Abstract
Background
Desmoid tumors are rare soft-tissue neoplasms with limited data on their management. We sought to determine the rates of recurrence following surgery for desmoid tumors and identify factors predictive of disease-free survival.
Methods
Between January 1983 and December 2011, 211 patients with desmoid tumors were identified from three major surgical centers. Clinicopathologic and treatment characteristics were analyzed to identify predictors of recurrence.
Results
Median age was 36 years; patients were predominantly female (68 %). Desmoid tumors most commonly arose in extremities (32 %), abdominal cavity (23 %) or wall (21 %), and thorax (15 %); median size was 7.5 cm. Most patients had an R0 surgical margin (60 %). The 1- and 5-year recurrence-free survival was 81.3 and 52.8 %, respectively. Factors associated with worse recurrence-free survival were: younger age (for each 5-year increase in age, hazard ratio [HR] = 0.90, 95 % confidence interval [95 % CI] 0.82–0.98) and extra-abdominal tumor location (abdominal wall referent: extra-abdominal site, HR = 3.28, 95 % CI, 1.46–7.36) (both P <0.05).
Conclusions
Recurrence remains a problem following resection of desmoid tumors with as many as 50 % of patients experiencing a recurrence within 5 years. Factors associated with recurrence included age, tumor location, and margin status. While surgical resection remains central to the management of patients with desmoid tumors, the high rate of recurrence highlights the need for more effective adjuvant therapies.
Desmoid tumors are rare soft-tissue tumors of mesenchymal origin, with an estimated incidence of 2–4 per million people per year.1–4 The term “desmoid” comes from the tumor’s macroscopic appearance and is derived from the Greek word “desmos,” meaning band or tendonlike.5,6 Desmoid tumors can arise from a variety of locations including the extremities, abdominal wall, and abdominal cavity—however, desmoid tumors have been reported to arise from virtually all parts of the human body.7–9 Certain familiar syndromes such as familial adenomatous polyposis (FAP) may predispose patients to desmoid tumors.1,10 Desmoids have also been possibly associated with local trauma, previous surgery, and high estrogen exposure.11
Although lacking metastatic potential, desmoid tumors can exhibit an aggressive locoregional behavior leading to invasion of adjacent structures. In turn, resection of desmoid tumors can be associated with residual disease at the surgical margin and high recurrence rates. The optimal treatment for patients with desmoid tumors is typically considered to be radical resection; however, there are relatively few data on the long-term outcomes of patients with desmoids following surgical resection. Specifically, few studies have rigorously examined the factors associated with recurrence and disease-free survival. In fact, previous studies often have had conflicting results, possibly due to their limited sample size and the heterogeneity of the study populations.7–9,12,13 In addition, most previous series used limited data from a single-center experience. As such, these studies may suffer from institutional treatment bias, as well as a lack of generalizability.14 In the current study, we sought to determine the rates of recurrence following surgery for desmoid tumors using data from three major surgical centers. Specifically, we examine the outcomes of patients who were managed with surgical resection and identify those factors predictive of overall recurrence and disease-free survival.
MATERIALS AND METHODS
Patients
Using a multi-institutional database, patients with histologically proven desmoid tumors at 1 of 3 institutions (Johns Hopkins School of Medicine, Baltimore, MD; Duke Medical Center, Durham, NC; Medical College of Wisconsin, Milwaukee, WI) between January 1983 and December 2011 were identified. The institutional review board of each respective institution approved this study. Only patients with histologically confirmed primary desmoid tumors who received their treatment at a study center were included.
Data Collection
Standard demographic and clinicopathologic data were collected, including age, sex, and race. Additional information on history of previous pregnancies, as well as family history (e.g., FAP, other family members with desmoids, etc.) was also ascertained. Data were collected on primary desmoid tumor location and size. Data on treatment-related variables, such as type of surgery and adjuvant therapy, were also obtained. Operative procedure details were collected, including intent of surgery (curative vs palliative/debulking) and need for soft tissue reconstruction when primary closure was not possible. Margin status was ascertained based on final pathologic assessment. Data on operative morbidity and mortality were recorded. Complications were classified according to the Clavien-Dindo classification.15 Data on the use of perioperative therapies were also recorded, including type of therapy (e.g., hormonal, chemotherapy, or radiotherapy). Date of last follow-up, vital status, and recurrence-related information were collected on all patients. Recurrence was defined as a lesion that was biopsy-proven recurrent desmoid tumor or a lesion that was deemed suspicious on cross-sectional imaging. Information regarding the location, as well as the disease-free interval from the date of initial operation to the development of recurrent disease was recorded. Dates of last follow-up and vital status were collected on all patients.
Data Analysis
Median values were used to describe continuous data, with discrete variables displayed as totals and frequencies. Comparisons of clinicopathologic characteristics were assessed using the χ2 test for dichotomous and categorical variables. Mann–Whitney U test was used to compare continuous variables. Cumulative event rates were calculated using the method of Kaplan and Meier, and survival curves were compared using the log-rank test. Recurrence-free survival was determined as the time from operation to either biopsy-proven or radiologic evidence of disease recurrence. Overall survival was calculated from the date of operation to last follow-up time or death. Cox’s proportional hazards regression model was used for multivariate modeling of recurrence-free survival. Statistical significance was defined as a 2-tailed P value less than 0.05. All data analyses were performed using SPSS version 17.0 for Microsoft Windows (LEAD Technologies, Inc., Chicago, IL) statistical software package.
RESULTS
Patient and Disease Characteristics
Characteristics of the 211 patients included in the current study are detailed in Table 1. The median age at presentation was 36 years (range, 1–82 years). Patients with desmoid tumors were predominantly female (n = 143; 67.8 %) and white (n = 145; 68.7 %). Nearly one-third (n = 61; 28.9 %) of patients had history of a surgical procedure at the site of the desmoid tumor, while 12 patients (5.7 %) recalled nonsurgical trauma at the site. Familial history of FAP was present in a small number of patients (n = 17; 8.1 %). The diagnosis of a desmoid tumor was made within 6 months of a recent pregnancy in 46 women (32.1 %). Desmoid tumors were located in the extremities (n = 67; 31.7 %), abdominal cavity (n = 48; 22.7 %), abdominal wall (n = 44; 20.9 %), or thorax (n = 32; 15.2 %). Most patients presented with solitary/focal disease (n = 192; 91.4 %). The median size of the desmoid tumor on cross-sectional imaging was 7.5 cm (range, 2.0–34.0 cm).
TABLE 1.
Patient and disease characteristics at baseline (n = 211)
| Patient characteristics | |
| Age at diagnosis, years; median (range) | 36 (1–82) |
| Female gender; n (%) | 143 (67.8) |
| Race | |
| White; n (%) | 145 (68.7) |
| Black; n (%) | 48 (22.7) |
| Other; n (%) | 18 (8.5) |
| Presence of syndromic risk factors or family history | |
| FAP | 17 (8.1) |
| Family history of desmoid tumors | 3 (1.4) |
| History of pregnancy in females with desmoid tumors | 46 (32.1) |
| History of trauma in the area of primary tumor | 12 (5.7) |
| History of surgery in the area of primary tumor | 61 (28.9) |
| Tumor characteristics | |
| Location | |
| Head and/or neck; n (%) | 11 (5.2) |
| Thorax; n (%) | 32 (15.2) |
| Abdominal cavity, mesenteric; n (%) | 34 (16.1) |
| Abdominal cavity, nonmesenteric; n (%) | 14 (6.6) |
| Abdominal wall, involving fascia; n (%) | 27 (12.8) |
| Abdominal wall, without fascia involvement; n (%) | 17 (8.1) |
| Upper extremities; n (%) | 29 (13.7) |
| Lower extremities; n (%) | 38 (18.0) |
| Other; n (%) | 8 (3.8) |
| Size, cm; median (range) | 7.5 (2–34) |
| Tumor focality | |
| Solitary; n (%) | 192 (91.4) |
| Multifocal; n (%) | 18 (8.6) |
| Symptomatic tumor; n (%) | 165 (78.2) |
Details of Management, Surgical Resection, and Postoperative Course
Of the 211 patients with a desmoid tumor, the overwhelming majority underwent surgical resection (n = 197; 93.4 %) with most being for curative intent (n = 179; 91.8 %) (Table 2). The surgical procedure involved radical/wide local excision of an extremity lesion (n = 49; 24.9 %), radical/wide local excision of an abdominal wall lesion (n = 38; 19.3 %), and resection of intra-abdominal lesion (n = 32; 16.2 %); 78 patients (39.6 %) had resection of desmoid tumors at other sites including the thorax (n = 32; 16.2 %), head/neck (n = 11; 5.6 %), or other (n = 35; 17.8 %). At the time of desmoid resection, 33 patients (16.8 %) underwent a concomitant procedure such as bone resection (n = 22; 11.2 %) or resection of contiguous intra-abdominal organs (n = 11; 5.6 %). Primary closure of the wound defect was not possible in 53 patients (27.5 %) and reconstruction was required. Reconstruction of the soft tissue defect involved prosthetic mesh (n = 25; 47.2 %), local soft tissue flap (n = 10; 18.9 %), rotational flap (n = 9; 17.0 %), free flap (n = 5; 9.4 %), and other/unspecified reconstruction procedure (n = 4; 7.5 %).
TABLE 2.
Treatment characteristics (n = 211)
| Treatment | n (%) |
|---|---|
| Surgery | 197 (93.4) |
| Intent of surgery | |
| Curative | 179 (91.8) |
| Debulking | 18 (8.2) |
| Surgical margins (curative intent surgery only)a | |
| R0 (microscopic negative) | 100 (59.9) |
| R1 (microscopic positive, macroscopic negative) | 41 (24.6) |
| R2 (macroscopic positive) | 26 (15.6) |
| Postoperative morbidity (all surgery) | |
| No complication | 163 (82.7) |
| Clavien grade 1–2 complications | 21 (10.7) |
| Clavien grade ≥3 complications | 13 (6.6) |
| Need for soft tissue reconstruction | 53 (27.5) |
| Plastics involved in soft tissue reconstruction | 34 (64.2) |
| Systemic medical therapy with curative intent surgery | 29 (16.2) |
Margin status missing for 12 patients who underwent surgery
Among the 179 patients operated on with curative intent, pathological margin status was available on 167 (93.3 %). While most patients (n = 100; 59.9 %) had a negative surgical margin (R0), a subset was noted to have microscopic disease at the margin (R1) (n = 41; 24.6 %) or have residual macroscopic disease (R2) (n = 26; 15.6 %). The incidence of R0 margin was comparable among patients who had a desmoid of the extremity (16.2 %), abdominal wall (15.6 %), or intra-abdominal site (12.6 %) (P = 0.55).
There were no postoperative deaths within 30 days of surgery. A total of 34 patients experienced a postoperative complication for a morbidity of 17.3 %. Most complications were mild (n = 21) grade I–II complications, while a smaller number were more serious grade III–V that required an intervention (n = 13). Patients who underwent resection of an intra-abdominal desmoid (5.8 %) were no more likely to experience a postoperative complication versus patients who had surgery for an extremity (2.7 %) or abdominal wall (4.8 %) desmoid (P = 0.13).
Of the 179 patients with desmoid tumors who underwent curative intent surgery, 29 (16.2 %) patients received perioperative systemic medical therapy. Specifically, 13 patients received preoperative therapy while 16 received adjuvant treatment. A total of 22 patients (12.3 %) underwent radiotherapy (external beam radiotherapy, n = 11; intensity modulated radiotherapy, n = 4; missing, n = 7). Most patients (n = 13) received radiotherapy in the adjuvant setting.
Recurrence and Overall Survival
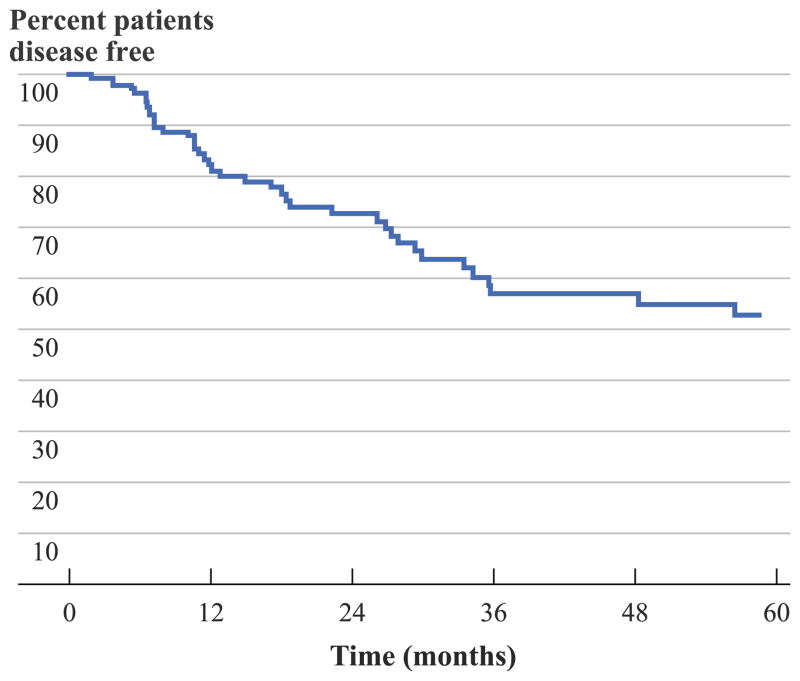
Among the 141 patients with complete surgical resection, the 1-, 3-, and 5-year actuarial recurrence-free survival was 81.3, 57.0, and 52.8 %, respectively; median disease-free survival was not reached during follow-up (Fig. 1). With a median follow-up of 25.7 months, 42 patients (29.8 %) had recurred within a median time interval of 13.6 months (range, 3–191 months). Among the 42 patients with recurrence, the pattern of recurrence was the same site in 31 patients (73.8 %) compared with a new anatomical site in 11 patients (26.2 %).
FIG. 1.
Overall recurrence-free survival of patients with desmoid tumors who underwent surgery with R0 or R1 margins
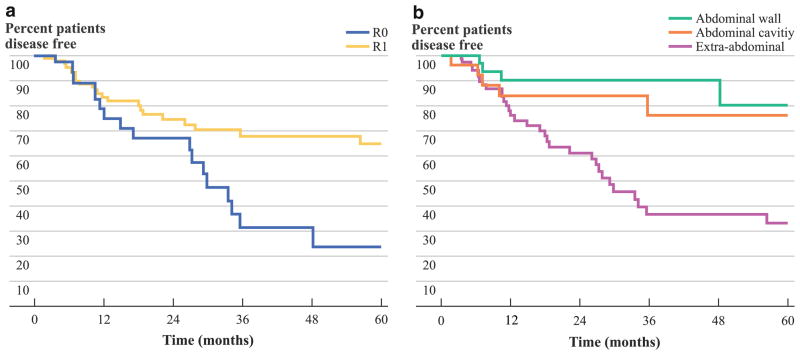
Several factors were associated with a worse recurrence-free survival: younger age (for each 5-year increase in age, hazard ratio [HR] = 0.91, 95 % confidence interval [95 % CI], 0.82–0.99), history of trauma in the area of primary tumor (HR = 2.88, 95 % CI, 1.20–6.90), margin status (R0 referent; R1, HR = 2.16, 95 % CI, 1.17–4.01), and extra-abdominal tumor location (abdominal wall referent; extra-abdominal site, HR = 4.66, 95 % CI, 1.65–13.19) (all P <0.05). Patients who had an R0 surgical margin did not reach median recurrence-free survival during follow-up compared with a median survival of 29.9 months for patients with R1 surgical margins, respectively (P <0.001) (Fig. 2a). Similarly, median recurrence-free survival was not reached following resection of either abdominal wall or intra-abdominal tumors; in contrast, median recurrence-free survival was 29.4 months for patients with extra-abdominal tumors (P <0.001) (Fig. 2b). Of note, median recurrence-free survival was different among patients following resection of an extra-abdominal tumor: thorax, not reached versus head/neck, 11.4 months versus extremities, 29.9 months (P <0.001). On multivariate analysis, younger age (HR = 0.90, 95 % CI, 0.82–0.98) and extra-abdominal tumor site (HR = 3.28, 95 % CI, 1.46–7.36) remained independently associated with a risk of worse recurrence-free survival (both P <0.05).
FIG. 2.
Recurrence-free survival of patients stratified by a surgical margin status and b tumor location
Disease-specific death following resection of desmoid was rare. Overall, both 1- and 5-year actuarial survival was 98.3 and 97.0 %, respectively. At the time of last follow-up, only 6 patients had died, with one death being directly attributable to the desmoid tumor. The patient who died was a 40-year-old, female patient who had initially presented with a multifocal tumor, predominantly located in the supraclavicular area. In the 7 years following initial diagnosis and surgical treatment, she had multiple recurrences, all in the area of the cervical and thoracic spine, and underwent multiple laminectomies for spinal decompression. She died 4 months after undergoing surgery for the fourth recurrence of desmoid tumor. Because death occurred in only one patient, factors associated with survival could not be assessed.
Therapeutic Management of Recurrences
Of the 42 patients who recurred, 31 (73.8 %) underwent repeat resection (curative intent, n = 30; 96.8 % vs debulking, n = 1; 3.2 %). Repeat surgery involved radical/wide local excision of an extremity lesion (n = 12; 38.7 %), radical/wide local excision of an abdominal wall lesion (n = 3; 9.7 %), resection of intra-abdominal lesion (n = 3; 9.7 %) or other wide local excision surgical procedure (n = 13; 41.9 %). Among the 29 patients (93.5 %) who had surgical margin status available, a majority was noted to have had an R0 resection (n = 20; 69.0 %); an R1 resection occurred less commonly (n = 9; 31.0 %). Intra-operative radiation therapy was used in three patients, while two patients received it solely in the adjuvant setting. There were six patients who received adjuvant medical therapy (systemic therapy, n = 2; hormonal therapy, n = 4). At follow-up, 7 of these 29 patients (24.1 %) recurred with a median interval of 10.7 months from the first to second recurrence among those who recurred for a second time. An attempt at another resection was undertaken in five patients. Of these patients, two developed a subsequent recurrence during follow-up.
DISCUSSION
Although often considered “benign,” desmoid tumors can be challenging to manage. In fact, surgical resection of desmoid tumors can sometimes require technically challenging operations with associated local recurrence rates as high as 20–45 %.7–9,12,13 While most clinicians recommend surgical resection of desmoid tumors, others have suggested that an expectant “wait-and-see” approach may be advisable in selected patients.9,16,17 Unfortunately, data on the surgical management of desmoid tumors has been relatively scarce. While large single-institutional series have been reported on occasion, most data on the surgical management comes from small case series.7–9,12,13 The current study is important because we report a large, multi-institutional experience with the surgical management of desmoid tumors. Perhaps more importantly, we detail the specific surgical approach to a wide range of patients with desmoid tumors. We noted that almost one-third of patients undergoing surgery for a desmoid required soft tissue reconstruction. Those factors predictive of recurrence included patient age and desmoid location.
With regard to patient age, we observed that older patients had a longer recurrence-free survival. Specifically, for each 5-year increase in age the hazard of recurrence incrementally decreased (HR = 0.90, 95 % CI, 0.82–0.98). The impact of age on outcome following surgical resection of desmoid tumors is ill defined. Several studies have reported that younger patient age was associated with a quicker time to recurrence; however, others failed to find an association between age and outcome.7,9,12,16,18–20 The reason for these disparate results are undoubtedly multi-factorial, but may be due in part to the manner in which age was analyzed in some studies. For example, the age cutoffs used were not consistent across the studies.9,20 One advantage of the current study was that we analyzed age using not a discrete cutoff value, but rather a range of values. Recently, a clinicopathologic study of desmoid tumors reported that different clinicogenetic subtypes of desmoid tumors may be responsible for the observed difference in recurrence patterns between older and younger patients.21 In a separate study, Lazar et al.18 reported that while there appeared to be no association of age with CTNNB1 gene (encoding the β-catenin protein) mutation, age remained an independent predictor of recurrence even after controlling for CTNNB1 gene mutation status. Risk of recurrence, and desmoid biology, is probably linked to both genetic alterations as well as host factors such as age and hormonal homeostasis. Some findings concerning the risk of recurrence in this study, such as age, reflect the same contradictory results of the literature and therefore need to be considered in light of this.7,9,12,16,18–20
Tumor location was also noted to be independently associated with recurrence-free survival in our multi-institutional dataset. Specifically, extra-abdominal tumors had a higher risk of recurrence (abdominal wall referent; extra-abdominal site, HR = 3.28, 95 % CI, 1.46–7.36). Gronchi et al.13 noted a similar association with tumor location and risk of recurrence. Specifically, patients with extremity/girdle tumors had a 72 % (95 % CI, 60–84 %) disease-free survival rate at 5 years and a 62 % (95 % CI, 49–76 %) disease-free survival rate at 10 years, whereas patients with wall/other tumors had an 88 % (95 % CI, 80–96 %) disease-free survival rate at 5 years, unchanged at 10 years (P <0.01). In the current study, 5-year disease-free survival was 28.8 % for patients with extremity tumors compared with 80.2 % for patients with abdominal wall tumors. Interestingly, the association of extremity location and worse recurrence-free survival persisted on multivariate analysis even after controlling for tumor size and margin status (Table 3). The specific reason for the worse outcome among patients with extremity desmoids remains to be elucidated and may have more to do with unknown underlying genetic differences.
TABLE 3.
Cox proportional hazards ratio estimates for the effect of covariates on recurrence-free survival in patients with surgically resected desmoids tumors
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Age at diagnosis (for each 5-year increase) | 0.91 (0.82–0.99) | 0.04 | 0.90 (0.82–0.98) | 0.02 |
| Race | ||||
| White | Reference | |||
| Black | 0.80 (0.47–1.36) | 0.68 | – | – |
| History of trauma in area of primary tumor | 2.88 (1.20–6.90) | 0.02 | 1.36 (0.57–3.24) | 0.48 |
| History of surgery in area of primary tumor | 0.69 (0.37–1.30) | 0.25 | – | – |
| Desmoid during or shortly after pregnancy | 0.73 (0.34–1.59) | 0.43 | – | – |
| Size >7 cm | 0.73 (0.40–1.33) | 0.30 | – | – |
| Tumor location | ||||
| Abdominal wall | Reference | Reference | ||
| Abdominal cavity | 1.51 (0.41–5.63) | 0.53 | – | |
| Extra-abdominal | 4.66 (1.65–13.19) | 0.01 | 3.28 (1.46–7.36) | 0.01 |
| Surgical margins | ||||
| R0 | Reference | Reference | ||
| R1 | 2.16 (1.17–4.01) | 0.01 | 1.51 (0.85–2.66) | 0.16 |
| Receipt of systemic medical therapy | 1.30 (0.46–3.66) | 0.62 | – | – |
| Receipt of radiotherapy | 1.63 (0.86–3.86) | 0.27 | – | – |
As with virtually all solid tumors, leaving residual disease at the surgical margin (R1) was associated with a shorter recurrence-free survival. Previous studies on the surgical management of desmoid tumors have inconsistently reported on margin status, and when examined, the effect of margin status has been conflicting. Several studies have noted that clear surgical margins were strongly associated with a better recurrence-free survival, while others have not noted an association.7,9,12,13,19,20 The lack of association among some studies may have been due to their inadequate sample size. However, other studies such as the one by Gronchi et al. 13 had a large sample size and still failed to find an association. In this study, the authors intriguingly suggested that surgical margins could be relevant in patients with already recurrent disease, but not in those with primary lesions.13 In a separate study, Merchant et al.22 analyzed a series of 105 patients and made a similar observation. Similar to other factors that we identified in the current study, recurrence may depend more on inherent characteristics of the disease, making it more or less aggressive, so that it might recur or not independent of the margin status in some circumstances.13 Notwithstanding, an R0 resection margin should in general be sought, but the decision should be tailored to each individual patient, taking into account the location of the tumor and the potential for local complications, as well as the morbidity associated with radical operations. As we note in the current study, the morbidity associated with surgery can approach 20 % and a subset of patients may have serious complications or require complex reconstructions. As such, depending on the clinical situation, not all patients may on balance benefit from an R0 operation and radical surgery should not be pursued at any price.
The current study had several limitations that should be considered. Like many studies on surgical management, it used retrospective data. As such, there may have been selection bias that affected which patients were chosen for surgical management. While the multi-institutional nature of the study is a strength, this may have led to treatment heterogeneity. In addition, we did not assess certain pre-operative radiological features such as whether desmoid tumors were stable or progressive, nor did we assess certain imaging characteristics such as whether the lesion was infiltrative versus well defined. Finally, despite amassing one of the largest experiences of surgical management of desmoid tumors by combining the experiences of three major centers, the overall cohort size was still relatively small. In addition, certain subsets (e.g., patients treated with hormonal therapy, radiation therapy, etc.) were even smaller. These limitations may have impacted the ability to detect factors that had smaller differences on outcome, and in some instances precluded us completely from doing subset analyses.
In conclusion, desmoid tumors remain a challenge for both the patient and the surgeon. Up to one-third of patients required resection that was not amenable to primary closure and necessitated more complex soft tissue closure. While morbidity and mortality following surgery for desmoid tumors is low, recurrence remains a problem; in fact, as many as 60 % of patients had experienced a recurrence within 5 years. In turn, these data call into question resection as an effective optimal sole therapy for desmoid tumors. Factors associated with recurrence included age and tumor location. While surgical resection remains important in managing a subset of patients with desmoid tumors, the high rate of recurrence, as well as the potential morbidity and need for complex surgical reconstruction, highlight the shortcomings of surgery alone and emphasize the need for more effective adjuvant therapies, as well as a better understanding of the underlying genetics and tumor biology of desmoid tumors.
Footnotes
Presented at the Society of Surgical Oncology Annual Meeting, Orlando, FL, 2012.
References
- 1.Latchford AR, Sturt NJ, Neale K, Rogers PA, Phillips RK. A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br J Surg. 2006;93:1258–64. doi: 10.1002/bjs.5425. [DOI] [PubMed] [Google Scholar]
- 2.Phillips SR, A’Hern R, Thomas JM. Aggressive fibromatosis of the abdominal wall, limbs and limb girdles. Br J Surg. 2004;91:1624–9. doi: 10.1002/bjs.4792. [DOI] [PubMed] [Google Scholar]
- 3.Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151:230–7. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 4.Rock MG, Pritchard DJ, Reiman HM, Soule EH, Brewster RC. Extra-abdominal desmoid tumors. J Bone Joint Surg Am. 1984;66:1369–74. [PubMed] [Google Scholar]
- 5.Müller J. Erste Lieferung. Berlin: G. Reimer; 1838. Über den feineren Bau und die Formen der krankhaften Geschwülste. [Google Scholar]
- 6.Sakorafas GH, Nissotakis C, Peros G. Abdominal desmoid tumors. Surg Oncol. 16:131–42. doi: 10.1016/j.suronc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Huang K, Fu H, Shi YQ, Zhou Y, Du CY. Prognostic factors for extra-abdominal and abdominal wall desmoids: a 20-year experience at a single institution. J Surg Oncol. 2009;100:563–9. doi: 10.1002/jso.21384. [DOI] [PubMed] [Google Scholar]
- 8.Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25:1785–91. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- 9.Salas S, Dufresne A, Bui B, Blay JY, Terrier P, Ranchere-Vince D, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol. 2011;29:3553–8. doi: 10.1200/JCO.2010.33.5489. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Bigas MA, Mahoney MC, Karakousis CP, Petrelli NJ. Desmoid tumors in patients with familial adenomatous polyposis. Cancer. 1994;74:1270–4. doi: 10.1002/1097-0142(19940815)74:4<1270::aid-cncr2820740415>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Kulaylat MN, Karakousis CP, Keaney CM, McCorvey D, Bem J, Ambrus JL., Sr Desmoid tumour: a pleomorphic lesion. Eur J Surg Oncol. 1999;25:487–97. doi: 10.1053/ejso.1999.0684. [DOI] [PubMed] [Google Scholar]
- 12.Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158–67. doi: 10.1200/JCO.1999.17.1.158. [DOI] [PubMed] [Google Scholar]
- 13.Gronchi A, Casali PG, Mariani L, Lo Vullo S, Colecchia M, Lozza L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21:1390–7. doi: 10.1200/JCO.2003.05.150. [DOI] [PubMed] [Google Scholar]
- 14.Sprague S, Matta JM, Bhandari M, et al. Multicenter collaboration in observational research: improving generalizability and efficiency. J Bone Joint Surg Am. 2009;91 (Suppl 3):80–6. doi: 10.2106/JBJS.H.01623. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiore M, Rimareix F, Mariani L, Domont J, Collini P, Le Péchoux C, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–93. doi: 10.1245/s10434-009-0586-2. [DOI] [PubMed] [Google Scholar]
- 17.Stoeckle E, Coindre JM, Longy M, Binh MB, Kantor G, Kind M, et al. A critical analysis of treatment strategies in desmoid tumours: a review of a series of 106 cases. Eur J Surg Oncol. 2009;35:129–34. doi: 10.1016/j.ejso.2008.06.1495. [DOI] [PubMed] [Google Scholar]
- 18.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–27. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensen A, Keller J, Nielsen OS, Jensen OM. Treatment of aggressive fibromatosis: a retrospective study of 72 patients followed for 1–27 years. Acta Orthop Scand. 2002;73:213–9. doi: 10.1080/000164702753671830. [DOI] [PubMed] [Google Scholar]
- 20.Bonvalot S, Eldweny H, Haddad V, Rimareix F, Missenard G, Oberlin O, et al. Extra-abdominal primary fibromatosis: aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34:462–8. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Salas S, Chibon F, Noguchi T, Terrier P, Ranchere-Vince D, Lagarde P, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer. 2010;49:560–8. doi: 10.1002/gcc.20766. [DOI] [PubMed] [Google Scholar]
- 22.Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86:2045–52. [PubMed] [Google Scholar]