Abstract
Background
The management of patients with liver metastasis from a gynecologic carcinoma remains controversial, as there is currently little data available. We sought to determine the safety and efficacy of liver-directed surgery for hepatic metastasis from gynecologic primaries.
Methods
Between 1990 and 2010, 87 patients with biopsy-proven liver metastasis from a gynecologic carcinoma were identified from an institutional hepatobiliary database. Fifty-two (60%) patients who underwent hepatic surgery for their liver disease and 35 (40%) patients who underwent biopsy only were matched for age, primary tumor characteristics, and hepatic tumor burden. Clinicopathologic, operative, and outcome data were collected and analyzed.
Results
Of the 87 patients, 30 (34%) presented with synchronous metastasis. The majority of patients had multiple hepatic tumors (63%), with a median size of the largest lesion being 2.5 cm. Of those patients who underwent liver surgery (n = 52), most underwent a minor hepatic resection (n = 44; 85%), while 29 (56%) patients underwent concurrent lymphadenectomy and 45 (87%) patients underwent simultaneous peritoneal debulking. Postoperative morbidity and mortality were 37% and 0%, respectively. Median survival from time of diagnosis was 53 months for patients who underwent liver-directed surgery compared with 21 months for patients who underwent biopsy alone (n = 35) (p = 0.01). Among those patients who underwent liver-directed surgery, 5-year survival following hepatic resection was 41%.
Conclusions
Hepatic surgery for liver metastasis from gynecologic cancer can be performed safely. Liver surgery may be associated with prolonged survival in a subset of patients with hepatic metastasis from gynecologic primaries and therefore should be considered in carefully selected patients.
Introduction
Gynecologic cancers affect over 80,000 females per year in the United States, with an annual estimated death rate of over 250,000 [1]. Within this group of cancers of the female genital tract, ovarian cancer is the most common subtype, as 1 in every 70 women in the Western World will develop this form of cancer [1]. While in the majority of cases the disease is limited to the primary gynecologic organ, distant metastasis can develop in up to 40% of patients, in either a synchronous or metachronous fashion [2–4]. Dissemination via the intraperitoneal route is generally considered the most common; however, lymphatic and hematogenous spread of the disease have also been reported [5]. Commonly affected sites include pleura [2, 3], lungs [6, 7], and liver [3]. Rose et al. [5] reported that 48% of gynecologic patients had hepatic metastasis at autopsy, perhaps indicating that liver metastasis from gynecologic primaries may be more common than estimated [8].
Data on management of patients with hepatic metastasis from a gynecologic primary tumor remain scarce. While several series on liver surgery for noncolorectal non-neuroendocrine metastasis have been published [9–12], few included many patients with gynecologic primary cancers. In fact, most—if not all—data on the role of hepatic surgery for gynecologic hepatic metastasis come from either case reports [13–17] or small studies with fewer than 25 patients [9, 18–28]. Due to the limited number of patients in previous studies, the role of liver surgery for hepatic metastasis from a gynecologic primary carcinoma remains ill-defined.
As surgical resection represents the only potentially curative therapeutic option for patients with metastatic disease to the liver, defining the role of hepatic resection for metastatic disease from gynecologic malignancies has important implications. Therefore, the objective of the current study was to assess the safety and efficacy of liver-directed surgery of hepatic metastasis from a gynecologic primary carcinoma. To accomplish this, we utilized a matched-pair analysis to compare outcomes among patients who underwent liver-directed surgery versus biopsy only for hepatic metastasis from a gynecologic primary carcinoma.
Methods
Data on 87 female patients diagnosed with liver metastasis from a primary gynecologic cancer from 1990 to 2010 were identified from our institutional hepatobiliary database. For the purpose of this study, a gynecologic cancer was defined as one of ovarian, cervical, uterine/endometrial, or fallopian origin [29, 30]. Only patients who had biopsy-proven hepatic metastasis from a gynecologic primary were included. Patients who underwent surgery (i.e., resection and/or ablation) for their liver metastasis and patients who underwent a nontherapeutic laparotomy during which liver biopsies were taken were included in the current study. The study was approved by the Johns Hopkins Hospital Institutional Review Board.
Data collection
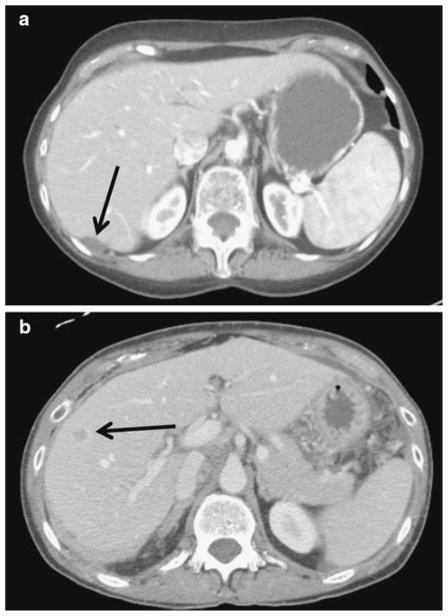
In addition to standard demographic data, the following data were collected for each patient: primary tumor characteristics (stage, grade, histology, and location of primary tumor), preoperative carbohydrate antigen-125 (CA-125) level, treatment-related variables, and presence and location of any extrahepatic disease. Details on the number, size, and location of the liver metastasis were also recorded. Location of the hepatic metastasis was characterized as “capsular/implant” or “parenchymal” based on re-review of operative notes and all preoperative cross-sectional imaging. Specifically, any lesion noted to be on the capsule of the liver or completely contained within 10 mm from the liver surface was defined as “capsular/ implant” (Fig. 1). Operative information included type of liver-directed therapy (i.e., resection, ablation, both), extent of resection, performance of concurrent procedures, and blood loss during surgery. Liver resection was defined as a minor (<3 segments) or major (≥3 segments) hepatectomy. Data on the postoperative course, including length of hospital stay, and perioperative occurrence and Clavien grade [31] of morbidity and mortality were recorded. For all patients, data on vital status and recurrence were noted and defined as intra- or extrahepatic.
Fig. 1.
Contrast-enhanced CT scans of a gynecologic primary tumor liver metastasis located (a) on the capsule of the liver (implant) versus (b) lesion situated within the hepatic parenchyma
Statistical analyses
Summary statistics were obtained using established methods and presented as percentages or median values. The χ2 test or Kruskal–Wallis test was used to compare categorical data, and the Mann–Whitney U test was used for continuous data. Disease-free intervals and survival times were estimated using the Kaplan–Meier method, both from time of surgery (for those patients who underwent hepatic resection) and from time of diagnosis (for all patients) [32]. Differences in survival were examined using the log-rank test. Cox regression analysis was used to identify factors associated with survival [33]. In order to compare the group of patients who underwent liver-directed surgery with those patients who underwent liver biopsy only, a matched-controlled analysis was performed. Specifically, patients were matched for age, primary tumor characteristics, and hepatic tumor burden (number of lesions, size of largest lesion). Hazard ratios and 95% confidence intervals were reported, and p <0.05 was considered significant. All tests were performed using SPSS ver. 18.0 (SPSS, Inc., Chicago, IL).
Results
Patient, tumor, and primary surgery characteristics
The patient and tumor characteristics of the 87 patients who were diagnosed with hepatic metastasis from gynecologic origin and who are included in the current study are detailed in Table 1. Most patients (n = 67; 77%) had a primary tumor located in the ovaries and about one half of the patients (n = 44; 51%) had stage III disease. All patients underwent surgical therapy of the primary gynecologic tumor, with the majority having a total abdominal hysterectomy and salphingo-oophorectomy (n = 71; 81%). Most patients (n = 53; 61%) underwent concurrent lymphadenectomy, of whom 30 (34%) were found to have lymph node metastasis. Of note, 71 (81%) patients had concurrent peritoneal debulking at the time of initial surgery for the primary tumor.
Table 1.
Overall patient and tumor characteristics
| Variable | No. of patients (%) (n = 87) |
|---|---|
| Patient characteristics | |
| Race, Caucasian | 78 (90) |
| Median age at diagnosis of liver metastasis [range] (years) | 60 [31–85] |
| Primary tumor and surgical details | |
| Location of primary tumor | |
| Ovary | 67 (77) |
| Uterus/endometrium | 15 (17) |
| Cervix | 3 (4) |
| Fallopian tube | 2 (2) |
| Stage of primary tumor | |
| I | 7 (8) |
| II | 6 (7) |
| III | 44 (51) |
| IV | 30 (34) |
| Grade of primary tumor | |
| Poorly differentiated | 50 (58) |
| Moderately differentiated | 15 (17) |
| Well differentiated | 9 (10) |
| Unknown | 13 (15) |
| Histology | |
| Papillary serous carcinoma | 41 (46) |
| Endometrioid carcinoma | 10 (12) |
| Papillary serous and endometrioid carcinoma | 10 (12) |
| Adenocarcinoma | 10 (12) |
| Other | 16 (18) |
| Type of primary surgery | |
| Total abdominal hysterectomy with bilateral salpingo-oopherectomy | 71 (82) |
| Other | 16 (18) |
| Concurrent lymphadenectomy | 53 (61) |
| Concurrent peritoneal debulking | 71 (82) |
| Hepatic metastasis | |
| Presentation, synchronous | 30 (34) |
| Number of lesions, multiple | 55 (63) |
| Size of largest liver lesion median [range] (cm) | 2.5 [0.1–10.5] |
| Distribution of liver lesions, bilobar | 21 (24) |
| Location of liver lesions | |
| Capsular/implant | 27 (31) |
| Deeply situated/parenchymal | 60 (69) |
| CA-125, >250 U/ml | 36 (41) |
| Concurrent extrahepatic disease | 72 (83) |
| Surgical resection of liver metastasis | 52 (60) |
While 30 (34%) patients had synchronous liver disease, 57 (66%) patients developed metachronous liver metastasis after a median disease-free interval of 25 months (95% CI: 19–32) from the time of surgery for the primary gynecologic tumor. Of the 57 with metachronous liver metastasis, 45 patients had postoperative chemotherapy following resection of the primary tumor. Of these 45 patients, 23 underwent subsequent resection of their liver metastasis while 22 patients did not (p = 0.11). Of those patients who developed metachronous liver metastasis, 14 (25%) patients underwent surgery for extrahepatic disease during the interval between their primary operation and the diagnosis of liver metastasis.
Overall, most patients (n = 55; 63%) had multiple liver lesions, but only 21 (24%) patients had bilobar hepatic metastasis. The median size of the largest lesion was 2.5 cm (range = 0.1–10.5). The majority of patients (n = 60; 69%) had deeply situated liver lesions located within the hepatic parenchyma; 27 (31%) patients had liver metastasis characterized as capsular/implant. Most patients (n = 72; 83%) had concurrent extrahepatic metastatic disease, with most having peritoneal disease (n = 71).
Of the 52 patients who underwent liver-directed surgery, 45 (87%) patients received chemotherapy. Pre- and postoperative chemotherapy was administered to 14 (27%) patients, whereas 31 patients received adjuvant chemotherapy only. All 35 patients who underwent liver biopsy only received systemic chemotherapy. Among the patients who underwent liver-directed surgery (n = 52), 30 (58%) patients also received intraperitoneal chemotherapy as part of their therapy.
Details of liver-directed surgery and postoperative course
Of the cohort of 87 patients included in the study, 52 (60%) underwent hepatic surgery for their liver metastasis (Table 2). Of the patients with synchronous liver metastasis (n = 30), 24 (80%) patients underwent a simultaneous procedure with hepatic resection at the same time as resection of the primary tumor; the other 6 (20%) patients underwent a staged liver-directed surgery.
Table 2.
Patient, tumor, liver-directed surgery, and postoperative characteristics of the 52 patients who underwent surgical management of their liver metastasis
| Variable | No. of patients (%)(n = 52) |
|---|---|
| Patient characteristics | |
| Race, Caucasian | 47 (90) |
| Median age at surgery [range] (years) | 60 [40–85] |
| Hepatic metastasis | |
| Presentation, synchronous | 23 (46) |
| Number of lesions, multiple | 2 [1] |
| Size of largest liver lesion median [range] (cm) | 3 [1–10.5] |
| Distribution of liver lesions, bilobar | 13 (25) |
| Location of liver lesions | |
| Capsular/implant | 20 (38) |
| Deeply situated/parenchymal | 32 (62) |
| CA-125, >250 U/ml | 15 (29) |
| Concurrent extrahepatic disease | 44 (85) |
| Details of liver-directed surgery | |
| Surgical approach | |
| Open | 31 (60) |
| Laparoscopic | 21 (40) |
| Median number of tumors treated [range] | 2 [1–8] |
| Type of liver-directed therapy | |
| Resection only | 46 (88) |
| Radiofrequency ablation only | 2 (4) |
| Both | 4 (8) |
| Type of liver resection | |
| Nonanatomic wedge resection/single segment | 30 (58) |
| Bisegmentectomy | 14 (27) |
| Hemihepatectomy | 8 (15) |
| Extent of resection | |
| Minor (<3 segments) | 44 (85) |
| Major (≥ 3 segments) | 8 (15) |
| Concurrent lymphadenectomy | 29 (56) |
| Concurrent peritoneal debulking | 45 (87) |
| Median estimated blood loss [range] (ml) | 600 [50–2900] |
| Median units of blood transfusion [range] | 2 [0–7] |
| Margin | |
| R0 | 33 (63) |
| R1 | 8 (15) |
| R2 | 1 (2) |
| Unknown | 10 (19) |
| Postoperative course | |
| Median length of stay median (days) | 7 [3–24] |
| Grade of complications | |
| None | 24 (46) |
| I | 4 (8) |
| II | 5 (10) |
| III | 8 (15) |
| IV | 2 (4) |
| V | 0 |
| Unknown | 9 (17) |
| Perioperative treatment | |
| Preoperative chemotherapy | 14 (27) |
| Preoperative radiotherapy | 0 |
| Postoperative chemotherapy | 45 (87) |
| Postoperative radiotherapy | 2 (4) |
The median number of liver lesions treated was 2 (range = 1–8). The majority (n = 46; 88%) of patients underwent resection only; radiofrequency ablation only (n = 2; 4%) or combined resection and ablation (n = 4; 8%) was utilized much less frequently. At the time of liver-directed surgery, most patients (n = 45, 87%) also underwent peritoneal debulking. On final pathological analysis of the liver specimen, most patients (n = 33; 63%) had a negative hepatic margin (R0), whereas 8 (15%) patients had microscopic disease at the margin (R1) and 1 (2%) patient had macroscopic disease left in situ (R2).
The median postoperative length of stay was 7 days (range = 3–24). There were no postoperative deaths within 90 days of surgery. Nineteen (37%) patients experienced a postoperative complication, most related to wound infection or pulmonary issues. One patient underwent a reoperation for repair of wound dehiscence. No patient had a liver-related complication (i.e., biloma, abscess, liver insufficiency or failure) (Table 2).
Recurrence and overall survival
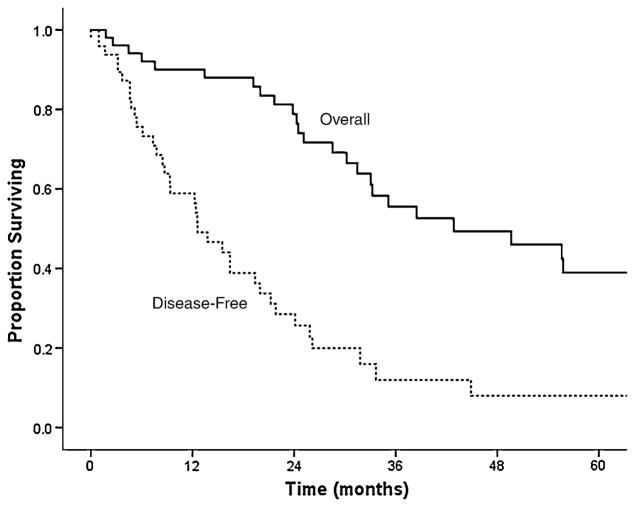
Following liver-directed surgery (n = 52), 39 (75%) patients recurred after a median disease-free interval of 13 months (95% CI: 9–16) (Fig. 2). Among the 39 patients with recurrence, the pattern of recurrence was intrahepatic only in 6 (15%) patients, extrahepatic only in 13 (33%) patients, and intra- and extrahepatic in 18 (46%) patients. In 2 patients (5%), the location of recurrence was unknown. Repeat surgery was undertaken in 3 (8%) patients, all of whom had intrahepatic recurrence only.
Fig. 2.
Kaplan–Meier curve depicting overall and recurrence-free survival of patients who underwent curative-intent surgery for gynecologic liver metastasis
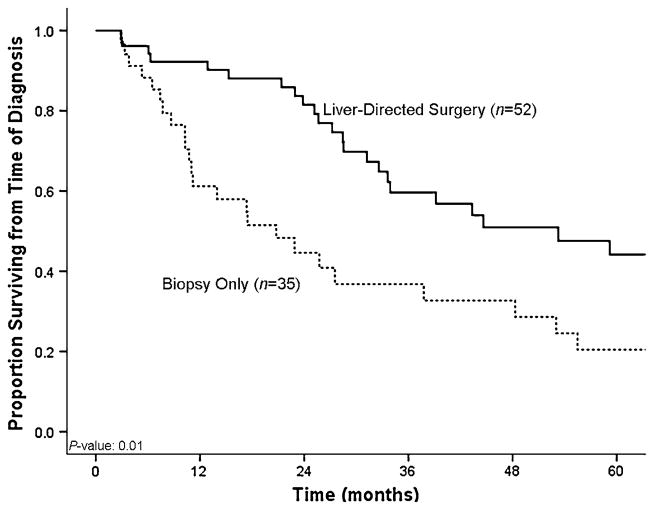
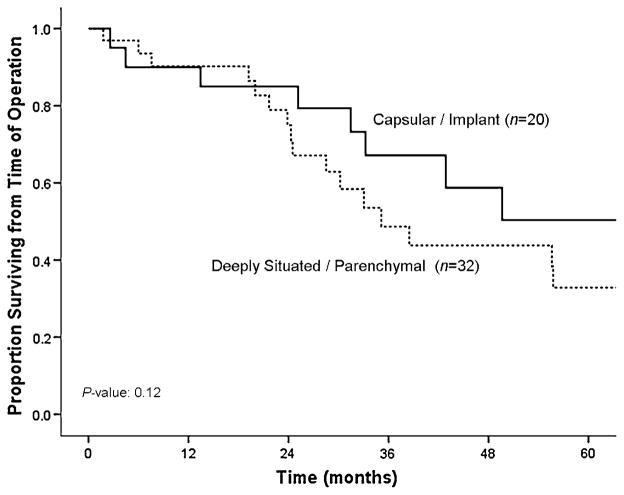
Median overall survival from time of diagnosis was 38 months (95% CI: 24–51) for the entire cohort. In order to assess the impact of liver-directed therapy for hepatic metastasis from a gynecologic primary tumor, we then performed a matched-paired analysis. Specifically, patients who underwent surgical therapy of their liver metastasis were matched for age, primary tumor characteristics, and hepatic tumor burden (number of lesions, size of largest lesion) with patients who had biopsy-proven gynecologic liver metastasis disease but did not undergo surgical treatment of their liver disease (Table 3). The median overall survival from time of diagnosis of liver metastasis was 53 months (95% CI: 58–78) for patients who underwent liver-directed surgery (n = 52; 60%) compared with 21 months (95% CI: 9–31) for patients who underwent biopsy only (n = 35; 40%) (p = 0.01) (Fig. 3). Moreover, when calculated from time of surgery (n = 52; 60%), the median overall survival was 50 months (95% CI: 24–75), with a 1-, 3-, and 5-year survival of 90, 57, and 41%, respectively (Fig. 1). On univariate analyses, no factor was found to be associated with survival following surgery (Table 4). Location of the primary tumor, histology of the primary tumor, presentation of the liver metastasis, and size, number, and distribution of the hepatic lesions were not found to correlate with survival on univariate analyses (all p >0.05). Specifically, the median survival following surgery was similar for patients with ovarian versus nonovarian primaries (21 vs. 22 months, respectively; p = 0.53). While not significant, patients with deeply situated liver lesions (i.e., within the hepatic parenchyma) tended to have a worse survival compared with patients who had liver metastasis characterized as capsular/implant (hazard ratio [HR]: 1.49; 95% CI: 0.90–2.48; p = 0.12) (Fig. 4).
Table 3.
Tumor characteristics for patients who underwent resection compared with patients who underwent a biopsy only
| Variable | No. of patients (%) (n = 87)
|
||
|---|---|---|---|
| Resection (n = 52) | Biopsy only (n = 35) | p | |
| Primary gynecologic cancer | |||
| Location of primary cancer | |||
| Ovarian carcinoma | 42 (81) | 25 (71) | 0.31 |
| Other gynecologic carcinoma | 10 (19) | 10 (29) | |
| Stage at diagnosis of primary tumor | |||
| I/II | 4 (8) | 9 (26) | 0.11 |
| III | 31 (59) | 13 (37) | |
| IV | 17 (33) | 13 (37) | |
| Grade | |||
| Poorly differentiated | 33 (63) | 17 (49) | 0.35 |
| Moderately/well differentiated | 13 (25) | 11 (31) | |
| Unknown | 6 (12) | 7 (20) | |
| Histology | |||
| Papillary serous | 27 (52) | 14 (40) | 0.39 |
| Other | 25 (48) | 21 (60) | |
| Type of primary surgery | |||
| Total abdominal hysterectomy with bilateral salpingo-oopherectomy | 42 (81) | 29 (83) | 0.81 |
| Other | 10 (19) | 6 (17) | |
| Concurrent lymphadenectomy | 33 (63) | 20 (57) | 0.55 |
| Concurrent peritoneal debulking | 45 (87) | 26 (74) | 0.15 |
| Liver metastasis | |||
| Median age at diagnosis [range] (years) | 60 [40–85] | 58 [31–83] | 0.30 |
| Overall presentation, synchronous | 23 (44) | 7 (20) | 0.02 |
| CA-125 at diagnosis ≥ 250 U/ml | 17 (33) | 10 (29) | 0.90 |
| Location of liver lesions | |||
| Capsular/implant | 20 (38) | 7 (20) | 0.07 |
| Deeply situated/parenchymal | 32 (62) | 28 (80) | |
| Distribution of liver lesions, unilobar | 39 (75) | 18 (51) | 0.59 |
| Presence of multiple lesions | 30 (58) | 25 (71) | 0.19 |
| Concurrent extrahepatic disease | 45 (87) | 27 (77) | 0.26 |
Fig. 3.
Kaplan–Meier curve comparing overall survival of patients who underwent curative-intent surgery for gynecologic liver metastasis with survival of patients who underwent liver biopsy only
Table 4.
Univariate analyses of factors proposed to be associated with survival from time of hepatic operation among the 52 patients who underwent liver-directed surgery
| Variable | Hazard ratio | 95% CI | p |
|---|---|---|---|
| Caucasian race | 0.68 | 0.20–2.53 | 0.55 |
| Location of primary tumor, ovaries | 0.83 | 0.35–1.96 | 0.66 |
| Stage at diagnosis of primary | |||
| I/II | Reference | ||
| III/IV | 1.56 | 0.36–6.07 | 0.55 |
| Grade of primary | |||
| Well/moderate differentiation | Reference | ||
| Poorly differentiated | 1.06 | 0.44–2.56 | 0.89 |
| Histology | |||
| All other reported histologies | Reference | ||
| Papillary serous | 0.76 | 0.36–1.61 | 0.48 |
| Type of primary surgery | |||
| Other | Reference | ||
| Total abdominal hysterectomy/bilateral salphingo-oophorectomy | 0.82 | 0.33–2.04 | 0.67 |
| Lymphadenectomy during primary surgery | 0.70 | 0.57–1.07 | 0.53 |
| Peritoneal debulking during primary surgery | 0.94 | 0.33–2.70 | 0.92 |
| Interval surgery | 1.37 | 0.61–3.03 | 0.44 |
| Synchronous presentation of liver metastasis | 0.79 | 0.37–1.45 | 0.54 |
| Multiple hepatic lesions | 1.61 | 0.74–3.47 | 0.23 |
| Size of largest hepatic lesion (cm) | 0.91 | 0.74–1.11 | 0.36 |
| Presence of concomitant extrahepatic disease | 0.72 | 0.45–1.14 | 0.16 |
| Location of liver lesions | |||
| Capsular/implant | Reference | ||
| Deeply situated/parenchymal | 1.49 | 0.90–2.48 | 0.12 |
| CA-125 ≥ 250 U/ml | 0.90 | 0.34–2.38 | 0.83 |
| Bilobar distribution | 1.05 | 0.69–1.59 | 0.83 |
| Major hepatic resection | 1.08 | 0.37–3.16 | 0.88 |
| Receipt of ablation | 0.56 | 0.13–2.38 | 0.44 |
| Lymphadenectomy during liver surgery | 0.83 | 0.40–1.75 | 0.64 |
| Peritoneal debulking during liver surgery | 0.53 | 0.21–1.33 | 0.18 |
| Positive hepatic resection margin | 1.10 | 0.45–3.23 | 0.76 |
Fig. 4.
Kaplan–Meier curve showing overall survival stratified by location of liver metastasis, capsular/implant versus deeply situated/ parenchymal
Discussion
Although the role of liver surgery in patients with hepatic metastasis from colorectal or neuroendocrine primary tumors is accepted, its role in treating liver metastasis from other primary malignancies is more controversial. Several institutions have reported series of patients who have undergone hepatic resection for noncolorectal and non-neuroendocrine metastases [9–12]. Data have also been published on hepatic surgery for liver metastasis from primary tumors, including sarcoma [34], squamous cell [35], and periampullary [36]. However, few previous studies focused exclusively on the role of hepatic surgery for metastasis derived from primary gynecologic malignancies [9, 13–28]. As such, the role of surgery to treat liver metastasis from gynecologic primary tumors is less clearly defined. The current study is important because it examined whether hepatic resection of metastasis from gynecologic primaries is warranted. Unlike many previous studies, only patients with liver metastasis from gynecologic primary tumors were included. In addition, we compared patients who underwent liver-directed surgery for gynecologic hepatic metastasis with comparably matched patients who underwent biopsy alone to assess the relative benefit of surgical intervention. Our data suggest that in well-selected patients liver-directed surgery for hepatic metastasis from a gynecologic primary can be associated with a 5-year survival of 40%.
An aggressive therapeutic approach that includes primary cytoreductive surgery and debulking procedures is considered part of the standard treatment for many gynecologic malignancies and has been shown to provide a survival benefit [37–39]. In a landmark study in 1975, Griffiths [40] reported on the association between residual tumor burden and patient survival. The inverse relationship between the amount of residual tumor and survival has subsequently been confirmed by other investigators [41–44]. Following cytoreductive surgery, systemic chemotherapy is typically administered. Most data on combined cytoreduction and chemotherapy have included either patients with stage III disease or those with stage IV disease characterized by peritoneal metastasis [45–47]. Among this group of patients, combined modality treatment of metastatic gynecologic malignancies with cytoreductive surgery and chemotherapy can achieve a median survival in the range of 29–36 months [45–47]. Specifically, patients with metastatic ovarian cancer have a better 5-year survival [48–50] following cytoreductive surgery compared with patients who have advanced metastatic uterine or cervical cancer [51–53]. The role of repeat surgical debulking, however, remains somewhat controversial. The Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer (EORTC) examined the role of debulking surgery for metastatic ovarian cancer [54]. In that study, patients who had residual lesions measuring more than 1 cm in diameter after primary surgery were randomized to repeat debulking surgery or no surgery. The authors noted that debulking surgery significantly lengthened progression-free and overall survival with the risk of death being reduced by one third. In a separate study, however, Rose et al. [55] reported no improvement in progression-free survival among patients who had advanced ovarian cancer and residual tumor exceeding 1 cm in diameter after primary surgery who subsequently went on to a secondary cytoreductive surgery. Some investigators [56] have suggested that the reason for the different findings in the two studies may have been related to the increased efficacy of the paclitaxel plus cisplatin regimen in the Rose et al. trial compared to the cyclo-phosphamide plus cisplatin in the EORTC trial, thereby perhaps blunting the effect of the second debulking in the Rose trial. In aggregate, the data suggest that while patients who have had an effective primary debulking operation probably do not benefit from a second operation, those patients whose initial surgical debulking was suboptimal may indeed benefit from a more thorough debulking.
Up to 6–28% of patients with gynecologic malignancies can also develop hepatic metastasis [21, 26]. In general, the development of distant metastatic disease in the lung or liver usually portends a worse prognosis. Several groups, however, have suggested that hepatic resection of liver metastasis from gynecologic malignancies may play a role in the treatment of a subset of patients [7, 18, 19, 26–28, 57]. For patients with liver metastasis, definitive therapy with surgical resection represents the only chance at cure. Despite the liver being the most common site of distant metastasis, resection of metastasis from gynecologic primary tumors is uncommon. In the current study, despite being a major referral center, we were able to amass only 52 patients who underwent surgery for hepatic metastasis from a gynecologic primary carcinoma. Regardless of the relative rarity of this indication for liver surgery, given the expanding indications for hepatic resection of noncolorectal and non-neuroendocrine tumors, data on liver-directed surgery for gynecologic malignancies is important to help inform clinical decision-making. In the current study, we report that liver surgery to treat hepatic metastasis from a gynecologic primary carcinoma was associated with overall 3- and 5-year survival rates of 57 and 41%, respectively. These data are consistent with those reported by Adam et al. [9], who reported a 5-year survival rate of 35–50% for patients who underwent resection of liver metastasis from a gynecologic primary. Other studies [19, 20, 27, 57–59] have reported median survival following liver surgery for gynecologic metastasis ranging from 26 to 62 months, which is similar to the median survival of 53 months that we report here. Unlike other studies, we also performed a matched-paired analysis. When examining patients with hepatic metastasis from gynecologic primary tumors who underwent liver-directed therapy (i.e., resection ± ablation) versus biopsy alone, there was a significant difference in long-term survival. In fact, patients who underwent liver-directed therapy had a near 2.5-fold increase in their median overall survival compared with patients who underwent biopsy alone (Fig. 3). Collectively, data from the current study and from previously published work suggest that liver surgery for hepatic metastasis from a gynecologic primary can result in long-term survival in a subset of patients.
It is important to identify the subset of patients with hepatic metastasis from gynecologic primary tumors who will benefit the most from surgical intervention. In the current study, the majority of patients who underwent liver surgery had a low volume of hepatic disease. In addition, while most patients had extrahepatic disease, the site of the extrahepatic disease was peritoneal in most patients. Although there is considerable data on the benefit of surgery for gynecologic metastasis disseminated via the peritoneal route [60–62], the benefit of surgery in patients with hematogenous spread of metastatic disease is more controversial. Interestingly, in the current study we noted a strong trend in the association between survival and location of the liver metastasis resected. Specifically, patients who underwent liver-directed surgery for metastasis characterized as capsular/implant disease seemingly benefitted more from surgery than patients who had deeply situated/ parenchymal liver metastasis. Other investigators have similarly suggested that location of the liver metastasis (capsular/implant versus parenchymal lesion) may impact outcome following surgery for metastatic gynecologic tumors [63]. These differences in outcome may reflect the underlying differences in prognosis for patients with peritoneal versus hematogenous spread of metastasis.
The current study had several limitations. Despite our institution having one of the largest hepatobiliary experiences in the country, only a small number of patients were included in the current series. This reflected the highly select nature of the cohort of patients with live metastasis from gynecologic primary tumors who were considered for resection. Due to the small sample size, the study had limited statistical power. Like all retrospective studies, selection bias may also have influenced choice of liver-directed surgery versus biopsy only. It is possible that resection/biopsy may simply be a surrogate for tumor biology and/or extent of disease. In turn, it is possible that the benefit of surgery may have been overstated. Causal inferences drawn from the data therefore need to be interpreted in light of these limitations.
In conclusion, resection of hepatic metastasis from gynecologic primary tumors can be performed with zero or near-zero mortality and low perioperative morbidity. Overall, a subset of patients with hepatic metastasis from gynecologic primary tumors can experience a 5-year survival approaching 40%. However, it must be kept in mind that most patients in the current study who underwent liver-directed therapy for gynecologic metastasis had a low burden of hepatic disease and were highly selected. While liver surgery for hepatic metastasis from a gynecologic primary tumor may be warranted and provide a survival benefit, the selection of patients needs to be carefully individualized and incorporated into a multidisciplinary approach.
Contributor Information
Sarah I. Kamel, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Mechteld C. de Jong, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Richard D. Schulick, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Teresa P. Diaz-Montes, The Kelly Gynecologic Oncology Service, Johns Hopkins University School of Medicine, Balimore, MD 21287, USA
Christopher L. Wolfgang, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Kenzo Hirose, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA.
Barish H. Edil, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Michael A. Choti, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
Robert A. Anders, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Timothy M. Pawlik, Email: tpawlik1@jhmi.edu, Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611, 600 N Wolfe Street, Baltimore, MD 21287, USA
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Dauplat J, Hacker NF, Nieberg RK, et al. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987;60(7):1561–1566. doi: 10.1002/1097-0142(19871001)60:7<1561::aid-cncr2820600725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoi H, A’Hern RP, Fisher C, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol. 1999;17(3):767–775. doi: 10.1200/JCO.1999.17.3.767. [DOI] [PubMed] [Google Scholar]
- 4.Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(2):125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989;64(7):1508–1513. doi: 10.1002/1097-0142(19891001)64:7<1508::aid-cncr2820640725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Kerr VE, Cadman E. Pulmonary metastases in ovarian cancer. Analysis of 357 patients. Cancer. 1985;56(5):1209–1213. doi: 10.1002/1097-0142(19850901)56:5<1209::aid-cncr2820560542>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Tangjitgamol S, Levenback CF, Beller U, Kavanagh JJ. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: a literature review. Int J Gynecol Cancer. 2004;14(3):399–422. doi: 10.1111/j.1048-891x.2004.14326.x. [DOI] [PubMed] [Google Scholar]
- 8.Guth U, Huang DJ, Bauer G, et al. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer. 2007;110(6):1272–1280. doi: 10.1002/cncr.22919. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244(4):524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ercolani G, Vetrone G, Grazi GL, et al. The role of liver surgery in the treatment of non-colorectal non-neuroendocrine metastases (NCRNNE). Analysis of 134 resected patients. Minerva Chir. 2009;64(6):551–558. [PubMed] [Google Scholar]
- 11.Choi EA, Abdalla EK. Patient selection and outcome of hepatectomy for noncolorectal non-neuroendocrine liver metastases. Surg Oncol Clin N Am. 2007;16(3):557–577. ix. doi: 10.1016/j.soc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Reddy SK, Barbas AS, Marroquin CE, et al. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204(3):372–382. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Chalkiadakis GE, Lasithiotakis KG, Petrakis I, et al. Major hepatectomy and right hemicolectomy at the time of primary cytoreductive surgery for advanced ovarian cancer: report of a case. Int J Gynecol Cancer. 2005;15(6):1115–1119. doi: 10.1111/j.1525-1438.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 14.Nair A, Pai DR. Single cystic liver metastasis in residual carcinoma of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):126–127. doi: 10.1016/j.ejogrb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Bojalian MO, Machado GR, Swensen R, Reeves ME. Radiofrequency ablation of liver metastasis from ovarian adenocarcinoma: case report and literature review. Gynecol Oncol. 2004;93(2):557–560. doi: 10.1016/j.ygyno.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Chi DS, Temkin SM, Abu-Rustum NR, et al. Major hepatectomy at interval debulking for stage IV ovarian carcinoma: a case report. Gynecol Oncol. 2002;87(1):138–142. doi: 10.1006/gyno.2002.6717. [DOI] [PubMed] [Google Scholar]
- 17.Garduno-Lopez AL, Mondragon-Sanchez R, Herrera-Goepfert R, Bernal-Maldonado R. Resection of liver metastases from a virilizing steroid (lipoid) cell ovarian tumor. Hepatogastroenterology. 2002;49(45):657–659. [PubMed] [Google Scholar]
- 18.Bristow RE, Montz FJ, Lagasse LD, et al. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol. 1999;72(3):278–287. doi: 10.1006/gyno.1998.5145. [DOI] [PubMed] [Google Scholar]
- 19.Merideth MA, Cliby WA, Keeney GL, et al. Hepatic resection for metachronous metastases from ovarian carcinoma. Gynecol Oncol. 2003;89(1):16–21. doi: 10.1016/s0090-8258(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 20.Chi DS, Fong Y, Venkatraman ES, Barakat RR. Hepatic resection for metastatic gynecologic carcinomas. Gynecol Oncol. 1997;66(1):45–51. doi: 10.1006/gyno.1997.4727. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim KS, Chung CW, et al. Hepatic resection of metastatic tumor from serous cystadenocarcinoma of the ovary. J Korean Med Sci. 2002;17(3):415–418. doi: 10.3346/jkms.2002.17.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaseki H, Yasui K, Niwa K, et al. Hepatic resection for metastatic squamous cell carcinoma from the uterine cervix. Gynecol Oncol. 1992;44(3):284–287. doi: 10.1016/0090-8258(92)90059-r. [DOI] [PubMed] [Google Scholar]
- 23.La Fianza A, Alberici E, Biasina AM, et al. Spontaneous hemorrhage of a liver metastasis from squamous cell cervical carcinoma: case report and review of the literature. Tumori. 1999;85(4):290–293. doi: 10.1177/030089169908500416. [DOI] [PubMed] [Google Scholar]
- 24.Savage AP, Malt RA. Survival after hepatic resection for malignant tumours. Br J Surg. 1992;79(10):1095–1101. doi: 10.1002/bjs.1800791035. [DOI] [PubMed] [Google Scholar]
- 25.Harrison LE, Brennan MF, Newman E, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121(6):625–632. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 26.Lim MC, Kang S, Lee KS, et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009;112(1):28–34. doi: 10.1016/j.ygyno.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Yoon SS, Jarnagin WR, Fong Y, et al. Resection of recurrent ovarian or fallopian tube carcinoma involving the liver. Gynecol Oncol. 2003;91(2):383–388. doi: 10.1016/j.ygyno.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Onda T, Yoshikawa H, Yasugi T, et al. Secondary cytoreductive surgery for recurrent epithelial ovarian carcinoma: proposal for patients selection. Br J Cancer. 2005;92(6):1026–1032. doi: 10.1038/sj.bjc.6602466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10(1):31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 30.Makhija S, Howden N, Edwards R, et al. Positron emission tomography/computed tomography imaging for the detection of recurrent ovarian and fallopian tube carcinoma: a retrospective review. Gynecol Oncol. 2002;85(1):53–58. doi: 10.1006/gyno.2002.6606. [DOI] [PubMed] [Google Scholar]
- 31.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 32.Silber JH, Rosenbaum PR, Polsky D, et al. Does ovarian cancer treatment and survival differ by the specialty providing chemotherapy? J Clin Oncol. 2007;25(10):1169–1175. doi: 10.1200/JCO.2006.08.2933. [DOI] [PubMed] [Google Scholar]
- 33.Zheng QQ, Wang P, Hui R, Yao AM. Prognostic analysis of ovarian cancer patients using the Cox regression model. Ai Zheng. 2009;28(2):170–172. [PubMed] [Google Scholar]
- 34.Pawlik TM, Vauthey JN, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141(6):537–543. doi: 10.1001/archsurg.141.6.537. discussion 543–544. [DOI] [PubMed] [Google Scholar]
- 35.Pawlik TM, Gleisner AL, Bauer TW, et al. Liver-directed surgery for metastatic squamous cell carcinoma to the liver: results of a multi-center analysis. Ann Surg Oncol. 2007;14(10):2807–2816. doi: 10.1245/s10434-007-9467-8. [DOI] [PubMed] [Google Scholar]
- 36.de Jong MC, Tsai S, Cameron JL, et al. Safety and efficacy of curative intent surgery for peri-ampullary liver metastasis. J Surg Oncol. 2010;102(3):256–263. doi: 10.1002/jso.21610. [DOI] [PubMed] [Google Scholar]
- 37.Eisenkop SM, Friedman RL, Spirtos NM. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer. 2000;88(1):144–153. doi: 10.1002/(sici)1097-0142(20000101)88:1<144::aid-cncr20>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 38.Goodman HM, Harlow BL, Sheets EE, et al. The role of cytoreductive surgery in the management of stage IV epithelial ovarian carcinoma. Gynecol Oncol. 1992;46(3):367–371. doi: 10.1016/0090-8258(92)90234-a. [DOI] [PubMed] [Google Scholar]
- 39.Zang RY, Zhang ZY, Cai SM, et al. Cytoreductive surgery for stage IV epithelial ovarian cancer. J Exp Clin Cancer Res. 1999;18(4):449–454. [PubMed] [Google Scholar]
- 40.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 41.Hacker NF, Berek JS, Lagasse LD, et al. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983;61(4):413–420. [PubMed] [Google Scholar]
- 42.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47(2):159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 43.Delgado G, Oram DH, Petrilli ES. Stage III epithelial ovarian cancer: the role of maximal surgical reduction. Gynecol Oncol. 1984;18(3):293–298. doi: 10.1016/0090-8258(84)90040-4. [DOI] [PubMed] [Google Scholar]
- 44.Del Campo JM, Felip E, Rubio D, et al. Long-term survival in advanced ovarian cancer after cytoreduction and chemotherapy treatment. Gynecol Oncol. 1994;53(1):27–32. doi: 10.1006/gyno.1994.1082. [DOI] [PubMed] [Google Scholar]
- 45.Chua TC, Robertson G, Liauw W, Morris DL. Salvage cytoreduction for chemorefractory ovarian cancer with peritoneal carcinomatosis: a last chance or futile efforts? Aust N Z J Obstet Gynaecol. 2010;50(5):478–484. doi: 10.1111/j.1479-828X.2010.01201.x. [DOI] [PubMed] [Google Scholar]
- 46.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 47.Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105(1):211–217. doi: 10.1016/j.ygyno.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Chi DS, Palayekar MJ, Sonoda Y, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. 2008;108(1):191–194. doi: 10.1016/j.ygyno.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Engelen MJ, Kos HE, Willemse PH, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106(3):589–598. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- 50.Yemelyanova AV, Cosin JA, Bidus MA, et al. Pathology of stage I versus stage III ovarian carcinoma with implications for pathogenesis and screening. Int J Gynecol Cancer. 2008;18(3):465–469. doi: 10.1111/j.1525-1438.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 51.Galaal K, Kew FM, Tam KF, et al. Evaluation of prognostic factors and treatment outcomes in uterine carcinosarcoma. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):88–92. doi: 10.1016/j.ejogrb.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Wu TI, Hsu KH, Huang HJ, et al. Prognostic factors and adjuvant therapy in uterine carcinosarcoma. Eur J Gynaecol Oncol. 2008;29(5):483–488. [PubMed] [Google Scholar]
- 53.Nordal RR, Kristensen GB, Stenwig AE, et al. An evaluation of prognostic factors in uterine carcinosarcoma. Gynecol Oncol. 1997;67(3):316–321. doi: 10.1006/gyno.1997.4875. [DOI] [PubMed] [Google Scholar]
- 54.van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995;332(10):629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 55.Rose PG, Nerenstone S, Brady MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351(24):2489–2497. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 56.Longo DL. Repeat surgical debulking after three cycles of chemotherapy does not improve outcomes in advanced ovarian cancer. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. McGraw-Hill; New York: 2004. [Google Scholar]
- 57.Abood G, Bowen M, Potkul R, et al. Hepatic resection for recurrent metastatic ovarian cancer. Am J Surg. 2008;195(3):370–373. doi: 10.1016/j.amjsurg.2007.12.012. discussion 373. [DOI] [PubMed] [Google Scholar]
- 58.Bosquet JG, Merideth MA, Podratz KC, Nagorney DM. Hepatic resection for metachronous metastases from ovarian carcinoma. HPB (Oxford) 2006;8(2):93–96. doi: 10.1080/13651820500472119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamy AP, Paineau JR, Mirallie EC, et al. Hepatic resections for non-colorectal metastases: forty resections in 35 patients. Hepatogastroenterology. 2000;47(34):1090–1094. [PubMed] [Google Scholar]
- 60.Eisenkop SM, Nalick RH, Wang HJ, Teng NN. Peritoneal implant elimination during cytoreductive surgery for ovarian cancer: impact on survival. Gynecol Oncol. 1993;51(2):224–229. doi: 10.1006/gyno.1993.1277. [DOI] [PubMed] [Google Scholar]
- 61.van Dam PA, Tjalma W, Weyler J, et al. Ultraradical debulking of epithelial ovarian cancer with the ultrasonic surgical aspirator: a prospective randomized trial. Am J Obstet Gynecol. 1996;174(3):943–950. doi: 10.1016/s0002-9378(96)70331-9. [DOI] [PubMed] [Google Scholar]
- 62.van der Burg ME, Vergote I. The role of interval debulking surgery in ovarian cancer. Curr Oncol Rep. 2003;5(6):473–481. doi: 10.1007/s11912-003-0008-8. [DOI] [PubMed] [Google Scholar]
- 63.Rahusen FD, Cuesta MA, Borgstein PJ, et al. Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg. 1999;230(1):31–37. doi: 10.1097/00000658-199907000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]