Abstract
Ducts of Luschka are a developmental abnormality found within the gallbladder fossa in up to 10% of cholecystectomy specimens. They are most often encountered by surgeons when injured during laparoscopic or open cholecystectomy, leading to bile leakage and subsequent peritonitis. Histologically, they are typically composed of lobular aggregates of small ductules lined by bland, cuboidal-to-columnar biliary-type epithelium, associated with centrally located, larger ductules surrounded by concentric fibrosis. We have identified 6 cases of florid Luschka duct proliferation in which the ductules demonstrated irregular growth pattern, loss of characteristic concentric fibrosis, and epithelial atypia that strongly suggested the diagnosis of invasive pancreatobiliary adenocarcinoma or metastatic adenocarcinoma involving the gallbladder serosa. Two of the cases were initially diagnosed as invasive adenocarcinoma, whereas the other 4 were sent for consultation to rule out adenocarcinoma. All cases were associated with marked acute and chronic cholecystitis with mucosal ulceration, cholelithiasis, and thickening of the gallbladder wall. The ducts of Luschka were located within the rim of adherent liver in all 6 cases and the gallbladder serosa in 5 cases. Limited follow-up information was available for all patients with no documentation of progressive disease. Awareness and proper recognition of the anatomic location and histologic features are imperative in distinguishing florid ducts of Luschka from both non-neoplastic conditions and most importantly adenocarcinoma.
Keywords: Luschka ducts, gallbladder, adenocarcinoma, cholangiocarcinoma, mimic
Ducts of Luschka represent a common developmental variant in gallbladder anatomy, present within the perimuscular connective tissue adjacent to the liver and/or less frequently along the serosal surface.3,19 They exist as either a single duct or network of ducts that communicate with intrahepatic bile ducts and possibly the gallbladder.3 Histologically, they are typically characterized by lobular aggregates of ductules that may vary in size. Centrally located larger ductules are lined by a columnar biliary epithelium surrounded by concentric rings of fibrous connective tissue. However, peripheral smaller ductules exhibit a more cuboidal epithelium and may lack the fibrous collar of larger ductules.1,6,17
We have observed that, when numerous and in a background of the marked inflammatory infiltrate of florid acute cholecystitis, the small ducts of Luschka may demonstrate reactive atypia that can be mistaken for invasive adenocarcinoma.16 This phenomenon is not well recognized by the published literature. The third edition of Armed Forces Institute of Pathology fascicle on Tumors of the Gallbladder Extrahepatic Bile Ducts and Ampulla of Vater states “when numerous, the small bile ducts that show reactive atypia may be mistaken for carcinoma”; however, it does not cite published references.3 Recently, a single case report in an internet pathology journal describes this issue, but whether a diagnosis of carcinoma was seriously considered in the reported case is not stated.16
The purpose of this study was to report 6 cases of florid ducts of Luschka, which mimicked invasive adenocarcinoma, and discuss the differential diagnosis.
MATERIALS AND METHODS
Study approval was obtained from The Johns Hopkins Hospital Medical Institutions Internal Review Board. The surgical pathology files of The Johns Hopkins Hospital were searched from the years 1995 to 2010 for cases of florid ducts of Luschka within the gallbladder. Three cases were identified, all of which involved cholecystectomy specimens. Three additional cholecystectomy cases were retrieved from the files of one author (N.V.A.). Overall, 4 cases were seen in consultation from other institutions and 2 were routine surgical cases. Patient demographic data, surgical pathology reports, and the hematoxylin and eosin-stained slides were reviewed for all cases. When present, immunohistochemical stains were also assessed. Clinical follow-up information was also obtained for all patients.
For comparison with the benign Lushka ducts, 21 cases of invasive well-to-moderately differentiated gallbladder adenocarcinoma were retrieved from the files of one author (P.A.). These cases represent a subset of a series of gallbladder carcinomas that have been described previously.15 For all cases of invasive adenocarcinoma, hematoxylin and eosin-stained slides were reviewed for the following features: haphazard growth pattern, incomplete glands, presence of desmoplasia, concentric fibrosis surrounding glands, variation in nuclear size by more than 4-to-1 in a single gland, mitotic figures, vascular invasion, perineural invasion, and extent of gallbladder wall involvement.
RESULT
Cases
The clinical and pathologic features of all 6 cases are summarized in Table 1. All 6 patients are white ranging in age from 50 to 89 years (mean, 72 y), and include 3 male and 3 female patients. All the patients presented clinically with the classic signs of acute cholecystitis, including right upper quadrant abdominal pain, nausea, and vomiting. Computed tomography and ultrasound examination revealed cholelithiasis in all cases; however, case 1 also had a mass in the head of the pancreas (see Follow-up section). All patients underwent laparoscopic cholecystectomy, and specimens were submitted for histopathologic examination.
TABLE 1.
Histologic Features of 6 Cases of Hyperplastic Ducts of Luschka
| Luschka Duct Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Age | Sex | Gallbladder Wall Thickness (cm) |
Calculi | Mucosal Ulceration |
Histologic Sections Submitted |
Serosa | Liver Resection Bed | Follow-up (mo) |
| 1 | 77 | F | 0.6 | Present | Present | 12 | Present | Present | NED (54)* |
| 2 | 70 | M | 0.7 | Present | Present | 20 | Present | Present | NED (19) |
| 3 | 50 | M | 0.5 | Present | Present | 14 | Absent | Present | NED (5) |
| 4 | 72 | M | 0.5 | Present | Present | 18 | Present | Present | NED (6) |
| 5 | 74 | F | 1.0 | Present | Present | 16 | Present | Present | NED (11) |
| 6 | 89 | F | 1.9 | Present | Present | 7 | Present | Present | NED (22) |
Radiographic studies were negative for disease outside of the pancreas.
F indicates female; M, male; NED, no evidence of disease.
Pathologic Findings
Grossly, the gallbladders demonstrated wall thickening within both the body and the fundus, ranging from 0.5 to 1.9 cm (mean, 0.9 cm). Multiple calculi of different sizes were found in all cases. No localized masses were present.
In all 6 cases, the gallbladder demonstrated florid acute and chronic cholecystitis with prominent fibrosis, ulceration, and microabscess formation. Regenerative changes were noted in the epithelium, but no evidence of dysplasia, metaplasia, or carcinoma was identified in these extensively sampled cases (mean of 15 sections per case). Rokitansky-Aschoff sinuses were present within the gallbladder wall in all cases, indicating a component of chronic cholecystitis.
Most notably, all of the gallbladders contained numerous small-to-medium-sized angulated ductules measuring 1 to 2mm in diameter. In all cases, the ductules were located within a thickened fibrous adventitia and did not involve the muscular wall, lamina propria, or mucosa. In all cases, a rim of adherent liver was present, and the ductules abutted the periphery of the liver parenchyma but were unassociated with portal triads. In 5 of 6 (83%) cases, the ductules also involved the gallbladder serosa.
At low magnification, the ductules were generally arranged in a linear manner along the subserosal surface or the liver bed, and in many areas, a lobular configuration could be appreciated (Figs. 1, 2). The ductules were generally lined by a cuboidal biliary-type epithelium, associated with larger ductules with more columnar epithelial lining within a dense concentric ring of fibroblasts. The majority of the cells lining the ductules had small, uniform nuclei with inconspicuous nucleoli, and the cytoplasm was abundant and eosinophilic. The lumens of the ductules lacked bile and were empty. These are the typical features of Luschka ducts. However, in some areas, particularly in those areas associated with ulceration, a lobular arrangement was less evident and the ductules appeared to irregularly infiltrate inflamed fibrous tissue. In these areas, the ductules demonstrated marked reactive atypia. These atypical ductules had enlarged vesicular nuclei with prominent nucleoli. Adding to the worrisome features, the typical concentric fibrosis seen around Luschka ducts was difficult to appreciate in these areas. Of note, variation in nuclear size by more than 4-to-1 within a single gland, mitotic figures, vascular invasion, and perineural invasion were all absent.
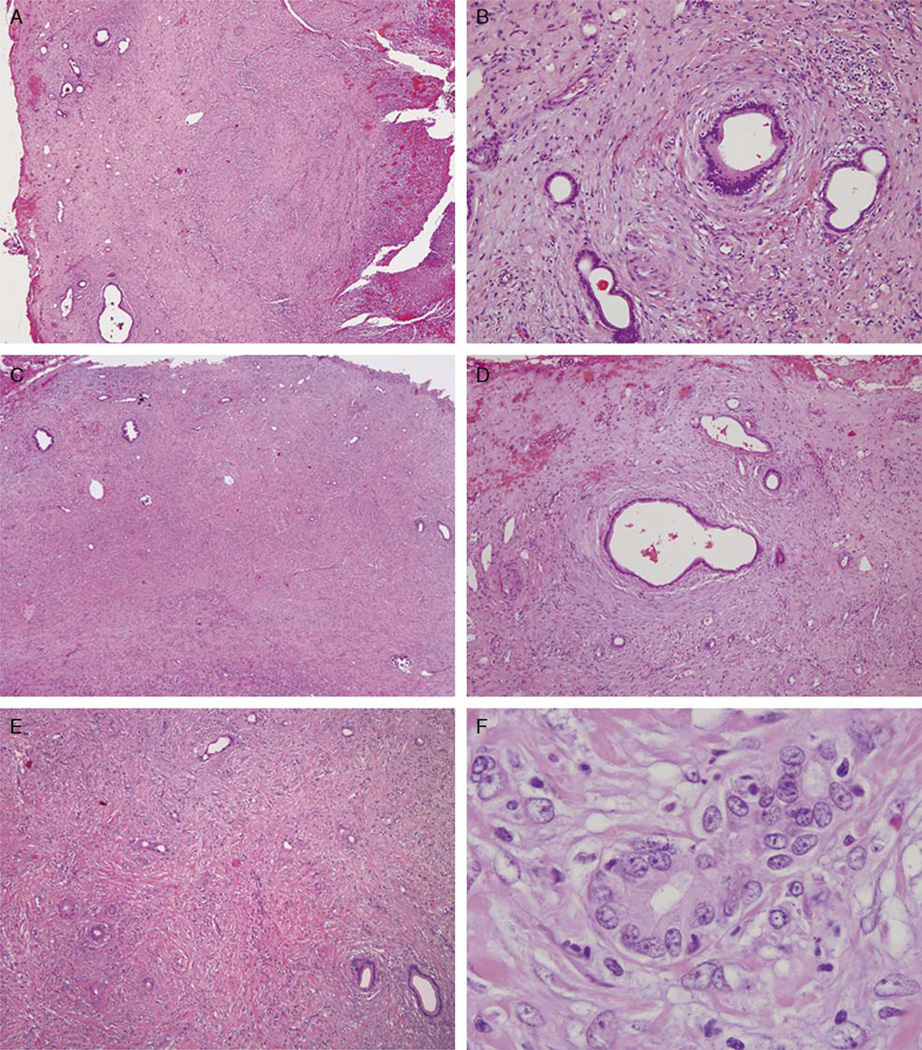
FIGURE 1.
Low-power view of the gallbladder (A) shows mucosal ulceration (right) and lobular aggregates of Luschka ducts in the subserosal soft tissue. At intermediate power (B), one can appreciate the bland cytology and concentric periductal fibrosis. Low-power view of another area in the gallbladder subserosa (C) shows usual Luschka ducts (left) merging with inflamed Luschka ducts showing florid reactive atypia (right). The more typical uninflamed Luschka ducts (D) maintain a lobular architecture and bland cytology. The inflamed Luschka ducts appear more disorganized in the inflamed nonconcentrically fibrotic stroma (E) and demonstrate significant cytologic atypia (F), best considered reactive.
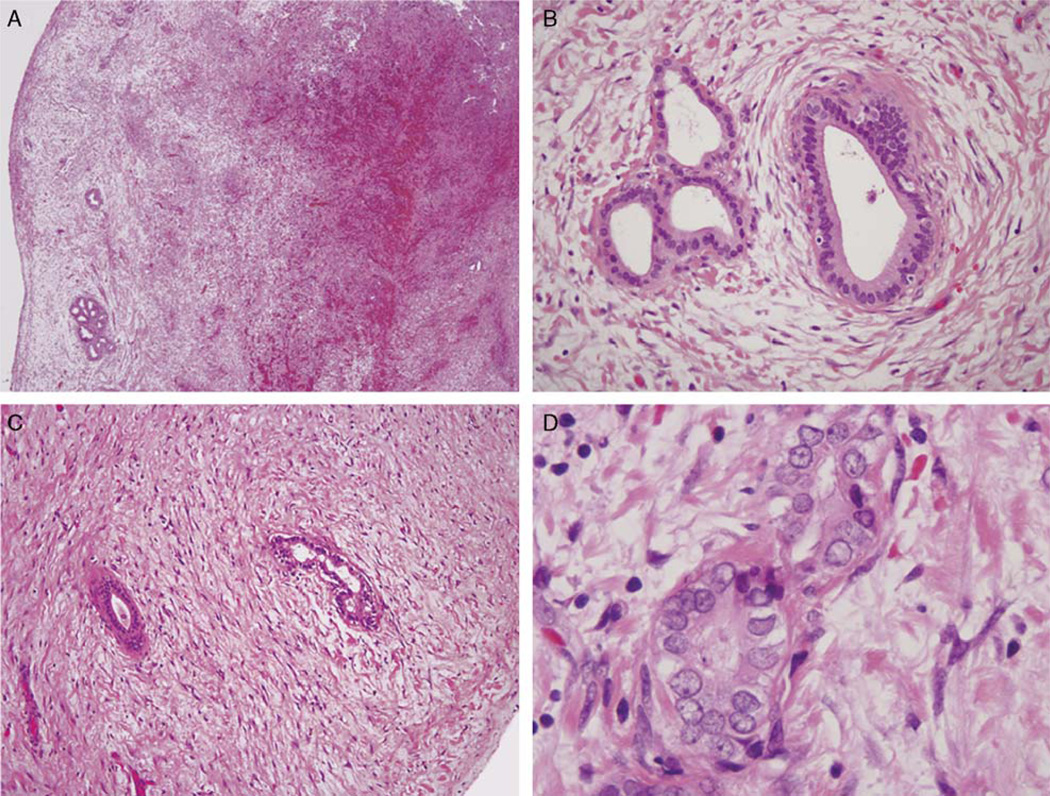
FIGURE 2.
At low power, the Luschka ducts appear as lobular aggregates of ducts within the gallbladder subserosa (A). At higher power magnification (B), the glands are surrounded by concentric fibrosis, maintain a lobular architecture, and demonstrate bland cytology. Individual fields of this case demonstrated distorting fibrosis (C) and mild cytologic atypia (D), which raised the possibility of neoplasia.
To better define the distinguishing features between Luschka ducts and adenocarcinoma of the gallbladder, we reviewed 21 cases of invasive well-to-moderately differentiated gallbladder adenocarcinomas from our previously published study15 for the following criteria: haphazard glandular growth pattern, incomplete lumen formation, presence of desmoplasia, concentric fibrosis surrounding glands, variation in nuclear size by more than 4-to-1 in a single gland, mitoses, vascular invasion, perineural invasion, and extent of gallbladder wall involvement. Haphazard growth pattern, incomplete glands, and desmoplasia were defining features in all cases (100%) of invasive gallbladder adenocarcinoma. Further, 19 of 21 (90%) cases involved the full thickness of the gallbladder wall. Mitoses, a 4-to-1 nuclear variation in a single gland, vascular invasion, and perineural invasion were identified in 90%, 71%, 71%, and 67% of cases, respectively. Interestingly, 3 of 21 (14%) cases demonstrated focal areas of concentric fibrosis surrounding individual cytologically malignant glands. These glands were also characterized by large, open lumens distinctly located within the gallbladder adventitia compared with the neighboring smaller, angulated glands found throughout the gallbladder wall. With careful consideration of the location and architecture, we favor that these glands represent cancerization of Luschka ducts. Thus, discriminating features of invasive gallbladder adenocarcinoma from Luschka ducts include an irregular growth pattern, incomplete glands, desmoplasia, and full-thickness gallbladder wall involvement. Other characteristics include mitotic figures, nuclear variation, vascular, and perineural invasion. In contrast, Luschka ducts demonstrate a lobular configuration of ductules confined to the gallbladder adventitia with larger ductules surrounded by a fibrous collar. The differences are summarized in Table 2.
TABLE 2.
Comparison of Histologic Features Between Luschka Ducts and Invasive Gallbladder Adenocarcinoma
| Histologic Features |
Luschka Ducts | Invasive Gallbladder Adenocarcinoma |
|---|---|---|
| Location in gallbladder wall | Adventitia only | Full thickness |
| Architecture | Lobular, linear | Haphazard |
| Fibrosis | Concentric | Irregular |
| Cytology | Reactive atypia associated with inflammation | Nuclear variation (4:1) within a single gland |
| Mitoses | Minimal/none | Usually present |
| Other findings | Vascular and perineural invasion |
Follow-up
In case 1, a laparotomy had been performed for a pancreatic mass. The gallbladder serosa was noted to be somewhat irregular; thus it was biopsied and sent for frozen section. The reactive ducts of Luschka were initially interpreted as metastatic adenocarcinoma given the history of a pancreatic mass and the absence of any histologic evidence of typical Luschka ducts, and the gallbladder was resected (Fig. 3). On permanent section, the architecture and cytology of the glands in the serosal biopsy were noted to be similar to those that were linearly arrayed in a more overtly lobular architecture with associated concentric fibrosis on the serosal surface of the resected gallbladder. Furthermore, immunohistochemical stains performed on the ductules present within the patient’s gallbladder demonstrated intact nuclear expression for DPC4 and absence of p53 overexpression. Thus, the immunoprofile supported a benign process within the gallbladder. The patient returned 2 months later for endoscopic ultrasound-guided biopsy of the pancreas. The biopsy revealed adenocarcinoma consistent with a pancreatic primary. However, disease outside the pancreas was not identified by radiographic studies.
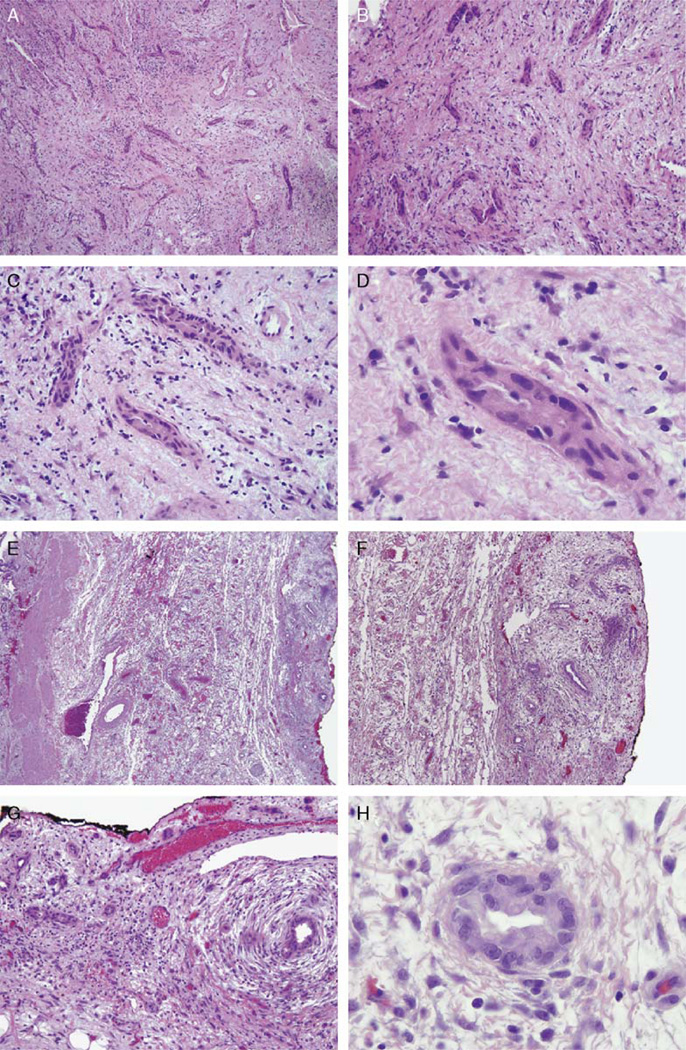
FIGURE 3.
The frozen section of the gallbladder fossa for case 1 shows a somewhat irregular proliferation of glands (A,B) with significant cytologic atypia (C,D) in a reactive stroma lacking concentric fibrosis, which suggested the diagnosis of metastatic adenocarcinoma involving the peritoneal cavity. The resected gallbladder specimen (E) contains similar glands now shown to be in a linear, lobular distribution along the gallbladder serosa (right), opposite the gallbladder lumen (left). These glands demonstrate concentric fibrosis (F) and show similar cytologic atypia (G,H). Given the stromal inflammation, these are consistent with as reactive Luschka ducts.
Case 2 was a gallbladder specimen submitted for consultation with the differential diagnosis of adenocarcinoma versus ducts of Luschka. As with case 1, immunostaining for p53 did not reveal overexpression, consistent with a benign diagnosis. Follow-up information was available 19 months after resection with no demonstration of neoplasia. Cases 3 and 4 were also cholecystectomy specimens submitted for consultation with the differential of adenocarcinoma versus benign. Although the follow-up interval for cases 3 and 4 were limited to 5 and 6 months, respectively, neither patient showed evidence of progressive disease.
Case 5 was a gallbladder specimen submitted for consultation with the preliminary diagnosis of adenocarcinoma. Case 6 was a routine surgical specimen, which was considered to be worrisome for cancer by the general surgical pathologist, and was thus sent for intradepartmental review. Neither patient showed further disease at last follow-up, 11 and 12 months, respectively. Hence, in limited follow-up, no patient in this study has developed progressive disease, supporting our interpretation of these lesions as benign.
DISCUSSION
In 1753, Ferrein, a human anatomist, initially identified the presence of bile ducts within the left lateral or triangular ligament of the liver. These ducts were later described by Weber in 1842 as “aberrant biliary ducts” located outside the hepatic parenchyma.19 However, it was not until 1863 that Luschka described these ducts in detail as “slender bile ducts running along the gallbladder fossa, draining into the right hepatic duct or common duct,” and thus these ducts were designated as ducts of Luschka.11,19 The ducts of Luschka are also known as accessory biliary ducts, vasa aberrantia, subvesicular ducts, or supravesicular ducts.
The ducts of Luschka are small biliary ducts, measuring up to 2mm in diameter, and typically originate within the right hepatic lobe. Microscopic examination shows that they may occur as a single duct or a meshwork of ductules. The ductules are lined by flattened-to-columnar biliary epithelium and are classically surrounded by a fibrous collar.6 However, the concentric fibrosis, which surrounds larger ductules, may be absent around smaller ductules. They run an intermediate course along the gallbladder bed, usually reaching the adventitia of the gallbladder. Often, they drain into the right hepatic duct or subsegmental branch of segment 4 or 5.8,10,12 These ducts have blind distal ends, and thus, do not drain a particular region of the liver. The ducts are usually not accompanied by arteries and veins. Furthermore, portal triads are absent, distinguishing them from other bile ducts that drain the liver.13
Embryologically, ducts of Luschka are thought to arise from anomalous and autonomic proliferation of the most distal biliary ducts formed from the pars hepatica as it develops in the septum transversum. These biliary ducts may persist in certain zones where the liver parenchyma should regress secondarily during development.9 Their incidence is fairly common, and they are reported in up to 10% of cholecystectomy specimens.6,17 The clinical significance of these ducts lies in the fact that they are often unnoticed during cholecystectomy and may be injured. Therefore, injury to the ducts of Luschka is well known to surgeons as a frequent cause of bile leak and biliary peritonitis.12,18
Although the ducts of Luschka are appreciated clinically, pathologists should also be aware of this normal variant of biliary histology. Confusion with neoplastic conditions can occur, especially in cases of florid proliferation in the setting of marked reactive inflammatory atypia. The problem is compounded when only a small serosal biopsy of the gallbladder fossa is performed (as in case 1), which may not allow the pathologist to appreciate the lobular configuration of the glands and their relationship with the liver and gallbladder. In this setting, the irregular growth pattern and epithelial atypia, and also the absence of characteristic concentric fibrosis, may strongly suggest the diagnosis of metastatic adenocarcinoma involving the peritoneal cavity. Metastatic adenocarcinoma typically demonstrates greater cytologic atypia, more irregular growth pattern, mitotic activity, and lacks the typical concentric fibrous collar seen in reactive Luschka ducts. However, in some cases, this distinction may be difficult to make. We urge caution when evaluating peritoneal biopsies of the gallbladder fossa (particularly on frozen section), recognizing that this specific site is home to the cancer mimic we have illustrated herein. Indeed, 2 of our cases were originally considered to represent invasive adenocarcinoma. At times, when encountering a bland but irregular ductal proliferation in the gallbladder fossa, it may be appropriate to defer the frozen section diagnosis and request additional tissue sampling intraoperatively or wait until permanent sections are available.
A comparison of 21 cases of invasive well-to-moderately differentiated gallbladder adenocarcinoma in our study was helpful in delineating key histologic features that may aid in distinguishing florid ducts of Luschka from invasive adenocarcinoma (Table 2). In fact, in contrast to Luschka ducts, invasive adenocarcinoma was characterized by a haphazard infiltrative growth pattern, glands with incomplete lumen formation, and irregular fibrosis surrounding individual glands consistent with desmoplasia. In addition, Luschka ducts are limited to the gallbladder adventitia, whereas the majority of invasive adenocarcinomas involved the entire gallbladder wall. Numerous mitotic figures, marked nuclear variation within a single gland, vascular invasion, and perineural invasion were frequently found in cases of adenocarcinoma, but were absent in benign Luschka ducts. A lobular configuration, confinement to the gallbladder adventitia, and a fibrous collar associated with larger glands were never appreciated in invasive adenocarcinoma. However, in a few cases, probable cancerization of Luschka ducts was identified. This may cause added confusion and difficulty; however in all cases, where this occurred, smaller, angulated, and incomplete glands diagnostic for invasive adenocarcinoma were found nearby.
Immunohistochemical markers discriminating benign ducts of Luschka from malignancy would be extremely helpful. Previous studies have shown that a limited immunohistochemical panel consisting of markers such as, p53, DPC4, and a Ki-67 has been very useful in making the distinction between benign versus malignant biliary disease. The overexpression of p53, loss of DPC4, and demonstration of a high proliferation index by Ki-67 have been shown to be associated with malignancy. However, this panel has not been specifically applied to hyperplastic Luschka ducts. Owing to the limited material available, immunohistochemical studies could not be conducted systematically on all the cases of florid ducts of Luschka presented herein. Therefore, future studies to determine their utility in differential diagnosis are warranted. We do note that case 1 of our series demonstrated a low Ki-67 index, whereas case 2 demonstrated a high Ki-67 index, suggesting that this single marker may not discriminate reactive Luschka ducts, especially in the context of severe acute cholecystitis from adenocarcinoma.
Benign diagnoses are also in the differential diagnosis of florid ducts of Lushka. For instance, Rokitansky-Aschoff sinuses represent herniations of the mucosa into the lamina propria, muscularis, and/or even the subserosa.5,6 They display smooth, undulating contours and are lined by a single layer of epithelium. In contrast to the cuboidal-to-columnar lining of ducts of Luschka, the epithelium of Rokitansky-Aschoff sinuses is typically composed of a distinctly columnar biliary epithelium. Rokitansky-Aschoff sinuses may be associated with hyperplastic muscularis, and once present in the subserosa, result in the fibrous distortion of the surrounding connective tissue. Furthermore, they may be associated with an inflammatory background and demonstrate marked reactive atypia. Adenomyomatous hyperplasia is essentially an exaggerated proliferation of Rokitansky-Aschoff sinuses. Three types of adenomyomatous hyperplasia are recognized: the localized form, also known as adenomyoma, and the segmental and diffuse types. As with Rokitansky-Aschoff sinuses, these are characterized by smooth muscle hyperplasia. Over time, the wall of the gallbladder may become fibrotic and the hyperplastic smooth muscle bundles may no longer be visible.2,4 Unlike florid ducts of Luschka, Rokitansky-Aschoff sinuses and adenomyomatous hyperplasia communicate through the thickened gallbladder muscular wall with the gallbladder lumen and are not restricted to the gallbladder subserosa. Moreover, they may contain bile and lack the concentric fibrosis surrounding larger ductules, which is typical of Luschka ducts.
For completeness, it is important to note that neoplastic transformation has been described within ducts of Luschka, but only 2 cases have been reported in the literature.7,14 Both cases demonstrated gross thickening of the fundic portion of the gallbladder and the neoplasms formed solid, gray-colored masses. Histologically, the neoplasms were characterized by both an intraductal and invasive component intermingled between benign ducts of Luschka. However, the intraductal component differed between the cases. One case exhibited prominent arborizing papillary fronds containing fibrovascular cores,14 whereas the other displayed a solid epithelial growth pattern.7 The invasive adenocarcinoma in both cases was composed of a haphazard arrangement of irregular, small glands within a desmoplastic stroma. The glands were lined by a single layer of markedly atypical cuboidal epithelium. Mitotic figures were rare. Lymphovascular invasion was absent in both cases, but perineural invasion was identified in a single case.7
In conclusion, the distinction between ducts of Luschka and other non-neoplastic and neoplastic conditions can be diagnostically challenging, especially in cases of florid proliferation and marked reactive inflammatory atypia. Special attention to the anatomic location and key histologic features are essential to establish the correct diagnosis.
Acknowledgments
Sources of Support: Gallbladder/Biliary Tract Research Fund at Johns Hopkins Hospital (PA).
REFERENCES
- 1.Adsay NV. Gallbladder, Extrahepatic Biliary Tree, and Ampulla. In: Mills SE, editor. Sternberg’s Diagnostic Surgical Pathology. Philadelphia, PA: Lippincott Williams and Wilkins; 2010. pp. 1600–1638. [Google Scholar]
- 2.Albores-Saavedra J, Henson DE. Adenomyomatous hyperplasia of the gallbladder with perineural invasion. Arch Pathol Lab Med. 1995;119:1173–1176. [PubMed] [Google Scholar]
- 3.Albores-Saavedra J, Henson DE, Klimstra DS. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 2000. Tumors of the gallbladder, extrahepatic bile ducts and ampulla of Vater; p. 159. 3rd series. [Google Scholar]
- 4.Albores-Saavedra J, Keenportz B, Bejarano PA, et al. Adenomyomatous hyperplasia of the gallbladder with perineural invasion: revisited. Am J Surg Pathol. 2007;31:1598–1604. doi: 10.1097/PAS.0b013e31804fa10e. [DOI] [PubMed] [Google Scholar]
- 5.Elfving G. Crypts and ducts in the gallbladder wall. Acta Pathol Microbiol Scand Suppl. 1960;49(Suppl 135):1–45. [PubMed] [Google Scholar]
- 6.Halpert B. Morphological studies on the gallbladder. II. The “true Luschka ducts” and ”Rokitansky-Aschoff sinuses” of the human gallbladder. Bull Johns Hopkins. 1927;41:77–103. [Google Scholar]
- 7.Jahan M, Xiao P, Go A, et al. Intraductal and invasive adenocarcinoma of duct of Luschka, mimicking chronic cholecystitis and cholelithiasis. World J Surg Oncol. 2009;7:4. doi: 10.1186/1477-7819-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitami M, Murakami G, Suzuki D, et al. Heterogeneity of subvesical ducts or the ducts of Luschka: a study using drip-infusion cholangiography-computed tomography in patients and cadaver specimens. World J Surg. 2005;29:217–223. doi: 10.1007/s00268-004-7652-5. [DOI] [PubMed] [Google Scholar]
- 9.Kocabiyik N, Yalcin B, Kilbas Z, et al. Anatomical assessment of bile ducts of Luschka in human fetuses. Surg Radiol Anat. 2009;31:517–521. doi: 10.1007/s00276-009-0473-3. [DOI] [PubMed] [Google Scholar]
- 10.Lin RK, Hunt GC. Left hepatic duct of Luschka. Gastrointest Endosc. 2004;60:984. doi: 10.1016/s0016-5107(04)02199-6. [DOI] [PubMed] [Google Scholar]
- 11.Luschka H. The anatomy of the human stomach. Tubingen Verlag der H Lauppschen, Buchhandlung. 1863:248–255. [in German]. [Google Scholar]
- 12.McQuillan T, Manolas SG, Hayman JA, et al. Surgical significance of the bile duct of Luschka. Br J Surg. 1989;76:696–698. doi: 10.1002/bjs.1800760715. [DOI] [PubMed] [Google Scholar]
- 13.Mergener K, Strobel JC, Suhocki P, et al. The role of ERCP in diagnosis and management of accessory bile duct leaks after cholecystectomy. Gastrointest Endosc. 1999;50:527–531. doi: 10.1016/s0016-5107(99)70077-5. [DOI] [PubMed] [Google Scholar]
- 14.Mori S, Kasahara M. Papillary adenocarcinoma of the subvesical duct. J Hepatobiliary Pancreat Surg. 2001;8:494–498. doi: 10.1007/s005340100016. [DOI] [PubMed] [Google Scholar]
- 15.Parwani AV, Geradts J, Caspers E, et al. Immunohistochemical and genetic analysis of non-small cell and small cell gallbladder carcinoma and their precursor lesions. Mod Pathol. 2003;16:299–308. doi: 10.1097/01.MP.0000062656.60581.AA. [DOI] [PubMed] [Google Scholar]
- 16.Rajab R, Meara N, Chang F. Florid ducts of Luschka mimicking a well differentiated adenocarcinoma of the gallbladder. The Int J Pathology. 2007;6:360–365. [Google Scholar]
- 17.Robertson HE, Ferguson WJ. The diverticula (Luschka’s crypts) of the gallbladder. Arch Pathol. 1945;40:312–333. [PubMed] [Google Scholar]
- 18.Sharif K, de Ville de Goyet J. Bile duct of Luschka leading to bile leak after cholecystectomy: revisiting the biliary anatomy. J Pediatr Surg. 2003;38:E21–E23. doi: 10.1016/j.jpedsurg.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Spanos CP, Syrakos T. Bile leaks from the duct of Luschka (subvesical duct): a review. Langenbecks Arch Surg. 2006;391:441–447. doi: 10.1007/s00423-006-0078-9. [DOI] [PubMed] [Google Scholar]