Abstract
We encountered two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. Both cases occurred in fishermen who became sick after fishing. They developed multiple organ failure within 20 to 36 h from the onset of initial symptoms despite intensive chemotherapy and surgical treatments.
CASE REPORTS
Case 1.
A 58-year-old fisherman with a history of diabetes mellitus noticed mild itching on his left wrist after fishing off the east coast of Okinawa on 16 December 1998. Complaints of developing hyperemia and swelling of the hand made the patient consult a local clinic. He received medications, but the symptoms were not improved. Twelve hours later, the patient consulted another hospital for a more precise diagnosis and further treatment. On initial physical examination, the patient showed almost clear consciousness, a blood pressure of 110 over 70 mmHg, a pulse rate of 127/min, a body temperature of 37.4°C, and hyperventilation. He felt exquisite pain in his left upper limb. Patchy purple-gray discoloration of the skin of the left arm was noted. His left three fingers (i.e., middle to little fingers) became cold and hypesthesic. Initial laboratory tests showed a leukocyte count of 21,000 cells/mm3 and a platelet count of 174,000/mm3. The results of standard coagulation tests, prothrombin time, activated partial thromboplastin time, and bleeding time were within normal limits. The C-reactive protein concentration was 3.14 mg/dl. Hyperglycemia (343 mg/dl), glycosuria, and ketonuria were noted. Ultrasonography of the left hand and forearm showed a low-echoic area. Compartment syndrome due to staphylococcal infection was strongly suspected, and 1 g of cefotiam per day was administered intravenously only for 1 day. Emergency surgical exploration was performed under general anesthesia. A skin incision was made to the level of the distal biceps. No bleeding or intravenous thrombi were observed in the purple-gray skin area. Two hours after anesthesia, the patient became anuric and hyperkalemic (6.6 mEq/liter). Emergency hemodialysis was prepared, but the patient went into cardiac arrest 20 h after the initial itchy feeling in his left wrist. Tubular necrosis without intravascular thrombosis was observed in the kidneys at autopsy, and myoglobin was not detected in the urine by immunohistochemical tests.
Case 2.
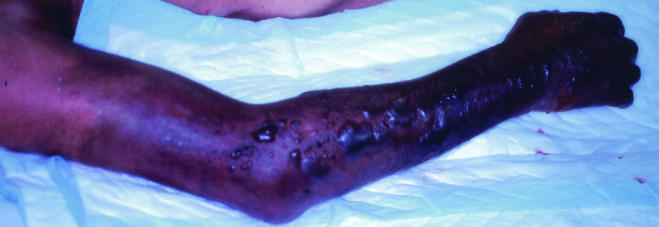
The second case was a 76-year-old fisherman with no special past medical history. He suffered a minor puncture wound to his right thumb from the fin of a sea bass on 18 September 2000 in Okayama. The wound was so slight that the patient did not seek any treatment. About 27 h later, he suffered from severe pain, swelling, and flaring of the palm, so he consulted a local clinic, where the wound was sanitized and an anti-inflammatory drug was prescribed. However, 36 h later, the severe pain and swelling persisted, and the symptoms had spread to the right shoulder. The patient was then transferred to an emergency medical center. On initial physical examination, he appeared to be sick and somnolent. The blood pressure was 96 over 60 mmHg, and the pulse rate was 136/min. His body temperature was 37.3°C, and the respirations were 18/min. The patient's right arm had turned dark red with subcutaneous hemorrhage, and bulla formation was observed (Fig. 1). Initial laboratory tests showed a leukocyte count of 3,000 cells/mm3 with 1% myelocytes, 6% metamyelocytes, 19% band form, and 55% segmented form and a platelet count of 23,000/mm3. The creatinine kinase level was 1,986 U/liter, that of myoglobin was 5,180 ng/ml, and the lactate dehydrogenase level was 4,139 U/liter. Gram-negative rods were seen by Gram staining of the bulla. At that time, necrotizing fasciitis due to Vibrio vulnificus was strongly suspected, so 4 g of ampicillin, 4 g of ceftazidime, 1,200 mg of clindamycin, and 120 mg of gentamicin per day were administered, and surgery was undertaken. Amputation of the right arm at the shoulder was accomplished after fasciotomy revealed extended necrosis of subcutaneous fat, deep fascia, and muscle extending to the shoulder. Debridement of necrotic tissue was also performed. The patient went into shock during the operation. Supportive therapy for shock was instituted postoperatively in the intensive care unit. The subsequent course was complicated by acute renal failure and disseminated intravascular coagulation. The patient subsequently died of multiorgan failure 4 days after the initial injury. No autopsy was performed.
FIG. 1.
Appearance of the right limb of case 2 upon arrival at the emergency center. Subcutaneous hemorrhage and bulla formation are evident.
Bacteriological findings.
Two strains of gram-negative rods were isolated from blood in the first case (PDA-1) and necrotic tissue of the right forearm in the second case (PDA-2). These strains were colonized with green colonies on thiosulfate-citrate-bile salts-sucrose (TCBS) agar (Eiken, Tokyo, Japan), and β-hemolysis appeared on 5% sheep blood agar (Eiken) after incubation for 24 h at 37°C. The phenotypes of these strains were analyzed biochemically by the Api20E test (BioMerieux, Marcy l'Etoile, France). The strains were positive for fermentation of glucose and maltose, the oxidase test, the urease test, and arginine dihydrolase activity, while the indole test was negative. These data accorded with the biochemical characteristics reported previously for Photobacterium damsela (8). DNA-DNA hybridization was studied by microplate methods (5) with photobiotin labeling and colorimetric detection as described previously (11). DNA samples prepared from both the PDA-1 and PDA-2 strains exhibited high hybridization levels, 97 and 100%, respectively, to the P. damsela control strain. These values were higher than the DNA similarity threshold value recommended for species delineation (70%) (15). The 16S rRNA sequences of both strains corresponded to the 16S rRNA sequence of P. damsela subsp. damsela ATCC 33539T (accession number X74700).
The MICs of various antimicrobial agents for these isolates were determined by the microbroth dilution method with 1% sodium chloride-Mueller-Hinton medium (Difco Laboratories, Detroit, Mich.) containing graded concentrations of antimicrobial agents. Both strains were susceptible to most of the antimicrobial agents used except ampicillin, amikacin, and sulfamethoxazole (Table 1).
TABLE 1.
Antibiotic susceptibility of P. damsela
| Agent | MIC (μg/ml)
|
|
|---|---|---|
| P. damsela PDA-1 | P. damsela PDA-2 | |
| Ampicillin | 64 | >64 |
| Piperacillin | 4 | 8 |
| Cefazolin | 2 | 4 |
| Cefotiam | 1 | 4 |
| Cefotaxime | 0.06 | 0.125 |
| Ceftazidime | 0.06 | 1 |
| Cefmetazol | 1 | 2 |
| Imipenem | 0.25 | 0.5 |
| Clindamycin | 2 | 4 |
| Erythromycin | 1 | 1 |
| Minocycline | 0.125 | 1 |
| Gentamicin | 4 | 4 |
| Amikacin | 32 | 32 |
| Levofloxacin | <0.06 | <0.06 |
| Sulfamethoxazole | 32 | >64 |
| Trimethoprim | 1 | 2 |
Discussion.
P. damsela was initially isolated in 1981 as a cause of skin ulcers in the temperate-water damselfish (9). This bacterium is a halophilic, facultatively anaerobic gram-negative rod. The species was earlier classified in the genus Vibrio and called Vibrio damsela and then reassigned to the genus Photobacterium, which was separated from Vibrio in 1991 on the basis of phenotypic data. Among the bacterial species belonging to the genus Vibrio, the most important and common cause of necrotizing fasciitis is Vibrio vulnificus. This species sometimes causes fatal infections among patients with an impaired immune system, such as those with liver cirrhosis or diabetes mellitus. Vibrio alginolyticus has also been reported as a microorganism causing necrotizing fasciitis after injury on a coral reef (7). Similar cases of necrotizing fasciitis caused by P. damsela have also been reported in the United States, Hong Kong, and Korea, although people without any special health problems sometimes suffer from this disease. P. damsela-associated necrotizing fasciitis in particular tended to demonstrate more serious complications and a higher mortality rate than observed in cases attributable to V. vulnificus. Thus, we describe here the first two cases of fatal necrotizing fasciitis caused by P. damsela reported in Japan.
All of the previous cases of severe P. damsela infections were reported as soft tissue infections, especially necrotizing fasciitis (1, 2, 3, 6, 10, 14, 16). These infections tend to initiate from a superior or inferior limb after a minor injury due to marine fish or contamination of a skin wound by brackish water. In the present report, the source of contagion in the first case was contamination of a minor wound by seawater, while in the second case it was a puncture injury by the fin of a sea bass. However, Shin et al. reported septicemia and necrosis of the left arm and hand in association with the ingestion of raw eels (13). This case had no obvious wounds or history of exposure to seawater. Therefore, the infectious route of the necrotizing fasciitis caused by P. damsela was presumably not only the skin wound infection but also the ingestion of raw seafood.
Only two cases of infection with P. damsela have been reported in immunocompromised hosts. The first case was a 61-year-old man suffering from diabetes mellitus and pancreatitis (2); the other case was a 63-year-old man with diabetes mellitus and alcoholic liver disease (13). Even healthy hosts can suffer from necrotizing fasciitis, and the average age of surviving patient was younger than that of dead patients. These facts suggest that immunocompromised or elderly hosts tend to easily progress to a severe state. P. damsela-associated necrotizing fasciitis tended to demonstrate more serious complications and a higher mortality rate than those associated with V. vulnificus. The typical clinical course of necrotizing fasciitis due to P. damsela was rapid progression of the infective process. The early stage of this disease is hardly distinguishable from cellulitis, with swelling and erythema. These areas often assume a dusky appearance with black patches and bulla formation, and the infection soon spreads to the surrounding normal skin. Patients develop septic shock and advance to multiorgan failure. Rapid deterioration and eventually high mortality rates are characteristic clinical features of this disease. One of the pathogenic factors identified in P. damsela is damselysin. This protein is a phospholipase D (a sphingomyelinase), an extracellular toxin with hemolytic activity, and it strongly correlated with pathogenicity in mice (4). No DNA sequence homologous to that of the cloned damselysin gene of P. damsela was found in V. vulnificus or other Vibrio species.
In antibiotic therapy for necrotizing fasciitis caused by V. vulnificus, administration of a maximum dosage of ceftazidime (or cefotaxime) and fluoroquinolone or tetracycline (or doxycycline) has been recommended (12). Indeed, P. damsela strains were susceptible to the above-mentioned agents, but the two strains isolated in this study were resistant to ampicillin, amikacin, and sulfamethoxazole. It has been deemed desirable to prescribe antimicrobial agents in the initial stage of infection in P. damsela infections, as recommended in cases of V. vulnificus infections, but medical management with antimicrobial agents alone seems insufficient, and surgical debridement should be done in P. damsela infections. This point of view is supported by the fact that many antimicrobial agents hardly penetrate the infected area in necrotic tissue in the presence of widespread intravascular thrombosis and peripheral circulation failure.
The two cases reported in this study and previously reported cases showed extremely destructive infections with necrotizing fasciitis due to P. damsela. The most prominent finding of P. damsela infection is a rapidly progressing infection with high mortality even in healthy persons with no evident history of medical problems. Early recognition of the fact of marine injury and signs of bulla formation or subcutaneous hemorrhage as well as prompt medical intervention, including chemotherapy and surgical debridement, appear to be very necessary for P. damsela infections.
Acknowledgments
This work was supported by a research fund from the Ministry of Health, Labor and Welfare, Japan.
REFERENCES
- 1.Barber, G. R., and J. S. Swygert. 2000. Necrotizing fasciitis due to Photobacterium damsela in a man lashed by a stingray. N. Engl. J. Med. 342:824. [DOI] [PubMed] [Google Scholar]
- 2.Clarridge, J. E., and S. Zighelboim-Daum. 1985. Isolation and characterization of two hemolytic phenotypes of Vibrio damsela associated with a fatal wound infection. J. Clin. Microbiol. 21:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey, J. A., Jr., R. L. Harris, M. L. Rutledge, M. W. Bradshaw, and T. W. Williams, Jr. 1986. Vibrio damsela: another potentially virulent marine vibrio. J. Infect. Dis. 153:800-802. [DOI] [PubMed] [Google Scholar]
- 4.Cutter, D. L., and A. S. Kreger. 1990. Cloning and expression of the damselysin gene from Vibrio damsela. Infect. Immun. 58:266-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezaki, T., Y. Hashimoto, and E. Yabuuchi. 1989. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol. 39:224-229. [Google Scholar]
- 6.Fraser, S. L., B. K. Purcell, B. Delgado, Jr., A. E. Baker, and A. C. Whelen. 1997. Rapidly fatal infection due to Photobacterium (Vibrio) damsela. Clin. Infect. Dis. 25:935-936. [DOI] [PubMed] [Google Scholar]
- 7.Gomez, J. M., R. Fajardo, J. F. Patino, and C. A. Arias. 2003. Necrotizing fasciitis due to Vibrio alginolyticus in an immunocompetent patient. J. Clin. Microbiol. 41:3427-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda, J. M., C. Powers, R. G. Bryant, and S. L. Abbot. 1988. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin. Microbiol. Rev. 1:245-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love, M., D. Teebken-Fisher, J. E. Hose, J. J. Farmer III, F. W. Hickman, and G. R. Fanning. 1981. Vibrio damsela, a marine bacterium, causes skin ulcers on the damselfish Chromis punctipinnis. Science 214:1139-1140. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Tirse, J., J. F. Levine, and M. Mecca. 1993. Vibrio damsela: a cause of fulminant septicemia. Arch. Intern. Med. 153:1838-1840. [DOI] [PubMed] [Google Scholar]
- 11.Satomi, M., B. Kimura, M. Mizoi, T. Satou, and T. Fujii. 1997. Tetragenococcus muriaticus sp. nov., a new moderately halophilic lactic acid bacterium isolated from fermented squid liver sauce. Int. J. Syst. Bacteriol. 47:832-836. [DOI] [PubMed] [Google Scholar]
- 12.Seal, D. V. 2001. Necrotizing fasciitis. Curr. Opin. Infect. Dis. 14:127-132. [DOI] [PubMed] [Google Scholar]
- 13.Shin, J. H., M. G. Shin, S. P. Suh, D. W. Ryang, J. S. Rew, and F. S. Nolte. 1996. Primary Vibrio damsela septicemia. Clin. Infect. Dis. 22:856-857. [DOI] [PubMed] [Google Scholar]
- 14.Tang, W. M., and J. W. K. Wong. 1999. Necrotizing fasciitis caused by Vibrio damsela. Orthopedics 22:443-444. [DOI] [PubMed] [Google Scholar]
- 15.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Truper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464.
- 16.Yuen, K. Y., L. Ma, S. S. Y. Wong, and W. F. Ng. 1993. Fatal necrotizing fasciitis due to Vibrio damsela. Scand J. Infect. Dis. 25:659-661. [DOI] [PubMed] [Google Scholar]