrated an AIS of ≥5, and 51% (56 of 110) of malignant lesions were above this threshold. Therefore, similar to findings with B7-H1 expression by melanocytes, the AIS did not correlate with the malignant potential of the lesion.
Association of B7-H1 expression by melanocytes with immune infiltrates
To explore a potential association between B7-H1 expression by melanocytes and the geographic proximity of infiltrating immune cells (Figs. 1, B and C, and 2 and figs. S1 and S2), we compared the degree of B7-H1 cell surface (membranous) expression based on IHC (negative, defined as <5% positive tumor cells; positive, ≥5%) to the intensity of associated infiltrates (Table 2). TILs and associated macrophages/histiocytes were scored as mild (rare), moderate (focal), or severe (diffuse), based on hematoxylin and eosin (H&E) staining. We found that 35% (14 of 40) of benign nevi (2 inflamed Spitz nevi, 2 severely dysplastic nevi, and 10 halo nevi) expressed B7-H1 (mean melanocyte expression in positive samples, 30%; range, 10 to 70%). Notably, 100% (14 of 14) of the B7-H1+ cases were associated with at least mild TILs compared to only 15% (4 of 26) of B7-H1− cases (P < 0.0001).
Table 2.
Correlation of B7-H1 expression by melanocytes with the presence of immune cell infiltration.
| Histology | Total | Number of cases/total cases (%) | P* | |||
|---|---|---|---|---|---|---|
| B7-H1+† | B7-H1− | |||||
| TIL+‡ | TIL− | TIL+ | TIL− | |||
| Benign nevi | 40 | 14/14 (100) | 0/14 (0) | 4/26 (15) | 22/26 (85) | <0.0001 |
| Primary melanomas (in situ or invasive) | 54 | 19/19 (100) | 0/19 (0) | 15/35 (43) | 20/35 (57) | <0.0001 |
| Metastases | 56 | 23/24 (96) | 1/24 (4) | 7/32 (22) | 25/32 (78) | <0.0001 |
| All | 150 | 56/57 (98) | 1/57 (2) | 26/93 (28) | 67/93 (72) | <0.0001 |
Fisher’s exact test, two-sided, was conducted on the 2 × 2 matrix defined by B7-H1 (±) expression and TIL (±) for each lesion type.
More than 5% melanocytes with membranous expression on IHC.
Including mild, moderate, and severe lymphocyte infiltrates and their associated histiocytes/macrophages.
Similarly, 35% (19 of 54) of malignant primary lesions (in situ and invasive melanomas) were B7-H1+ (mean melanocyte expression, 14%; range, 5 to 50%), and 100% (19 of 19) of the positive cases were associated with at least mild TILs compared to 43% (15 of 35) of B7-H1− cases (P < 0.0001). Among metastatic melanoma lesions, 43% (24 of 56) were B7-H1+ (mean melanoma cell expression in positive samples, 15%; range, 5 to 60%), and 96% of those samples (23 of 24) were associated with at least mild TILs compared to 22% (7 of 32) of B7-H1− cases (P < 0.0001). In total, we found that 98% (56 of 57) of B7-H1+ cases were associated with at least mild TILs compared to 28% (26 of 93) of B7-H1− cases (P < 0.0001).
When all 150 specimens were analyzed, B7-H1 expression of ≥5% was significantly associated with the presence of inflammatory infiltrates (P < 0.0001, Fisher’s exact test) (Table 2). This association was confirmed in subset analyses for nevi, primary in situ and invasive melanoma, and metastatic disease. The degree of the inflammatory infiltrate (mild, moderate, severe) did not vary by melanoma stage (in situ, invasive, or metastatic) (P = 0.60, Fisher’s exact test) but was significantly correlated with the amount of B7-H1 expression by tumor (Spearman’s rho = 0.71, P < 0.001). These data demonstrate a strong association between B7-H1 expression and immune cell infiltration in both nevi and malignant melanoma.
We also assessed whether immune infiltrates associated with melanocytes expressed B7-H1, as indicated by the AIS. By univariate analysis, the AIS was highly associated with B7-H1 expression by all types of melanocytic lesion studied (P < 0.001). When analyzed by subtype of malignant lesion, this association was statistically significant for invasive primary melanomas (P < 0.001) and metastatic melanomas (P < 0.001) and had borderline significance for in situ disease (P = 0.08). Therefore, when a melanocytic lesion is associated with a host immune response, both components are likely to express B7-H1.
IFN-γ detected at the interface of TILs and B7-H1+ tumor cells by laser capture microdissection and quantitative reverse transcription–polymerase chain reaction
The high correlation between TIL infiltration and B7-H1 expression by melanocytes among 150 lesions of various malignant potentials, and geographically within individual tumors, suggested that, as with inflamed tissues, B7-H1 expression was induced by inflammatory cytokines such as IFN-γ. To investigate the presence of IFN-γ–producing immune cells that could potentially drive B7-H1 expression in melanomas, we performed quantitative reverse transcription–polymerase chain reaction (qRT-PCR) for the leukocyte common antigen CD45 (protein tyrosine phosphatase receptor type C) and IFN-γ in laser capture microdissection (LCM)–dissected specimens from both B7-H1− and B7-H1+ tumors at the interface with TILs (Table 3). Fresh peripheral blood mononuclear cells (PBMCs) and activated T cells, and cultured melanoma cells, were used as positive and negative controls for IFN-γ and CD45 expression, respectively. Although CD45 mRNA was detected by qRT-PCR in all five B7-H1− specimens examined, indicating the presence of hematopoietically derived cells, IFN-γ was not detected in these specimens. In contrast, both CD45 and IFN-γ were detected in all five B7-H1+ tumors. These results strongly support the notion that functional production by TIL of IFN-γ, a cytokine shown to rapidly induce B7-H1 expression by melanoma cells (fig. S3), is an important inducer of B7-H1 in melanomas in vivo.
Table 3.
Specific expression of IFN-γ in B7-H1+ melanomas. IFN-γ was detected by qRT-PCR as described in Materials and Methods. These results are representative of two separate experiments. UD, undetectable (CT greater than 45 cycles); NA, not applicable.
| Patient number | Tissue source |
CT (mean)* |
SEM† |
CT (mean) |
SEM |
|---|---|---|---|---|---|
| B7-H1− tumors | |||||
| 1 | Lymph node metastasis | UD‡ | NA | 33.19 | 0.45 |
| 2 | Small bowel metastasis | UD§ | NA | 34.46 | 0.38 |
| 3 | Lymph node metastasis | UD | NA | 32.93 | 0.11 |
| 4 | Lymph node metastasis | UD | NA | 35.53 | 0.28 |
| 5 | Cutaneous primary lesion | UD | NA | 36.11 | 0.13 |
| B7-H1+ tumors | |||||
| 6 | Lymph node metastasis | 34.67 | 0.49 | 32.41 | 0.16 |
| 7 | Lymph node metastasis | 35.00 | 0.32 | 33.55 | 0.36 |
| 8 | Lung metastasis | 33.15 | 0.30 | 31.91 | 0.78 |
| 9 | Lung metastasis | 31.80 | 0.07 | 28.43 | 0.02 |
| 10 | Lymph node metastasis | 35.17 | 0.22 | 32.51 | 0.22 |
| Controls | |||||
| NA | PBMCs | 33.12 | 0.35 | 28.55 | 0.66 |
| NA | Activated CD3+ T cells | 23.60 | 0.08 | 28.33 | 0.11 |
| NA | 1359-mel | UD | NA | UD | NA |
Lower values reflect greater expression.
SEM of triplicate samples.
One of three triplicate reactions for patient 1 tumor had CT = 41.7, and two of three were undetectable and average undetectable.
One of three triplicate reactions for patient 2 tumor had CT = 43.1, and two of three were undetectable and average undetectable.
Association of B7-H1 expression by melanocytes and host infiltrating cells, with melanoma subtypes, Breslow thickness, pT stage, and TNM stage
To gain further insights into the clinical biology of B7-H1 expression in melanoma, we examined the relationship between B7-H1 expression patterns and tumor subtype and stage. Forty-five percent (13 of 29) of superficial spreading and nodular melanomas were B7-H1+ (mean melanocyte expression, 16%), whereas other subtypes, including lentigo maligna melanoma (n = 3), acral lentiginous melanoma (n = 7), and desmoplastic melanoma (n = 4), were B7-H1−, suggesting an association of B7-H1 expression with histologic subtype (P = 0.033, Fisher’s exact test).
When the Breslow thickness of primary invasive melanomas was examined, there was no correlation with B7-H1 expression by univariate analysis (P = 0.23, Wilcoxon rank-sum test). In addition, for 54 in situ or invasive primary melanoma lesions examined, B7-H1 expression did not vary by pathologic primary tumor stage (T1 to T4) or the highest clinicopathologic TNM stage of the patient during a median 8-year follow-up (P = 0.43 and 0.33, respectively, Fisher’s exact test, one-sided) (table S1), indicating that B7-H1 expression is not linked to melanoma stage. Furthermore, the AIS for primary tumors did not associate with Breslow thickness (P = 0.30), pathologic tumor stage, or clinicopathologic TNM stage (P = 0.64 and 0.66, respectively). When B7-H1 expression in metastases was analyzed by anatomic location, there was no association between expression and location in lymph nodes, subcutaneous tissue, lung, or other visceral sites (P = 0.516, Fisher’s exact test, one-sided).
Association of B7-H1 expression in melanomas with overall patient survival
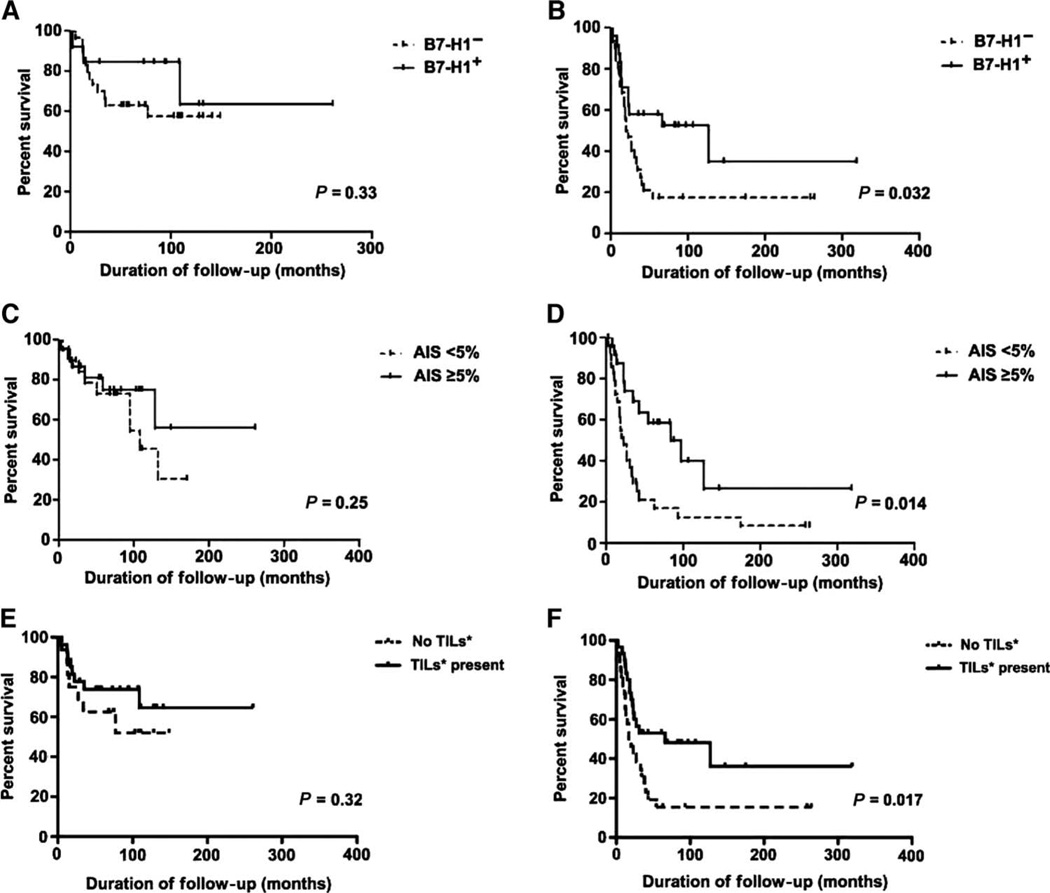
If indeed B7-H1 expression marked the existence of an active antitumor response, it might be predicted that, even though this resistance mechanism ultimately allowed tumors to persist, tumor growth might be partially restrained, leading to improved survival relative to patients with little antitumor response (and hence no B7-H1 expression). We therefore performed a survival analysis for the 99 patients with primary invasive or metastatic melanoma included in our study. As described above, the clinicopathologic factors such as Breslow depth, pathologic T stage, and TNM stage were not associated with B7-H1 expression by univariate analysis; thus, a multivariate analysis was not indicated. Among 43 patients who had primary invasive melanoma, those whose melanoma cells expressed B7-H1 showed no significant difference in overall survival when compared to those with B7-H1− lesions (P = 0.33, Fig. 4A). In contrast, there was a significant difference in survival for 56 patients with metastatic melanoma, where B7-H1 expression by tumor cells (≥5%) correlated with improved survival (P = 0.032, Fig. 4B).
Fig. 4.
Correlation of overall patient survival with B7-H1 expression in melanomas. (A) There was no significant difference in survival related to melanoma cell B7-H1 expression in 43 patients with primary invasive melanomas (P = 0.33). (B) However, among 56 patients with metastatic disease, those whose metastatic tumors expressed B7-H1 had significantly improved survival compared to the B7-H1− cohort (P = 0.032). Similar results were seen in analyzing the AIS, representing B7-H1 expression in lymphohistiocytic tumor infiltrates, as well as in analyzing the presence of TILs (mild, moderate, and severe lymphocyte infiltrates with associated histiocytes/macrophages). (C and E) No correlation of AIS or TILs was observed with survival in patients with invasive primary melanomas [P = 0.25 (C) and 0.32 (E)]. (D and F) However, there was a significant correlation in patients with metastatic disease [P = 0.014 (D) and 0.017 (F)]. B7-H1+, ≥5% of tumor cells with membranous expression; B7-H1−, <5% membranous tumor cell expression.
Because of the association between B7-H1 expression and inflammatory infiltrates, we examined the relationship of the AIS to melanoma patient survival. By univariate analysis, patients with primary melanomas with an AIS of ≥5 demonstrated no significant difference in survival when compared to those with a score of <5 (P = 0.25, Fig. 4C). However, significantly improved overall survival was noted for patients whose metastases were associated with an AIS of ≥5 (P = 0.014, Fig. 4D). When the impact of TILs alone on overall survival was determined, a similar result was obtained. Patients with primary melanomas demonstrated no difference in survival as related to the presence or absence of immune infiltrates (P = 0.32, Fig. 4E), whereas improved survival was seen in patients with metastatic disease who had TILs present (P = 0.017, Fig. 4F). The close parallels among the influence of TILs, the AIS, and tumor cell B7-H1 expression on survival are consistent with the hypothesis that these factors are interrelated and may influence patient outcomes.
The correlation of B7-H1+ tumor cells and AIS scores of ≥5 with improved survival for patients with metastatic, but not localized, melanoma may reflect the subsequent therapies received by these patients. Among the 56 patients with metastatic melanoma (stage III or IV) in this study, treatment records were available for 53. Forty-three percent of these patients (23 of 53) received some form of systemic immunotherapy [IFN-α, high-dose interleukin-2 (IL-2), vaccine, anti–PD-1 mAb, or combinations thereof] after their diagnostic biopsy demonstrating metastatic disease. Among those patients whose tumors were B7-H1−, 8 of 22 (36%) received immunotherapy compared to 15 of 31 (48%) with B7-H1+ tumors. These differences were not significant (P = 0.415), suggesting that patients with “inflamed” tumors expressing B7-H1 might be more likely to respond to immunotherapy.
The potential impact of high-aggregate B7-H1 expression on overall survival was examined in 99 patients with invasive or metastatic melanoma lesions. There was no difference in survival for patients whose tumors demonstrated >20% B7-H1 expression on melanoma cells and/or an AIS of >50 compared to those with lower-aggregate B7-H1 expression (P = 0.58). Together, these findings validate our selection of expression thresholds by tumor cells and infiltrating immune cells, and indicate that even focal B7-H1 expression in the immediate environment of metastatic melanoma is associated with improved survival.
DISCUSSION
Our study reveals a highly significant concordance of membranous expression of B7-H1 by nevus and melanoma cells with the presence of immune infiltrates and provides direct evidence of geographic colocalization. Furthermore, expression of the inflammatory cytokine IFN-γ, known to be a primary cytokine driving B7-H1 expression, was detected specifically at the interface of B7-H1+ tumor cells and infiltrating immune cells, but not in B7-H1− tumors. These findings suggest that infiltrating immune cells can produce factors driving B7-H1 expression as a negative feedback mechanism, resulting in what may be considered adaptive immune resistance exerted by the tumor. This mechanism is distinct from the conventional view of immune resistance by either immune escape via mutation of tumor-specific epitopes (31, 32) or constitutive oncogene-driven up-regulation of immune-inhibitory ligands (33). IFN-γ is secreted by human melanoma–specific T lymphocytes, and this cytokine rapidly induces B7-H1 in cultured melanomas. Although our studies associate IFN-γ production with B7-H1+ melanomas in vivo, other secreted factors in the tumor microenvironment such as IL-10, IL-6, and common γ-chain cytokines may also be involved (34, 35). These findings help to explain why immunogenic melanoma fails to be controlled by the immune system, even when boosted by antigen-based cancer vaccines, and implicate a new immune escape mechanism in the cancer microenvironment.
The up-regulation of B7-H1 in the cancer microenvironment should not be interpreted simply as the dominance of immune suppression because this up-regulation is also associated with endogenous inflammatory immune responses. The balance of the host’s immune response and negative feedback inhibition will therefore determine outcome. We did not detect a significant correlation between overall survival and B7-H1 expression in patients with primary invasive melanomas. In contrast, we documented a significantly improved overall survival in patients with B7-H1+ metastatic lesions. With the exception of one published report in cervical cancer (36), this finding is different from a number of reports from other tumor types that have claimed B7-H1 expression as a negative overall prognostic factor (24–26).
This seemingly paradoxical observation, in which expression of an immunosuppressive molecule correlates with improved outcomes, may be resolved if B7-H1 expression is viewed as reflecting the presence of endogenous antitumor immunity (infiltrating lymphocytes producing cytokines in response to tumor-specific or tumor-selective antigens) among melanoma patients who are candidates for immunotherapy. Indeed, 43% of the patients with metastatic disease in our study received some form of immunotherapy after diagnostic tumor biopsy, including IFN-α, high-dose IL-2, vaccine, PD-1 blockade, or combinations thereof. A growing literature suggests the positive correlation of an inflamed tumor microenvironment with response to immunotherapies such as cancer vaccines or ipilimumab (37, 38), and our findings further suggest that these immunotherapies should be interpreted as protection or potentiation of ongoing immunity rather than the generation of de novo immunity against cancer. Note that medical center–specific treatment practices may be critical to these observations. Indeed, about one-third of patients with metastatic melanoma show evidence of objective tumor regressions in response to anti–PD-1 therapy (39). Ongoing studies in our laboratories are investigating whether tumor regressions in this series of patients correlate with tumor cell B7-H1 expression and the presence and type of TILs.
Although intratumoral lymphocytes are a common feature in many different types of cancer, the prognostic significance of this finding varies by tumor type. For example, TILs are associated with a better prognosis in patients with colorectal, ovarian, pancreatic, esophageal, and small-cell lung carcinoma (40–44), but are an adverse prognostic feature in patients with renal cell carcinoma (45). Notably, in colon cancer, the type, density, and location of immune infiltrates have been shown to be a better predictor of survival than TNM classification (46). In melanoma, TILs may be seen in all stages of disease, but are not prognostically significant in early stages, that is, in in situ or radial growth phase melanomas; they do reach significance in vertical growth phase melanomas and those that have metastasized (47, 48). Our findings provide an interpretation for these seemingly contradictory observations. Although the presence of TILs may indicate immunogenicity of tumors and ongoing immune responses, clinical outcomes may depend on B7-H1 expression in addition to other factors such as composition of infiltration, persistence of inflammation, and the treatment received.
We observed only rare cases showing diffuse tumor cell B7-H1 expression, unrelated or out of proportion to TILs. These may represent melanomas that have developed intrinsic pathways driving B7-H1 gene expression. PTEN (phosphatase and tensin homolog deleted from chromosome 10) deficiency has been shown to support posttranscriptional B7-H1 expression in gliomas (34), but factors driving constitutive expression in melanoma have not been defined. Distinct histologic subtypes of melanoma are associated with characteristic tumorigenic signaling pathways: for example, superficial spreading and nodular melanomas are more likely to have activating BRAF mutations than acral lentiginous melanomas, which are often associated with c-Kit mutations. In our study, 45% of superficial spreading and nodular melanomas expressed B7-H1, whereas other subtypes including acral lentiginous melanoma, lentigo maligna melanoma, and desmoplastic melanoma did not, suggesting the presence of distinct signaling pathways. Studies focused on the transcriptional regulation of B7-H1 expression in nodular and superficial spreading melanomas and metastatic lesions, as well as studies of cytokine expression profiles in microdissected boundary areas between tumor cells and TILs, are currently under way in our laboratories to explore these issues. Notably, the correlation between IFN-γ and B7-H1 expression was 100% among the microdissected samples tested. However, roughly one-third of lesions with observable TIL (on IHC and/or H&E staining) did not express B7-H1, suggesting that a T helper 1 (TH1) pattern of cytokine expression characterized by IFN-γ may be needed to induce B7-H1 expression.
The clinical significance of B7-H1 expression in melanoma has not yet been firmly established. Two reports have been published to date, with conflicting results (49, 50), perhaps because of different histologic subtypes studied or methodologies used. Gadiot et al. used the polyclonal antibody 4059 for IHC detection, scored a 1% threshold of expression by melanocytes as a positive result, and did not distinguish membranous and cytoplasmic patterns of cellular expression. In that study, most patients had superficial spreading or nodular-type melanomas, and B7-H1 expression by melanocytes was associated with a trend toward better survival. In contrast, Hino et al. used mAb clone 27A2 for IHC and compared “high” to “low” cytoplasmic expression based on automated color density measurements. Most patients had the acral lentiginous subtype of melanoma, which is associated with c-Kit mutations, rather than with BRAF mutations, which predominate in cohorts of European descent (51, 52). Hino et al. reported high-intensity B7-H1 expression as an independent risk factor for decreased survival. Both studies had about a 4- to 5-year median follow-up time. Neither reported the geographic association of B7-H1 expression with TILs or B7-H1 expression by other cells in the tumor microenvironment.
Both cytoplasmic and membranous B7-H1 staining have been reported in multiple tumor types; however, B7-H1 is a type I transmembrane molecule (6). Cytoplasmic staining may represent intracellular stores of B7-H1, which may be deployed to the cell surface depending on appropriate stimulation. Our previous study describing B7-H1 expression in various human cancers included a small sample size of melanomas (n = 22), among which all were deemed at least focally positive in frozen sections (5); however, the previous study assessed both cytoplasmic and membranous positivity, whereas the current study assesses only membranous expression. We hypothesize that cell surface expression of B7-H1 is the most immediately biologically relevant as a potential biomarker predicting clinical response to PD-1 blockade (29).
These findings highlight a therapeutic opportunity in administering agents blocking the PD-1/B7-H1 pathway to patients with metastatic melanoma. B7-H1 is a critical immunomodulating component within the melanoma microenvironment. Expression of B7-H1 on tumor cells at the interface with immune infiltrates, in comparison to constitutive tumor expression in areas devoid of infiltrates, may represent distinct underlying immune resistance mechanisms and warrant further study on the molecular level. A deeper understanding of mechanisms driving the observed expression patterns is essential for developing rational treatment combinations with mAbs blocking PD-1 or B7-H1, which are already in clinical trials, and other potentially synergistic therapeutic agents.
MATERIALS AND METHODS
Case selection
After Johns Hopkins Institutional Review Board approval, 150 melanocytic lesions of various histologic subtypes and stages were identified from 150 individual patients through a search of the Johns Hopkins Hospital surgical pathology archives. Nevi of various histologic subtypes (n = 40, including 10 common, 2 blue, 13 halo, 8 Spitz, and 7 dysplastic nevi), melanomas in situ (n = 11), invasive melanomas of different histologic subtypes (n = 43, including 14 superficial spreading, 15 nodular, 3 lentigo maligna, 4 desmoplastic, and 7 acral lentiginous melanomas), and metastases to various sites (n = 56, including 34 lymph node, 8 cutaneous/soft tissue, 6 lung, and 8 other visceral sites) were evaluated.
Clinical and pathologic features
The original histologic diagnosis was confirmed on archival H&E-stained slides, and pathologic features such as histologic subtype, Breslow thickness, and pathologic T stage were collected. The pathologic T stage is based on microstaging of the primary tumor and includes the parameters of Breslow thickness, ulceration, and mitotic activity. A single representative paraffin block for each case was chosen. Where necessary, cases were restaged to current American Joint Committee on Cancer criteria (53). The highest TNM stage and overall survival data were obtained for each patient. For patients with metastatic disease, information on treatment regimens was gathered. The duration of follow-up was calculated from the date of the diagnostic procedure to the date of cancer progression, last follow-up, or death.
Immunohistochemistry
For the purpose of this retrospective study, archival FFPE specimens were cut into 5-µm sections and mounted on glass slides. IHC for B7-H1 was performed with a murine anti-human B7-H1 mAb [clone 5H1, isotype mouse IgG1 (immunoglobulin G1)] (5) at a concentration of 2 µg/ml according to a standard protocol. See the Supplementary Materials and fig. S4 for additional details. IHC for B7-H1 was also performed on select slides with a rabbit polyclonal antibody to B7-H1 designated 4059 (ProSci) as previously described (50). Additional details about 4059 are provided in figs. S5 and S6. IHC for select cell lineage markers including CD3, S100, and CD68 for T cells, melanocytes, and macrophages, respectively, was performed on adjacent 5-µm sections according to standard automated protocols.
Quantification of B7-H1 expression by melanocytes/melanoma cells and the associated host inflammatory response
Percentages of tumor cells exhibiting a membranous staining pattern for B7-H1 were independently quantified as <5%, 5 to 9%, and then in 10% increments up to 100% by two pathologists (J.M.T. and R.A.A.) who were blinded to patient outcomes. Differences in scoring were adjudicated. The host response to tumor, composed predominantly of lymphocytes with scattered histiocytes (tissue macrophages, dendritic cells, and Langerhans cells), referred to collectively as “immune cell infiltrates,” was also scored for percent B7-H1 membranous expression. The intensity of the infiltrate was graded as none (0), mild (score of 1, rare lymphocytes), moderate (2, focal infiltration of tumor by lymphohistiocytic aggregates), or severe (3, diffuse infiltration). An AIS was defined as the intensity of intratumoral inflammation multiplied by the percent of inflammatory cells expressing B7-H1 (27). B7-H1 expression by inflammatory cells that were not immediately associated with melanocytic cells in a given tumor section was not included in calculating the AIS. Percent B7-H1 expression by tumor cells and the AIS, reflecting B7-H1 in the inflammatory host response, as well as the presence of TILs were correlated with overall patient survival.
LCM and qRT-PCR for IFN-γ
Specimens were cut into 7-µm sections on Arcturus PEN membrane glass slides (Applied Biosystems). LCM was performed with a Leica LMD6000 Laser Capture Microdissection microscope. In melanoma biopsies that were B7-H1−, central areas containing nonnecrotic tumor cells were captured. In tumors that were B7-H1+, tumor cells were excised along with associated infiltrating immune cells (fig. S7). RT-PCR was performed with primer/probe preparations for either human IFN-γ or CD45, according to standard protocols. Triplicate CT averages and SEs of the mean are reported. See the Supplementary Materials for additional details.
Western blotting
537-mel was cultured in the presence or absence of IFN-γ (500 IU/ml) for 3 days. Lysates of whole cells as well as fractions of cytosolic/nuclear proteins and membrane proteins were prepared with M-PER and MEM-PER Reagent, respectively (Pierce). Cell lysates (10 µg of total protein per lane) were separated by 10% bis-tris SDS-PAGE (polyacrylamide gel electrophoresis) under reducing conditions (Invitrogen). Human IgG1 (Sigma) and recombinant B7 Fc chimeric proteins (B7.1, B7.2, B7-H1, and B7-DC from R&D Systems Inc.) were loaded at 300 ng per lane and resolved by 4 to 12% bis-tris SDS-PAGE under reducing conditions. Proteins were transferred to a polyvinylidene difluoride membrane, which was blocked for 1 hour with 5% milk in tris-buffered saline–Tween 20. Membranes were incubated with either rabbit anti-human B7-H1 polyclonal antibody (ProSci 4059) or mouse anti-human B7-H1 mAb (clone 5H1) (0.5 µg/ml) at 4°C overnight. The secondary antibody was either anti-rabbit IgG-HRP (horseradish peroxidase) (1:10,000 dilution) or anti-mouse IgG-HRP (1:5000), respectively. Proteins were detected by the ECL Plus Chemiluminescent Detection kit (GE Healthcare).
Statistical analysis
Overall survival was calculated from the date of the diagnostic biopsy to the date of last follow-up or death with the Kaplan-Meier method and analyzed with the log-rank test. Associations of B7-H1 expression and clinicopathologic features were evaluated with Fisher’s exact test, Student’s t tests, and χ2 tests. Statistical analyses were performed with the STATA V11 software package. All tests were two-sided except as indicated, and P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank R. Li, C. Umbricht, Y. Liu, and F. Housseau for helpful discussions, as well as J. Califano and J. Handa for their support (all Johns Hopkins University School of Medicine). Funding: This study was supported by grants from the National Cancer Institute (CA016359, CA97085, and CA85721 to L.C.), the NIH (R01DK080736 and R01DK081417 to R.A.A.), the Melanoma Research Alliance (to D.M.P., S.L.T., and L.C.), the Barney Family Foundation (to S.L.T.), the Michael Rolfe Foundation for Pancreatic Cancer Research (to R.A.A.), and the Dermatology Foundation (to J.M.T.).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/4/127/127ra37/DC1
Materials and Methods
Fig. S1. Geographic patterns of CD3+ TILs corresponding to the patterns of B7-H1 expression in cases shown in Fig. 1.
Fig. S2. Immunohistochemical characterization of cell types and architecture at the interface of B7-H1 expression and immune infiltrates in a melanoma lesion.
Fig. S3. Kinetics of B7-H1 induction by IFN-γ in cultured human melanoma cells.
Fig. S4. Comparison of B7-H1 detection in fresh and FFPE tissues using mAb 5H1.
Fig. S5. Comparison of B7-H1 detection by the mAb 5H1 versus the polyclonal antibody 4059.
Fig. S6. Comparative specificities of anti–B7-H1 mAb 5H1 and polyclonal antibody 4059 by Western blotting.
Fig. S7. B7-H1+ tumor and associated TILs sampled by laser capture microdissection.
Table S1. B7-H1 expression by melanocytes and infiltrating immune cells in 54 in situ and invasive primary melanomas does not correlate with pT or TNM stage.
Author contributions: J.M.T., R.A.A., G.D.Y., S.C., D.M.P., S.L.T., and L.C. conceived and designed the experiments. J.M.T., R.A.A., G.D.Y., H.X., R.S., T.L.M., and S.C. performed the experiments. J.M.T., R.A.A., G.D.Y., T.L.M., S.C., A.P.K., D.M.P., S.L.T., and L.C. analyzed the data. J.M.T., D.M.P., S.L.T., and L.C. wrote the manuscript.
Competing interests: S.L.T. is a consultant to (uncompensated) and S.L.T., J.M.T., R.A.A., and H.X. receive research support from Bristol-Myers Squibb. The 5H1 antibody (made in L.C.’s laboratory, U.S. 7,797,710 and U.S. 7,892,540) will be distributed via a standard University Material Transfer Agreements for research, which is consistent with Science Translational Medicine’s material sharing policy. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Benlalam H, Labarrière N, Linard B, Derré L, Diez E, Pandolfino MC, Bonneville M, Jotereau F. Comprehensive analysis of the frequency of recognition of melanoma-associated antigen (MAA) by CD8 melanoma infiltrating lymphocytes (TIL): Implications for immunotherapy. Eur. J. Immunol. 2001;31:2007–2015. doi: 10.1002/1521-4141(200107)31:7<2007::aid-immu2007>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2011;78:112–126. doi: 10.1016/j.critrevonc.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 4.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 7.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian ST, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J. Clin. Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzales R, Robert C, Schadendorf D, Hassel JC, Akerly W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 12.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte–associated antigen 4. J. Clin. Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 15.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 16.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki T, Akiba H, Iwai H. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 19.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand-interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 21.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2011;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 23.Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J. Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin. Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 31.Schrieber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 33.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 34.Wölfle SJ, Strebovsky J, Bartz H, Sähr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 35.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 36.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin. Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: A potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 38.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 2011 doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sznol M, Powderly JD, Smith DC, Brahmer JR, Drake CG, McDermott DF, Lawrence DP, Wolchok JD, Topalian SL, Lowy I. Safety and antitumor activity of biweekly MDX-1106 (anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies [abstract 2506] J. Clin. Oncol. (Meet. Abstr.) 2010;28:15s. [Google Scholar]
- 40.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Eerola AK, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin. Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 43.Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 44.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 45.Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML, Cheville JC, Kwon ED. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107:46–53. doi: 10.1002/cncr.21951. [DOI] [PubMed] [Google Scholar]
- 46.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 47.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab. Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 49.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 50.Gadiot J, Hooijkaas AI, Kaiser ADM, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117:2192–2201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 51.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 52.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 53.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.